Abstract
We identified a case of breakthrough candidemia in a 25-year-old patient receiving micafungin prophylaxis (50 mg/day). Five Candida glabrata isolates were obtained from blood cultures and were classified as multidrug-resistant isolates, since all of them exhibited high MICs for echinocandin and azole drugs. A mutation (S663F) in hot spot 1 of the FKS2 gene was found in all five isolates. This mutation yielded a 1,3-β-d-glucan synthase enzyme with highly reduced sensitivities to echinocandin drugs.
TEXT
In recent years, the epidemiology of candidemia in Latin American hospitals has been changing, with a trend toward an increase in the incidence of candidemia due to Candida glabrata, as documented in recently published multicenter studies (1, 2, 3). C. glabrata is naturally less susceptible to azoles than other Candida species (4), and this has led to the expanded use of echinocandins for fungemia treatment caused by this Candida species (5, 6, 7). This class of drug is now reported as the first-line therapy for candidemia in several guidelines of medical societies (5, 6, 7).
Acquired echinocandin resistance among Candida spp. is largely infrequent. Yet, episodes of invasive infection due to echinocandin-resistant Candida isolates are increasingly being reported in U.S. and European medical centers (8, 9, 10, 11). We describe one case of C. glabrata breakthrough candidemia documented in a patient with Burkitt lymphoma and prolonged neutropenia who received antifungal prophylaxis with low doses of micafungin (50 mg/day) in a tertiary care hospital in Curitiba, Paraná, Brazil. This is the first documented case of multidrug resistance among clinical strains of C. glabrata from a Latin American medical center.
Case.
A 25-year-old man was diagnosed with stage IV adult sporadic Burkitt lymphoma in November 2011, and due to profound neutropenia, he received fluconazole prophylactically at doses of 200 mg/day for 20 days (27 November to 16 December), followed by micafungin at doses of 50 mg/day for 12 days (17 December to 28 December). In January 2012 (3 January), the patient was admitted for a third round of chemotherapy treatment with a cyclophosphamide, vincristine, doxorubicin, dexamethasone (hyper-CVAD) regimen, having presented with profound neutropenia since December 2011. Levofloxacin and sulfamethoxazole-trimethoprim were initiated as prophylaxis therapy. Due to prolonged neutropenia, antifungal prophylaxis was restarted with micafungin at doses of 50 mg/day (posaconazole is not available in Brazil). At day 12 of hospitalization, the patient developed fever during a neutropenic period, without a defined focus of infection, and cefepime treatment was initiated empirically. A blood culture was collected and revealed growth of Candida (isolate 1), despite 24 days of exposure to micafungin. Initially, the decision was made to increase the micafungin dose to 100 mg/day. The patient continued to have a fever for 6 days after initiation of the micafungin treatment (100 mg/day), with sequential blood cultures positive for Candida (isolates 2 to 5). Possible infectious foci for persistent fungemia (endocarditis, hepatosplenic candidiasis, and fungal meningitis) were ruled out by clinical, imaging, and laboratory tests. Echinocandin resistance was suspected, and a conventional formulation of amphotericin B was initiated with good clinical response. Blood cultures were negative after 5 days of treatment.
A total of five clinical Candida isolates were obtained from sequential blood cultures collected during breakthrough candidemia that occurred during antifungal prophylaxis with micafungin (50 mg/day). All isolates tested were identified as Candida glabrata by internal transcribed spacer (ITS) sequencing, as previously described by our group (12, 13).
Antifungal susceptibility testing was performed using the broth microdilution method according to Clinical and Laboratory Standards Institute (CLSI) document M27-A3 (14), using current CLSI MIC interpretative criteria (CLSI, M27-S4) (15). The antifungal compounds were kindly provided as pure powders by their manufacturers. All five C. glabrata bloodstream isolates tested exhibited resistance to fluconazole (FLC), voriconazole (VRC), and all echinocandins. The MIC values for echinocandins were 16- to 33-fold higher than those for the control strain (ATCC 90030). Table 1 summarizes the MIC values obtained for the five C. glabrata isolates for the six antifungal agents tested.
TABLE 1.
In vitro activities of six antifungal agents and GS inhibition profiles for echinocandin drugs against five C. glabrata isolates harboring FKS mutations
| Strain/mutation | FKS2 hot spot genotype | MIC (mg/liter) for: |
IC50 (ng/ml) for: |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMB | FLC | VRC | ANF | CSF | MCF | ANF | CSF | MCF | ||
| ATCC 90030 | FKS1/FKS1 (wild type) | 0.25 | 4 | 0.03 | 0.06 | 0.03 | 0.03 | 46.1 | 29.7 | 15.29 |
| 8622 A | fks1/fks1 (S663F) | 0.25 | >64.0 | 4.0 | 1.0 | 1.0 | 0.5 | 2,655 | 1,263 | 61,760 |
| 8622 B | fks1/fks1 (S663F) | 0.25 | >64.0 | 4.0 | 1.0 | 1.0 | 0.5 | NDa | ND | ND |
| 8622 C | fks1/fks1 (S663F) | 0.25 | >64.0 | 4.0 | 1.0 | 1.0 | 0.5 | ND | ND | ND |
| 8622 D | fks1/fks1 (S663F) | 0.25 | >64.0 | 4.0 | 1.0 | 1.0 | 0.5 | ND | ND | ND |
| 8622 E | fks1/fks1 (S663F) | 0.25 | >64.0 | 4.0 | 1.0 | 1.0 | 0.5 | ND | ND | ND |
ND, not determined.
DNA sequencing analyses of the two hot spot (HS) regions of the drug target genes FKS1 and FKS2 were performed on the five C. glabrata clinical isolates, as previously described (16). The two HS regions of the FKS1 gene corresponded to nucleotides (nt) 1873 to 1902 (amino acids [aa] 625 to 633) and nt 4018 to 4041 (aa 1340 to 1347) of the published strain C. glabrata ATCC 90030 (DNA Data Bank of Japan [DDBJ] accession no. HM366440.1), and the two HS regions of the FKS2 gene corresponded to nt 1975 to 2001 (aa 659 to 667) and nt 4122 to 4143 (aa 1374 to 1381) of the published strain C. glabrata ATCC 90030 (DDBJ accession no. HM366442.1). The sequencing of the HS regions of the FKS genes revealed only a nonsynonymous mutation in HS1 in the FKS2 gene, which led to an S663F amino acid substitution (Table 1).
We performed an in vitro glucan synthase inhibition assay, as previously described (17, 18, 19). The echinocandin inhibition parameter 50% inhibitory concentration (IC50) was determined for the wild-type strain (C. glabrata ATCC 90030) and the 8622A clinical isolate (Table 1). The fks mutant enzyme extracted from the clinical isolate showed significantly higher IC50 values than did the corresponding enzyme isolated from the wild-type strain (58-, 43-, and >4,000-fold on average for anidulafungin [ANF], caspofungin [CSF], and micafungin [MCF], respectively) (Table 1 and Fig. 1). These results confirm that the point mutation S663F encoded in the FKS2 gene of C. glabrata yielded a 1,3-β-d-glucan synthase enzyme with highly reduced sensitivities to echinocandin drugs, resulting in elevated MICs with a strong potential for clinical failure. In the present investigation, a patient with Burkitt lymphoma and severe neutropenia that was expected to persist for a longer period of time was subjected to antifungal prophylaxis with micafungin at a dosage of 50 mg daily. The patient developed C. glabrata breakthrough candidemia after 19 and 24 days of exposure to fluconazole and micafungin, respectively. In vitro antifungal susceptibility testing confirmed the resistance to fluconazole, voriconazole, and all echinocandins.
FIG 1.
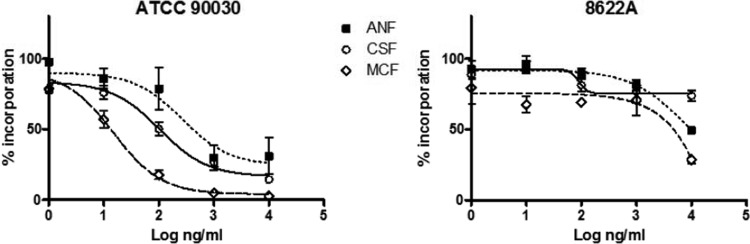
Echinocandin inhibition profiles for product-entrapped 1,3-β-d-glucan synthase enzyme complexes assessed by the incorporation of [3H]glucose into radiolabeled product. Titration curves are shown for anidulafungin (ANF), caspofungin (CSF), and micafungin (MCF) for the wild type and clinical isolate 8622A of C. glabrata.
The epidemiologic impact of using an echinocandin for antifungal prophylaxis in neutropenic patients in terms of promoting the development of multidrug-resistant Candida isolates remains unclear, but it is concerning. Prominent C. glabrata echinocandin resistance has recently been reported by Alexander et al. (8) and Pfaller et al. (9), whose studies suggested significant emergence of multidrug resistance over time to both azoles and echinocandins in C. glabrata isolates.
As echinocandins are more frequently used for the treatment of invasive candidiasis, sporadic cases of echinocandin breakthrough candidemia associated with nonsusceptible Candida isolates and treatment failure have been reported (10, 11, 19). In the present study, all five C. glabrata isolates recovered from sequential blood cultures collected during prophylaxis therapy with micafungin showed increases in the echinocandin MIC values, and all of them showed a point mutation in HS1 in the FKS2 gene that led to a S663F amino acid substitution, which has been previously described as being involved in treatment failure and echinocandin resistance in C. glabrata isolates (20). Notably, other studies have shown that a point mutation in the same position of the FKS2 gene, though with a different amino acid substitution (S663P), is the most frequently observed mutation related to echinocandin resistance in C. glabrata isolates (21, 22). It is important to note that in our case, in accordance with previous publications, the long-term echinocandin exposure (24 days) resulted in the development of FKS gene mutations and echinocandin resistance (10). On the other hand, one case of rapid echinocandin resistance in C. glabrata was reported by Lewis et al. (23) in a patient without previous or prolonged echinocandin exposure (8 days of treatment).
Our findings emphasize that the widespread use of echinocandins may increase the occurrence of echinocandin resistance, especially when using low doses, as in the present case in which the neutropenic patient was exposed to 50 mg daily. In this scenario, the use of echinocandin as prophylactic therapy should be recommended cautiously in patients with a high risk of candidemia development, now that previous exposure to echinocandins has been clearly recognized as a risk factor for the development of resistance.
Nucleotide sequence accession numbers.
The sequences generated in this study have been deposited in the GenBank (NCBI) database under accession numbers KF211452, KF211447, KF211442, KF211437, KF211456, KF211448, KF211438, KF211443, KF211455, KF211449, KF211444, KF211439, KF211454, KF211450, KF211445, KF211440, KF211453, KF211451, KF211446, KF211441, KF305827, KF305828, KF305829, KF305830, and KF305831.
ACKNOWLEDGMENTS
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil (grants 2007/08575-1 and 2012/04767-1), and the Conselho Nacional de Pesquisas Científicas e Tecnológicas (CNPq), Brazil (grant 308011/2010-4). A.C.R.S. received a doctoral fellowship from FAPESP (2012/04769-4). F.C.B. received a postdoctoral fellowship from FAPESP (grant 2010/17179-5). A.L.C. received grants from FAPESP and CNPq. D.S.P. was supported by grants from the NIH (AI069397), Pfizer, and Merck.
A.L.C. and F.L.Q. have received research and/or educational grants from Astellas, MSD, Pfizer, and United Medical in the last 2 years. D.S.P. has received research support from Merck, Pfizer, and Astellas and serves on opinion leader panels for these companies. The other authors report no conflicts of interest.
The opinions expressed in this paper are those of the authors and do not necessarily represent those of the pharmaceutical companies listed above.
Footnotes
Published ahead of print 27 January 2014
REFERENCES
- 1.Colombo AL, Garnica M, Aranha Camargo LF, Da Cunha CA, Bandeira AC, Borghi D, Campos T, Senna AL, Valias Didier ME, Dias VC, Nucci M. 2013. Candida glabrata: an emerging pathogen in Brazilian tertiary care hospitals. Med. Mycol. 51:38–44. 10.3109/13693786.2012.698024 [DOI] [PubMed] [Google Scholar]
- 2.Moretti ML, Trabasso P, Lyra L, Fagnani R, Resende MR, de Oliveira Cardoso LG, Schreiber AZ. 2013. Is the incidence of candidemia caused by Candida glabrata increasing in Brazil? Five-year surveillance of Candida bloodstream infection in a university reference hospital in southeast Brazil. Med. Mycol. 51:225–230. 10.3109/13693786.2012.708107 [DOI] [PubMed] [Google Scholar]
- 3.Nucci M, Queiroz-Telles F, Alvarado-Matute T, Tiraboschi IN, Cortes J, Zurita J, Guzman-Blanco M, Santolaya ME, Thompson L, Sifuentes-Osornio J, Echevarria JI, Colombo AL, Latin American Invasive Mycosis Network 2013. Epidemiology of candidemia in Latin America: a laboratory-based survey. PLoS One 8:e59373. 10.1371/journal.pone.0059373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfaller MA, Diekema DJ, International Fungal Surveillance Participant Group 2004. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin. Microbiol. Infect. 10(Suppl 1):11–23. 10.1111/j.1470-9465.2004.t01-1-00844.x [DOI] [PubMed] [Google Scholar]
- 5.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious Diseases Society of America 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535. 10.1086/596757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ullmann AJ, Cornely OA, Donnelly JP, Akova M, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Calandra T, Castagnola E, Garbino J, Groll AH, Herbrecht R, Hope WW, Jensen HE, Kullberg BJ, Lass-Flörl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Cuenca-Estrella M, ESCMID Fungal Infection Study Group 2012. ESCMID guideline for the diagnosis and management of Candida diseases 2012: developing European guidelines in clinical microbiology and infectious diseases. Clin. Microbiol. Infect. 18(Suppl 7):1–8. 10.1111/1469-0691.12037 [DOI] [PubMed] [Google Scholar]
- 7.Colombo AL, Guimarães T, Aranha Camargo LF, Richtmann R, Queiroz-Telles F, Salles MJC, da Cunha CA, Yasuda MAS, Moretti ML, Nucci M, Consenso Brasileiro de Infecções por Candida Group 2012. Brazilian guidelines for the management of candidiasis: a joint meeting report of three medical societies: Sociedade Brasileira de Infectologia, Sociedade Paulista de Infectologia, Sociedade Brasileira de Medicina Tropical. Braz J. Infect. Dis. 17:283–312. 10.1016/j.bjid.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer AS, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 57:1724–1732. 10.1093/cid/cit136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaller MA, Castanheira M, Lockhart SR, Ahlquist AM, Messer SA, Jones RN. 2012. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J. Clin. Microbiol. 50:1199–1203. 10.1128/JCM.06112-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeiffer CD, Garcia-Effron G, Zaas AK, Perfect JR, Perlin DS, Alexander BD. 2010. Breakthrough invasive candidiasis in patients on micafungin. J. Clin. Microbiol. 48:2373–2380. 10.1128/JCM.02390-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Effron G, Kontoyiannis DP, Lewis RE, Perlin DS. 2008. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob. Agents Chemother. 52:4181–4183. 10.1128/AAC.00802-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Souza ACR, Ferreira RC, Gonçalves SS, Quindós G, Eraso E, Bizerra FC, Briones MRS, Colombo AL. 2012. Accurate identification of Candida parapsilosis (sensu lato) using mitochondrial DNA and real-time PCR. J. Clin. Microbiol. 50:2310–2314. 10.1128/JCM.00303-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White TJ, Bruns TD, Lee SB, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322 In Innis MA, Gelfand DH, Sninsky JJ, White TJ. (ed), PCR protocols: a guide to methods and applications. Academic Press, Inc., San Diego, CA [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—3rd ed. CLSI document M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement. CLSI document M27-S4 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 16.Castanheira M, Woosley LN, Diekema DJ, Messer SA, Jones RN, Pfaller MA. 2010. Low prevalence of fks1 hot spot 1 mutations in a worldwide collection of Candida strains. Antimicrob. Agents Chemother. 54:2655–2659. 10.1128/AAC.01711-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Effron G, Lee S, Park S, Cleary JD, Perlin DS. 2009. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-beta-d-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother. 53:3690–3699. 10.1128/AAC.00443-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Effron G, Park S, Perlin DS. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob. Agents Chemother. 53:112–122. 10.1128/AAC.01162-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S, Kelly R, Kahn JN, Robles J, Hsu MJ, Register E, Li W, Vyas V, Fan H, Abruzzo G, Flattery A, Gill C, Chrebet G, Parent SA, Kurtz M, Teppler H, Douglas CM, Perlin DS. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264–3273. 10.1128/AAC.49.8.3264-3273.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimbeck AJ, Iqbal N, Ahlquist AM, Farley MM, Harrison LH, Chiller T, Lockhart SR. 2010. FKS mutations and elevated echinocandin MIC values among Candida glabrata isolates from U.S. population-based surveillance. Antimicrob. Agents Chemother. 54:5042–5047. 10.1128/AAC.00836-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Effron G, Chua DJ, Tomada JR, Dipersio J, Perlin DS, Ghannoum M, Bonilla H. 2010. Novel FKS mutations associated with echinocandin resistance in Candida species. Antimicrob. Agents Chemother. 54:2225–2227. 10.1128/AAC.00998-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa-de-Oliveira S, Marcos Miranda I, Silva RM, Pinto e Silva A, Rocha R, Amorim A, Gonçalves Rodrigues A, Pina-Vaz C. 2011. FKS2 mutations associated with decreased echinocandin susceptibility of Candida glabrata following anidulafungin therapy. Antimicrob. Agents Chemother. 55:1312–1314. 10.1128/AAC.00589-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis JS, Jr, Wiederhold NP, Wickes BL, Patterson TF, Jorgensen JH. 2013. Rapid emergence of echinocandin resistance in Candida glabrata resulting in clinical and microbiologic failure. Antimicrob. Agents Chemother. 57:4559–4561. 10.1128/AAC.01144-13 [DOI] [PMC free article] [PubMed] [Google Scholar]


