Abstract
Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae strains have spread worldwide and become a major threat in health care facilities. Transmission of blaKPC, the plasmid-borne KPC gene, can be mediated by clonal spread and horizontal transfer. Here, we report the complete nucleotide sequences of two novel blaKPC-3-harboring IncFIA plasmids, pBK30661 and pBK30683. pBK30661 is 74 kb in length, with a mosaic plasmid structure; it exhibits homologies to several other plasmids but lacks the plasmid transfer operon (tra) and the origin of transfer (oriT) that are required for plasmid transfer. pBK30683 is a conjugative plasmid with a cointegrated plasmid structure, comprising a 72-kb element that highly resembles pBK30661 (>99.9% nucleotide identities) and an extra 68-kb element that harbors tra and oriT. A PCR scheme was designed to detect the distribution of blaKPC-harboring IncFIA (pBK30661-like and pBK30683-like) plasmids in a collection of clinical Enterobacteriaceae isolates from 10 hospitals in New Jersey and New York. KPC-harboring IncFIA plasmids were found in 20% of 491 K. pneumoniae isolates, and all carried blaKPC-3. pBK30661-like plasmids were identified mainly in the epidemic sequence type 258 (ST258) K. pneumoniae clone, while pBK30683-like plasmids were widely distributed in ST258 and other K. pneumoniae sequence types and among non-K. pneumoniae Enterobacteriaceae species. This suggests that both clonal spread and horizontal plasmid transfer contributed to the dissemination of blaKPC-harboring IncFIA plasmids in our area. Further studies are needed to understand the distribution of this plasmid group in other health care regions and to decipher the origins of pBK30661-like and pBK30683-like plasmids.
INTRODUCTION
Carbapenem-resistant Enterobacteriaceae (CRE), especially Klebsiella pneumoniae, are a major threat in health care facilities, both in the United States and worldwide (1). According to a recent CDC report, 3.9% of short-stay hospitals and 17.8% of long-term acute-care facilities documented at least one carbapenem-resistant infection (2). Accordingly, the prevalence of carbapenem-resistant Klebsiella species increased from 1.6% in 2001 to 10.4% in 2011 (2). Notably, a single enzyme, namely, K. pneumoniae carbapenemase (KPC), is responsible for a majority of the cases of carbapenem resistance in the United States.
The KPC gene, blaKPC, is commonly carried on numerous transferable plasmids, thereby facilitating its interspecies and intraspecies dissemination (3–5). Presently, blaKPC is identified on plasmids of different incompatibility (Inc) replicon groups, including IncFII, IncI2, IncL/M, IncN, IncA/C, IncR, IncX, and ColE1 (4, 6–9). Among them, certain plasmids appear to be epidemic, contributing significantly to the spread of KPC. For example, the first K. pneumoniae sequence type 258 (ST258)-associated plasmid, pKpQIL, which was originally found in Israel, has spread to several countries, including Italy, Poland, the United Kingdom, Columbia, Canada, and the United States (1, 7, 10, 11). An IncI2 plasmid, pBK15692, has spread widely in New Jersey and New York hospitals, accounting for 23% of a collection of 256 KPC-bearing K. pneumoniae isolates collected in 2007 to 2012 (8). Here, we report the complete sequences of two novel blaKPC-harboring IncFIA plasmids, pBK30661 and pBK30683. Additionally, a PCR-based approach was developed to explore their prevalence among a collection of KPC-positive K. pneumoniae isolates from hospitals in New York and New Jersey.
MATERIALS AND METHODS
Bacterial strains.
Two K. pneumoniae isolates, BK30661 and BK30683, were identified in a retrospective study of carbapenem-resistant K. pneumoniae isolates from New Jersey and New York hospitals. They were isolated from urine samples obtained from patients with urinary tract infections, in two separate New Jersey hospitals, in June 2010. A total of 491 K. pneumoniae unique clinical isolates, collected from 10 hospitals in New Jersey and New York in 2001 to 2012, were included to determine the distribution of pBK30661-like and pBK30683-like plasmids, using a PCR approach (described below). In addition, 52 KPC-producing non-K. pneumoniae Enterobacteriaceae isolates, including 33 Enterobacter isolates, 15 Escherichia coli isolates, and 4 Citrobacter isolates, collected from four of the 10 hospitals between 2009 and 2012 were subjected to PCR screening.
Characterization of strains and manipulation of plasmids.
Isolates BK30661 and BK30683 were initially screened with a multiplex real-time PCR for the presence of blaKPC (12), and its sequence type (ST) was determined by multilocus sequence typing (MLST) (13). Plasmid DNA in strains BK30683 and BK30661 was extracted and electroporated into Escherichia coli DH10B (Invitrogen) by using the method described previously (6). E. coli DH10B transformants were selected on lysogeny broth (LB) agar plates containing 100 μg/ml ampicillin, and the presence of blaKPC genes was confirmed by multiplex real-time PCR (12). The plasmid size of the transformants was estimated by S1 nuclease digestion of plasmid DNA, followed by pulsed-field gel electrophoresis (PFGE) using a Bio-Rad CHEF-DR III variable-angle system (14). Transformants with single plasmids were then selected and subjected to susceptibility testing and sequencing.
MICs for the two parental isolates and their E. coli DH10B transformants were determined by broth microdilution in cation-adjusted Mueller-Hinton broth (MHB), using Sensititre GNX2F panels (Thermo Fisher Scientific, Waltham, MA), according to Clinical and Laboratory Standards Institute methods and interpretations (15, 16). Transferability of blaKPC-bearing plasmids of BK30661 and BK30683 was examined by conjugation experiments using the E. coli J53AzR strain as the recipient, as described previously (17).
Plasmid sequencing and bioinformatics.
Plasmid DNA from the E. coli DH10B transformants of BK30661 and BK30683 was extracted using a Qiagen plasmid maxikit (Qiagen, Valencia, CA). The plasmid DNA was sequenced using a Roche 454 GS-FLX system. Plasmid sequence assembly, closure, and annotation were performed as described elsewhere (6).
PCR screening for pBK30661-like and pBK30683-like plasmids.
A PCR scheme with four duplex PCRs was designed to detect pBK30661-like and pBK30683-like plasmids (Fig. 1). Duplex I (PCR-1 and PCR-2) was designed to target the IncFIA plasmid-specific replication gene repA (in both pBK30661 and pBK30683) and the second IncFII replication gene repA in pBK30683. Duplex II (PCR-3 and PCR-4) was designed to target the upstream and downstream junctions between Tn4401 and the neighboring regions of the plasmid. Duplex III (PCR-5 and PCR-6) was designed to detect both junctions of the 68-kb integrated fragment in pBK30683. Duplex IV (PCR-7 and PCR-8) was used to detect the pBK30661- and pBK30683-associated Tn4401d isoform. pBK30661-like plasmids are positive with PCR-1, PCR-3, PCR-4, PCR-6, PCR-7, and PCR-8, while pBK30683-like plasmids are positive with all eight PCRs. The primer sequences are shown in Table 1, and the primer locations are illustrated in Fig. 1. The PCR cycling conditions were as follows: an initial denaturation step of 95°C for 4 min, 35 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min, and a final extension step of 72°C for 7 min. DNA samples from strains BK30661, BK30683, and E. coli DH10B were used as positive and negative controls in each PCR run.
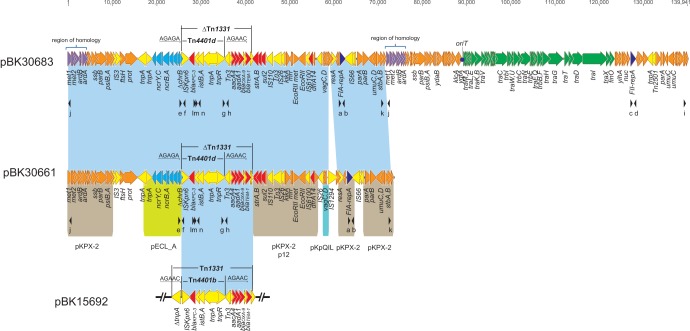
FIG 1.
Plasmid structures of pBK30661 (GenBank accession no. KF954759), pBK30683 (GenBank accession no. KF954760), and the Tn4401/Tn1331 nested transposon in pBK15692 (GenBank accession no. KC845573). Light blue shading, shared regions of homology among pBK30661, pBK30683, and pBK15692. Open reading frames (ORFs) are portrayed by arrows and colored based on predicted gene function. Orange arrows, plasmid scaffold regions; green arrows, genes associated with the tra locus; dark-blue arrows, replication-associated genes; red arrows, antimicrobial resistance genes; light-blue arrows, nickel resistance-associated genes; yellow arrows, accessory genes; purple arrows, genes located in the 4-kb repeated homologous regions in pBK30683. The 5-bp Tn4401 adjacent sequences are underlined. Small black arrowheads beneath the plasmids indicate the locations of primers used for PCR screening. The primer sequences are shown in Table 1.
TABLE 1.
Oligonucleotide primers used in this study
| PCR | Ordera | Primer | Sequence (5′ to 3′) | Length (bp) | Target |
|---|---|---|---|---|---|
| Duplex I | |||||
| PCR-1 | a | IA-1f | GCCGTCCTTTCTGTGACAAATCA | IncFIA repA of pBK30661 and pBK30683 | |
| b | IA-1r | GGATGGACTGTGGGCACGTT | 516 | ||
| PCR-2 | c | IA-2f | CCGTTTCTGTGTCATTTGCTCCT | Second IncFII repA in pBK30683 | |
| d | IA-2r | CTTATAGTGAGACGGCCGGAACC | 250 | ||
| Duplex II | |||||
| PCR-3 | e | IA-3f | ATACCGGTGCCGCCATGCTGCG | Tn4401 upstream junction between chrB gene and ISKpn6 in both pBK30661 and pBK30683 | |
| f | IA-3r | TCGTCATGCCGCGGACCACCCC | 213 | ||
| PCR-4 | g | IA-4f | CCGGCATCACCGGCCCTCACCT | Tn4401 downstream junction between Tn4401 tnpR gene and neighboring Tn3 tnpA gene in both pBK30661 and pBK30683 | |
| h | IA-4r | ACACTCCCGGCTGTGCGCCTGA | 515 | ||
| Duplex III | |||||
| PCR-5 | i | IA-5f | CGATGACGTGGAGAGCAGTA | 534 | Region between putative cytoplasmic protein gene and adenine-specific methyltransferase gene (met1) in pBK30683 |
| j | IA-56r | TCCCGAGAATGAATCTGGAC | |||
| PCR-6 | k | IA-6f | CGTGCATTCGGTGACTAAAA | 768 | Region between hypothetical protein gene and adenine-specific methyltransferase gene (met1) in both pBK30661 and pBK30683 |
| Duplex IVb | |||||
| PCR-7 | l | 4401v-r (3781L) | CACAGCGGCAGCAAGAAAGC | 635 | Tn4401d isoform in pBK30661 and pBK30683 |
| PCR-8 | m | 4401v-r1 | GCAAGCCGCTCCCTCTCCAG | 314 | |
| n | 4401v-f (3098U) | TGACCCTGAGCGGCGAAAGC |
Currently, there are at least 5 defined Tn4401 isoforms (isoforms a to e) that differ by deletions found upstream of blaKPC (isoform a, 99-bp deletion; isoform b, no deletion; isoform c, 215-bp deletion; isoform d, 68-bp deletion; isoform e, 255-bp deletion), as well as some other forms with other deletions or insertions (3, 9, 18, 19). The pBK30661- and pBK30683-associated Tn4401d isoform in this study refers to the variant with a 68-bp deletion upstream of blaKPC. Different Tn4401 isoforms are distinguished by a PCR spanning the upstream ISKpn7 istB and blaKPC regions (20). We introduced a new reverse primer specific for the Tn4401b and Tn4401d isoforms into the previously published PCR method (Fig. 1 and Table 1), in order to resolve Tn4401a and Tn4401d isoforms (31-bp difference) by agarose gel electrophoresis. The K. pneumoniae ST258 and blaKPC variants of all isolates were characterized by two multiplex real-time PCR methods developed by our laboratory (12, 21).
Nucleotide sequence accession numbers.
The complete nucleotide sequences of pBK30661 and pBK30683 were deposited in GenBank with accession numbers KF954759 and KF954760, respectively.
RESULTS
Microbiological characterization of isolates BK30661 and BK30683.
MLST analysis revealed that BK30661 belongs to the epidemic K. pneumoniae ST258 clone (allelic profile 3-3-1-1-1-1-79), while BK30683 belongs to ST963 (allelic profile 2-9-2-1-13-1-25), which is genetically distinct from ST258. Multiplex real-time PCR assays for blaKPC variants showed that both isolates carried blaKPC-3. Susceptibility testing showed that both BK30661 and BK30683 were resistant to imipenem (MIC, >8 μg/ml), ertapenem (MIC, ≥4 μg/ml), meropenem (MIC, ≥8 μg/ml), doripenem (MIC, ≥4 μg/ml), cefepime (MIC, ≥32 μg/ml), cefotaxime (MIC, ≥32 μg/ml), ceftazidime (MIC, >16 μg/ml), aztreonam (MIC, >16 μg/ml), ticarcillin-clavulanate (MIC, ≥128 and ≥2 μg/ml), piperacillin-tazobactam (MIC, ≥128 and ≥4 μg/ml), levofloxacin (MIC, >8 μg/ml), ciprofloxacin (MIC, ≥4 μg/ml), tobramycin (MIC, ≥16 μg/ml), and co-trimoxazole (MIC, ≥4 and ≥76 μg/ml). BK30661 was also resistant to gentamicin (MIC, ≥16 μg/ml), amikacin (MIC, ≥64 μg/ml), and colistin (MIC, 4 μg/ml).
With selection on ampicillin-containing agar (100 μg/ml), we were successful in transferring carbapenem resistance from K. pneumoniae BK30683 to E. coli J53 by conjugation and to E. coli DH10B by electroporation. In contrast, multiple attempts to transfer the blaKPC-bearing plasmid from BK30661 by conjugation were not successful; transfer was achieved only by electroporation. Representative E. coli DH10B transformants of BK30661 and BK30683 displayed resistance profiles similar to those of the parent strains but were susceptible to ciprofloxacin (MIC, ≤0.25 μg/ml), levofloxacin (MIC, ≤1 μg/ml), and colistin (MIC, 0.5 μg/ml).
Structure of blaKPC-3-harboring plasmids pBK30661 and pBK30683.
pBK30661 is 73,635 bp in length, with an average G+C content of 53.9%, and harbors 84 predicted open reading frames (ORFs) (Fig. 1). It carries a single replication gene, repA, with 99.7% nucleotide similarity to the replication gene of plasmid pKPX-2, which was identified from a multidrug-resistant K. pneumoniae strain in Taiwan (22). Plasmid sequence queries against the plasmid MLST databases (http://pubmlst.org/plasmid) showed that the replication region of pBK30661 was close to IncFIA allele 8, with 94.0% nucleotide identity, identifying pBK30661 as a member of the IncFIA plasmid group.
pBK30661 has a mosaic plasmid structure, with regions homologous to several other plasmids (pKPX-2, pECL_A, pBK15692, p12, and pKpQIL) (Fig. 1). The plasmid backbone genes are separated by several insertion sequence (IS) elements (IS3, IS26, IS1294, and IS66), suggesting that the IS elements play important roles in the architecture of the pBK30661 genome. Full plasmid BLAST queries against NCBI GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi) showed that pBK30661 has 55% query coverage and overall 97% nucleotide identity to pKPX-2 (Fig. 1A). Notably, the plasmid transfer operon (tra) and the origin-of-transfer site (oriT) regions were absent in the pBK30661 genome.
pBK30661 harbors nine antimicrobial resistance determinants, including blaKPC-3, blaTEM-1, blaOXA-9 (β-lactam resistance), aacA4, aadA1, strA, strB (aminoglycoside resistance), sul2 (sulfonamide resistance), and dfrA14 (trimethoprim resistance), and a nickel resistance operon (Fig. 1A). The blaKPC-3 gene is carried on a truncated Tn4401/Tn1331 nested transposon, closely resembling the nested transposon in the IncI2 plasmid pBK15692 we described recently (8). The main difference is that blaKPC-3 is carried by a Tn4401d variant with a 68-bp deletion located upstream of the blaKPC gene in pBK30661, while it is carried by a Tn4401b variant without deletions upstream of the blaKPC gene in pBK15692. Tn4401 is approximately 10 kb in size, delimited by two 39-bp imperfect inverted repeat (IR) sequences, and usually is associated with 5-bp target site duplications (TSDs) at both ends, adjacent to the IR sequences, as a result of integration (3, 23). In this study, Tn4401d was integrated into the Tn1331 tnpA gene at the same site in pBK30661 as in pBK15692, generating a 5-bp target sequence (AGAAC) (Fig. 1). However, the upstream region of Tn1331 (ΔtnpA) in pBK30661 was truncated by an 8-kb nickel resistance operon (nic operon), which showed 98% nucleotide similarity to that of plasmid pECL_A from Enterobacter cloacae subsp. cloacae strain ATCC 13047 (24). The insertion of the nic operon also deleted the corresponding 5-bp target sequence (AGAAC) of Tn4401d in pBK30661, leaving a 5-bp unique sequence (AGAGA) adjacent to the upstream IR sequence of Tn4401d (Fig. 1). To our knowledge, this is the first completely characterized plasmid showing deletion of the Tn4401-associated TSD.
pBK30683 is 139,941 bp in length, with an average G+C content of 54.0%, and has 181 predicted ORFs (Fig. 1). The upstream 72-kb sequence in pBK30683 is almost identical to that in pBK30661, sharing >99.9% nucleotide similarity, except that pBK30661 harbors an extra IS1294 (Fig. 1). pBK30683 carries an identical IncFIA repA gene, the truncated Tn1331/Tn4401d nested transposon, and the nic operon, in comparison with pBK30661. Interestingly, pBK30683 carries an extra 68-kb sequence that is unrelated to pBK30661, with the full machinery for genes that are responsible for plasmid replication, stability, and conjugation. As an example, pBK30683 harbors a second set of genes for plasmid replication (a novel IncFII repA), partition (parA and parB), SOS inhibition (psiA and psiB), antirestriction (ardA and ardB), and error-prone repair (umuC and umuD) (Fig. 1). Notably, pBK30683 carries a 35-kb tra operon that contributes to plasmid conjugation, similar to the conjugative region of several other IncFII plasmids (e.g., pKPN3 and pKPN4), with more than 97% nucleotide similarities. The tra operon-associated oriT sites were identified upstream of the traM gene (Fig. 1). Inspection of the junctions between the 72-kb pBK30661 homologous region and the remaining 68-kb sequence in pBK30683 revealed a 4-kb region of homology (>97% nucleotide similarity), containing the adenine-specific methyltransferase genes (met1 and met2) and plasmid antirestriction genes (ardA and ardB) (Fig. 1).
Prevalence and dissemination of pBK30661-like and pBK30683-like plasmids.
As part of an ongoing surveillance project, hospitals in New York and New Jersey routinely submit carbapenem-resistant and -susceptible Enterobacteriaceae isolates to our laboratory for genotyping. A total of 491 KPC-producing K. pneumoniae clinical isolates collected between 2001 and 2012 from 10 hospitals in our area were evaluated by PCR for the presence of pBK30661-like and pBK30683-like plasmid markers. Among them, 228 (46.4%) were blaKPC-2 positive, while 263 (53.6%) were blaKPC-3 positive; 411 (83.7%) belonged to ST258 (Table 2). Among these 491 isolates, 79 (16.1%) contained pBK30683-like plasmids and 18 (3.7%) harbored pBK30661-like plasmids. Interestingly, pBK30683-like and pBK30661-like plasmids were found to be exclusively associated with KPC-3-positive isolates, accounting for 30.0% and 6.8%, respectively, of the 263 KPC-3-producing isolates. Among the 79 pBK30683-like plasmids, 55 (69.6%) were in ST258 isolates, while 24 (30.4%) were in non-ST258 isolates. In contrast, all 18 pBK30661-like plasmids were in ST258 isolates.
TABLE 2.
Distributions of pBK30683-like and pBK30661-like plasmids among clinical KPC-producing Enterobacteriaceae isolates collected between 2001 and 2012 from 10 hospitals in the New York-New Jersey region
| Isolate type | Distribution (n [%]) of: |
Total distribution (n) | |
|---|---|---|---|
| pBK30683-like plasmid | pBK30661-like plasmid | ||
| K. pneumoniae | |||
| KPC-2 vs KPC-3 | |||
| KPC-2 | 0 (0) | 0 (0) | 228 |
| KPC-3 | 79 (30.0) | 18 (6.8) | 263 |
| ST258 vs non-ST258 | |||
| Non-ST258 | 24 (30.0) | 0 (0) | 80 |
| ST258 | 55 (13.4) | 18 (4.4) | 411 |
| Non-K. pneumoniae, KPC-2 vs KPC-3 | |||
| KPC-2 | 0 (0) | 0 (0) | 10 |
| KPC-3 | 22 (52.4) | 2 (4.8) | 42 |
Of 52 non-K. pneumoniae KPC-carrying Enterobacteriaceae isolates collected from four of the 10 hospitals, 22 were found to carry pBK30683-like plasmids (17 Enterobacter isolates, 4 E. coli isolates, and 1 Citrobacter isolate), and they all harbored blaKPC-3. Surprisingly, two non-K. pneumoniae KPC-carrying Enterobacteriaceae isolates (1 E. cloacae isolate and 1 E. coli isolate) were found to harbor pBK30661-like plasmids (Table 2).
DISCUSSION
Transmission of antibiotic resistance genes, e.g., blaKPC, can be mediated by different molecular mechanisms, from mobility of small genetic elements (e.g., Tn4401 transposon) to horizontal transfer of plasmids and clonal spread (25). The complexity of different transmission modes challenges our ability to identify, to track, and to control the spread of carbapenem-resistant Enterobacteriaceae strains as they become a global problem (26).
Since its initial emergence in 1996, KPC has spread in the United States and across the world, predominantly via a single K. pneumoniae sequence type, ST258, suggesting that the clonal spread of a single strain underlies the worldwide dissemination of KPC (1). An example of this is in a study from the Clinical Center of the U.S. National Institutes of Health (NIH) in 2011; an outbreak of drug-resistant ST258 Klebsiella pneumoniae infected 18 patients, causing the death of 6 of them, presumably due to the same strain introduced by an index patient (27). Other mechanisms, including horizontal transfer of blaKPC-bearing plasmids and Tn4401 transposons, have also been documented (28). Plasmid conjugation is dependent on the presence of oriT and the tra operon (29). Conjugation is initiated by the activity of the relaxase enzyme, which creates a nick in one of the strands of the conjugative plasmid at the oriT site. Consequently, both oriT and an intact tra operon are required for plasmid conjugation.
In this study, we sequenced to closure two blaKPC-3-bearing plasmids. The first one, pBK30661, which could not be transferred in conjugation experiments, lacks both the tra operon and the oriT site. In contrast, plasmid pBK30683, harboring the transfer-associated tra operon and the oriT site, was successfully transferred by conjugation. Interestingly, a comparison between these two plasmids revealed that they share a nearly identical 72-kb region, encompassing the full length of pBK30661. In this case, pBK30683 appears to be a cointegrate of pBK30661 with an extra 68-kb genetic element.
The origins of pBK30661 and pBK30683 remain unknown. One possible explanation is that pBK30683 originated from the cointegration of pBK30661 and a second 68-kb plasmid or genetic element. This 68-kb element found in pBK30683 harbors a complete set of genes for plasmid replication, stability, and conjugation, and we presume it is from an independent plasmid that likely coexisted with pBK30661 in the same isolate. Notably, this element is flanked by a highly similar homologous 4-kb region, and it is possible that cointegration was achieved through homologous recombination among the large reiterated sequences. The 68-kb element carries the tra and oriT regions that are necessary for plasmid conjugation. This novel cointegrated plasmid, pBK30683, therefore acquired the ability to transfer by conjugation, facilitating dissemination of carbapenem resistance among different strains and species. Plasmid cointegration is not rare in Gram-negative or Gram-positive bacteria and has been associated with the spread of antimicrobial resistance genes, including genes for β-lactamases (30, 31). For example, Lin et al. recently reported the cointegration of a nonconjugative blaCTX-M-17-bearing plasmid, pIP843, with an ∼73-kb conjugative plasmid that may be responsible for the spread of CTX-M-17 (31).
An alternative hypothesis is that pBK30661 was formed by excision from pBK30683 through homologous recombination at the repeat regions. This argument appears to be supported by the evidence that pBK30661-like plasmids were found in other non-K. pneumoniae Enterobacteriaceae, including E. cloacae and E. coli (Table 2). As we described above, pBK30661 lacks the oriT site and the tra operon, disabling its capacity for lateral transfer or mobilization. In this case, the spread of pBK30661-like plasmids should be primarily through clonal spread of pBK30661-bearing ST258 strains. However, we also found two isolates, one E. cloacae and one E. coli, carrying pBK30661-like plasmids. A plausible explanation is that the pBK30661-like plasmids in E. cloacae and E. coli were transferred by a pBK30683-like plasmid, followed by excision of the 68-kb tra-harboring element. Routine sequencing and comparative analysis of resistance plasmids will ultimately increase our database of plasmid architectures and sequences and contribute to a better understanding of how plasmids such as pBK30661 and pBK30683 arose.
In this study, blaKPC-3-bearing IncFIA plasmids (including both pBK30661-like and pBK30683-like plasmids) were identified in 19.8% of KPC-producing K. pneumoniae isolates from 10 hospitals, suggesting the widespread nature of this group of plasmids in our region. In K. pneumoniae, pBK30661-like plasmids are found only in ST258, indicating their clonal dissemination with ST258 isolates. In contrast, pBK30683-like plasmids have spread beyond the K. pneumoniae ST258 clone into other STs as well as into other species, presumably due to the transconjugal ability encoded by the tra operon. The finding of pBK30683-like plasmids in other STs and other species from the same hospitals suggested that interstrain and intraspecies transfers contribute significantly to the spread of this plasmid group.
In conclusion, this report presents the first complete sequences of two blaKPC-3-harboring IncFIA plasmids, pBK30661 and pBK30683, in KPC-bearing strains. A screening study from 10 New Jersey and New York hospitals reveals that KPC-producing IncFIA (pBK30661-like and pBK30683-like) plasmids are spread widely in the New York-New Jersey region. The architecture of the plasmids described in this study also highlights the plasticity of KPC-carrying extrachromosomal elements. Further studies are required to determine the origins of the pBK30661 and pBK30683 plasmids and the distributions of this plasmid group in other geographic areas, in order to understand their contributions to international KPC epidemiology.
ACKNOWLEDGMENTS
This study was supported by a grant (to B.N.K.) from the National Institutes of Health (grant R01AI090155). This work was also supported by grants R01AI072219 and R01AI063517 (to R.A.B.) from the National Institutes of Health and by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, the Veterans Affairs Merit Review Program, and the Geriatric Research Education and Clinical Center VISN 10 (to R.A.B.).
B.N.K. discloses that he holds two patents that focus on using DNA sequencing to identify bacterial pathogens.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Published ahead of print 3 February 2014
REFERENCES
- 1.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 13:785–796. 10.1016/S1473-3099(13)70190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacob JT, Klein E, Laxminarayan R, Beldavs Z, Lynfield R, Kallen AJ, Ricks P, Edwards J, Srinivasan A, Fridkin S, Rasheed KJ, Lonsway D, Bulens S, Herrera R, McDonald LC, Patel J, Limbago B, Bell M, Cardo D. 2013. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb. Mortal. Wkly. Rep. 62:165–170 [PMC free article] [PubMed] [Google Scholar]
- 3.Naas T, Cuzon G, Villegas M-V, Lartigue M-F, Quinn JP, Nordmann P. 2008. Genetic structures at the origin of acquisition of the β-lactamase blaKPC gene. Antimicrob. Agents Chemother. 52:1257–1263. 10.1128/AAC.01451-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gootz TD, Lescoe MK, Dib-Hajj F, Dougherty BA, He W, Della-Latta P, Huard RC. 2009. Genetic organization of transposase regions surrounding blaKPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital. Antimicrob. Agents Chemother. 53:1998–2004. 10.1128/AAC.01355-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen P, Wei Z, Jiang Y, Du X, Ji S, Yu Y, Li L. 2009. Novel genetic environment of the carbapenem-hydrolyzing β-lactamase KPC-2 among Enterobacteriaceae in China. Antimicrob. Agents Chemother. 53:4333–4338. 10.1128/AAC.00260-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Chavda KD, Fraimow HS, Mediavilla JR, Melano RG, Jacobs MR, Bonomo RA, Kreiswirth BN. 2013. Complete nucleotide sequences of blaKPC-4- and blaKPC-5-harboring IncN and IncX plasmids from Klebsiella pneumoniae strains isolated in New Jersey. Antimicrob. Agents Chemother. 57:269–276. 10.1128/AAC.01648-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leavitt A, Chmelnitsky I, Carmeli Y, Navon-Venezia S. 2010. Complete nucleotide sequence of KPC-3-encoding plasmid pKpQIL in the epidemic Klebsiella pneumoniae sequence type 258. Antimicrob. Agents Chemother. 54:4493–4496. 10.1128/AAC.00175-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Chavda KD, Al Laham N, Melano RG, Jacobs MR, Bonomo RA, Kreiswirth BN. 2013. Complete nucleotide sequence of a blaKPC-harboring IncI2 plasmid and its dissemination in New Jersey and New York hospitals. Antimicrob. Agents Chemother. 57:5019–5025. 10.1128/AAC.01397-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant KA, Van Schooneveld TC, Thapa I, Bastola D, Williams LO, Safranek TJ, Hinrichs SH, Rupp ME, Fey PD. 2013. KPC-4 is encoded within a truncated Tn4401 in an IncL/M plasmid, pNE1280, isolated from Enterobacter cloacae and Serratia marcescens. Antimicrob. Agents Chemother. 57:37–41. 10.1128/AAC.01062-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Fernandez A, Villa L, Carta C, Venditti C, Giordano A, Venditti M, Mancini C, Carattoli A. 2012. Klebsiella pneumoniae ST258 producing KPC-3 identified in Italy carries novel plasmids and OmpK36/OmpK35 porin variants. Antimicrob. Agents Chemother. 56:2143–2145. 10.1128/AAC.05308-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baraniak A, Grabowska A, Izdebski R, Fiett J, Herda M, Bojarska K, Zabicka D, Kania-Pudlo M, Mlynarczyk G, Zak-Pulawska Z, Hryniewicz W, Gniadkowski M. 2011. Molecular characteristics of KPC-producing Enterobacteriaceae at the early stage of their dissemination in Poland, 2008–2009. Antimicrob. Agents Chemother. 55:5493–5499. 10.1128/AAC.05118-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Mediavilla JR, Endimiani A, Rosenthal ME, Zhao Y, Bonomo RA, Kreiswirth BN. 2011. Multiplex real-time PCR assay for detection and classification of Klebsiella pneumoniae carbapenemase gene (blaKPC) variants. J. Clin. Microbiol. 49:579–585. 10.1128/JCM.01588-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43:4178–4182. 10.1128/JCM.43.8.4178-4182.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barton BM, Harding GP, Zuccarelli AJ. 1995. A general method for detecting and sizing large plasmids. Anal. Biochem. 226:235–240. 10.1006/abio.1995.1220 [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M07-A9, 9th ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing; 23rd informational supplement. M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 17.Wang M, Tran JH, Jacoby GA, Zhang Y, Wang F, Hooper DC. 2003. Plasmid-mediated quinolone resistance in clinical isolates of Escherichia coli from Shanghai, China. Antimicrob. Agents Chemother. 47:2242–2248. 10.1128/AAC.47.7.2242-2248.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naas T, Cuzon G, Truong HV, Nordmann P. 2012. Role of ISKpn7 and deletions in blaKPC gene expression. Antimicrob. Agents Chemother. 56:4753–4759. 10.1128/AAC.00334-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Chavda KD, Mediavilla JR, Jacobs MR, Levi MH, Bonomo RA, Kreiswirth BN. 2012. Partial excision of blaKPC from Tn4401 in carbapenem-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 56:1635–1638. 10.1128/AAC.06182-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuzon G, Naas T, Truong H, Villegas MV, Wisell KT, Carmeli Y, Gales AC, Venezia SN, Quinn JP, Nordmann P. 2010. Worldwide diversity of Klebsiella pneumoniae that produce β-lactamase blaKPC-2 gene. Emerg. Infect. Dis. 16:1349–1356. 10.3201/eid1609.091389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Chavda KD, Mediavilla JR, Zhao Y, Fraimow HS, Jenkins SG, Levi MH, Hong T, Rojtman AD, Ginocchio CC, Bonomo RA, Kreiswirth BN. 2012. Multiplex real-time PCR for detection of an epidemic KPC-producing Klebsiella pneumoniae ST258 clone. Antimicrob. Agents Chemother. 56:3444–3447. 10.1128/AAC.00316-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang TW, Chen TL, Chen YT, Lauderdale TL, Liao TL, Lee YT, Chen CP, Liu YM, Lin AC, Chang YH, Wu KM, Kirby R, Lai JF, Tan MC, Siu LK, Chang CM, Fung CP, Tsai SF. 2013. Copy number change of the NDM-1 sequence in a multidrug-resistant Klebsiella pneumoniae clinical isolate. PLoS One 8:e62774. 10.1371/journal.pone.0062774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuzon G, Naas T, Nordmann P. 2011. Functional characterization of Tn4401, a Tn3-based transposon involved in blaKPC gene mobilization. Antimicrob. Agents Chemother. 55:5370–5373. 10.1128/AAC.05202-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren Y, Ren Y, Zhou Z, Guo X, Li Y, Feng L, Wang L. 2010. Complete genome sequence of Enterobacter cloacae subsp. cloacae type strain ATCC 13047. J. Bacteriol. 192:2463–2464. 10.1128/JB.00067-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munoz-Price LS, Quinn JP. 2009. The spread of Klebsiella pneumoniae carbapenemases: a tale of strains, plasmids, and transposons. Clin. Infect. Dis. 49:1739–1741. 10.1086/648078 [DOI] [PubMed] [Google Scholar]
- 26.Adler A, Carmeli Y. 2011. Dissemination of the Klebsiella pneumoniae carbapenemase in the health care settings: tracking the trails of an elusive offender. mBio 2:e00280–11. 10.1128/mBio.00280-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, Palmore TN, Segre JA. 2012. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci. Transl. Med. 4:148ra116. 10.1126/scitranslmed.3004129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathers AJ, Cox HL, Kitchel B, Bonatti H, Brassinga AK, Carroll J, Scheld WM, Hazen KC, Sifri CD. 2011. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. mBio 2:e00204–11. 10.1128/mBio.00204-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de la Cruz F, Frost LS, Meyer RJ, Zechner EL. 2010. Conjugative DNA metabolism in Gram-negative bacteria. FEMS Microbiol. Rev. 34:18–40. 10.1111/j.1574-6976.2009.00195.x [DOI] [PubMed] [Google Scholar]
- 30.Leelaporn A, Firth N, Paulsen IT, Skurray RA. 1996. IS257-mediated cointegration in the evolution of a family of staphylococcal trimethoprim resistance plasmids. J. Bacteriol. 178:6070–6073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin DL, Ramirez MS, Tran T, Tolmasky ME. 2013. A cointegrate-like plasmid that facilitates dissemination by conjugation of the extended-spectrum β-lactamase CTX-M-17. Antimicrob. Agents Chemother. 57:5191–5192. 10.1128/AAC.01365-13 [DOI] [PMC free article] [PubMed] [Google Scholar]