Abstract
Objectives
We sought to evaluate the impact of coronary artery calcium (CAC) burden and regional distribution on the need for and type of future coronary revascularization (percutaneous [PCI] vs. surgical [CABG]) among asymptomatic individuals.
Background
The need for coronary revascularization and the chosen mode of revascularization are thought to be a function of disease burden and anatomic distribution. The association between the baseline burden and regional distribution of CAC and the risk and type of future coronary revascularization remains unknown.
Methods
6,540 MESA participants (individuals aged 45-84 years, free of known baseline cardiovascular disease) with vessel-specific CAC measurement were followed for median 8.5 (7.7 – 8.6) years. Annualized rates and multivariable adjusted hazard ratios for revascularization and revascularization type were analyzed according to CAC score category, number of vessels with CAC (0-4, including the left main), and by involvement of individual coronary arteries.
Results
A total of 265 revascularizations (4.2%) occurred during follow-up, and 206 (78% of total) were preceded by adjudicated symptoms. Revascularization was uncommon when CAC=0 (0.6%), with graded increase over both rising CAC burden and increasingly diffuse CAC distribution. The revascularization rate per 1,000 person-years for CAC 1-100, 101-400, and >400 was 4.9, 11.7 and 25.4; for 1, 2, 3, and 4 vessels with CAC the rates were 3.0, 8.0, 16.1, and 24.8. In multivariable models adjusting for CAC score, number of vessels with CAC remained predictive of mode of revascularization. Independent predictors of CABG vs. PCI included 3 or 4 vessel CAC, higher CAC burden, and involvement of the left main. Risk for CABG was extremely low with <3 vessel baseline CAC. Results were similar when considering only symptom-driven revascularizations.
Conclusions
In this multi-ethnic cohort of asymptomatic individuals, baseline CAC was highly predictive of future coronary revascularization procedures, with measures of CAC burden and distribution each independently predicting need for PCI vs. CABG over 8.5 year follow-up.
Keywords: cardiac CT, coronary artery calcium, coronary artery disease, revacularization
Introduction
Measurement of the total coronary artery calcium (CAC) score (Agatston score) using non-contrast cardiac gated computed tomography provides an excellent estimation of cardiovascular risk via its strong correlation with total coronary atherosclerotic burden. Moderate to high CAC is a strong independent predictor of hard cardiovascular events including myocardial infarction and death1-4. In contrast, absence of CAC among asymptomatic patients identifies a low risk population with <1% estimated 10-year risk of cardiovascular mortality5-7 and a low probability of significant coronary artery disease on invasive coronary angiography5. When added to traditional risk prediction scores, CAC scoring provides significant improvement in risk discrimination and risk reclassification across gender and ethnic groups2, 8, 9, 10.
While CAC is a strong marker of future cardiovascular risk, the extent to which regional distribution of CAC provides additional risk information beyond the Agatston score has not been fully explored. A prior analysis from a registry of more than 25,000 individuals suggested that left main or multi-vessel CAC may identify a higher risk group independent of the overall CAC score11. Further supporting the potential importance of CAC distribution, there is a significant association between the burden of CAC within an individual coronary artery and severity of angiographic stenosis within the same artery12.
Revascularization remains an important clinical endpoint, and the need for and chosen method of revascularization are directly influenced by the overall burden and distribution of angiographic coronary artery disease. For example, coronary artery bypass grafting (CABG) is commonly associated with more diffusely distributed angiographic coronary atherosclerosis compared to use of percutaneous coronary intervention (PCI). Thus, mode of revascularization provides an excellent opportunity to test the importance of the anatomical information inherent in measures of regional CAC distribution that are not accounted for in the traditional Agatston score.
We first sought to evaluate whether the distribution of subclinical atherosclerosis, as measured by CAC, was independently and incrementally associated with risk of future revascularization. We then sought to evaluate whether the overall burden and distribution of CAC was associated with a specific mode of revascularization (percutaneous vs. surgical), hypothesizing that increasingly diffuse CAC would be preferentially associated with future surgical revascularization.
Methods
Study Population
Full details of the MESA study design have been published previously13. In brief, MESA is a prospective observational cohort of 6,814 men and women age 45-84 years from different ethnic origins (White, Black, Hispanic, and Chinese) with no known baseline clinical cardiovascular disease who were asymptomatic at the time or enrollment. Individuals were enrolled between July 2000 and September 2002 at six field centers across the United States (Baltimore; Chicago; Forsyth County, North Carolina; Los Angeles; New York City; and St. Paul, Minnesota). The study protocol was approved by the institutional review board at each site and all participants provided written informed consent.
Risk Factor Measurement
As part of the baseline examination, staff at each of the six centers collected information about cardiovascular risk factors including medical history, smoking history, blood pressure measurement, anthropometric measurements, and laboratory data as previously described13. A central laboratory (University of Vermont, Burlington, VT) measured levels of total and HDL cholesterol, triglycerides, plasma glucose, and high sensitivity C-reactive protein after a 12-hour fast.
CAC Measurement
As previously described by Carr et al.14, all MESA study participants underwent measurement of CAC by cardiac CT. Participants were scanned twice and CAC was reported as the average CAC (Agatston)15 score. Vessel-specific CAC measurements were performed in 6,540 (96%) MESA participants. Individuals were told after the baseline visit (2000-2002) whether they had no CAC, less than average, average, or greater than average CAC and encouraged to discuss the results with their physician.
Regional CAC was analyzed according to: 1) number of vessels with CAC, defined as the number of main coronary arteries (left main, left anterior descending, left circumflex, and right coronary) with calcification (values ranging from 0 – 4); and 2) involvement of individual coronary arteries. “Three-vessel CAC” was defined as involvement of either the left main or LAD in addition to CAC in the left circumflex and the right coronary artery.
Follow-up
Participants were followed for a median of 8.5 (interquartile range [IQR] 7.7 - 8.6) years. At intervals of 9 to 12 months, an interviewer contacted each participant or family member by telephone to inquire about interim revascularization, hospital admission, or death. To verify self-reported diagnoses, MESA obtained medical records for approximately 98% of hospital events and 95% of outpatient diagnoses. Two physicians from the MESA mortality and morbidity review committee independently classified events. In the event of disagreement, the full committee made the final classification.
The primary endpoints for this study were time-to-first revascularization, time-to-first CABG, and time-to-first PCI. In mode of revascularization analyses, all CABG events were considered. For these analyses, we excluded PCIs that followed a CABG procedure.
At the time of hospitalization, prior to the revascularization procedure, individuals were classified as having preceding adjudicated myocardial infarction, angina, or neither. The diagnosis of myocardial infarction was based on a combination of symptoms, electrocardiographic findings, and levels of cardiac biomarkers. A classification of angina required symptoms of chest pain (or other related symptoms), a physician diagnosis of angina, and medical treatment for the symptoms. Revascularization or a physician diagnosis of angina/CHD without documented symptoms was not considered angina.
For sensitivity analyses, “symptom-driven revascularization” was defined as having adjudicated myocardial infarction or angina within 365 days prior to revascularization.
Statistical Analysis
The baseline characteristics of the study population were analyzed according to revascularization status. Frequencies and proportions were calculated for categorical variables, and either means with standard deviations or medians with interquartile ranges calculated for continuous variables. Differences between the two groups were calculated using Chi-square test, t-test, or non-parametric testing where appropriate.
Kaplan-Meier curves were constructed expressing time-to-revascularization as a function of number of vessels with CAC (0, 1, 2, 3, and 4) and CAC score groups (0, 1-100, 101-400 and > 400).
To evaluate the predictive value of CAC score and CAC distribution on need for subsequent revascularization and revascularization type, annualized absolute event rates (number of events divided by number of person-years at risk) were calculated after stratification by CAC distribution (0, 1, 2, 3, and 4 vessels with CAC) and CAC score category (0, 1-100, 101-400, and > 400). Multivariable adjusted Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CI) with CAC=0 as the reference group. Models were adjusted for age, gender, race/ethnicity, smoking, diabetes mellitus, hypertension, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, C-reactive protein, family history of family history of coronary heart disease, anti-hypertensive medication use, lipid lowering medication use, education level, and MESA study site. To test for an additive value of regional measures of CAC beyond traditional CAC scoring, additional models were constructed in the subgroup of participants with CAC>0 further adjusting for the CAC score group.
We then compared specific CAC characteristics among those who underwent revascularization, according to the chosen mode of revascularization (PCI vs. CABG). Among the few participants with baseline CAC=0 who ultimately underwent revascularization, we produced a descriptive analysis summarizing the demographic, risk factor, serial CAC scanning, adjudicated symptomatology, and mode of revascularization data.
Sensitivity Analyses
Sensitivity analyses were performed excluding individuals who underwent early revascularization within 90 days of CAC scanning (2 individuals). Among individuals who underwent revascularization, there were a small number of individuals (22% of revascularizations) without MESA-adjudicated angina or myocardial infarction within 365 days revascularization. To confirm that inclusion of these individuals did not cause biased results that could be directly attributable to CAC testing, sensitivity analyses were performed among only those individuals with a MESA-adjudicated diagnosis of angina or myocardial infarction at hospitalization prior to revascularization.
Additionally, to evaluate whether change in medication usage had an impact on revascularization rates, sensitivity analysis was performed adjusting for change in medication (ACE inhibitors, angiotensin receptor blockers, amlodipine, thiazide diuretics, beta blockers, fibrates, niacin, statins, and aspirin) prior to revascularization. This analysis required exclusion of individuals who underwent revascularization prior to exam 2 (in whom change in medications was likely a result of revascularization).
Results
Clinical characteristics of the study cohort
The average age for the study cohort was 62.2 ± 10.2 years, with slightly more than half female (52.8%). A total of 265 participants (4.1%) underwent revascularization, including 206 (78%) symptom-driven revascularizations, with the majority of the occurring shortly after the diagnosis of myocardial infarction or angina (Figure 1). Individuals undergoing revascularization were more likely to be men and Caucasian. As expected, the cardiovascular risk profile was less favorable for those requiring revascularization compared to those without revascularization (Table 1). Participants treated with revascularization had a significantly higher median CAC score (254 [IQR 66-741] vs. 0 [IQR 0-70] p< 0.001) and number of vessels with CAC (2.7 ± 1.2 vs. 1.0 ± 1.3 p<0.001) than those not requiring revascularization.
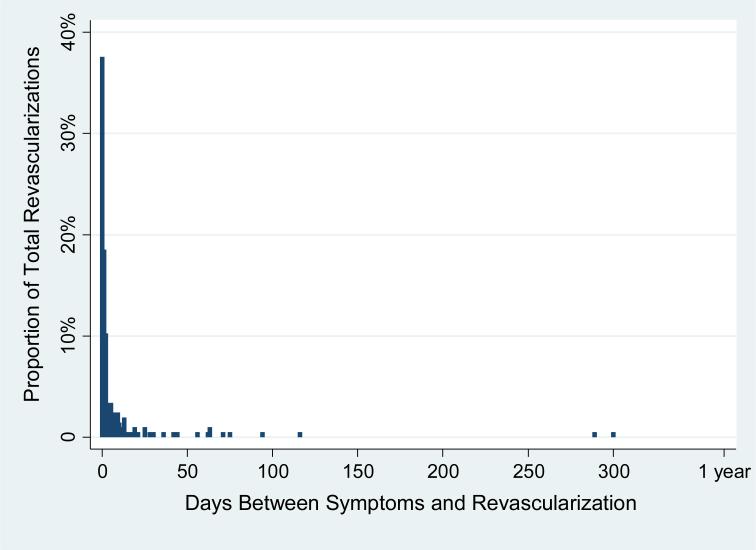
Figure 1. Time Elapsed Between MESA Adjudicated MI or Angina and Revascularization.
A majority of all revascularizations occurred within 10 days after adjudicated symptoms.
Table 1.
Baseline Characteristics by Coronary Revascularization Status.
| Characteristics | No Revascularization | Revascularization | p-value |
|---|---|---|---|
| N=6,275 (95.9%) | N=265 (4.1%) | ||
| Age (mean, years) | 62 ± 10 | 66 ± 9 | <0.001 |
| Gender (%, female) | 54 | 24 | <0.001 |
| Race/ethnicity (%) | <0.001 | ||
| White | 38 | 52 | |
| Black | 28 | 20 | |
| Hispanic | 23 | 20 | |
| Chinese | 12 | 8 | |
| Smoking (%, current) | 13 | 15 | 0.004 |
| Pack years (mean) | 11 ± 22 | 17 ± 28 | <0.001 |
| Hypertension (%) | 44 | 62 | <0.001 |
| Systolic blood pressure (mean) | 126 ± 21 | 132 ± 22 | <0.001 |
| Diastolic blood pressure (mean) | 72 ± 10 | 73 ± 11 | 0.05 |
| Anti-hypertensive medication (%) | 37 | 54 | <0.001 |
| Diabetes (%) | 12 | 24 | <0.001 |
| LDL (mg/dL, mean) | 117 ± 31 | 123 ± 35 | <0.001 |
| HDL (mg/dL, mean) | 51 ± 15 | 45 ± 14 | <0.001 |
| Triglycerides (mg/dL, median, IQR) | 111 (77 – 159) | 123 (90 – 189) | 0.001 |
| Lipid lowering medication (%) | 16 | 25 | <0.001 |
| hsCRP (mg/dL, median, IQR) | 1.9 (0.8 – 4.2) | 2.0 (1.0 – 4.7) | 0.08 |
| Family history of CHD (%) | 42 | 59 | <0.001 |
| Education (%) | 0.22 | ||
| Bachelor's degree | 35 | 41 | |
| No Bachelor's degree | 65 | 59 | |
| CAC presence (%) | 48 | 93 | <0.001 |
| CAC score (% total) | <0.001 | ||
| 0 | 52 | 7 | |
| 1-100 | 27 | 24 | |
| 100-400 | 13 | 29 | |
| >400 | 9 | 40 | |
| Agatston score (median, IQR) | 0 (0 – 70) | 254 (66 – 741) | <0.001 |
| Mean # vessels with CAC | 1.03 ± 1.3 | 2.7 ± 1.2 | <0.001 |
CAC and revascularization
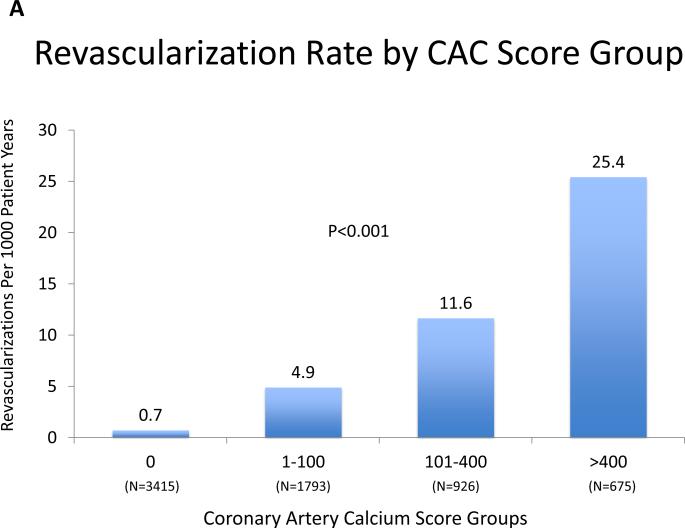
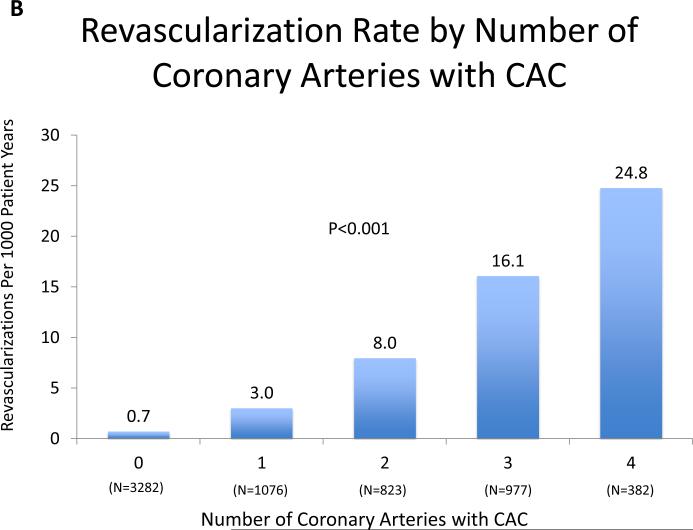
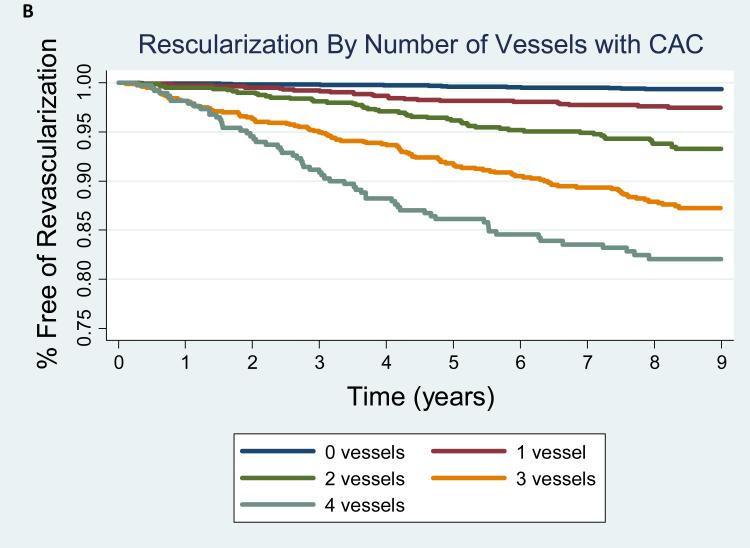
The frequency of revascularization increased with increasing CAC score (CAC 0 [19 events, 0.6%), CAC 1-100 [64 events, 3.8%), CAC 101-400 [76 events, 9.4%], CAC>400 [106 events, 19.8%] and with the total number of vessels with CAC (0 vessels [19, 0.6%), 1 vessel [25 events, 2.4%], 2 vessels [49 events, 6.3%], 3 vessels [110 events, 12.7%], 4 vessels [62 events, 19.4%]. The annualized revascularization rates increased according to CAC score category from 0.7 events per 1000 person-years for CAC = 0 to 25.4 events per 1000 person-years for CAC score >400 (Figure 2A). Similarly, the rate of revascularization increased proportionally to the number of vessels with CAC from an annualized event rate of 0.7 events per 1000 person-years to 24.8 events per 1000 person-years when 4 vessels were involved (Figure 2B). Kaplan-Meier estimates of revascularization-free survival according to CAC score and number of vessels with CAC are shown in Figure 3A and 3B, respectively.
Figure 2AB. Revascularization Rate by CAC Score Group and Number of Coronary Arteries with CAC.
There is a strong, statistically significant increase in revascularization with both increasing CAC score group and increasingly diffuse CAC.
Figure 3AB. Kaplan Meier Estimates of Revascularization-Free Survival by CAC Burden and Distrubution.
Logrank p<0.001 for both models.
Table 2 shows the risk of revascularization associated with CAC score and number of vessels with CAC after comprehensive adjustment for cardiovascular risk factors. The hazard ratio increases stepwise to >16-fold risk for both CAC score >400 and 4 vessel CAC compared to CAC = 0. CAC distribution remained a significant predictor even after concomitant adjustment for the CAC score. Among those individuals with CAC >0, the number of coronary vessels with CAC remained a strong, independent predictor of revascularization with a more than 4-fold increased risk associated with 4 vessel CAC compared to CAC in 1 vessel. Supplemental tables 1A and 1B repeat these analyses for individual revascularization types (PCI and CABG), with similar results for each revascularization type.
Table 2.
Multivariable-Adjusted Hazard Ratios (HR) for Incident Coronary Revascularization
| HR | 95% CI | p-value | |
|---|---|---|---|
| All Subjects (N=6540)* | |||
| CAC Group | |||
| 0 | 1.0 | ||
| 1-100 | 4.4 | 2.6 – 7.5 | <0.001 |
| 101-400 | 9.8 | 5.8 – 16.8 | <0.001 |
| >400 | 17.5 | 10.1 – 30.3 | <0.001 |
| # of vessels with CAC | |||
| 0 | 1.0 | ||
| 1 | 2.6 | 1.4 – 4.8 | 0.004 |
| 2 | 7.0 | 4.0 – 12.3 | <0.001 |
| 3 | 12.2 | 7.2 – 20.7 | <0.001 |
| 4 | 16.8 | 9.6 – 29.6 | <0.001 |
| Subjects with CAC>0 (N=3259)** | |||
| # of vessels with CAC | |||
| 1 | 1.0 | ||
| 2 | 2.5 | 1.5 – 4.4 | 0.001 |
| 3 | 3.4 | 1.9 – 6.0 | <0.001 |
| 4 | 4.1 | 2.2 – 7.7 | <0.001 |
Model adjusted for age, gender, race/ethnicity, diabetes, smoking, hypertension, LDL-C, HDL-C, triglycerides, CRP, family history, anti-hypertensive medication use, lipid-lowering medication use, education level, and MESA study site.
Model additionally adjusted for CAC group (1-100, 101-400, >400)
Mode of revascularization according to CAC distribution
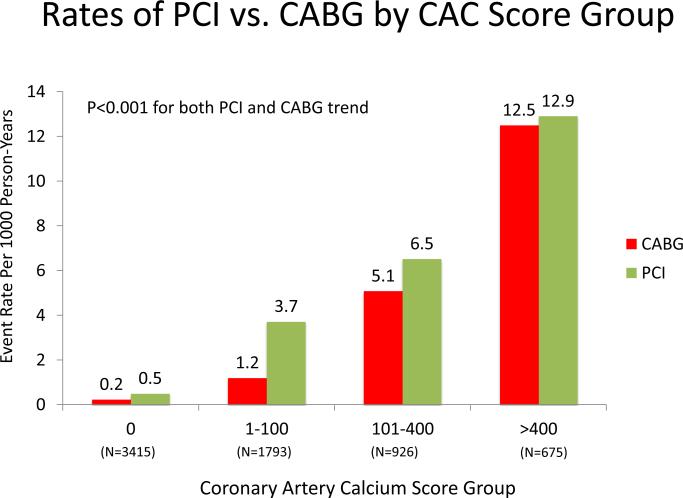
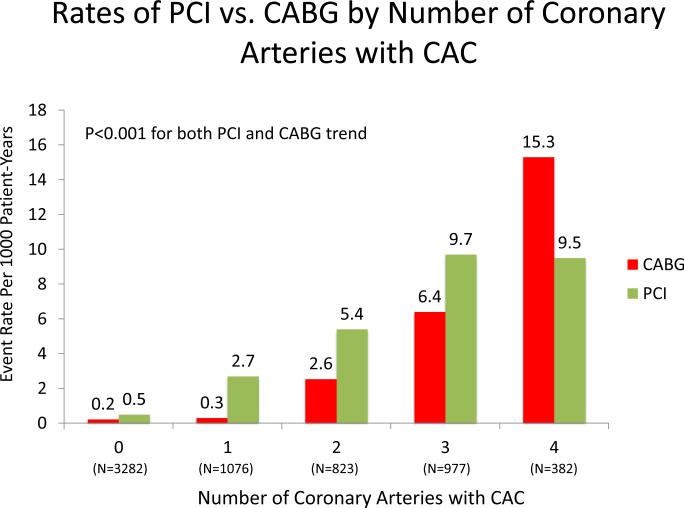
Among individuals who underwent revascularization, PCI was more common than CABG (154 vs. 111). Figure 4A and 4B compare the absolute annualized event rates of PCI vs. CABG by CAC score category and number of vessels, respectively. Rates for both PCI and CABG increase with higher CAC scores; however, at all CAC score categories, PCI was more common. In contrast, when the population was stratified by number of vessels with CAC, rates of CABG were higher than for PCI among individuals with baseline 4-vessel CAC.
Figure 4AB. Rates of CABG vs. PCI by CAC Burden and Distribution.
There is a strong, statistically significant increase in both PCI and CABG with both increasing CAC score group and increasing number of coronary arteries with CAC.
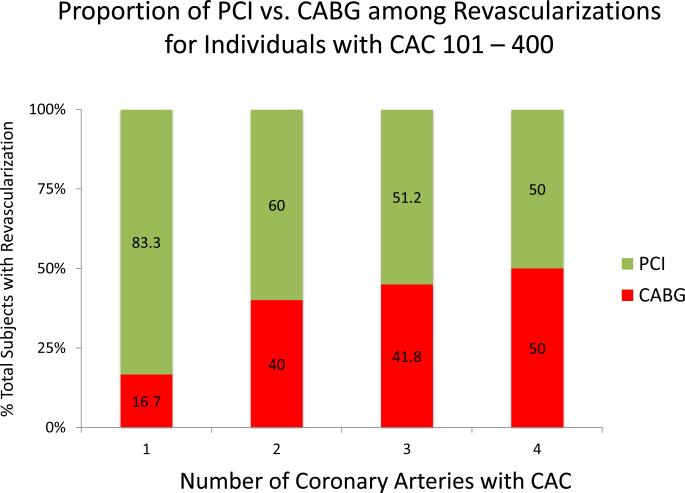
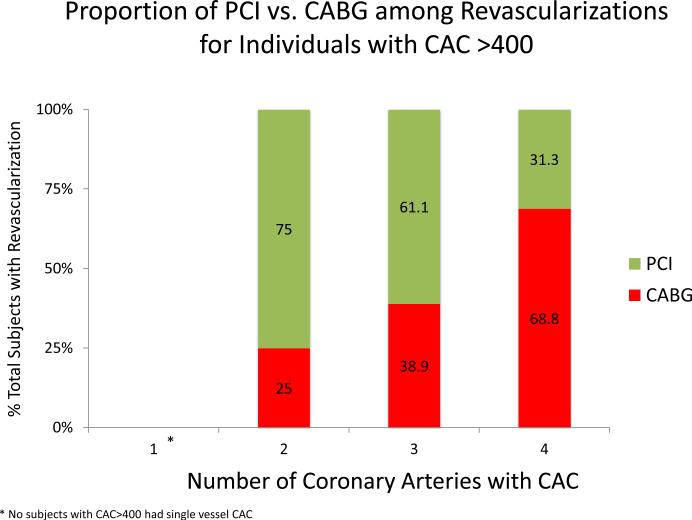
Figure 5 demonstrates the impact of CAC distribution on types of revascularization across CAC score categories (CAC 1-100, 101-400, and >400). Within all CAC score categories, more diffuse CAC was associated with an increased proportion of CABG revascularizations. Among individuals with CAC > 400, 4-vessel CAC is associated with a greater proportion of individuals undergoing CABG than PCI.
Figure 5ABC. Proportion of Revascularizations that are PCI vs. CABG by CAC Burden and Distribution.
CABG is more frequent with increasing CAC score group and with more diffusely distributed CAC. CABG becomes the predominant mode of revasculariation when all coronary arteries are diseased at baseline.
Table 3 demonstrates the specific characteristics of CAC distribution according to mode of revascularization. Individuals who underwent CABG in general had a significantly higher CAC score in the left main, left anterior descending, left circumflex, and right coronary arteries (p<0.01). Among participants who underwent CABG, 42% had left main involvement compared to 22% of participants who underwent PCI (p=0.001). A total of 74% of individuals who underwent CABG had “3-vessel CAC” compared to only 51% of those who underwent PCI (p<0.001). Just 8% of all CABGs occurred in participants with no CAC or 1-vessel CAC, whereas these individuals accounted for 23% of all those treated with PCI (p<0.001).
Table 3.
CAC characteristics of participants who underwent incident coronary revascularization stratified by mode of revascularization
| CAC distribution | PCI | CABG | p-value |
|---|---|---|---|
| N=154 | N=111 | ||
| Time from CAC measurement to revascularization (years, mean) | 3.6 ± 2.3 | 4.0 ± 2.4 | 0.19 |
| By vessel, CAC prevalence and score | |||
| Left Main (%) | 22 | 42 | <0.001 |
| • LM CAC score (mean) | 17.0 ± 63.8 | 26.8 ± 72.8 | 0.008* |
| Left Anterior Descending (%) | 86 | 92 | 0.12 |
| • LAD CAC score (mean) | 159 ± 210 | 298 ± 300 | <0.001* |
| Left Circumflex (%) | 66 | 86 | <0.001 |
| • LCx CAC score (mean) | 95 ± 166 | 244 ± 382 | <0.001* |
| Right Coronary Artery (%) | 66 | 80 | 0.009 |
| • RCA score (mean) | 125 ± 300 | 333 ± 592 | <0.001* |
| Total CAC score, all vessels (mean) | 396 ± 550 | 901 ± 1131 | <0.001 |
| Number of vessels (mean) | 2.4 ± 1.2 | 3.0 ± 1.1 | <0.001 |
| ≥3 vessel CAC (%) | 51 | 74 | <0.001 |
P-values calculated using non-parametric Kruskal-Wallis testing.
Revascularization among individuals with baseline CAC = 0.
Revascularization during median 8.5 years of follow-up was extremely rare among participants with a baseline CAC score = 0 (19/3281, 0.6%). Median time to revascularization for this group was 4.6 (1.7-6.0) years. Table 4 shows person-level characteristics of individuals with a baseline CAC score = 0 who ultimately required revascularization. 15 of the 19 participants were classified as having either angina or a myocardial infarction within 365 days of revascularization. Of the 12 participants with baseline CAC = 0 who had repeat a MESA protocol-driven CAC measurement prior to revascularization, 6 (50%) had developed interim CAC>0.
Table 4.
Description of participants with baseline CAC=0 who subsequently required coronary revascularization
| Age | Gender | Race/ethnicity | Smoking | Diabetes | HTN | Family History | Time (years) from baseline to most proximal CAC scan | CAC on repeat scan? | Time of revasc (years) from baseline | Revasc type | MI or Angina prior to revasc? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 63 | Male | White | Former | No | Yes | Yes | 3.5 | No | 4.6 | PCI | No |
| 67 | Male | White | Yes | No | No | Yes | - | - | 1.0 | PCI | Yes |
| 48 | Male | Black | No | No | Yes | No | 4.3 | No | 5.1 | PCI | Yes |
| 57 | Male | Hispanic | Former | Yes | Yes | No | 1.8 | No | 7.4 | PCI | No |
| 52 | Female | White | Former | No | No | Yes | 2.8 | Yes | 4.9 | PCI | Yes |
| 78 | Male | Black | No | Yes | Yes | No | - | - | 3.0 | PCI | No |
| 46 | Male | Hispanic | Yes | No | No | No | 4.8 | Yes | 6.0 | PCI | Yes |
| 56 | Female | White | No | No | No | Yes | - | - | 0.4 | PCI | Yes |
| 59 | Female | Black | Former | Yes | No | No | - | - | 0.3 | PCI | Yes |
| 68 | Female | Hispanic | No | Yes | Yes | Yes | - | - | 1.7 | PCI | Yes |
| 55 | Male | White | Former | No | No | Yes | - | - | 3.0 | PCI | Yes |
| 65 | Male | White | Former | No | Yes | No | 4.3 | Yes | 4.8 | PCI | Yes |
| 59 | Male | White | Former | No | Yes | Yes | 3.3 | No | 7.5 | PCI | Yes |
| 59 | Female | White | Yes | No | No | Yes | 1.2 | Yes | 7.3 | CABG | Yes |
| 71 | Female | White | Former | No | No | Yes | 3.3 | Yes | 4.3 | CABG | Yes |
| 54 | Female | Black | Former | No | Yes | Yes | 1.7 | No | 5.8 | CABG | Yes |
| 51 | Male | Hispanic | Yes | Yes | Yes | Yes | 1.5 | No | 4.0 | CABG | Yes |
| 70 | Female | Black | Former | No | No | No | - | - | 1.5 | CABG | Yes |
| 65 | Male | Hispanic | No | Yes | Yes | No | 4.6 | Yes | 7.9 | CABG | No |
Sensitivity Analyses
The results of the sensitivity analysis excluding the 2 individuals who underwent early revascularization within 90 days of CAC scanning were identical to the main analysis, and thus are not shown.
A total of 206 out of 265 individuals undergoing revascularization (78%) were classified as having symptom-driven revascularization. Within this subset, revascularization rates and multivariable adjusted risks of revascularization associated with increasing CAC score and number of vessels with CAC were similar to the trends noted for the overall population (supplemental figures 1 and 2, and supplemental table 2).
The risk of revascularization associated with increasing CAC score and number of vessels with CAC was similar to the primary analysis after adjustment for change in medication use between MESA visit 1 and visit 2 (supplemental table 3).
Discussion
In this multi-ethnic cohort of asymptomatic individuals, we demonstrate that the distribution of CAC on baseline scans provides incremental risk information to the Agatston score for predicting need for future coronary revascularization over 9 year follow-up. Individuals with a baseline CAC score of >400 or CAC in all 4 coronary vessels had an approximately 25% risk of revascularization at 8.5 year follow-up, compared to a rate of < 1% among those with zero CAC. Importantly, we also found that both the total burden and distribution of CAC were predictive of the mode of revascularization. A higher CAC burden, more diffuse distribution of CAC, and left main involvement were all strongly associated with need for CABG vs. PCI. CABG was particularly uncommon among participants with zero CAC or 1 vessel CAC (8%), whereas 74% of the 111 total CABGs occurred in people with at least 3-vessel CAC at baseline. To our knowledge, this is the first study evaluating the relationship between CAC distribution and subsequent need for surgical versus percutaneous revascularization.
Predictors of Revascularization
Since Agatston et al15 first demonstrated the utility of computed tomography in detecting and quantifying CAC, multiple studies have established the value of CAC in asymptomatic individuals in predicting cardiovascular events including myocardial infarction and death1-4. The utility of CAC has been shown to extend across gender and ethnic groups and adds significant improvement in risk reclassification when added to standard risk factors or risk factor scores2, 8, 9. Recently CAC has been shown to provide superior discrimination and risk reclassification compared with other common markers of cardiovascular risk when added to the Framingham Risk Score or the Reynolds score16.
Some prior analyses have included revascularization as part of a combined composite cardiovascular endpoint, but the relationship between CAC and revascularization has not been independently evaluated. Although revascularization is often considered a “soft” endpoint, it accounts for large health care expenditures, exceeding $28 billion annually in the United States17,18 and remains an important outcome for both patients and physicians. Furthermore, there is increasing interest in exploring the additional prognostic utility of measuring regional or vessel-specific CAC above and beyond the total burden of CAC.
Regional Measures of CAC Distribution and Coronary Stenosis
CAC has been shown to correlate with myocardial perfusion defects5, 19-21, a marker of ischemia and a surrogate for anatomic stenosis that is a common indication for revascularization. Schuif et al21 performed a comparative regional analysis of CAC scores versus vessel-specific myocardial perfusion imaging in 140 patients with clinically suspected coronary artery disease. The average calcium score in coronary arteries with normal myocardial perfusion on SPECT was 69 +/- 167, whereas a significantly higher calcium score of 272 +/- 646 was noted for coronary arteries with abnormal myocardial perfusion (P < 0.001).
Furthermore, previous studies have evaluated the relationship between CAC and angiographic coronary artery stenosis12,21. Budoff et al22 previously reported in symptomatic individuals that both increasing CAC burden and number of vessels with CAC are each independently associated with increased likelihood of angiographically significant disease. The reported sensitivity of CAC to detect significant angiographic disease (>50% stenosis) was 95%, with a specificity of 44%. Notably, the specificity of CAC increased substantially with increasing number of vessels with CAC22.
In addition to total CAC score and number of vessels with CAC, vessel-specific CAC score has been shown to be a robust predictor of angiographic coronary stenosis12,23. Voros et al have shown that lesion- and vessel-specific CAC scoring are superior to total Agatston scores for the prediction of obstructive CAD23.
In our study, although we do not directly evaluate the relationship between CAC and myocardial perfusion defects or angiographic stenosis, we do evaluate the association between CAC and revascularization, which is often driven by anatomic stenosis or ischemia.
Zero Coronary Artery Calcium Score
There has been a tremendous amount of interest in the potential clinical utility of CAC=05-7. Blaha et al.6 studied more than 44,000 asymptomatic patients referred for CAC scoring. More than 19,000 individuals had CAC=0 and had an excellent prognosis with an estimated 10-year mortality of approximately 1%. In addition, individuals with CAC >10 had a 4- to 8-fold increased risk of dying over 10 year compared to those with CAC=0. The excellent prognosis of CAC=0 in asymptomatic individuals persists among the elderly, women, people with diabetes, and across ethnic groups24.
In our current analysis of individuals who were asymptomatic and free of known cardiovascular disease at enrollment, we found that the rate of revascularization during 8.5 years of follow-up was extraordinarily low among individuals with a baseline CAC score = 0 (0.7%). Interestingly, 50% of individuals with baseline CAC=0 who were rescanned before revascularization had incident CAC on a subsequent protocol-driven CAC scan, suggesting that measurable interval progression of coronary atherosclerosis occurred. Progression of coronary artery calcium has been shown to be associated with diabetes, metabolic syndrome, other traditional risk factors,25,26 and has been shown to predict total and hard CHD events in asymptomatic individuals both with and without baseline CAC >027.
Limitations
MESA participants received limited information about their baseline CAC scans and were instructed to share these limited results with their physicians. It is possible that this knowledge may have influenced the clinical evaluation, thus potentially biasing the results toward a stronger relationship between CAC and revascularization. However, in our sensitivity analysis excluding revascularizations performed within 90 days of CAC scoring, we oberved an equally significant relationship between CAC and revascularization. Furthermore the Kaplan-Meier estimates show that the majority of revascularization events occurred proportionally and remotely. This suggests that the limited communication of CAC scores in MESA had little impact on revascularization.
Alternatively, as a result of the limited knowledge of the CAC scan results, individuals with elevated CAC may have had more aggressive risk factor modification thereby reducing revascularizations and possibly weakening the relationship between CAC and revascularization. However, there was no significant difference in the change in use of aspirin or statins by CAC score groups. Furthermore, when adjusted for change in medication use, the risk of revascularization was similar to the primary analysis.
Additionally, revascularization as an outcome is subject to differences in physician preference and regional practice, and in some cases may have been done in the absence of symptoms. Furthermore, there has likely been a significant temporal shift in the application of PCI during the time of the study whereas the indications for CABG have remained largely constant. Despite these concerns, rates for both PCI and CABG were proportional during the course of the study, and remain increasingly important outcomes for patients and physicians.
Conclusions
In a multi-ethnic cohort of individuals free of baseline CVD, the overall burden and distribution of CAC are each highly predictive of future coronary revascularization including both PCI and CABG. Among individuals undergoing revascularization, more diffuse CAC was predictive of CABG compared to PCI. Additional research is necessary to further define whether the anatomic distribution of CAC can add to the risk stratification of other cardiovascular endpoints.
Supplementary Material
Acknowledgements
We thank the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding
This research was supported by the National Institutes of Health / National Heart, Lung, and Blood Institute [N01-HC-95159 through N01-HC-95169], National Institutes of Health / National Heart, Lung, and Blood Institute T32-HL-7227-36 to MGS, JRH and MJBlaha, and National Institutes of Health L30 HL110027 to MJBlaha.
Abbreviations
- CAC
Coronary artery calcium
- PCI
Percutaneous coronary intervention
- CABG
Coronary artery bypass surgery
- MESA
Multi-Ethnic Study of Atherosclerosis
- CHD
Coronary heart disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 2.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291(2):210–5. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 3.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158–65. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 4.LaMonte MJ, FitzGerald SJ, Church TS, Barlow CE, Radford NB, Levine BD, et al. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol. 2005;162(5):421–9. doi: 10.1093/aje/kwi228. [DOI] [PubMed] [Google Scholar]
- 5.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Hoffman U, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675–88. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Budoff MJ, McClelland RL, Nasir K, Greenland P, Kronmal RA, Kondos GT, et al. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J. 2009;158(4):554–61. doi: 10.1016/j.ahj.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303(16):1610–6. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erbel R, Möhlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56(17):1397–406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122(25):2748–64. doi: 10.1161/CIR.0b013e3182051bab. [DOI] [PubMed] [Google Scholar]
- 11.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49(18):1860–70. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 12.Rosen BD, Fernandes V, McClelland RL, Carr JJ, Detrano R, Bluemke DA, Lima JA. Relationship between baseline coronary calcium score and demonstration of coronary artery stenoses during follow-up MESA (Multi-Ethnic Study of Atherosclerosis). JACC Cardiovasc Imaging. 2009;2:1175–1183. doi: 10.1016/j.jcmg.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. American Journal of Epidemiology. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 14.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 15.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 16.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308(8):788–95. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagal PC, Smith AW. Review of Recent US Cost Estimates of Revascularization. Am J Manag Care. 2004;10:S370–S376. [PubMed] [Google Scholar]
- 18.Shaw LJ, Min JK, Budoff M, Gransar H, Rozanski A, Hayes SW, Friedman JD, Miranda R, Wong ND, Berman DS. Induced cardiovascular procedural costs and resource consumption patterns after coronary artery calcium screening: results from the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) study. J Am Coll Cardiol. 2009;54:1258–67. doi: 10.1016/j.jacc.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 19.He ZX, Hedrick TD, Pratt CM, Verani MS, Aquino V, Roberts R, et al. Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000;101(3):244–51. doi: 10.1161/01.cir.101.3.244. [DOI] [PubMed] [Google Scholar]
- 20.Berman DS, Wong ND, Gransar H, Miranda-Peats R, Dahlbeck J, Hayes SW, et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44(4):923–30. doi: 10.1016/j.jacc.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 21.Schuijf JD, Wijns W, Jukema JW, Decramer I, Atsma DE, de Roos A, et al. A comparative regional analysis of coronary atherosclerosis and calcium score on multislice CT versus myocardial perfusion on SPECT. J Nucl Med. 2006;47(11):1749–55. [PubMed] [Google Scholar]
- 22.Budoff MJ, Georgiou D, Brody A, Agatston AS, Kennedy J, Wolfkiel C, et al. Ultrafast Computed Tomography as a Diagnostic Modality in the Detection of Coronary Artery Disease: A Multicenter Study. Circulation. 1996;93:898–904. doi: 10.1161/01.cir.93.5.898. [DOI] [PubMed] [Google Scholar]
- 23.Qian Z, Anderson H, Marvasty I, Akram K, Vazquez G, Rinehart S, Voros S. Lesion- and vessel-specific coronary artery calcium scores are superior to whole-heart Agatston and volume scores in the diagnosis of obstructive coronary artery disease. J Cardiovasc Comput Tomogr. 2010;4:391–399. doi: 10.1016/j.jcct.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Blaha MJ, Blumenthal RS, Budoff MJ, Nasir K. Understanding the utility of zero coronary calcium as a prognostic test: a Bayesian approach. Circ Cardiovasc Qual Outcomes. 2011;4:253–6. doi: 10.1161/CIRCOUTCOMES.110.958496. [DOI] [PubMed] [Google Scholar]
- 25.DeFilippis A, Blaha MJ, Ndumele CE, Budoff MJ, Lloyd-Jones DM, McClelland RL, et al. The association of Framingham and Reynolds risk scores with incidence and progression of coronary artery calcification in MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2011;58(20):2076–83. doi: 10.1016/j.jacc.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okwuosa TM, Greenland P, Burke GL, Eng J, Cushman M, Michos ED, et al. Prediction of coronary artery calcium progression in individuals with low Framingham Risk Score: the Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2012;5(2):144–53. doi: 10.1016/j.jcmg.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, Shea S, Szklo M, Post W, Lima J, Bertoni A, Wong ND. Progression of Coronary Calcium and Incident Coronary Heart Disease Events: The Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2013;61:1231–1239. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.