Abstract
Passive immunotherapy with monoclonal antibodies is an indispensable cornerstone of clinical oncology. Notably, all FDA-approved antibodies comprise the IgG class, although numerous research articles proposed monoclonal antibodies of the IgM, IgG, IgA and IgE classes directed specifically against tumor-associated antigens. In particular, for the IgE isotype class, several recent studies could demonstrate high tumoricidic efficacy. Therefore, this review specifically highlights the latest developments toward IgE-based immunotherapy of cancer. Possible mechanisms and safety aspects of IgE-mediated tumor cell death are discussed with special focus on the attracted immune cells. An outlook is given on how especially comparative oncology could contribute to further developments. Humans and dogs have a highly comparable IgE biology, suggesting that translational AllergoOncology studies in patients with canine cancer could have predictive value for the potential of IgE-based anticancer immunotherapy in human clinical oncology.
Keywords: AllergoOncology, comparative oncology, IgE, passive immunotherapy
The nascent field of AllergoOncology 1,2 aims to reveal the inverse associations between atopic and malignant diseases, which have in particular been seen in pancreatic cancer, glioma, and childhood leukemia 3–6, to harness allergic mechanisms, such as degranulation of mast cells or basophils and Fcε receptor (FcεR)-mediated immune effects for therapy of cancer.
Cancer research has aimed for decades to overcome tumor tolerance and instead engage the immune system in defense of cancer. Strategies that have been pursued cover basically the whole spectrum of the immune repertoire, such as vaccines against tumorigenic viruses 7, vaccinations with tumor cells or tumor-associated antigens (TAAs) 8, pulsing of patients’ antigen-presenting cells 9, and activating antitumor immunity via blockade of immune checkpoints 10 to passive immunotherapy with monoclonal antibodies 11. More recent experimental approaches propose to use genetically modified immune cells such as natural killer cells (NK cells) to specifically target tumor-associated antigens (TAAs) 12 or to engage cytotoxic T cells for identification and vaccination against TAA T-cell epitopes 13.
In spite of promising in vitro and in vivo data of several experimental immunotherapeutic trials and numerous immunotherapeutic approaches in the pipeline (http://www.cancer.gov/clinicaltrials), only two approaches are at the moment of practical relevance in public health: prophylactic vaccines against tumorigenic viruses and passive antibody therapy against tumor-associated antigens.
State of the art: passive immunotherapy of cancer with monoclonal antibodies
Immunotherapy using monoclonal antibodies has found its place in several treatment regimens of malignancies and is at the moment standard of care in, for example, therapy of metastatic breast cancer overexpressing HER-2 14, metastatic colon cancer overexpressing EGFR 15, or B-cell non-Hodgkin's lymphoma with autonomous growth of CD20-positive B cells 16. More recent approaches even try to modulate the immune system by attacking immune checkpoint inhibitors such as the anti-CTLA-4 (cytotoxic T-lymphocyte antigen-4) antibody ipilimumab, which displayed encouraging results in clinical studies of advanced metastatic melanoma 17–21 or the PD-1 (programmed death-1) 22 targeting antibodies nivolumab and lambrolizumab 23. In particular for lambrolizumab, safety and efficacy could be already demonstrated in patients with advanced metastatic melanoma 24.
The target molecules of the established therapies, however, represent either specific markers of malignantly transformed cells, such as CD20, CD33, or CD52 in hematologic malignancies 25, signal molecules promoting the growth of tumors, such as vascular endothelial growth factor (VEGF) 26, as well as growth factor receptors such as epidermal growth factor receptor (EGFR) 27 or human epidermal growth factor receptor-2 (HER-2) 28. An overview of current FDA-approved monoclonal antibody therapies is depicted in Table 1 (adapted from 29).
Table 1.
Overview of FDA-approved monoclonal antibody therapies (adapted from 29)
| Antibody | Conjugate | Subtype | Brand name | Target |
|---|---|---|---|---|
| Cetuximab | – | Mouse/human chimeric IgG1 | Erbitux® | EGFR |
| Panitumumab | – | Human IgG2 | Vectibix® | EGFR |
| Trastuzumab | – | Humanized IgG1 | Herceptin® | HER-2 |
| Bevacizumab | – | Humanized IgG1 | Avastin® | VEGF |
| Ipilimumab | – | Human IgG1 | Yervoy® | CTLA-4 |
| Rituximab | – | Mouse/human chimeric IgG1 | Rituxan®/MabThera® | CD20 |
| Ofatumumab | – | Human IgG1 | Arzerra® | CD20 |
| 90Y-Ibritumomab Tiuxetan | 90Yttrium | Murine IgG1 | Zevalin® | CD20 |
| 131I-Tositumomab | 131Iodine | Murine IgG2 | Bexxar® | CD20 |
| Brentuximab Vedotin | Monomethyl auristatin E | Mouse/human chimeric IgG1 | Adcetris® | CD30 |
| Gemtuzumab Ozogamicin | Ozogamicin | Humanized IgG4 | Mylotarg® | CD33 |
| Alemtuzumab | – | Humanized IgG1 | Campath® | CD52 |
Monoclonal antibodies can thus act in two ways: first by interfering via their Fab regions with binding of growth factors to receptors and thus silencing proliferation signals 30,31 and second by interacting with immune cells via their Fc domains 32, conferring active tumor cell killing by immune cells via antibody-dependent cell-mediated cytotoxicity (ADCC) 33 and antibody-dependent cell-mediated phagocytosis (ADCP) 34. Moreover, the Fc regions do mediate not only cellular responses, but also humoral immune responses like complement activation 35,36, ultimately resulting in tumor cell lysis 37,38.
Fcγ-receptor-mediated tumor cell killing
As all monoclonal antibodies currently applied in clinical oncology comprise the IgG class (39, Table 1), attracted immune cells are Fc-gamma-receptor-bearing cells, such as monocytes, macrophages, granulocytes, NK cells (CD32, CD16) 40, and dendritic or Langerhans cells 41. These cells can lead to ADCC 33 or ADCP 34 of tumor cells, furthermore to antigen-processing, transport, and presentation to T cells.
In humans, three groups of Fc gamma receptors were identified: CD64 (FcγRI), CD32 (FcγRIIa, FcγRIIb, FcγRIIc), and CD16 (FcγRIIIa, FcγRIV) 33. They can be divided into activating and inhibiting receptors, depending on the transduction of their signals via immunoreceptor tyrosine-based activation (ITAM) or immunoreceptor tyrosine-inhibitory motifs (ITIMs), respectively. In humans, only FcγRIIb acts inhibitory, whereas all others are activating receptors 42. In early studies with monoclonal antibodies directed against TAAs, different efficacy of murine IgG1 or IgG2a could be observed with respect to ADCC 43. This can be explained by the net result of binding capacities to either activating or inhibitory receptors of the two subclasses 44.
These findings are also valid in humans, but as the nomenclature of IgG subclasses differs between the murine and human IgG system, differently labeled subclasses were investigated. How functionally mouse and human IgGs correspond to each other is depicted in Table 2 45. When Bruhns et al. investigated the binding capacities of different human IgG subclasses to Fc gamma receptors, they could elucidate that IgG1 and IgG3 can bind to all Fc-gamma receptors and that the inhibitory receptor FcγRIIb has a lower affinity for IgG1, IgG2, and IgG3 than other human FcγRs (i.e. KA ∼ 2 × 105/M compared with, for example, KA ∼ 6.5 × 107/M for IgG1 to FcγRI). However, IgG4 has a relatively higher affinity toward FcγRIIb than to FcγRIIa and FcγRIIIa 46, which led to the present understanding of IgG4 being an anti-inflammatory antibody, supporting the immune system in dampening inappropriate inflammatory reactions 47,48. In particular, in allergy, IgG4 mediates allergy-blocking effects (either on the mast cell or at the antigen-presenting cell), accompanied by increased production of IL-10, induction of T-regulatory (Treg) cells 49, and a decrease in symptoms 48. Hence, in malignant disease, the same IgG4-mediated mechanism rendering IL-10 production and Treg induction could likely prompt tumors to escape immunosurveillance. As demonstrated recently, a monoclonal IgG4 directed against chondroitin sulfate proteoglycan 4 (CSPG4), a surface antigen expressed by >80% of malignant melanomas, was ineffective in triggering effector cell-mediated tumor cell killing in vitro. Moreover, when competitively applied with an IgG1 of the same specificity, this IgG4 significantly impaired the tumoricidic impact of anti-CSPG4-IgG1 in a human melanoma xenograft mouse model 50. In line with these findings is another study by Huang et al. 51: when a carcinoembryonic antigen (CEA)-specific IgG4 antibody was converted to IgG1, it significantly gained CDC and ADCC capacity against CEA-expressing tumor cells.
Table 2.
Functional correspondence between human and mouse IgG subclasses
| Human | Mouse |
|---|---|
| IgG1 | IgG2a |
| IgG2 | IgG3 |
| IgG3 | IgG2b |
| IgG4 | IgG1 |
ADCC is one of the most important killing mechanisms harnessed in passive immunotherapy of cancer, underlined by findings that mice deficient for activating receptors FcγRI and FcγRIII were unable to mount protective immune responses against a challenge with tumor cells presenting a virus-encoded tumor-specific antigen 52. In contrast, mice deficient for the inhibitory receptor FcγRIIb showed high capacity of ADCC, resulting in tumor growth arrest of subcutaneously grafted BT474 breast cancer cells. Similar effects could be observed in these knockout mice in a pulmonary metastasis model with B16 melanoma cells, where antibody treatment mediated a 100-fold reduction in pulmonary metastasis load compared with untreated animals 53. In humans, binding of IgG1 is affected by a genetic polymorphism of FcγRIIIa on position 158 in the IgG-binding domain (phenylalanine F or valine V, with significantly better binding to FcγRIIIa185V) 54. Accordingly, in a subpopulation analysis of 54 trastuzumab-treated patients with breast cancer, Musolino et al. could depict that individuals homozygous for FcγRIIIa185V/V showed significantly better objective response rates (ORR) and significantly better progression-free survival (PFS) than heterozygous FcγRIIIa185V/F or homozygous for FcγRIIIa185F/F. These findings correlated with significantly higher levels of ADCC in a cytotoxicity assay using peripheral blood mononuclear cells (PBMCs) purified from FcγRIIIa185V/V patients. For other polymorphisms of FcγRIIa (histidine H or arginine R on position 131) and FcγRIIb (isoleucine I or threonine T on position 232), no clinically significant difference could be found but only a trend toward better ORR and longer PFS for the FcγRIIa131H/H genotype 55. Similar effects could be demonstrated in 49 patients with follicular non-Hodgkin's lymphoma treated with the anti-CD20 IgG1 antibody rituximab 56. Also in a patient cohort with metastatic irinotecan-refractory colorectal cancer, treatment with cetuximab resulted in significantly better outcome rates in FcγRIIIa158V/V homozygous patients with respect to PFS; but also this study failed to display a significant difference for the FcγRIIa131 genotype 57.
Another recently discovered regulation mechanism of FcγR function is high copy number variation in their respective gene loci, which is in clear contrast with the gene loci for other Fc receptors 40. It could be shown that there is an association between gene copy number and surface expression of FcγRIIIb in neutrophils 58, resulting in enhanced uptake of and adherence to immune complexes 59. Consistently, it was also demonstrated for NK cells from individuals with two or three copies of the FCGR3A gene that also a gene dosage effect for FcγRIIIa receptor levels as well as for ADCC function exists 60. Several recent studies associated copy number variations of FCGR genes to autoimmune diseases such as systemic lupus erythematosus (SLE), low copy number of FCG3B 61,62, Sjogren's syndrome (low copy number of FCG3B) 63, rheumatoid arthritis (low copy number of FCG3B) 64, and antiglomerular basement membrane antibody disease (anti-GBM disease, high copy number of FCGR3A) 65. In particular for SLE and the deletion of FCG3B, the evidence is clear, as a meta-analysis could confirm this association 66. Clearly, such observations should be included in further studies attempting to identify genetic risk factors for autoimmunity 67. However, the effect of these copy number variations concerning tumor immunology and tumor immunotherapy has not been investigated yet and might also contribute to success or failure of IgG-based immunotherapies.
Altogether, epigenetic modification should be considered as a very important factor in all antibody strategies currently applied for immunotherapy of cancer.
Antibody optimization approaches: trials and pitfalls
Different approaches to use the documented effects of Fcγ receptor polymorphisms have been pursued therapeutically, for example, by modulation of IgG binding to Fcγ receptors via site-directed mutagenesis, mediating significantly higher rates of tumor cell lysis via ADCC 68.
Biochemical studies could reveal that variations in post-translational glycosylation of constant regions in antibodies’ heavy chains are also of high relevance for binding to different Fcγ receptors 44. So-called glycoengineering of monoclonal antibodies such as the modification of the N-glycosylation pattern at Asn 297 of the IgG heavy chain into reduced fucose content in Fc glycan seems to enhance binding to FcγRIIIa resulting in higher ADCC levels of cancer cells as well as mediating survival benefits in a CEA-overexpressing xenograft model 69. Another chimeric antibody with low fucose content in its Fc region, ublituximab, directed against CD20, had a marked antitumor effect in intracerebral and intraocular mouse models of lymphoma, resulting again in significantly increased survival rates 70.
First examples of glycoengineered antibodies even made their way into clinical testing, like the humanized anti-CD20 antibody, obinutuzumab (GA101). A recently finished phase I study in Japanese patients with relapsed or refractory B-cell non-Hodgkin's lymphoma exhibited an acceptable safety profile for obinutuzumab, with no dose-limiting toxicities observed up to doses of 2000 mg (cmax = 1910 ± 156 μg/ml), while end-of-treatment response rates were 58% 71. In a different phase I clinical trial, obinutuzumab was administered as maintenance therapy for 2 years, which was again well tolerated 72, leading to current phase III testing of this compound. Another example for a glycoengineered monoclonal antibody in clinical testing is the EGFR-targeting RG7160 (GA201), for which a dose-escalating study showed acceptable safety while exhibiting efficacy in a study cohort of 75 patients with advanced EGFR-positive solid tumors 73.
This also indicates that the expression system for anticancer antibodies is of crucial importance, not just because of efficacy but also for safety aspects. Recently, Platts-Mills et al. observed for cetuximab that it contains galactose-α-1,3-galactose (α-Gal), an immunodominant glyco-epitope derived from SP2/0 cells used as expression system, leading to a risk of anaphylaxis 74. SP2/0 cells, a murine hybridoma cell line 75, encodes, in contrast to other mammalian expression systems, the gene for α-1,3-galactosyltransferase (α-1,3GT), thereby modifying cetuximab post-translationally with α-Gal residues. Interestingly, nonprimate mammals and New World monkeys decorate glycolipids and glycoproteins with α-Gal, but not humans, apes, and Old World monkeys, as α-1,3GT became inactivated in ancestral Old World primates 76,77. As antigens of the AB0 blood group system are also oligosaccharide moieties, which are closely related to α-Gal, preformed antibodies against α-Gal exist in humans 78,79. Moreover, Platts-Mills et al. could demonstrate that a subgroup of patients with cancer already harbored IgE against α-Gal prior to cetuximab treatment. Interestingly, a series of those patients also reported episodes of anaphylaxis or severe angioedema 1–3 h after eating red meat 80. Additionally, there was a striking geographic difference in the prevalence of IgE antibodies against α-Gal with high numbers in the southeast of the USA (Tennessee, Arkansas, and North Carolina) compared with northern or western areas (Massachusetts and California) 74. In a follow-up study, the same group could identify tick bites as the cause of these phenomena. They identified a strong epidemiologic correlation with histories of tick bites and could correlate it with IgE antibodies specific for tick salivary proteins, being α-Gal decorated, which are potent immunogens 81. The expression of alpha-Gal in red meat explains the potential for associated food-related symptoms.
Overall, oncologists are more and more confronted with hypersensitivity reactions to monoclonal antibodies as well as chemotherapeutics, and pretreatments with antihistamines and cortison belong to their clinical routine. Specifically for that, precise desensitization protocols have been elaborated 82,83.
Anticancer IgM, IgA, and IgE
Other optimization approaches aim at engaging different classes of immunoglobulins than IgG. IgM antibodies, physiologically representing the first line of immune response to foreign antigens, could be one option. In particular, it was discovered that the majority of natural antibodies against cancer cells are IgMs, directed against new carbohydrates on post-translationally modified cell surface receptors of malignant cells 84,85. Although research in this field is young and recombinant IgMs to peptide epitopes not far developed yet, first results are promising. In a model of metastasizing malignant melanoma, a tumor entity with very limited treatment options, Dobroff et al. 86 could demonstrate that monoclonal IgM antibodies reactive to histone 1 can reduce the number of lung nodules in mice.
Generally therapeutic antibodies are directed against epitopes on cell surfaces; however, especially in autoimmune diseases, early studies suggested an uptake of autoantibodies into viable cells 87, leading to apoptosis 88. As many oncoproteins are located intracellular, for example, phosphatase of regenerating liver-3 (PRL-3) or the polyomavirus middle T (mT) oncoprotein, novel targeting approaches via intracellular antibodies have been evolved recently 89. In addition, combination therapies as antibody–drug conjugates (ADCs) could be highly beneficial using these antibodies as vehicles 90.
As IgM antibodies are formed upon the primary encounter with antigens, their affinity is in general low before affinity maturation occurs during an isotype switch from IgM to IgG, IgA, or IgE 45,91. For monoclonal antibodies against tumor-associated antigens, affinity values to glycan epitopes have been measured in the range of 0.5 nM/l (anti-human embryonic stem cell monoclonal antibody Hesca 2) 92 to 0.04 nM/l (anti-Sialyl-Lewisa, also known as tumor-associated antigen CA19.9) 93.
IgA, however, either in monomeric 94 or dimeric form 95, can attract a similar panel of effector cells as IgG. NK cells, granulocytes, monocytes, or macrophages express the Fc alpha receptor CD89 96, but IgA could lead to diverse effector mechanisms 94,97,98. IgA can trigger substantial amounts of ADCC via FcαRI, which could be demonstrated elaborately for immature neutrophils, mobilized from the bone marrow upon stimulation with G-CSF 99.
Not only IgA, but also IgE antibodies could be beneficial in this aspect, as IgE is able to mediate high levels of ADCC. Fu et al. 100 could demonstrate that IgE antibodies purified from patients suffering from pancreatic cancer act in vitro cytotoxic against pancreatic cancer cell lines. Additionally, IgE can engage a broad panel of effector cells in tumor defense, with a high cytotoxic and phagocytic potential upon binding to IgE receptors 101, as well as restimulate the immune system via IgE-mediated facilitated antigen uptake and consecutive presentation 102.
Fcε-receptor-mediated tumor cell killing
Fcε receptors comprise, in contrast to Fcγ receptors, only of two classes: FcεRI and FcεRII (CD23), whereupon FcεRI is also termed ‘high-affinity IgE receptor’, and CD23 is known as ‘low-affinity IgE receptor’ 103.
Additionally, galectin-3 has IgE-binding properties, but its entire function in the context of IgE remains to be determined. So far it is known that it can have pro-inflammatory functions in a mouse asthma model 104, via activating mast cells or basophils by cross-linking receptor-bound IgE 105.
However, both ‘high-affinity’ FcεRI and ‘low-affinity’ CD23 show outstanding affinity to the Fc domains of IgE. For FcεRI, the affinity is in the range of Ka∼1010/M. CD23 belongs to the C-type (calcium dependent) lectin superfamily of receptors and displays three lectin domains each having a Ka∼106–107/M to IgE, thus ranging in the average affinity of Fcγ receptors 106. The avidity of the CD23 trimer increases the affinity to a Ka∼108–109/M approaching the high affinity of FcεRI 107 and again exceeding the affinity of IgG to its high-affinity receptor FcγRI 106.
Using recombinant IgE antibodies specific for folate receptor-α on ovarian cancer cells, Karagiannis et al. 101 could demonstrate that monocytic killing of tumor cells via ADCC is FcεRI-dependent: blocking of IgE binding to FcεRI on monocytes with monoclonal antibodies or with a soluble α-chain of FcεRI 108, resulting in substantially decreased ADCC. CD23 on monocytic cells, however, which is upregulated upon incubation with IL-4 and IL-13, has the function to clear IgE–antigen complexes from the circulation, and it could be demonstrated that this mechanism can lead to IgE-mediated phagocytosis (ADCP) of tumor cells 107. In this ovarian cancer model, IgE-armed monocytes killed tumor cells via FcεRI-mediated cytotoxicity, followed by CD23-mediated phagocytosis of the remaining cell fragments 101,108.
Subsequently, side-by-side comparison studies of ADCC and ADCP of the clinically applied anti-HER-2 antibody trastuzumab (Herceptin®, IgG1) and a trastuzumab-like IgE were performed in a breast cancer model, using HER-2-overexpressing cells as targets and the monocytic cell line U937 as effector cells. In this setting, indeed ADCP was the major mechanism of trastuzumab IgG killing, whereas IgE rather triggered monocytes to ADCC of tumor cells 34. The same effect could be observed in a recent study where the clinically used antibody cetuximab (Erbitux®, again IgG1) and cetuximab-like IgE were compared in the same ADCC/ADCP assay using this time EGFR-overexpressing A431 cells as targets (Plum et al., unpublished observations). The classical cetuximab (IgG1) mediated phagocytosis, as well as cytotoxicity, concentration dependently. In contrast, cetuximab-like IgE samples caused much less phagocytosis, but significantly higher ADCC levels than those of the IgG, in a concentration-dependent manner 109.
IgE effector cells
Eosinophils
The IgE-mediated tumoricidic mechanisms of monocytic cells are also valid for eosinophilic granulocytes, being among the most classical IgE effector cells. For long, they were just known for their role in allergy or defense of helminthic parasitic infections 110. However, when human eosinophils were purified from venous blood and armed with the antifolate receptor-α-specific IgE described above, ADCC of ovarian cancer cells could be measured. In contrast to killing by monocytes, no phagocytosis of ovarian cancer cells could be determined 108. This could be due to low constitutive expression of CD23 on the surface of eosinophils 111 or lack of CD23 expression on the surface of the eosinophils used in these assays 108. Upon IgE activation, eosinophils can release cytotoxic mediators such as eosinophil cationic protein (ECP), major basic protein (MBP), eosinophil peroxidase (EPO), and eosinophil-derived neurotoxin (EDN). These proteins are well investigated in their cytotoxic action against bacteria, parasites, and viruses, but also respiratory epithelium and cancer cells 112. Synthetic eosinophil-derived neurotoxin, slightly modified by adding four extra residues, has even been studied as a therapeutic agent on its own in Kaposi's sarcoma in vitro 113,114. Moreover, eosinophilic granulocytes are able to release TNF-α 115,116, and it could be demonstrated in a recent study by Legrand et al. 117 that cytotoxic killing of colon cancer cells by eosinophils can be mediated through TNF-α and granzyme A. On the other hand, eosinophils could be ambivalent 118, as they play a role in tissue remodeling in allergic and malignant diseases via mediators such as basic fibroblast growth factor (b-FGF), IL-6, IL-8, granulocyte-macrophage colony-stimulating factor (GM-CSF), platelet-derived growth factor (PDGF), and transforming growth factor-beta (TGF-β) 112.
Eosinophilic peroxidase (EPO) is a haloperoxidase enzyme, whose catalyzed metabolites have been shown to promote oxidative stress and subsequent cell death by apoptosis or necrosis 119. However, even for this eosinophilic enzyme, it could be demonstrated that at noncytotoxic levels, it can drive cell cycle progression and proliferation by signaling via the tumor-associated receptor tyrosine kinase HER-2 120.
As eosinophils were described to be found in several cancer entities including malignancies of the head and neck region 121, uterine cervix, esophagus, or the gastro-intestinal tract 122, the term ‘tumor-associated tissue eosinophilia’ (TATE) was introduced 123. It is not yet clear what TATE means with regard to prognosis 124; studies in oral squamous cell carcinoma range from higher overall survival 121, across no significant association with respect to tumor differentiation, perineural, vascular, and muscular invasion or locoregional metastasis 125, to unfavorable prognosis for heavy eosinophilic infiltration and expression of HLA-DR antigen 126. What has been accepted so far is that blood eosinophilia (tumor-associated blood eosinophilia, TABE 123) in patients with oral squamous cell carcinoma indicates disseminated carcinoma, resulting in poor outcome 127,128.
Only further studies with recombinant antitumor IgG vs IgE antibodies will give a definite picture about the ambivalent role of eosinophils in cancer.
Mast cells
The controversy described above is even bigger for another type of IgE effector cells in and around tumors, mast cells 129. Mast cells, named and discovered by Paul Ehrlich in the 19th century 130, have been identified as eminent players in allergic and anaphylactic reactions of type I hypersensitivity 131. Upon activation via bi- or multivalent antigen–IgE complex binding, which leads to cross-linking of FcεRI, mast cells released within minutes preformed histamine, heparin, and other proteoglycans, several proteases, and cytoplasmic granule-associated cytokines 132, but also a variety of immunomodulatory mediators, such as histamine, serotonin, IL-2, IL-4, IL-21, TNF, G-CSF, and prostaglandins 133,134. Clinical symptoms of this mediator release include vasodilatation, increase in vascular permeability, contraction of bronchial smooth muscle, mucus secretion, sneezing, itching, and coughing 135. Activation via FcεRI cross-linking also induces the production of cytokines, chemokines, and growth factors, leading to a second wave of allergic symptoms, also called late-phase reactions that typically develop 2–6 h after allergen encounter and peak after 6–9 h 132,135. Chronic exposure to allergens results in constitutive activation of mast cells leading to tissue remodeling, for example, an increase in mucus-producing goblet cells in the airway epithelium, subepithelial membrane thickening through increased lung collagen deposition, neoangiogenesis, and an increased bronchial smooth muscle mass 132,136. Similar effects could be demonstrated in a model of human skin, where sonicates of mast cells significantly increased fibroblast proliferation, collagen synthesis, and collagen contraction; surrogates for skin remodeling; and fibrosis 137. These remodeling effects, especially the induction of angiogenesis and neovascularization, are detrimental in malignant diseases 138.
Besides their role in allergy, mast cells are important players in the defense of parasitic infections, such as nematodes and protozoa 139. Furthermore, mast cells contribute to an efficient immune response to bacteria, as it could be demonstrated in an in vivo model of skin infection with Pseudomonas aeruginosa, where mast cell-deficient mice showed increased lesions due to impaired neutrophil recruitment and bacterial clearance 140.
With respect to tumors, mast cells were reported early in tumor-surrounding tissues of different malignant lesions, even by Paul Ehrlich himself. He assumed that mast cells directly fulfill nutritional requirements of malignant tissues 130,141. This is definitely not the case, but the distinct role of mast cells in oncology is still a matter of debate 142.
One big research topic is how mast cell-derived proteases act on tumor progression. Aromando et al. 143 could demonstrate in a hamster cheek pouch carcinogenesis model that tumor growth was stimulated by mast cell-specific serine protease-6 (MCP-6, tryptase) through activation of protease-activated receptor-2 (PAR-2) on the surface of carcinoma cells. This finding is in line with a previous in vitro study demonstrating that mast cell tryptase stimulates the growth of DLD-1 colon adenocarcinoma cells through PAR-2 and mitogen-activated protein kinase (MAPK)-dependent manner 144. Similar tumor-promoting effects could be demonstrated when investigating mast cell-specific serine protease-4 (MCP-4, chymase), which can activate progelatinase B, thus acting as proangiogenic 145. But when de Souza et al. investigated in a recent study the expression of mast cell proteases MCP-4, MCP-5, MCP-6, MCP-7, and carboxypeptidase A, they could correlate that all proteases increased during tumor progression in a chemically induced skin tumor model, with the exception of MCP-4. Moreover, they could demonstrate that MCP-6 and MCP-7 were able to induce blood vessel formation in vitro 146. Recapitulating these studies, the function of mast cell proteases still remains not fully clear, as it has to be considered that cancerogenesis studies use different chemical compounds for tumor initiation and promotion and different sites, which could also affect the overall susceptibility of the animal to the tumor.
However, there is strong evidence for mast cell-related angiogenesis in tumor growth 138,145,146, and also, the multiple immunomodulatory effects of mast cells are intensively investigated, which will clarify the enigmatic role of mast cells in malignant disease.
Basophils
Other major players of Th2-driven immune responses as well as possible potent effector cells of IgE-based immunotherapies are basophils. Basophils share many features with mast cells, both were initially described by Paul Ehrlich, both express FcεRI, and both release histamine upon IgE binding. Whereas mast cells are located primarily in the tissue, basophils can be found in circulation, but with less than 1% of leukocytes in healthy human beings; basophils are the least abundant immune cell population 147. Apart from their contribution to allergic 148 and anaphylactic reactions 149,150, basophils play a crucial and nonredundant role in defense of endo- and ectoparasites such as helminths 151 or ticks 152.
As basophils are one of the major sources of histamine and anaphylactic mediators in the circulation during an anaphylactic shock 153, one of the major concerns of passive immunotherapy of cancer with monoclonal IgE antibodies is that intravenously applied IgE sensitizes FcεRI on basophils and could potentially be cross-linked by soluble tumor-associated antigens in the circulation, which are shed by tumors. Therefore, it is crucial to target only epitopes, which are not repetitively expressed on the target antigen and do not occur complexed in the circulation. Such antigens, which form tumor-associated molecular patterns 154 on the cell, would solely lead to degranulation in the tissue, but not in the circulation. Tumor-associated antigens that fulfill these requirements are, for example, EGFR and HER-2, for which we could demonstrate that only the dense and rigid antigen display on the surface of cancer cells leads to degranulation of IgE-loaded rat basophilic leukemia cells (RBL-SX38, transfected with human FcεRI), whereas the soluble, monomeric protein shows no effect 34,109. Rudman et al. could demonstrate in a recent study that patients with ovarian cancer displayed elevated levels of folate receptor-α not only on cancer cells but also in the circulation (up to 35 ng/ml). Still, sera of these patients could neither trigger degranulation of RBL-SX38 cells loaded with antifolate receptor-α-specific IgE, nor activate basophils of healthy donors in an ex vivo setting again preloaded with antifolate receptor-α IgE 155,156.
Although this study is very promising, future work in this direction is required, as there are many reports of circulating tumor cells in serum of patients 157–159, and so far it was not investigated how this could affect possible applications of IgE-based immunotherapies.
How to approach translation – from bench to bedside
Clearly, more studies with respect to safety and clinical efficacy are needed to clarify the advantages or complementary effects of IgE-based immunotherapy of cancer (Fig. 1). In particular, side-by-side comparison studies with ‘next-generation’ antibodies such as ‘glycoengineered’ IgG or IgA and IgE antibodies of the same specificity could assess the different potential of antibody classes with particular respect to ADCC and ADCP. There is also great demand for further in vivo studies above preclinical proof-of-concept in mice, given the fact that in vivo efficacy of TAA-specific IgE antibodies has already been demonstrated in severe combined immunodeficiency (SCID) mice with xenografted tumors 101,160,161. However, these observed effects do not fully represent the natural picture, which can be expected in cancer patients with spontaneous tumors. To overcome this experimental limitation is not trivial. In contrast to Fcγ receptors, which are similarly distributed on human and murine immune cells, the distribution patterns of Fcε receptors differ considerably. Whereas FcεRI is expressed on human mast cells, basophils, eosinophils, monocytes, Langerhans cells, and dendritic cells, in mice, it could be only found on mast cells and basophils 162. Therefore, mouse strains transgenic for human FcεRI have been generated by introducing the human α-chain of FcεRI, which displays the IgE-binding site. Functionality of the receptor could be shown on mast cells 163,164, as well as monocytes, epidermal Langerhans cells, basophilic and eosinophilic granulocytes 162, making these transgenic mice important models to study the biologic function of IgE 165,166 and models to investigate the effects and potential of passively applied IgE against grafted tumors 167.
Figure 1.
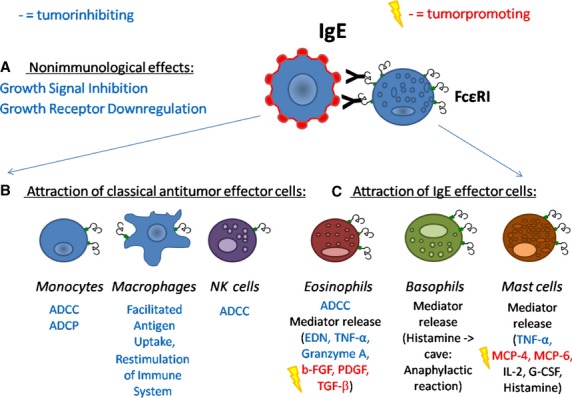
Effects of IgE-based immunotherapy of cancer. (A) Immunotherapy with IgE antibodies can lead to nonimmunologic effects such as growth signal silencing or growth receptor downregulation, due to their epitope specificity. (B) Immunologic effects comprise the attraction of classical antitumor effector cells such as monocytes, macrophages, or NK cells, leading to antibody-dependent cell-mediated cytotoxicity (ADCC) or phagocytosis (ADCP) of cancer cells. Macrophages are also employed to restimulate the immune system, due to their ability for facilitated antigen uptake via Fcε receptors. (C) Moreover, classical IgE effector cells are allured to the site of the tumor, that is, eosinophils, basophils, and mast cells. These cells lead again to ADCC of tumor cells, but release additionally specific mediators, which have been shown to act tumor-inhibiting and/or tumoricidic, such as eosinophil-derived neurotoxin (EDN), tumor necrosis factor-α (TNF-α), or granzyme A. As these cells are also involved in tissue remodeling, known tumor-promoting agents can be released as well, such as basic fibroblast growth factor (b-FGF), platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), or mast cell-specific serine proteases (MCP-4 and MCP-6).
In this context, it is important to note that the dog (Canis lupus familiaris) shares a much more similar FcεRI expression pattern with humans, with functional FcεRI expression not only on mast cells 168, but also on Langerhans cells 169. This results in similar prevalence and pathophysiology of atopic and anaphylactic reactions, underlined by the fact that the historically first described anaphylactic reaction has been observed in a dog model by Paul Jules Portier and Charles Robert Richet 130,170. In recent years, the value of the dog as a research model has been rediscovered for food allergy and atopic dermatitis 171,172. As a coincidence, dogs also spontaneously develop tumors, again of striking homology to human disease 173.
Combining both aspects – IgE pathophysiology and cancer biology – it can thus be anticipated that canine patients would be an ideal natural model, independent of tumor transplants, but developing spontaneous tumors like human patients. Dog patients suffering from cancer simultaneously offer the same IgE effector cell panel, being an ideal model for a potential AllergoOncology trial. They have the same risk of side-effects, but also potentially the same therapeutic benefits as human oncology patients. Such a study could overcome the limitations of the human FcεRI mouse as well as other rodent model organisms, for example, the rat, which although shares IgE receptor biology with humans and is therefore a valuable model in allergy research 174–176, but again would have to get tumors either grafted or artificially induced.
For dogs, however, almost 400 inherited disorders are characterized 177, many of those leading to cancer 178. Moreover, there are several hundred isolated breeds of dogs, and each has a vastly reduced genetic variation. Therefore, several breeds prone to certain malignancies, for example, Golden Retrievers for hemangiosarcoma or Irish Wolfhounds, Siberian Huskies, and Shih Tzus for T-cell lymphoma, could be easily investigated and treated 177.
As dogs live in the same environment like their owners, they share similar risk factors for cancer: age, obesity in early life, and a diet rich of red meat are all associated with higher incidence of mammary carcinoma 179. Also hormonal factors, like reproductive cycles, appear to be similar 180, resulting in expression of estrogen receptors on canine breast cancer cells 181,182. However, it long seemed that the dog is distinct in its sensitivity to a mammary tumor-promoting effect of progestins. This was due to the fact that the progestin induced growth hormone (GH) in the mammary gland 183, a mechanism that could later also be detected in human mammary tumors 184,185. But it is still not fully clear, how hormone replacement therapy including progestins changes the risk of breast cancer in women treated with hormone replacement therapy including progestins 186–188.
Furthermore, it could be demonstrated that these malignancies also share biologic properties, as canine homologues of the tumor-associated-antigens EGFR and HER-2 could be detected on canine mammary carcinoma 189–192. This perception that malignancies in companion dogs and humans occur according to very similar biologic principles has attracted attention because it offers a chance to speed up drug development for both, humans and animals, for now peaking in the establishment of the comparative oncology trial consortium (COTC) by the National Cancer Institute (NCI; http://ccr.cancer.gov/resources/cop/COTC.asp; an overview of the most recent clinical comparative trials initiated by the COTC is depicted in Table 3). In line with this concept, we could show in a recent work that the canine EGFR and HER-2 homologues are susceptible to cetuximab and trastuzumab targeting, leading to growth arrest due to growth signal inhibition 173. Combining both aspects – that dogs resemble similar biologic properties according to the development of malignancies as well as to develop atopic diseases – we suggest the ‘caninization’ of cetuximab and trastuzumab antibodies to canine IgG and IgE antibodies, respectively, in order to more accurately assess side by side the full potential of IgE-based immunotherapies against cancer and important therapy-related safety issues.
Table 3.
Overview of current comparative oncology trials initiated by the Comparative Oncology Trials Consortium of the National Cancer Institute
| Study No. | Name | Status |
|---|---|---|
| COTC001 | Evaluation of RGD Targeted Delivery of Phage Expressing TNF-alpha to Tumor Bearing Dogs | Closed trial |
| COTC003 | Evaluation of the MTOR inhibitor Rapamycin in dogs with osteosarcoma | Closed trial |
| COTC005 | Evaluation of immunocytokine fusion protein in tumor-bearing dogs | Closed trial |
| COTC006 | Evaluation Cryobiopsy Instrumentation And Cellsave Blood Collections In Dogs With Lymphoma | Closed trial |
| COTC007a | A Pilot Study of Topotecan in Dogs with Lymphoma | Closed trial |
| COTC007b | Preclinical Comparison of Three Indenoisoquinolines Candidates in Tumor Bearing Dogs | Open |
| COTC008 | Evaluation of the mTOR inhibitor Rapamycin in Dogs with Metastatic Osteosarcoma | Closed trial |
| COTC010 | Evaluation of two immunocytokine fusion proteins in tumor bearing dogs | Closed trial |
| COTC013 | Evaluation of Orally Administered mTOR inhibitor Rapamycin in Tumor Bearing Dogs | Closed trial |
| COTC016 | A Pilot Study to Assess Feasibility of Tissue Collections and Molecular Profiling for future Comparative Oncology Personalized Medicine Studies | Closed trial |
| COTC018 | Evaluation of a novel anticancer agent in tumor bearing dogs to define its pharmacokinetic profile and biological activity | Open |
Acknowledgments
This work was supported by grant P 23398-B11 of the Austrian Science Fund (FWF), by SFB F4606-B19, and JS by the CCHD PhD program, FWF project APW01205FW.
Conflicts of interest
Both authors declare that they have no conflicts of interest related to this manuscript.
References
- 1.Jensen-Jarolim E, Achatz G, Turner MC, Karagiannis S, Legrand F, Capron M, et al. AllergoOncology: the role of IgE-mediated allergy in cancer. Allergy. 2008;63:1255–1266. doi: 10.1111/j.1398-9995.2008.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen-Jarolim E, Pawelec G. The nascent field of AllergoOncology. Cancer Immunol Immunother. 2012;61:1355–1357. doi: 10.1007/s00262-012-1315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner MC, Chen Y, Krewski D, Ghadirian P, Thun MJ, Calle EE. Cancer mortality among US men and women with asthma and hay fever. Am J Epidemiol. 2005;162:212–221. doi: 10.1093/aje/kwi193. [DOI] [PubMed] [Google Scholar]
- 4.Turner MC, Chen Y, Krewski D, Ghadirian P. An overview of the association between allergy and cancer. Int J Cancer. 2006;118:3124–3132. doi: 10.1002/ijc.21752. [DOI] [PubMed] [Google Scholar]
- 5.Turner MC. Epidemiology: allergy history, IgE, and cancer. Cancer Immunol Immunother. 2012;61:1493–1510. doi: 10.1007/s00262-011-1180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlehofer B, Siegmund B, Linseisen J, Schuz J, Rohrmann S, Becker S, et al. Primary brain tumours and specific serum immunoglobulin E: a case-control study nested in the European Prospective Investigation into Cancer and Nutrition cohort. Allergy. 2011;66:1434–1441. doi: 10.1111/j.1398-9995.2011.02670.x. [DOI] [PubMed] [Google Scholar]
- 7.Plymoth A, Viviani S, Hainaut P. Control of hepatocellular carcinoma through hepatitis B vaccination in areas of high endemicity: perspectives for global liver cancer prevention. Cancer Lett. 2009;286:15–21. doi: 10.1016/j.canlet.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Bilusic M, Madan RA. Therapeutic cancer vaccines: the latest advancement in targeted therapy. Am J Ther. 2012;19:e172–e181. doi: 10.1097/MJT.0b013e3182068cdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carballido E, Fishman M. Sipuleucel-T: prototype for development of anti-tumor vaccines. Curr Oncol Rep. 2011;13:112–119. doi: 10.1007/s11912-011-0152-5. [DOI] [PubMed] [Google Scholar]
- 10.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyiadzis M, Foon KA. Approved monoclonal antibodies for cancer therapy. Expert Opin Biol Ther. 2008;8:1151–1158. doi: 10.1517/14712598.8.8.1151. [DOI] [PubMed] [Google Scholar]
- 12.Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100:1265–1273. [PubMed] [Google Scholar]
- 13.Anderson LD, Jr, Cook DR, Yamamoto TN, Berger C, Maloney DG, Riddell SR. Identification of MAGE-C1 (CT-7) epitopes for T-cell therapy of multiple myeloma. Cancer Immunol Immunother. 2011;60:985–997. doi: 10.1007/s00262-011-1009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comen EA, Fornier MN. Algorithms for the treatment of patients with metastatic breast cancer and prior exposure to taxanes and anthracyclines. Clin Breast Cancer. 2010;10(Suppl 2):S7–S19. doi: 10.3816/CBC.2010.s.008. [DOI] [PubMed] [Google Scholar]
- 15.Javle M, Hsueh CT. Recent advances in gastrointestinal oncology–updates and insights from the 2009 annual meeting of the American society of clinical oncology. J Hematol Oncol. 2010;3:11. doi: 10.1186/1756-8722-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Zhu Z. Monoclonal antibody-based therapeutics for leukemia. Expert Opin Biol Ther. 2007;7:319–330. doi: 10.1517/14712598.7.3.319. [DOI] [PubMed] [Google Scholar]
- 17.Weber JS, O'Day S, Urba W, Powderly J, Nichol G, Yellin M, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–5956. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 18.Luke JJ, Callahan MK, Postow MA, Romano E, Ramaiya N, Bluth M, et al. Clinical activity of ipilimumab for metastatic uveal melanoma: a retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer. 2013;119 doi: 10.1002/cncr.28282. 3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postow MA, Luke JJ, Bluth MJ, Ramaiya N, Panageas KS, Lawrence DP, et al. Ipilimumab for patients with advanced mucosal melanoma. Oncologist. 2013;18:726–732. doi: 10.1634/theoncologist.2012-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rughani MG, Gupta A, Middleton MR. New treatment approaches in melanoma: current research and clinical prospects. Ther Adv Med Oncol. 2013;5:73–80. doi: 10.1177/1758834012463260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer–preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37:430–439. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Robert C, Soria JC, Eggermont AM. Drug of the year: Programmed Death-1 receptor/Programmed Death-1 Ligand-1 receptor monoclonal antibodies. Eur J Cancer. 2013;49:2968–2971. doi: 10.1016/j.ejca.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris JC, Waldmann TA. Antibody-based therapy of leukaemia. Expert Rev Mol Med. 2009;11:e29. doi: 10.1017/S1462399409001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pirrotta MT, Bernardeschi P, Fiorentini G. Targeted-therapy in advanced renal cell carcinoma. Curr Med Chem. 2011;18:1651–1657. doi: 10.2174/092986711795471293. [DOI] [PubMed] [Google Scholar]
- 27.Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2006;94:184–188. doi: 10.1038/sj.bjc.6602941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 29.Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012;12:14. [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 32.Martinelli E, De Palma R, Orditura M, De Vita F, Ciardiello F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol. 2009;158:1–9. doi: 10.1111/j.1365-2249.2009.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–4399. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karagiannis P, Singer J, Hunt J, Gan SK, Rudman SM, Mechtcheriakova D, et al. Characterisation of an engineered trastuzumab IgE antibody and effector cell mechanisms targeting HER2/neu-positive tumour cells. Cancer Immunol Immunother. 2009;58:915–930. doi: 10.1007/s00262-008-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuberger MS, Rajewsky K. Activation of mouse complement by monoclonal mouse antibodies. Eur J Immunol. 1981;11:1012–1016. doi: 10.1002/eji.1830111212. [DOI] [PubMed] [Google Scholar]
- 36.Bindon CI, Hale G, Bruggemann M, Waldmann H. Human monoclonal IgG isotypes differ in complement activating function at the level of C4 as well as C1q. J Exp Med. 1988;168:127–142. doi: 10.1084/jem.168.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang SY, Weiner G. Complement and cellular cytotoxicity in antibody therapy of cancer. Expert Opin Biol Ther. 2008;8:759–768. doi: 10.1517/14712598.8.6.759. [DOI] [PubMed] [Google Scholar]
- 38.Kim MK, Breitbach CJ, Moon A, Heo J, Lee YK, Cho M, et al. Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci Transl Med. 2013;5:185ra163. doi: 10.1126/scitranslmed.3005361. [DOI] [PubMed] [Google Scholar]
- 39.Reichert JM, Valge-Archer VE. Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov. 2007;6:349–356. doi: 10.1038/nrd2241. [DOI] [PubMed] [Google Scholar]
- 40.Bournazos S, Woof JM, Hart SP, Dransfield I. Functional and clinical consequences of Fc receptor polymorphic and copy number variants. Clin Exp Immunol. 2009;157:244–254. doi: 10.1111/j.1365-2249.2009.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iannello A, Ahmad A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev. 2005;24:487–499. doi: 10.1007/s10555-005-6192-2. [DOI] [PubMed] [Google Scholar]
- 42.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Masui H, Moroyama T, Mendelsohn J. Mechanism of antitumor activity in mice for anti-epidermal growth factor receptor monoclonal antibodies with different isotypes. Cancer Res. 1986;46:5592–5598. [PubMed] [Google Scholar]
- 44.Schroeder HW, Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125(Suppl 2):S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mix E, Goertsches R, Zett UK. Immunoglobulins–basic considerations. J Neurol. 2006;253(Suppl 5):V9–V17. doi: 10.1007/s00415-006-5002-2. [DOI] [PubMed] [Google Scholar]
- 46.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 47.Aalberse RC, Schuurman J. IgG4 breaking the rules. Immunology. 2002;105:9–19. doi: 10.1046/j.0019-2805.2001.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39:469–477. doi: 10.1111/j.1365-2222.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 49.Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy. 2008;63:1455–1463. doi: 10.1111/j.1398-9995.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 50.Karagiannis P, Gilbert AE, Josephs DH, Ali N, Dodev T, Saul L, et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest. 2013;123:1457–1474. doi: 10.1172/JCI65579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang J, Shibaguchi H, Zhao J, Luo N, Kuroki M, Kinugasa T, et al. IgG isotype conversion of a novel human anti-carcinoembryonic antigen antibody to increase its biological activity. Anticancer Res. 2006;26:1057–1063. [PubMed] [Google Scholar]
- 52.Lowe DB, Shearer MH, Jumper CA, Bright RK, Kennedy RC. Fc gamma receptors play a dominant role in protective tumor immunity against a virus-encoded tumor-specific antigen in a murine model of experimental pulmonary metastases. J Virol. 2007;81:1313–1318. doi: 10.1128/JVI.01943-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 54.Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90:1109–1114. [PubMed] [Google Scholar]
- 55.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 56.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 57.Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, et al. Impact of FcγRIIa-FcγRIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27:1122–1129. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 58.Robinson JI, Carr IM, Cooper DL, Rashid LH, Martin SG, Emery P, et al. Confirmation of association of FCGR3B but not FCGR3A copy number with susceptibility to autoantibody positive rheumatoid arthritis. Hum Mutat. 2012;33:741–749. doi: 10.1002/humu.22031. [DOI] [PubMed] [Google Scholar]
- 59.Willcocks LC, Lyons PA, Clatworthy MR, Robinson JI, Yang W, Newland SA, et al. Copy number of FCGR3B, which is associated with systemic lupus erythematosus, correlates with protein expression and immune complex uptake. J Exp Med. 2008;205:1573–1582. doi: 10.1084/jem.20072413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Breunis WB, van Mirre E, Geissler J, Laddach N, Wolbink G, van der Schoot E, et al. Copy number variation at the FCGR locus includes FCGR3A, FCGR2C and FCGR3B but not FCGR2A and FCGR2B. Hum Mutat. 2009;30:E640–E650. doi: 10.1002/humu.20997. [DOI] [PubMed] [Google Scholar]
- 61.Molokhia M, Fanciulli M, Petretto E, Patrick AL, McKeigue P, Roberts AL, et al. FCGR3B copy number variation is associated with systemic lupus erythematosus risk in Afro-Caribbeans. Rheumatology (Oxford) 2011;50:1206–1210. doi: 10.1093/rheumatology/keq456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mueller M, Barros P, Witherden AS, Roberts AL, Zhang Z, Schaschl H, et al. Genomic pathology of SLE-associated copy-number variation at the FCGR2C/FCGR3B/FCGR2B locus. Am J Hum Genet. 2013;92:28–40. doi: 10.1016/j.ajhg.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nossent JC, Rischmueller M, Lester S. Low copy number of the Fc-gamma receptor 3B gene FCGR3B is a risk factor for primary Sjogren's syndrome. J Rheumatol. 2012;39:2142–2147. doi: 10.3899/jrheum.120294. [DOI] [PubMed] [Google Scholar]
- 64.Graf SW, Lester S, Nossent JC, Hill CL, Proudman SM, Lee A, et al. Low copy number of the FCGR3B gene and rheumatoid arthritis: a case-control study and meta-analysis. Arthritis Res Ther. 2012;14:R28. doi: 10.1186/ar3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou XJ, Lv JC, Bu DF, Yu L, Yang YR, Zhao J, et al. Copy number variation of FCGR3A rather than FCGR3B and FCGR2B is associated with susceptibility to anti-GBM disease. Int Immunol. 2010;22:45–51. doi: 10.1093/intimm/dxp113. [DOI] [PubMed] [Google Scholar]
- 66.McKinney C, Merriman TR. Meta-analysis confirms a role for deletion in FCGR3B in autoimmune phenotypes. Hum Mol Genet. 2012;21:2370–2376. doi: 10.1093/hmg/dds039. [DOI] [PubMed] [Google Scholar]
- 67.Olsson LM, Holmdahl R. Copy number variation in autoimmunity–importance hidden in complexity? Eur J Immunol. 2012;42:1969–1976. doi: 10.1002/eji.201242601. [DOI] [PubMed] [Google Scholar]
- 68.Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res. 2007;67:8882–8890. doi: 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- 69.Ashraf SQ, Umana P, Mossner E, Ntouroupi T, Brunker P, Schmidt C, et al. Humanised IgG1 antibody variants targeting membrane-bound carcinoembryonic antigen by antibody-dependent cellular cytotoxicity and phagocytosis. Br J Cancer. 2009;101:1758–1768. doi: 10.1038/sj.bjc.6605355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ben Abdelwahed R, Donnou S, Ouakrim H, Crozet L, Cosette J, Jacquet A, et al. Preclinical study of a glycoengineered anti-human Cd20 antibody in murine models of primary cerebral and intraocular B-cell lymphomas. Invest Ophthalmol Vis Sci. 2013;54:3657–3665. doi: 10.1167/iovs.12-10316. [DOI] [PubMed] [Google Scholar]
- 71.Ogura M, Tobinai K, Hatake K, Uchida T, Suzuki T, Kobayashi Y, et al. Phase I study of obinutuzumab (GA101) in Japanese patients with relapsed or refractory B-cell non-Hodgkin lymphoma. Cancer Sci. 2013;104:105–110. doi: 10.1111/cas.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sehn LH, Assouline SE, Stewart DA, Mangel J, Gascoyne RD, Fine G, et al. A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012;119:5118–5125. doi: 10.1182/blood-2012-02-408773. [DOI] [PubMed] [Google Scholar]
- 73.Paz-Ares LG, Gomez-Roca C, Delord JP, Cervantes A, Markman B, Corral J, et al. Phase I pharmacokinetic and pharmacodynamic dose-escalation study of RG7160 (GA201), the first glycoengineered monoclonal antibody against the epidermal growth factor receptor, in patients with advanced solid tumors. J Clin Oncol. 2011;29:3783–3790. doi: 10.1200/JCO.2011.34.8888. [DOI] [PubMed] [Google Scholar]
- 74.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shulman M, Wilde CD, Kohler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978;276:269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- 76.Galili U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 2005;83:674–686. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 77.Koike C, Uddin M, Wildman DE, Gray EA, Trucco M, Starzl TE, et al. Functionally important glycosyltransferase gain and loss during catarrhine primate emergence. Proc Natl Acad Sci USA. 2007;104:559–564. doi: 10.1073/pnas.0610012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milland J, Sandrin MS. ABO blood group and related antigens, natural antibodies and transplantation. Tissue Antigens. 2006;68:459–466. doi: 10.1111/j.1399-0039.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- 79.Galili U. Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology. 2013;140:1–11. doi: 10.1111/imm.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Commins SP, Kelly LA, Ronmark E, James HR, Pochan SL, Peters EJ, et al. Galactose-alpha-1,3-galactose-specific IgE is associated with anaphylaxis but not asthma. Am J Respir Crit Care Med. 2012;185:723–730. doi: 10.1164/rccm.201111-2017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2011;127:1286–1293. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castells M, Sancho-Serra Mdel C, Simarro M. Hypersensitivity to antineoplastic agents: mechanisms and treatment with rapid desensitization. Cancer Immunol Immunother. 2012;61:1575–1584. doi: 10.1007/s00262-012-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bluth MH. IgE and chemotherapy. Cancer Immunol Immunother. 2012;61:1585–1590. doi: 10.1007/s00262-011-1170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vollmers HP, Brandlein S. Natural antibodies and cancer. N Biotechnol. 2009;25:294–298. doi: 10.1016/j.nbt.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 85.Schwartz-Albiez R. Naturally occurring antibodies directed against carbohydrate tumor antigens. Adv Exp Med Biol. 2012;750:27–43. doi: 10.1007/978-1-4614-3461-0_3. [DOI] [PubMed] [Google Scholar]
- 86.Dobroff AS, Rodrigues EG, Juliano MA, Friaca DM, Nakayasu ES, Almeida IC, et al. Differential antitumor effects of IgG and IgM monoclonal antibodies and their synthetic complementarity-determining regions directed to new targets of B16F10-Nex2 melanoma cells. Transl Oncol. 2010;3:204–217. doi: 10.1593/tlo.09316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alarcon-Segovia D, Ruiz-Arguelles A, Fishbein E. Antibody to nuclear ribonucleoprotein penetrates live human mononuclear cells through Fc receptors. Nature. 1978;271:67–69. doi: 10.1038/271067a0. [DOI] [PubMed] [Google Scholar]
- 88.Sun KH, Tang SJ, Lin ML, Wang YS, Sun GH, Liu WT. Monoclonal antibodies against human ribosomal P proteins penetrate into living cells and cause apoptosis of Jurkat T cells in culture. Rheumatology (Oxford) 2001;40:750–756. doi: 10.1093/rheumatology/40.7.750. [DOI] [PubMed] [Google Scholar]
- 89.Guo K, Li J, Tang JP, Tan CP, Hong CW, Al-Aidaroos AQ, et al. Targeting intracellular oncoproteins with antibody therapy or vaccination. Sci Transl Med. 2011;3:99ra85. doi: 10.1126/scitranslmed.3002296. [DOI] [PubMed] [Google Scholar]
- 90.Harper J, Mao S, Strout P, Kamal A. Selecting an optimal antibody for antibody-drug conjugate therapy: internalization and intracellular localization. Methods Mol Biol. 2013;1045:41–49. doi: 10.1007/978-1-62703-541-5_3. [DOI] [PubMed] [Google Scholar]
- 91.Kracker S, Durandy A. Insights into the B cell specific process of immunoglobulin class switch recombination. Immunol Lett. 2011;138:97–103. doi: 10.1016/j.imlet.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 92.Shoreibah MG, Jackson CL, Price PW, Meagher R, Godwin AK, Cai Q, et al. Anti-human embryonic stem cell monoclonal antibody Hesca-2 binds to a glycan epitope commonly found on carcinomas. Stem Cells Dev. 2011;20:515–525. doi: 10.1089/scd.2010.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sawada R, Sun SM, Wu X, Hong F, Ragupathi G, Livingston PO, et al. Human monoclonal antibodies to sialyl-Lewis (CA19.9) with potent CDC, ADCC, and antitumor activity. Clin Cancer Res. 2011;17:1024–1032. doi: 10.1158/1078-0432.CCR-10-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dechant M, Vidarsson G, Stockmeyer B, Repp R, Glennie MJ, Gramatzki M, et al. Chimeric IgA antibodies against HLA class II effectively trigger lymphoma cell killing. Blood. 2002;100:4574–4580. doi: 10.1182/blood-2002-03-0687. [DOI] [PubMed] [Google Scholar]
- 95.Lohse S, Derer S, Beyer T, Klausz K, Peipp M, Leusen JH, et al. Recombinant dimeric IgA antibodies against the epidermal growth factor receptor mediate effective tumor cell killing. J Immunol. 2011;186:3770–3778. doi: 10.4049/jimmunol.1003082. [DOI] [PubMed] [Google Scholar]
- 96.Monteiro RC. Role of IgA and IgA fc receptors in inflammation. J Clin Immunol. 2010;30:1–9. doi: 10.1007/s10875-009-9338-0. [DOI] [PubMed] [Google Scholar]
- 97.Dechant M, Beyer T, Schneider-Merck T, Weisner W, Peipp M, van de Winkel JG, et al. Effector mechanisms of recombinant IgA antibodies against epidermal growth factor receptor. J Immunol. 2007;179:2936–2943. doi: 10.4049/jimmunol.179.5.2936. [DOI] [PubMed] [Google Scholar]
- 98.Lohse S, Peipp M, Beyer T, Valerius T, Dechant M. Impact of human IgA antibodies on complement-dependent cytotoxicity mediated by combinations of EGF-R-directed antibodies. Arch Immunol Ther Exp (Warsz) 2010;58:303–312. doi: 10.1007/s00005-010-0081-2. [DOI] [PubMed] [Google Scholar]
- 99.Otten MA, Rudolph E, Dechant M, Tuk CW, Reijmers RM, Beelen RH, et al. Immature neutrophils mediate tumor cell killing via IgA but not IgG Fc receptors. J Immunol. 2005;174:5472–5480. doi: 10.4049/jimmunol.174.9.5472. [DOI] [PubMed] [Google Scholar]
- 100.Fu SL, Pierre J, Smith-Norowitz TA, Hagler M, Bowne W, Pincus MR, et al. Immunoglobulin E antibodies from pancreatic cancer patients mediate antibody-dependent cell-mediated cytotoxicity against pancreatic cancer cells. Clin Exp Immunol. 2008;153:401–409. doi: 10.1111/j.1365-2249.2008.03726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karagiannis SN, Bracher MG, Beavil RL, Beavil AJ, Hunt J, McCloskey N, et al. Role of IgE receptors in IgE antibody-dependent cytotoxicity and phagocytosis of ovarian tumor cells by human monocytic cells. Cancer Immunol Immunother. 2008;57:247–263. doi: 10.1007/s00262-007-0371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Turcanu V, Stephens AC, Chan SM, Rance F, Lack G. IgE-mediated facilitated antigen presentation underlies higher immune responses in peanut allergy. Allergy. 2010;65:1274–1281. doi: 10.1111/j.1398-9995.2010.02367.x. [DOI] [PubMed] [Google Scholar]
- 103.Riffo-Vasquez Y, Pitchford S, Spina D. Murine models of inflammation: role of CD23. Allergy. 2000;55(Suppl 61):21–26. doi: 10.1034/j.1398-9995.2000.00503.x. [DOI] [PubMed] [Google Scholar]
- 104.Zuberi RI, Hsu DK, Kalayci O, Chen HY, Sheldon HK, Yu L, et al. Critical role for galectin-3 in airway inflammation and bronchial hyperresponsiveness in a murine model of asthma. Am J Pathol. 2004;165:2045–2053. doi: 10.1016/S0002-9440(10)63255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu FT. Regulatory roles of galectins in the immune response. Int Arch Allergy Immunol. 2005;136:385–400. doi: 10.1159/000084545. [DOI] [PubMed] [Google Scholar]
- 106.Fridman WH. Fc receptors and immunoglobulin binding factors. FASEB J. 1991;5:2684–2690. doi: 10.1096/fasebj.5.12.1916092. [DOI] [PubMed] [Google Scholar]
- 107.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 108.Karagiannis SN, Bracher MG, Hunt J, McCloskey N, Beavil RL, Beavil AJ, et al. IgE-antibody-dependent immunotherapy of solid tumors: cytotoxic and phagocytic mechanisms of eradication of ovarian cancer cells. J Immunol. 2007;179:2832–2843. doi: 10.4049/jimmunol.179.5.2832. [DOI] [PubMed] [Google Scholar]
- 109.Spillner E, Plum M, Blank S, Miehe M, Singer J, Braren I. Recombinant IgE antibody engineering to target EGFR. Cancer Immunol Immunother. 2012;61:1565–1573. doi: 10.1007/s00262-012-1287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shamri R, Xenakis JJ, Spencer LA. Eosinophils in innate immunity: an evolving story. Cell Tissue Res. 2011;343:57–83. doi: 10.1007/s00441-010-1049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lantero S, Alessandri G, Spallarossa D, Scarso L, Rossi GA. Stimulation of eosinophil IgE low-affinity receptor leads to increased adhesion molecule expression and cell migration. Eur Respir J. 2000;16:940–946. doi: 10.1183/09031936.00.16594000. [DOI] [PubMed] [Google Scholar]
- 112.Pereira MC, Oliveira DT, Kowalski LP. The role of eosinophils and eosinophil cationic protein in oral cancer: a review. Arch Oral Biol. 2011;56:353–358. doi: 10.1016/j.archoralbio.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 113.Newton DL, Rybak SM. Unique recombinant human ribonuclease and inhibition of Kaposi's sarcoma cell growth. J Natl Cancer Inst. 1998;90:1787–1791. doi: 10.1093/jnci/90.23.1787. [DOI] [PubMed] [Google Scholar]
- 114.Dricu A, Sergiu-Bogdan C, Brismar K, Biberfeld P, Andersson LC. A synthetic peptide derived from the human eosinophil-derived neurotoxin induces apoptosis in Kaposi's sarcoma cells. Anticancer Res. 2004;24:1427–1432. [PubMed] [Google Scholar]
- 115.Bheekha-Escura R, MacGlashan DW, Langdon JM, MacDonald SM. Human recombinant histamine-releasing factor activates human eosinophils and the eosinophilic cell line, AML14-3D10. Blood. 2000;96:2191–2198. [PubMed] [Google Scholar]
- 116.Melo RC, Liu L, Xenakis JJ, Spencer LA. Eosinophil-derived cytokines in health and disease: unraveling novel mechanisms of selective secretion. Allergy. 2013;68:274–284. doi: 10.1111/all.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Legrand F, Driss V, Delbeke M, Loiseau S, Hermann E, Dombrowicz D, et al. Human eosinophils exert TNF-alpha and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J Immunol. 2010;185:7443–7451. doi: 10.4049/jimmunol.1000446. [DOI] [PubMed] [Google Scholar]
- 118.Roth N, Stadler S, Lemann M, Hosli S, Simon HU, Simon D. Distinct eosinophil cytokine expression patterns in skin diseases – the possible existence of functionally different eosinophil subpopulations. Allergy. 2011;66:1477–1486. doi: 10.1111/j.1398-9995.2011.02694.x. [DOI] [PubMed] [Google Scholar]
- 119.Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol. 2009;101:81–121. doi: 10.1016/S0065-2776(08)01003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Walsh MT, Connell K, Sheahan AM, Gleich GJ, Costello RW. Eosinophil peroxidase signals via HER2 to induce cell proliferation. Am J Respir Cell Mol Biol. 2011;45:946–952. doi: 10.1165/rcmb.2010-0454OC. [DOI] [PubMed] [Google Scholar]
- 121.Dorta RG, Landman G, Kowalski LP, Lauris JR, Latorre MR, Oliveira DT. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology. 2002;41:152–157. doi: 10.1046/j.1365-2559.2002.01437.x. [DOI] [PubMed] [Google Scholar]
- 122.Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother. 2007;30:16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]
- 123.Lowe D, Jorizzo J, Hutt MS. Tumour-associated eosinophilia: a review. J Clin Pathol. 1981;34:1343–1348. doi: 10.1136/jcp.34.12.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gatault S, Legrand F, Delbeke M, Loiseau S, Capron M. Involvement of eosinophils in the anti-tumor response. Cancer Immunol Immunother. 2012;61:1527–1534. doi: 10.1007/s00262-012-1288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tadbir AA, Ashraf MJ, Sardari Y. Prognostic significance of stromal eosinophilic infiltration in oral squamous cell carcinoma. J Craniofac Surg. 2009;20:287–289. doi: 10.1097/SCS.0b013e318199219b. [DOI] [PubMed] [Google Scholar]
- 126.Horiuchi K, Mishima K, Ohsawa M, Sugimura M, Aozasa K. Prognostic factors for well-differentiated squamous cell carcinoma in the oral cavity with emphasis on immunohistochemical evaluation. J Surg Oncol. 1993;53:92–96. doi: 10.1002/jso.2930530209. [DOI] [PubMed] [Google Scholar]
- 127.Horie N, Shimoyama T, Kaneko T, Ide F. Multiple oral squamous cell carcinomas with blood and tissue eosinophilia. J Oral Maxillofac Surg. 2007;65:1648–1650. doi: 10.1016/j.joms.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 128.Martinelli-Klay CP, Mendis BR, Lombardi T. Eosinophils and oral squamous cell carcinoma: a short review. J Oncol. 2009;2009:310132. doi: 10.1155/2009/310132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Theoharides TC, Conti P. Mast cells: the Jekyll and Hyde of tumor growth. Trends Immunol. 2004;25:235–241. doi: 10.1016/j.it.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 130.Beaven MA. Our perception of the mast cell from Paul Ehrlich to now. Eur J Immunol. 2009;39:11–25. doi: 10.1002/eji.200838899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Descotes J, Choquet-Kastylevsky G. Gell and Coombs's classification: is it still valid? Toxicology. 2001;158:43–49. doi: 10.1016/s0300-483x(00)00400-5. [DOI] [PubMed] [Google Scholar]
- 132.Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immunol. 2010;40:1843–1851. doi: 10.1002/eji.201040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Walker ME, Hatfield JK, Brown MA. New insights into the role of mast cells in autoimmunity: evidence for a common mechanism of action? Biochim Biophys Acta. 2012;1822:57–65. doi: 10.1016/j.bbadis.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nechushtan H. The complexity of the complicity of mast cells in cancer. Int J Biochem Cell Biol. 2010;42:551–554. doi: 10.1016/j.biocel.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 135.Kneilling M, Rocken M. Mast cells: novel clinical perspectives from recent insights. Exp Dermatol. 2009;18:488–496. doi: 10.1111/j.1600-0625.2009.00860.x. [DOI] [PubMed] [Google Scholar]
- 136.Bara I, Ozier A, Tunon de Lara JM, Marthan R, Berger P. Pathophysiology of bronchial smooth muscle remodelling in asthma. Eur Respir J. 2010;36:1174–1184. doi: 10.1183/09031936.00019810. [DOI] [PubMed] [Google Scholar]
- 137.Garbuzenko E, Nagler A, Pickholtz D, Gillery P, Reich R, Maquart FX, et al. Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: a direct role for mast cells in skin fibrosis. Clin Exp Allergy. 2002;32:237–246. doi: 10.1046/j.1365-2222.2002.01293.x. [DOI] [PubMed] [Google Scholar]
- 138.Ribatti D, Crivellato E. Mast cells, angiogenesis, and tumour growth. Biochim Biophys Acta. 2012;1822:2–8. doi: 10.1016/j.bbadis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 139.Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol. 2007;19:31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 140.Siebenhaar F, Syska W, Weller K, Magerl M, Zuberbier T, Metz M, et al. Control of Pseudomonas aeruginosa skin infections in mice is mast cell-dependent. Am J Pathol. 2007;170:1910–1916. doi: 10.2353/ajpath.2007.060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Crivellato E, Beltrami C, Mallardi F, Ribatti D. Paul Ehrlich's doctoral thesis: a milestone in the study of mast cells. Br J Haematol. 2003;123:19–21. doi: 10.1046/j.1365-2141.2003.04573.x. [DOI] [PubMed] [Google Scholar]
- 142.Dalton DK, Noelle RJ. The roles of mast cells in anticancer immunity. Cancer Immunol Immunother. 2012;61:1511–1520. doi: 10.1007/s00262-012-1246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aromando RF, Perez MA, Heber EM, Trivillin VA, Tomasi VH, Schwint AE, et al. Potential role of mast cells in hamster cheek pouch carcinogenesis. Oral Oncol. 2008;44:1080–1087. doi: 10.1016/j.oraloncology.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 144.Yoshii M, Jikuhara A, Mori S, Iwagaki H, Takahashi HK, Nishibori M, et al. Mast cell tryptase stimulates DLD-1 carcinoma through prostaglandin- and MAP kinase-dependent manners. J Pharmacol Sci. 2005;98:450–458. doi: 10.1254/jphs.fpj05002x. [DOI] [PubMed] [Google Scholar]
- 145.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.de Souza DA, Jr, Toso VD, Campos MR, Lara VS, Oliver C, Jamur MC. Expression of mast cell proteases correlates with mast cell maturation and angiogenesis during tumor progression. PLoS ONE. 2012;7:e40790. doi: 10.1371/journal.pone.0040790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Siracusa MC, Comeau MR, Artis D. New insights into basophil biology: initiators, regulators, and effectors of type 2 inflammation. Ann N Y Acad Sci. 2011;1217:166–177. doi: 10.1111/j.1749-6632.2010.05918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy. 2011;66:1107–1113. doi: 10.1111/j.1398-9995.2011.02570.x. [DOI] [PubMed] [Google Scholar]
- 149.Voehringer D. Basophils in allergic immune responses. Curr Opin Immunol. 2011;23:789–793. doi: 10.1016/j.coi.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 150.Simons FE. Anaphylaxis pathogenesis and treatment. Allergy. 2011;66(Suppl 95):31–34. doi: 10.1111/j.1398-9995.2011.02629.x. [DOI] [PubMed] [Google Scholar]
- 151.Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, et al. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Karasuyama H, Wada T, Yoshikawa S, Obata K. Emerging roles of basophils in protective immunity against parasites. Trends Immunol. 2011;32:125–130. doi: 10.1016/j.it.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 153.Peavy RD, Metcalfe DD. Understanding the mechanisms of anaphylaxis. Curr Opin Allergy Clin Immunol. 2008;8:310–315. doi: 10.1097/ACI.0b013e3283036a90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jensen-Jarolim E, Mechtcheriakova D, Pali-Schoell I. The targets of IgE: allergen-associated and tumor-associated molecular patterns. In: Penichet ML, Jensen-Jarolim E, editors. Cancer and IgE, Introducing the Concept of AllergoOncology. Cham, Switzerland: Springer Science+Business Media; 2010. pp. 231–254. [Google Scholar]
- 155.Rudman SM, Josephs DH, Cambrook H, Karagiannis P, Gilbert AE, Dodev T, et al. Harnessing engineered antibodies of the IgE class to combat malignancy: initial assessment of FcvarepsilonRI-mediated basophil activation by a tumour-specific IgE antibody to evaluate the risk of type I hypersensitivity. Clin Exp Allergy. 2011;41:1400–1413. doi: 10.1111/j.1365-2222.2011.03770.x. [DOI] [PubMed] [Google Scholar]
- 156.Jensen-Jarolim E, Singer J. Why could passive immunoglobulin E antibody therapy be safe in clinical oncology? Clin Exp Allergy. 2011;41:1337–1340. doi: 10.1111/j.1365-2222.2011.03764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fan T, Zhao Q, Chen JJ, Chen WT, Pearl ML. Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer. Gynecol Oncol. 2009;112:185–191. doi: 10.1016/j.ygyno.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nel I, Baba HA, Ertle J, Weber F, Sitek B, Eisenacher M, et al. Individual profiling of circulating tumor cell composition and therapeutic outcome in patients with hepatocellular carcinoma. Transl Oncol. 2013;6:420–428. doi: 10.1593/tlo.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Williams SC. Circulating tumor cells. Proc Natl Acad Sci USA. 2013;110:4861. doi: 10.1073/pnas.1304186110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gould HJ, Mackay GA, Karagiannis SN, O'Toole CM, Marsh PJ, Daniel BE, et al. Comparison of IgE and IgG antibody-dependent cytotoxicity in vitro and in a SCID mouse xenograft model of ovarian carcinoma. Eur J Immunol. 1999;29:3527–3537. doi: 10.1002/(SICI)1521-4141(199911)29:11<3527::AID-IMMU3527>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 161.Kershaw MH, Darcy PK, Trapani JA, MacGregor D, Smyth MJ. Tumor-specific IgE-mediated inhibition of human colorectal carcinoma xenograft growth. Oncol Res. 1998;10:133–142. [PubMed] [Google Scholar]
- 162.Dombrowicz D, Lin S, Flamand V, Brini AT, Koller BH, Kinet JP. Allergy-associated FcRbeta is a molecular amplifier of IgE- and IgG-mediated in vivo responses. Immunity. 1998;8:517–529. doi: 10.1016/s1074-7613(00)80556-7. [DOI] [PubMed] [Google Scholar]
- 163.Fung-Leung WP, De Sousa-Hitzler J, Ishaque A, Zhou L, Pang J, Ngo K, et al. Transgenic mice expressing the human high-affinity immunoglobulin (Ig) E receptor alpha chain respond to human IgE in mast cell degranulation and in allergic reactions. J Exp Med. 1996;183:49–56. doi: 10.1084/jem.183.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dombrowicz D, Brini AT, Flamand V, Hicks E, Snouwaert JN, Kinet JP, et al. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol. 1996;157:1645–1651. [PubMed] [Google Scholar]
- 165.Dombrowicz D, Nutten S, Desreumaux P, Neut C, Torpier G, Peeters M, et al. Role of the high affinity immunoglobulin E receptor in bacterial translocation and intestinal inflammation. J Exp Med. 2001;193:25–34. doi: 10.1084/jem.193.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Platzer B, Dehlink E, Turley SJ, Fiebiger E. How to connect an IgE-driven response with CTL activity? Cancer Immunol Immunother. 2012;61:1521–1525. doi: 10.1007/s00262-011-1127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Daniels TR, Martinez-Maza O, Penichet ML. Animal models for IgE-meditated cancer immunotherapy. Cancer Immunol Immunother. 2012;61:1535–1546. doi: 10.1007/s00262-011-1169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brazis P, De Mora F, Ferrer L, Puigdemont A. IgE enhances Fc epsilon RI expression and IgE-dependent TNF-alpha release from canine skin mast cells. Vet Immunol Immunopathol. 2002;85:205–212. doi: 10.1016/s0165-2427(01)00428-7. [DOI] [PubMed] [Google Scholar]
- 169.Bonkobara M, Miyake F, Yagihara H, Yamada O, Azakami D, Washizu T, et al. Canine epidermal langerhans cells express alpha and gamma but not beta chains of high-affinity IgE receptor. Vet Res Commun. 2005;29:499–505. doi: 10.1007/s11259-005-2494-7. [DOI] [PubMed] [Google Scholar]
- 170.Portier D, Richet C. De l'action anaphylactique de certains venins. Comptes rendus de la Société de biologie. 1902;54:170–172. [Google Scholar]
- 171.Ermel RW, Kock M, Griffey SM, Reinhart GA, Frick OL. The atopic dog: a model for food allergy. Lab Anim Sci. 1997;47:40–49. [PubMed] [Google Scholar]
- 172.Helm RM, Ermel RW, Frick OL. Nonmurine animal models of food allergy. Environ Health Perspect. 2003;111:239–244. doi: 10.1289/ehp.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Singer J, Weichselbaumer M, Stockner T, Mechtcheriakova D, Sobanov Y, Bajna E, et al. Comparative oncology: ErbB-1 and ErbB-2 homologues in canine cancer are susceptible to cetuximab and trastuzumab targeting. Mol Immunol. 2012;50:200–209. doi: 10.1016/j.molimm.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kucharewicz I, Bodzenta-Lukaszyk A, Buczko W. Experimental asthma in rats. Pharmacol Rep. 2008;60:783–788. [PubMed] [Google Scholar]
- 175.Pauluhn J, Mohr U. Experimental approaches to evaluate respiratory allergy in animal models. Exp Toxicol Pathol. 2005;56:203–234. doi: 10.1016/j.etp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 176.Rashid A, Sadroddiny E, Ye HT, Vratimos A, Sabban S, Carey E, et al. Review: diagnostic and therapeutic applications of rat basophilic leukemia cells. Mol Immunol. 2012;52:224–228. doi: 10.1016/j.molimm.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 177.Rowell JL, McCarthy DO, Alvarez CE. Dog models of naturally occurring cancer. Trends Mol Med. 2011;17:380–388. doi: 10.1016/j.molmed.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Casal M, Haskins M. Large animal models and gene therapy. Eur J Hum Genet. 2006;14:266–272. doi: 10.1038/sj.ejhg.5201535. [DOI] [PubMed] [Google Scholar]
- 179.Perez Alenza MD, Pena L, del Castillo N, Nieto AI. Factors influencing the incidence and prognosis of canine mammary tumours. J Small Anim Pract. 2000;41:287–291. doi: 10.1111/j.1748-5827.2000.tb03203.x. [DOI] [PubMed] [Google Scholar]
- 180.Concannon PW. Reproductive cycles of the domestic bitch. Anim Reprod Sci. 2011;124:200–210. doi: 10.1016/j.anireprosci.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 181.Yang WY, Liu CH, Chang CJ, Lee CC, Chang KJ, Lin CT. Proliferative activity, apoptosis and expression of oestrogen receptor and Bcl-2 oncoprotein in canine mammary gland tumours. J Comp Pathol. 2006;134:70–79. doi: 10.1016/j.jcpa.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 182.Kumaraguruparan R, Prathiba D, Nagini S. Of humans and canines: immunohistochemical analysis of PCNA, Bcl-2, p53, cytokeratin and ER in mammary tumours. Res Vet Sci. 2006;81:218–224. doi: 10.1016/j.rvsc.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 183.Rutteman GR. Contraceptive steroids and the mammary gland: is there a hazard?–Insights from animal studies. Breast Cancer Res Treat. 1992;23:29–41. doi: 10.1007/BF01831473. [DOI] [PubMed] [Google Scholar]
- 184.van Garderen E, Schalken JA. Morphogenic and tumorigenic potentials of the mammary growth hormone/growth hormone receptor system. Mol Cell Endocrinol. 2002;197:153–165. doi: 10.1016/s0303-7207(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 185.Mol JA, van Garderen E, Rutteman GR, Rijnberk A. New insights in the molecular mechanism of progestin-induced proliferation of mammary epithelium: induction of the local biosynthesis of growth hormone (GH) in the mammary glands of dogs, cats and humans. J Steroid Biochem Mol Biol. 1996;57:67–71. doi: 10.1016/0960-0760(95)00251-0. [DOI] [PubMed] [Google Scholar]
- 186.Chlebowski RT, Anderson GL. Changing concepts: menopausal hormone therapy and breast cancer. J Natl Cancer Inst. 2012;104:517–527. doi: 10.1093/jnci/djs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chlebowski RT, Manson JE, Anderson GL, Cauley JA, Aragaki AK, Stefanick ML, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women's Health Initiative Observational Study. J Natl Cancer Inst. 2013;105:526–535. doi: 10.1093/jnci/djt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Schairer C, Brinton LA. The effect of estrogen plus progestin hormone therapy on breast cancer mortality: still unresolved. J Natl Cancer Inst. 2013;105:513–514. doi: 10.1093/jnci/djt058. [DOI] [PubMed] [Google Scholar]
- 189.Gama A, Gartner F, Alves A, Schmitt F. Immunohistochemical expression of Epidermal Growth Factor Receptor (EGFR) in canine mammary tissues. Res Vet Sci. 2009;87:432–437. doi: 10.1016/j.rvsc.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 190.Ahern TE, Bird RC, Bird AE, Wolfe LG. Expression of the oncogene c-erbB-2 in canine mammary cancers and tumor-derived cell lines. Am J Vet Res. 1996;57:693–696. [PubMed] [Google Scholar]
- 191.Dutra AP, Granja NV, Schmitt FC, Cassali GD. c-erbB-2 expression and nuclear pleomorphism in canine mammary tumors. Braz J Med Biol Res. 2004;37:1673–1681. doi: 10.1590/s0100-879x2004001100013. [DOI] [PubMed] [Google Scholar]
- 192.Peruzzi D, Mesiti G, Ciliberto G, La Monica N, Aurisicchio L. Telomerase and HER-2/neu as targets of genetic cancer vaccines in dogs. Vaccine. 2010;28:1201–1208. doi: 10.1016/j.vaccine.2009.11.031. [DOI] [PubMed] [Google Scholar]


