Abstract
Familial idiopathic basal ganglia calcification (IBGC) or Fahr’s disease is a rare neurodegenerative disorder characterized by calcium deposits in the basal ganglia and other brain regions, which is associated with neuropsychiatric and motor symptoms. Familial IBGC is genetically heterogeneous and typically transmitted in an autosomal dominant fashion. We performed a mutational analysis of SLC20A2, the first gene found to cause IBGC, to assess its genetic contribution to familial IBGC. We recruited 218 subjects from 29 IBGC-affected families of varied ancestry and collected medical history, neurological exam, and head CT scans to characterize each patient’s disease status. We screened our patient cohort for mutations in SLC20A2. Twelve novel (nonsense, deletions, missense, and splice site) potentially pathogenic variants, one synonymous variant, and one previously reported mutation were identified in 13 families. Variants predicted to be deleterious cosegregated with disease in five families. Three families showed nonsegregation with clinical disease of such variants, but retrospective review of clinical and neuroimaging data strongly suggested previous misclassification. Overall, mutations in SLC20A2 account for as many as 41 % of our familial IBGC cases. Our screen in a large series expands the catalog of SLC20A2 mutations identified to date and demonstrates that mutations in SLC20A2 are a major cause of familial IBGC. Non-perfect segregation patterns of predicted deleterious variants highlight the challenges of phenotypic assessment in this condition with highly variable clinical presentation.
Keywords: Basal ganglia calcification, Fahr’s, Genetics, Sequencing, Mutations
Introduction
Familial idiopathic basal ganglia calcification (IBGC) or Fahr’s disease is an enigmatic neurodegenerative disorder characterized by calcium deposits in the basal ganglia and other brain regions in the absence of metabolic abnormalities or other causes of secondary calcification, such as infectious disease. Approximately 0.5–1.0 % of CT scans in patients over age 50 exhibit incidental sporadic calcification of the basal ganglia. In contrast, familial IBGC is typically transmitted in an autosomal dominant fashion and is genetically heterogeneous. More than 30 families with Mendelian forms of IBGC have been reported in the literature. However, its true prevalence remains unknown [1]. Clinical features include a variable combination of neuropsychiatric and motor symptoms including dystonia, parkinsonism, ataxia, psychosis, dementia, chorea, and frontal–subcortical cognitive dysfunction [2, 3]. The variability in clinical presentation and penetrance, as well as the presence of phenocopies and relatively high prevalence of other causes of secondary calcifications, have been significant confounding factors in elucidating the genetic basis of familial IBGC [4–6]. Efforts to ascertain a genetic location responsible for IBGC have resulted in the identification of three genetic loci through linkage analysis: IBGC1 on chromosome 14 (14q13), IBGC2 on chromosome 2 (2q37), and IBGC3 on chromosome 8 (8p21.1–8q11.23) [7–9]. Recently, Wang et al. reported the first causative gene linked to IBGC by identifying seven IBGC families with mutations in SLC20A2, a gene located in the IBGC3 region that encodes for type III sodium-dependent phosphate transporter 2 (PiT2) [10].
Here, we present a mutational analysis of SLC20A2 in 218 patients from 29 IBGC families of varied ancestry to further examine the genetic contribution of SLC20A2 mutations in a large cohort of IBGC families. We identified 12 novel sequence variants predicted to be deleterious, one rare synonymous variant, and one previously known mutation in 13 of these families. Our findings show that mutations in SLC20A2 are a major cause of familial IBGC and expand the catalog of SLC20A2 mutations identified to date.
Patients and methods
Patient recruitment and assessment
We identified 218 individuals belonging to 29 IBGC-affected families collected through the UCLA Medical Center and from a number of collaborating institutions. Some of these families were included in previous clinical or genetic studies (Table 1). Informed consent was obtained, and the investigation was approved by the UCLA Institutional Review Board. Medical history and neurological examinations were performed in all probands and additional family members for most families. Serum calcium and parathormone levels were assayed in at least one proband from most families to exclude calcium dysregulation and other metabolic disorders that would cause brain calcifications unrelated to familial IBGC.
Table 1.
Main clinical and neuroimaging features of IBGC families
| IBGC family | Geographic origin/descent | N. of available members | Affected | Unaffected | Unknown | Clinical symptoms | Diagnostic workup | Calcifications | Reference |
|---|---|---|---|---|---|---|---|---|---|
| F1 | North American | 31 | 11 | 7 | 13 | Parkinsonism, focal dystonia, tremor, and dysphagia | Serum calcium and PTH | +++ (BBG, globus pallidus, thalamus, white matter) | [7] |
| F2 | European | 30 | 10 | 6 | 14 | 8 individuals normal neurological, cognitive, psychiatric features; 2 individuals dementia, parkinsonism, bipolar I | Dense calcification in at least one area | [3] | |
| F3 | 30 | 7 | 5 | 18 | Parkinsonism, dizziness, slurred speech, and balance disorder | Serum calcium and PTH | NP | ||
| F4 | North American/Swedish | 17 | 10 | 3 | 4 | Dementia, chorea, slurred speech, palilalia, gait disturbance, 5 asymptomatic | Serum calcium and PTH | +++ (BBG, white matter, cerebellum) | [15] |
| F5 | North American/Irish | 16 | 10 | 2 | 4 | Parkinsonism and dystonia, 3 asymptomatic | Serum calcium and PTH | +++ (BBG, thalamus, white matter) | [15] |
| F6 | Serbian | 14 | 6 | 8 | 0 | Parkinsonism, severe gait disturbances with freezing of gait, and dyskinesia; 2 asymptomatic | [16] | ||
| F7 | 12 | 6 | 0 | 6 | Cramps and headaches | Serum calcium and PTH | NP | ||
| F8 | German | 10 | 6 | 1 | 3 | Dizziness, epilepsy, headaches | Serum calcium and PTH | +++ (BBG) | [15] |
| F9 | North American/Chinese | 9 | 6 | 0 | 3 | Dizziness, dementia, muscle spasms, and cramps; 2 asymptomatic | Serum calcium and PTH | +++ (BBG, cerebellum) | |
| F10 | 6 | 3 | 0 | 3 | Serum calcium and PTH | NP | |||
| F11 | German/Irish | 5 | 2 | 0 | 3 | Dizziness and syncope, speech delay, cognitive delay, tremor, global developmental delay, family member with bipolar disorder | Serum calcium and PTH | +++ (BBG, thalamus, white matter) | NP |
| F12 | Spanish | 5 | 2 | 2 | 1 | No cognitive or movement disorder, neurosensorial hearing loss, myopia, astigmatism, migraine headache, scoliosis, pescavus | +++ (BBG, thalamus, cerebellum, brainstem, cortico-subcortical) | ||
| F13 | Finnish/Swedish | 4 | 4 | 0 | 0 | Migraine, speech difficulties, essential tremor, and neuropsychiatric symptoms | Serum calcium and PTH | +++ (BBG, thalamus, white matter, and cerebellum) | NP |
| F14 | 4 | 3 | 0 | 1 | Dysarthria, micrographia, balance disorder | Serum calcium and PTH | NP | ||
| F15 | 3 | 2 | 0 | 1 | Depression and muscle spasms | Serum calcium and PTH | NP | ||
| F16 | Portuguese | 3 | 3 | 0 | 0 | Migraine, vertigo, anxiety, depression, personality and behavioral problems, intellectual and language delay | ++ (BBG, dentate nuclei calcification by fifth decade), + (earlier decades) | NP | |
| F17 | Thai | 3 | 2 | 0 | 1 | Parkinsonism, dystonia, ataxia, cognitive impairment, psychosis, paraparesis | Serum calcium and PTH | +++ (BBG, subcortical) | NP |
| F18 | Libyan | 2 | 1 | 0 | 1 | Serum calcium and PTH | NP | ||
| F19 | 2 | 1 | 1 | 0 | Clawed hand and slurred speech, dopa-responsive parkinsonism | Serum calcium and PTH | +++ (BBG, cerebellum, subcortical white matter, bilateral temporal lobes) | NP | |
| F20 | English/Irish, German | 2 | 2 | 0 | 0 | Fainting episodes, complaint of headache, no cognitive impairment | Serum calcium and PTH | NP | |
| F21 | Spanish | 2 | 2 | 0 | 0 | Dopa-responsive parkinsonism, dysarthria, subcortical cognitive impairment, stroke | +++ (BBG, cerebellum) | NP | |
| F22 | 1 | 1 | 0 | 0 | Dystonia, cramps, depression, insomnia, headaches, and muscle spasms | Serum calcium and PTH | +++ (BBG, dentate nucleus) | NP | |
| F23 | Ashkenazi Jewish/Russian | 1 | 1 | 0 | 0 | Asymptomatic (1), tremor, psychosis, behavioral problems (1), parkinsonism (1) | Serum calcium and PTH | NP | |
| F24 | French | 1 | 1 | 0 | 0 | Severe migraine | Serum calcium and PTH | ++ (white matter, thalamus) | NP |
| F25 | (Asian) Indian | 1 | 1 | 0 | 0 | Headaches, movement disorders | Serum calcium and PTH | NP | |
| F26 | Scottish | 1 | 1 | 0 | 0 | Serum calcium and PTH | NP | ||
| F27 | Spanish | 1 | 1 | 0 | 0 | Dementia, psychiatric disorder, parkinsonism, facial palsy, leukemia | +++ (BBG, cerebellum) | NP | |
| F28 | Spanish | 1 | 1 | 0 | 0 | Mild cognitive impairment, ataxia | +++ (BBG, subcortical, cerebellum) | NP | |
| F29 | European (Irish, English, Hungarian) | 1 | 1 | 0 | 0 | Segmental dystonia (cervical and torso); mild frontal and subcortical findings on neuropsychological testing (slowed processing speed, difficulty with learning and retrieval); family history of dementia and multiple sclerosis | Serum calcium and PTH | +++ (BBGC, dentate) | NP |
BBG bilateral basal ganglia, NP unpublished, PTH parathormone
Neuroimaging
Head CT scans were performed as part of the diagnostic workup or reviewed for the presence of calcifications or other brain abnormalities. Subjects with CT scans positive for calcification were given an affected disease status, while CT-negative patients >50 years who remained asymptomatic until their death were assigned an unaffected disease status. Subjects whose CT scans were negative but were under the age of 50, or whose CT scan results were not available, were classified as unknown.
Molecular genetics and analytical methods
Blood samples were obtained from the participants, and genomic DNA was extracted using standard methods. Using published primer pairs [10], we amplified all of the exonic and flanking intronic regions of SLC20A2 by PCR in two CT-positive affected patients from each family. The PCR solution and touchdown PCR cycling conditions were prepared and optimized using standard procedures. The final purified amplicons were sequenced in both forward and reverse directions by Sanger sequencing on the ABI 3730 platform (Applied Biosystems) to produce chromatogram traces that were analyzed using the CodonCode software (CodonCode Corporation). When variants were identified, all available family members in each family were screened using variant-specific primer pairs following the protocol described above. Online databases of human genetic variation were used to assess the novelty of the variants identified: the National Heart, Lung, and Blood Institute (NHLBI) Exome Variant Server (http://evs.gs.washington.edu/EVS/, accessed July 2012), dbSNP135 as reported in the UCSC Genome Browser (http://genome.ucsc.edu/), and the 1000 Genomes Project (http://www.1000genomes.org, 20100804 release 12 May 2012). The pathogenic potential of the identified variants was predicted using Sorting Intolerant from Tolerant (SIFT; http://sift.bii.a-star.edu.sg/), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), and the Human Splicing Finder software (http://www.umd.be/HSF/HSF.html, May 2009 release) [11–13].
Role of the funding source
The sponsors had no role in the study design, data collection, analysis, or interpretation.
Results
A total of 218 subjects from 29 families of various ancestries were included in the study. Major clinical features and CT findings of our IBGC family cohort are summarized in Table 1. At least one affected subject from each family exhibited movement, psychiatric, and/or cognitive symptoms typical of familial IBGC. In five families, several asymptomatic individuals were also classified as affected because of significant bilateral basal ganglia calcifications identified on CT scans. Sequence analysis of the 29 probands identified 1 previously published mutation, 1 rare synonymous variant, and 12 novel variants in SLC20A2 in 13 families, including three nonsense variants, three deletions, three splice site variants, and three missense variants (Figs. 1 and 2, Table 2). None of the 12 novel variants had been reported in dbSNP135, the 1000 Genomes database, the NHLBI Exome Sequencing Project, or in the previous study from Wang et al. [10]. We did not identify SLC20A2 variants or mutations in 16 of the 29 IBGC families screened. There was no clear correlation between age of symptom onset, severity of disease symptoms, or any particular clinical phenotype in IBGC families with SLC20A2 mutations compared to IBGC families without SLC20A2 mutations.
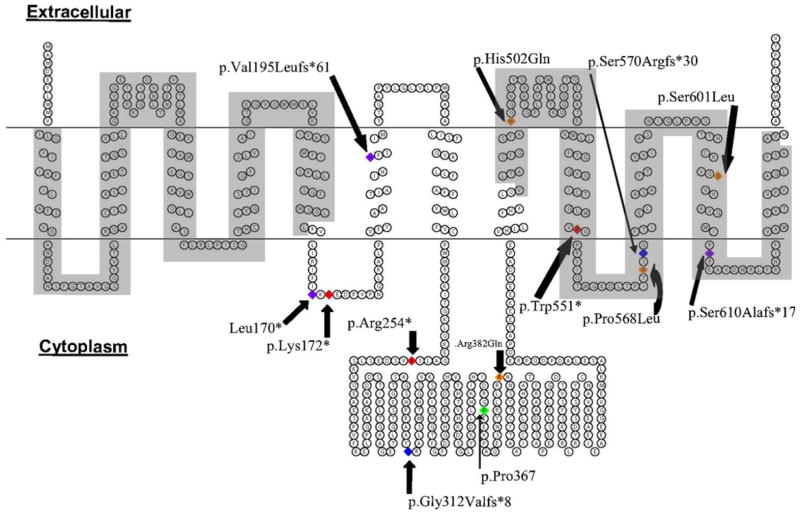
Fig. 1.
Structure model of PiT2 protein with the variant locations. Red residues denote nonsense variants, orange residues denote missense variants, blue residues denote splice site variants, purple residues denote insertions/deletions, and green residues denote synonymous variants. ProDom domains (I11–L161 and V492–V640) are highlighted in gray
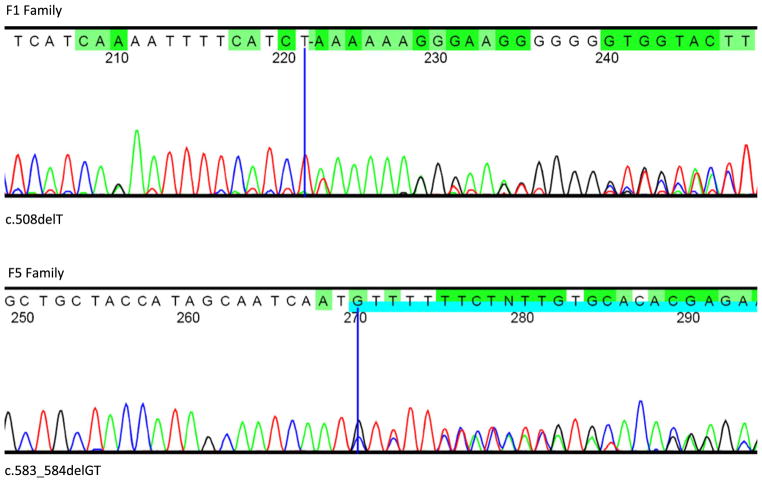
Fig. 2.
DNA sequence chromatograms of the SLC20A2 variants identified in IBGC-affected families. Representative partial sequence chromatograms for family F1 (mutation: c.508delT) and F5 (p.Val195Leufs*61). The dark blue cursor denotes the position of the indicated mutation and the subsequent frameshift is shown with the blue and green highlighted bases
Table 2.
SLC20A2 variants and mutations identified in 13 IBGC families
| N. | cDNAa | Amino acidb | Location | IBGC family | Mutation type | Polyphen-2 prediction | SIFT prediction | Human Splicing Finder |
|---|---|---|---|---|---|---|---|---|
| 1 | c.508delT | p.Leu170* | Exon 4 | F1 | Nonsense | Not available | Damaging subject to nonsense decay | |
| 2 | c.514A>T | p.Lys172* | Exon 4 | F22 | Nonsense | Not available | Damaging | |
| 3 | c.583 584delGT | p.Val195Leufs*61 | Exon 5 | F5 | Frameshift | Not available | Damaging and subject to nonsense-mediated decay | |
| 4 | c.760C>T | p.Arg254* | Exon 7 | F15 | Nonsense | Not available | Damaging due to stop | |
| 5c | c.1101C>G | p.Pro367Pro | Exon 8 | F18 | Synonymous | Not available | Not available | |
| 6 | c.1145G>A | p.Arg382Gln | Exon 8 | F7 | Missense | Probably damaging | Tolerated | |
| 7 | c.1506C>A | p.His502Gln | Exon 8 | F24 | Missense | Probably damaging | Damaging | |
| 8 | c.1523+1G>A | p.Gly312Valfs*8 | IVS 8 | F7 | Splice site | Natural 5′ donor site | ||
| 9 | c.1652G>A | p.Trp551* | Exon 9 | F20 | Nonsense | Not available | Damaging | |
| 10 | c.1703C>T | p.Pro568Leu | Exon 9 | F19 | Missense | Probably damaging | Damaging | |
| 11 | c.1794+1G>A | p.Ser570Argfs*30 | IVS 10 | F9 | Splice site | Natural 5′ donor site | ||
| 12 | c.1794+1G>C | p.Ser570Argfs*30 | IVS 10 | F29 | Splice site | Natural 5′ donor site | ||
| 13d | c.1802C>T | p.Ser601Leu | Exon 11 | F23 | Missense | Probably damaging | Damaging | |
| 14 | c.1828 1831delTCC | p.Ser610Alafs*17 | Exon 11 | F2 | Frameshift | Not available | Damaging (nonsense-mediated decay not predicted) |
Numbering according to GenBank reference build NM_006749.3 starting at the translation initiation codon
Numbering according to GenPept reference build NP_006740.1
Rare variant with allele frequency of 0·0009 in the 1000 Genomes Project database
Previously reported by Wang et al. [10]
To further explore pathogenicity, we studied the 12 novel variants for segregation and predicted deleteriousness. Three nonsense variants were discovered: (1) c.514A>T (family F22), introducing a stop codon in exon 4 (p.Lys172*); (2) c.760C>T (family F15), introducing a stop codon in exon 7 (p.Arg254*); and (3) c.1652G>A (family F20) introducing a stop codon in exon 9 (p.Trp551*). Three deletions altering the protein reading frame were identified: (1) c.508delT (family F1) leading to a premature stop codon in exon 4 (p.Leu170*); (2) c.583 584delGT (family F5), predicted to substitute a leucine for a valine followed by a frame shift terminating after 61 aberrant amino acids (p.Val195Leufs*61); and (3) c.1828 1831delTCCC (family F2) which substitutes an alanine for a serine followed by a frameshift and premature termination after 17 amino acids (p.Ser610Alafs*17). Three of the identified variants were located at natural 5′ donor splice sites: (1) c.1523+1G>A (family F7) was located one base pair immediately flanking the 3′ end of exon 8; (2) c.1794+1G>A (family F9); and (3) c.1794+1G>C (family F29), were both located at the same position, one base pair immediately flanking the 3′ end of exon 10. The substitution of a guanine for an adenine or cytosine one nucleotide adjacent to the exon changes the highly critical GU dinucleotide essential for splicing and would most likely result in skipping of the affected exon equating to a large deletion in the final protein product. For the F7 family, the splice site variant would likely result in the loss of exon 8, the largest exon in SLC20A2, while in the F9 and F29 families, the splice site variant would likely result in the loss of exon 10. In both cases, exon exclusion is predicted to introduce an early stop codon (p.Gly312-Valfs*8 and p.Ser570Argfs*30). In family F7, the splicing variant (c.1523+1G>A) was in linkage disequilibrium with a missense variant in the coding region of exon 8 (c.1145G>A) substituting an arginine for a glutamine (p.Arg382Gln) predicted as probably damaging by Polyphen-2 and tolerated by SIFT (Table 2). Two additional novel missense variants were identified: (1) c.1506C>A (family F24) substituting a glutamine for a histidine at residue 502 (p.His502Gln), predicted to be a critical region for the transport function [14], and (2) c.1703C>T (family F19) causing the change of a leucine to a proline at codon 568 (p.Pro568Leu). We also identified a previously known single base pair mutation (c.1802C>T) resulting in the amino acid substitution of a leucine for a serine at residue 601 (p.Ser601Leu) in family F23 [10]. Currently, this is the only SLC20A2 mutation that has been reported in more than one IBGC-affected family. It is not clear at this point if this mutation arose independently in both families or if the families share a common founder ancestor.
Finally, we identified a rare synonymous sequence variant with an allele frequency of 0.0009 in the 1000 Genomes Project database (c.1101C>G, in the F18 family, p.Pro367Pro) of unknown pathogenic significance. The pathogenic potential of the nonsynonymous coding variants was analyzed using both PolyPhen-2 and SIFT, all were predicted to be damaging to the protein product in at least one out of the two prediction software packages, and several variants were predicted to be damaging by both (Table 2). In particular, the variants p.Leu170* and p.Val195Leufs*61 were predicted to induce nonsense-mediated decay, a surveillance mechanism that would result in a degradation of the aberrant RNA product analogous to a complete deletion of one copy of the SLC20A2 gene [11]. Cosegregation analysis was performed in the families for which DNA was available for more than one affected subject (8 of 13). Five families (F7, F19, F9, F15, and F20) demonstrated perfect segregation with disease status (Table 3). In contrast, two putatively affected subjects in F1, two affected subjects in F5, and one affected subject in the F2 family did not carry the SLC20A2 variant found in all other respective affected family members suggesting that they were phenocopies. Review of CT scans for family F1 (Fig. 3) revealed a strong contrast between the subjects who tested positive for mutations, who presented with clearly abundant and symmetrical calcification typical of IBGC-affected individuals, and those mutation negative, who presented only minimal calcifications, consistent with a phenocopy. For the remaining five families, only one affected subject was available in four families (F22, F23, F24, and F29), and we were not able to ascertain a segregation pattern, whereas in the family with the synonymous change (F18), one affected and one subject of unknown status shared the mutation. Excluding the three families with non-perfect segregation, 5 out of 23 families (22 %) where a segregation analysis was possible have segregating deleterious mutations in SLC20A2. Overall, considering the likelihood of phenocopies in families F2 and F5 and the predicted pathogenicity of the other variants, SLC20A2 variants and mutations may account for as many as 41 % (12 out of 29) of IBGC-affected families in our patient population.
Table 3.
Cosegregation analysis of variants in SLC20A2
| Family members with variant | Family members without variant | ||||||
|---|---|---|---|---|---|---|---|
| IBGC Family | Proband Variant | Affected | Unaffected | Unknown | Affected | Unaffected | Unknown |
| F1 | c.508delT | 9 | 2 | 2 | 8 | 10 | |
| F2 | c.1828 1831delTCCC | 9 | 1 | 1 | 6 | 13 | |
| F5 | c.583 584delGT | 8 | 2 | 2 | 4 | ||
| F7 | c.1523+1G > A c.1145G > A | 6 | 1 | 5 | |||
| F9 | c.1794+1G > A | 6 | 1 | 2 | |||
| F15 | c.760C> T | 2 | 1 | ||||
| F19 | c.1703C> T | 1 | 1 | ||||
| F18 | c.1101C> G | 1 | 1 | ||||
| F20 | c.1652G> A | 2 | |||||
| F22 | c.514A> T | 1 | |||||
| F23 | c.1802C> T | 1 | |||||
| F24 | c.1506C> A | 1 | |||||
| F29 | c.1794+1G > C | 1 | |||||
Numbers of subjects in each category are reported by SLC20A2 variant status. Shaded cells highlight possible cosegregation mismatches
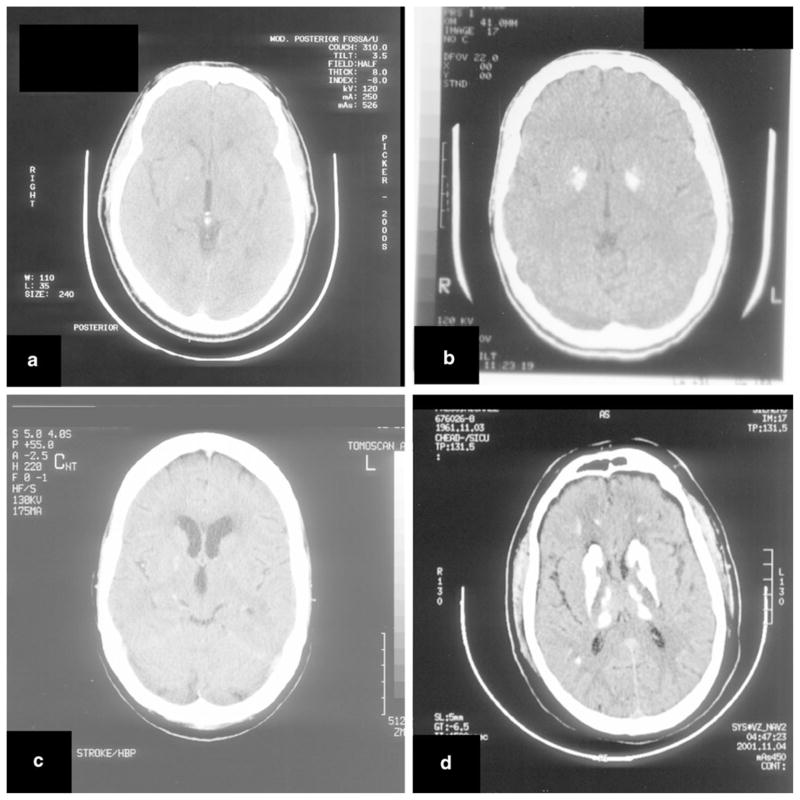
Fig. 3.
Brain CT images of nonsegregating individuals and positive controls. a CT classified as positive for a F1 family member but tested negative for the variant; b CT-positive F1 family member with the variant showing abundant calcifications; c CT classified as positive for F5 family member at age 75 but tested negative for the variant; d CT-positive F5 family member with the variant showing abundant calcifications
Discussion
The recent identification of loss-of-function SLC20A2 mutations in familial IBGC-affected patients finally advances the understanding of the molecular etiology of IBGC by establishing the first genetic location responsible for this disease [10]. Our systematic screen of 29 IBGC families identified 1 previously reported mutation, 1 rare synonymous variant, and 12 novel SLC20A2 variants predicted to be deleterious, with at least five showing full segregation with disease status, indicating that mutations in SLC20A2 are a major cause of familial IBGC. Furthermore, we identified SLC20A2 variants in IBGC families of multiple ancestries, across different countries, supporting the conclusion that SLC20A2 mutations are linked to IBGC worldwide. Nine out of the 14 mutations we identified are predicted to introduce a stop codon, pointing to haploinsufficiency as a causal mechanism for IBGC due to mutations in SLC20A2. Additionally, previous studies identifying the histidine at residue 502 [14], a position found to harbor a variant in family F24 (p.His502Gln), as critical for transport functionality in PiT2 highlights the loss of phosphate transport capacity as a major factor in the molecular etiology of IBGC. Also notable is that three of the four missense variants identified in our IBGC cohort are located within the ProDom domain (PD001131) shared by all PiT transporters (Fig. 1).
Defining disease status in IBGC is complicated by several factors that have likely hampered identification of clear genetic linkage signals: (1) the broad variability of symptom manifestations, ranging from migraine and minor psychiatric symptoms to severe movement and cognitive disorders; (2) the number of additional neurologic and systemic diseases that may cause secondary brain calcifications; and (3) the common occurrence of age-related, idiopathic calcium deposits in the basal ganglia. While some IBGC family members with basal ganglia calcification are asymptomatic, others reporting neuropsychiatric or motor symptoms are CT negative for calcifications. This poses the question as to whether the onset age of basal ganglia calcifications is variable in these patients or whether their symptoms have a different etiology. The minimum age at which absence of calcifications on a CT scan excludes the disease remains unknown, contributing to ambiguity in identifying patients that harbor a pathogenic mutation but are asymptomatic and CT negative at the time of data collection. Although CT status may not completely reflect disease status, both because it can be normal in younger family members and because nonspecific calcifications are often present in older individuals, it is currently the most reliable test for diagnosing IBGC.
We found cosegregation of mutations with disease in five out of the eight families where a cosegregation analysis was possible (Table 3), consistent with the families reported by Wang et al. [10]. We did not identify variant carriers who were not affected, suggesting 100 % sensitivity of the clinical/CT evaluation. In contrast, 2 out of 11 affected family members from the F1 family, 2 out of 10 affected from the F5 family, and 1 out of the 10 affected from the F2 family had received affected disease status based upon clinical examination and/or CT scan, but did not carry predicted–deleterious SLC20A2 sequence variants. Possible reasons for this finding include (1) incorrect clinical evaluation or CT scan analysis and therefore suboptimal specificity of the clinical/CT-based diagnosis. Consistent with this is the observation that CT calcifications in some of these patients are minor (Fig. 3) and are compatible with an incidental finding that appears in 0.5–1.0 % of routine CT scans and that is unrelated to familial IBGC [4–6], as well as the observation that both of the F5 individuals who did not harbor the variant identified in the F5 family were asymptomatic and had been classified as affected based solely on CT scan; (2) noncausality of the identified sequence variants, which is unlikely given the predicted deleteriousness of the sequence variants (all microdeletions leading to frameshift) and the cosegregation with disease in the vast majority of other family members; and (3) possible technical factors, including false-negative mutation detection (nonamplification or degradation of the mutated allele), which is unlikely since the mutation is detected in other family members, or sample identification errors.
Importantly, the discovery of a novel and predicted deleterious SLC20A2 variant in the F1 family, which we previously reported to have significant linkage to disease at the 14q13 locus (designated IBGC1), suggests that the genetic mutation responsible for IBGC in this family was not on chromosome 14 but rather on chromosome 8 within SLC20A2. Notably, both individuals with discordant disease and genetic status contributing to the non-perfect segregation pattern observed in this family were also included in the initial cohort of 11 patients enrolled in the linkage mapping previously performed in this family. It is likely that the discordant disease status of these two individuals is due to clinical ascertainment and/or phenocopy, highlighting once again the importance of accurate phenotypic assessment when performing linkage studies, as linkage analysis relies heavily upon correct identification of affected individuals. The exclusion of IBGC1 from linkage analysis studies in larger cohorts of distinct IBGC families has also demonstrated that IBGC1 is not a major genetic locus for this disease [15]. The discovery of deleterious mutations in SLC20A2 as a cause of familial IBGC greatly advances our understanding of this complex disease and will be crucial in the development of future treatments for IBGC patients as well as other conditions associated with brain calcification. Our assessment of the genetic contribution of SLC20A2 mutations in our cohort of 218 familial IBGC patients demonstrates that as many as 41 % (or 12 out of 29) of the families studied have predicted deleterious sequence variants or mutations in SLC20A2. This finding strongly suggests causality and establishes SLC20A2 as a key gene for familial IBGC. Furthermore, the identification of 12 novel variants—all predicted to be highly disruptive to protein function—broadens the spectrum of known SLC20A2 mutations and adds to the genetic knowledge of this relatively unknown disease-causing gene. More work is still needed to explain the variability in penetrance and expressivity within families. Identifying additional causal genes for IBGC will provide valuable insight for understanding the molecular etiology responsible for the clinical heterogeneity observed in patients with this disease and will ultimately contribute to the identification of therapeutic targets.
Acknowledgments
We would like to acknowledge and thank all of the participants and families for their valuable contribution to our study. This work was funded by NIH/NINDS (R01 NS040752 to DHG), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico to JRMO and MZ), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior to JRMO), FAPESP/CEPID (State of São Paulo Research Foundation to MZ), FACEPE (Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco to JRMO), Australian NHMRC (program grant 510135 to PBM), Ministry of Education and Science, Republic of Serbia (grant no. 175090 to VK, MJ, VD, and IN), and NIMH (K08 MH86297 to BLF). MJS and BQ are supported by research contracts from the Institute of Health Carlos III, European Regional Development Funds (FEDER) and the Botin Foundation. JG is supported by NIH (PSO AG008702-22 to M. Shelanski).
Footnotes
Conflict of interest AEL declares that he has served as an advisor for Abbott, Allon Therapeutics, Astra Zenica, Avanir Pharmaceuticals, Biovail, Boerhinger-Ingelheim, BMS Cephalon, Ceregene, Eisai, GSK, Lundbeck A/S, Medtronic, Merck Serono, MSD, Novartis, Santhera, Solvay, and Teva; received grants from Canadian Institutes of Health Research, Dystonia Medical Research Foundation, Michael J. Fox Foundation, National Parkinson Foundation, Parkinson Society of Canada, and Ontario Problem Gambling Research Centre; received publishing royalties from Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press; and has served as an expert witness in cases related to the welding industry. All the other authors have no conflicts to disclose.
Contributor Information
Sandy Chan Hsu, Program in Neurogenetics, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Renee L. Sears, Program in Neurogenetics, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA
Roberta R. Lemos, Fundación Pública Galega de Medicina Xenómica and Clinical University Hospital of Santiago de Compostela-SERGAS, Santiago de Compostela, Spain. Keizo Asami Laboratory and Biological Sciences Graduate Program, Federal University of Pernambuco, Recife, Brazil
Beatriz Quintáns, Fundación Pública Galega de Medicina Xenómica and Clinical University Hospital of Santiago de Compostela-SERGAS, Santiago de Compostela, Spain. Center for Biomedical Research on Rare Diseases (CIBERER) Institute of Health Carlos III, Valencia, Spain.
Alden Huang, Program in Neurogenetics, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Elizabeth Spiteri, Program in Neurogenetics, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Lisette Nevarez, Program in Neurogenetics, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Catherine Mamah, Program in Neurogenetics, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Mayana Zatz, Human Genome Center, University of São Paulo, São Paulo, Brazil.
Kerrie D. Pierce, Neuroscience Research Australia, Sydney, Australia
Janice M. Fullerton, Neuroscience Research Australia, Sydney, Australia. School of Medical Sciences, The University of New South Wales, Sydney, Australia
John C. Adair, Department of Neurology, University of New Mexico, Albuquerque, NM, USA
Jon E. Berner, Woodinville Psychiatric Association, Woodinville, WA, USA
Matthew Bower, Division of Genetics and Metabolism, University of Minnesota Medical Center, Fairview, Minneapolis, MN, USA.
Henry Brodaty, Centre for Healthy Brain Ageing, School of Psychiatry, The University of New South Wales, Sydney, Australia.
Olga Carmona, Department of Neurology, Hospital of Figueres, Girona, Spain.
Valerija Dobricić, Neurology Clinic, University Clinical Center, Belgrade, Serbia.
Brent L. Fogel, Program in Neurogenetics, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA
Daniel García-Estevez, Department of Neurology, Monforte de Lemos Hospital-SERGAS, Lugo, Spain.
Jill Goldman, The Center for Parkinson’s Disease & Related Disorders, Columbia University Medical Center, New York, NY, USA.
John L. Goudreau, Department of Neurology, Michigan State University, East Lansing, MI, USA
Suellen Hopfer, Program in Neurogenetics, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Milena Janković, Neurology Clinic, University Clinical Center, Belgrade, Serbia.
Serge Jaumà, Department of Neurology, Hospital of Bellvitge, Barcelona, Spain.
Joanna C. Jen, Program in Neurogenetics, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA
Suppachok Kirdlarp, Division of Medicine, Buriram Hospital, Buriram, Thailand.
Joerg Klepper, Klinikum Aschaffenburg, Aschaffenburg, Germany.
Vladimir Kostić, Neurology Clinic, University Clinical Center, Belgrade, Serbia.
Anthony E. Lang, The Movement Disorders Center and the Edomond J. Safra Program in Parkinson’s Disease, Toronto Western Hospital, Toronto, Canada
Agnès Linglart, APHP, Center for Rare Disorders of the Calcium and Phosphorus Metabolism, Bicêtre-Paris-Sud Hospital; INSERM U986, Paris, France.
Melissa K. Maisenbacher, Department of Pediatrics, University of Florida, Gainesville, FL, USA
Bala V. Manyam, Department of Neurology, Penn State Milton S. Hershey College of Medicine, Odessa, FL, USA
Pietro Mazzoni, The Center for Parkinson’s Disease & Related Disorders, Columbia University Medical Center, New York, NY, USA.
Zofia Miedzybrodzka, Medical Genetics Group, School of Medicine & Dentistry, University of Aberdeen, Polwarth Building, Foresterhill, Aberdeen, UK AB25 2ZD.
Witoon Mitarnun, Division of Medicine, Buriram Hospital, Buriram, Thailand.
Philip B. Mitchell, School of Psychiatry, The University of New South Wales and Black Dog Institute, Sydney, Australia
Jennifer Mueller, Division of Genetics and Metabolism, University of Florida, Gainesville, FL, USA.
Ivana Novaković, Neurology Clinic, University Clinical Center, Belgrade, Serbia.
Martin Paucar, Translational Neuropharmacology, Clinical Neuroscience, Center for Molecular Medicine, Karolinska Institute and Neurology Clinic, Karolinska Hospital, Huddinge, Stockholm, Sweden.
Henry Paulson, Department of Neurology, University of Michigan, Ann Arbor, MI, USA.
Sheila A. Simpson, Medical Genetics Group, School of Medicine & Dentistry, University of Aberdeen, Polwarth Building, Foresterhill, Aberdeen, UK AB25 2ZD
Per Svenningsson, Translational Neuropharmacology, Clinical Neuroscience, Center for Molecular Medicine, Karolinska Institute and Neurology Clinic, Karolinska Hospital, Huddinge, Stockholm, Sweden.
Paul Tuite, Department of Neurology, University of Minnesota Medical Center, Fairview, MN, USA.
Jerrold Vitek, Department of Neurology, University of Minnesota Medical Center, Fairview, MN, USA.
Suppachok Wetchaphanphesat, Division of Medicine, Buriram Hospital, Buriram, Thailand.
Charles Williams, Department of Pediatrics, University of Florida, Gainesville, FL, USA.
Michele Yang, Program in Neurogenetics, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Peter R. Schofield, Neuroscience Research Australia, Sydney, Australia. School of Medical Sciences, The University of New South Wales, Sydney, Australia
João R. M. de Oliveira, Neuropsychiatry Department and Keizo Asami Laboratory, Federal University of Pernambuco, Recife, Brazil
María-Jesús Sobrido, Fundación Pública Galega de Medicina Xenómica and Clinical University Hospital of Santiago de Compostela-SERGAS, Santiago de Compostela, Spain. Center for Biomedical Research on Rare Diseases (CIBERER) Institute of Health Carlos III, Valencia, Spain.
Daniel H. Geschwind, Email: dhg@ucla.edu, Program in Neurogenetics, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA. Department of Human Genetics, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA. Department of Psychiatry and Semel Institute for Neuroscience and Human Behavior, University of California Los Angeles, Los Angeles, CA, USA. Program in Neurogenetics, Department of Neurology, 2306 Gonda, 695 Charles Young Drive South, Los Angeles, CA 90095, USA
Giovanni Coppola, Email: gcoppola@ucla.edu, Program in Neurogenetics, Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA. Department of Psychiatry and Semel Institute for Neuroscience and Human Behavior, University of California Los Angeles, Los Angeles, CA, USA. Semel Institute for Neuroscience and Human Behavior, 1524 Gonda, 695 Charles Young Drive South, Los Angeles, CA 90095, USA.
References
- 1.Sobrido MJ, Hopfer S, Geschwind DH. Familial idiopathic basal ganglia calcification. In: Pagon R, Bird T, Dolan C, editors. GeneReviews [Internet] Seattle WA: 2007. [Google Scholar]
- 2.Moskowitz M, Winickoff R, Heinz E. Familial calcification of the basal ganglions: a metabolic and genetic study. N Engl J Med. 1971;285(2):72–77. doi: 10.1056/NEJM197107082850202. [DOI] [PubMed] [Google Scholar]
- 3.Brodaty H, Mitchell P, Luscombe G, Kwok JJ, Badenhop RF, McKenzie R, et al. Familial idiopathic basal ganglia calcification (Fahr’s disease) without neurological, cognitive and psychiatric symptoms is not linked to the IBGC1 locus on chromosome 14q. Hum Genet. 2002;110(1):8–14. doi: 10.1007/s00439-001-0650-x. [DOI] [PubMed] [Google Scholar]
- 4.Koller WC, Cochran JW, Klawans HL. Calcification of the basal ganglia: computerized tomography and clinical correlation. Neurology. 1979;29(3):328–333. doi: 10.1212/wnl.29.3.328. [DOI] [PubMed] [Google Scholar]
- 5.Harrington MG, Macpherson P, McIntosh WB, Allam BF, Bone I. The significance of the incidental finding of basal ganglia calcification on computed tomography. J Neurol Neurosurg Psychiatry. 1981;44(12):1168–1170. doi: 10.1136/jnnp.44.12.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Förstl H, Krumm B, Eden S, Kohlmeyer K. What is the psychiatric significance of bilateral basal ganglia mineralization? Biol Psychiatry. 1991;29(8):827–833. doi: 10.1016/0006-3223(91)90201-v. [DOI] [PubMed] [Google Scholar]
- 7.Geschwind D, Loginov M, Stern J. Identification of a locus on chromosome 14q for idiopathic basal ganglia calcification (Fahr disease) Am J Hum Genet. 1999;65(3):764–772. doi: 10.1086/302558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volpato CBB, De Grandi A, Buffone E, Facheris M, Gebert U, Schifferle G, et al. 2q37 as a susceptibility locus for idiopathic basal ganglia calcification (IBGC) in a large South Tyrolean family. J Mol Neurosci. 2009;39(3):346–353. doi: 10.1007/s12031-009-9287-3. [DOI] [PubMed] [Google Scholar]
- 9.Dai X, Gao Y, Xu Z, Cui X, Liu J, Li Y, et al. Identification of a novel genetic locus on chromosome 8p21.1–q11.23 for idiopathic basal ganglia calcification. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(7):1305–1310. doi: 10.1002/ajmg.b.31102. [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Li Y, Shi L, Ren J, Patti M, Wang T, et al. Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nature Genetics. 2012;44:254–256. doi: 10.1038/ng.1077. [DOI] [PubMed] [Google Scholar]
- 11.Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucl Acids Res. 2003;31(13):3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desmet FO, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucl Acids Res. 2009;37(9):e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bøttger P, Pedersen L. Mapping of the minimal inorganic phosphate transporting unit of human PiT2 suggests a structure universal to PiT-related proteins from all kingdoms of life. BMC Biochem. 2011;12(1):21. doi: 10.1186/1471-2091-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliveira JR, Spiteri E, Sobrido MJ, Hopfer S, Klepper J, Voit T, et al. Genetic heterogeneity in familial idiopathic basal ganglia calcification (Fahr disease) Neurology. 2004;63(11):2165–2167. doi: 10.1212/01.wnl.0000145601.88274.88. [DOI] [PubMed] [Google Scholar]
- 16.Kostić VS, Lukić-Ječmenica M, Novaković I, Dobričić V, Brajković L, Krajinović M, et al. Exclusion of linkage to chromosomes 14q, 2q37 and 8p21.1–q11.23 in a Serbian family with idiopathic basal ganglia calcification. J Neurol. 2011;258(9):1637–1642. doi: 10.1007/s00415-011-5985-1. [DOI] [PubMed] [Google Scholar]