Abstract
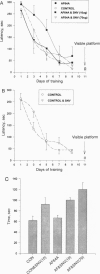
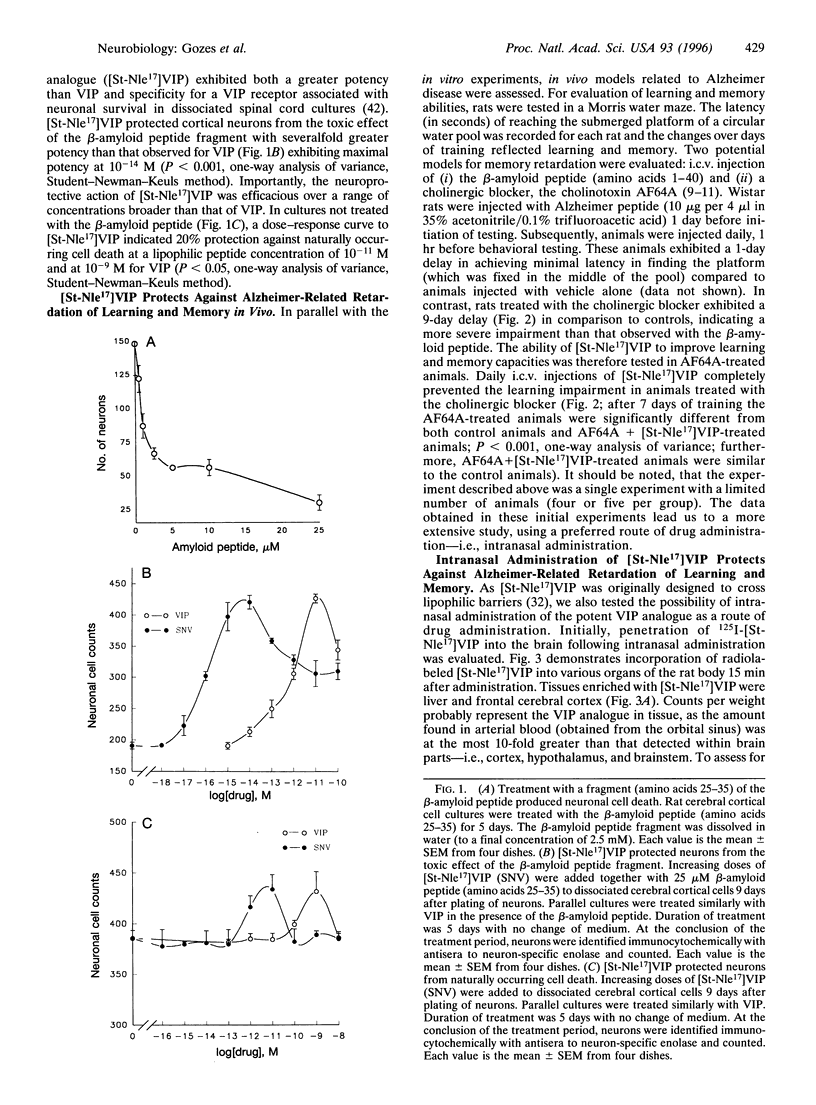
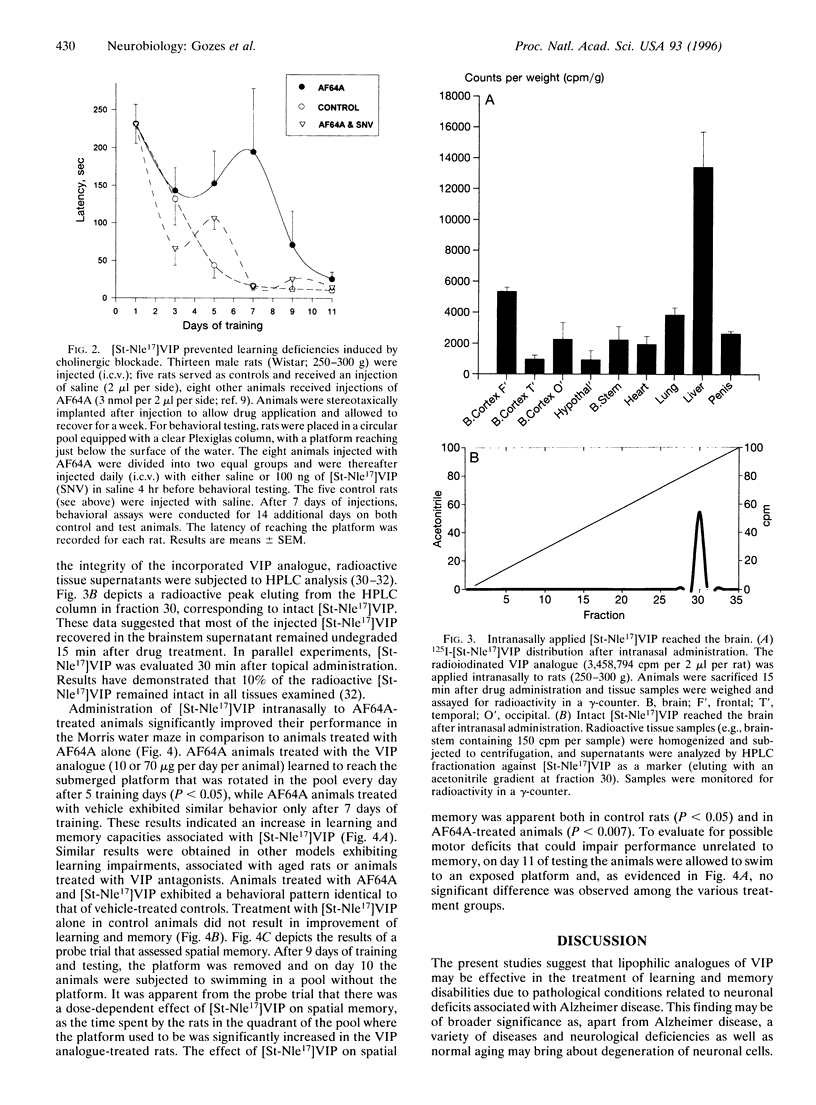
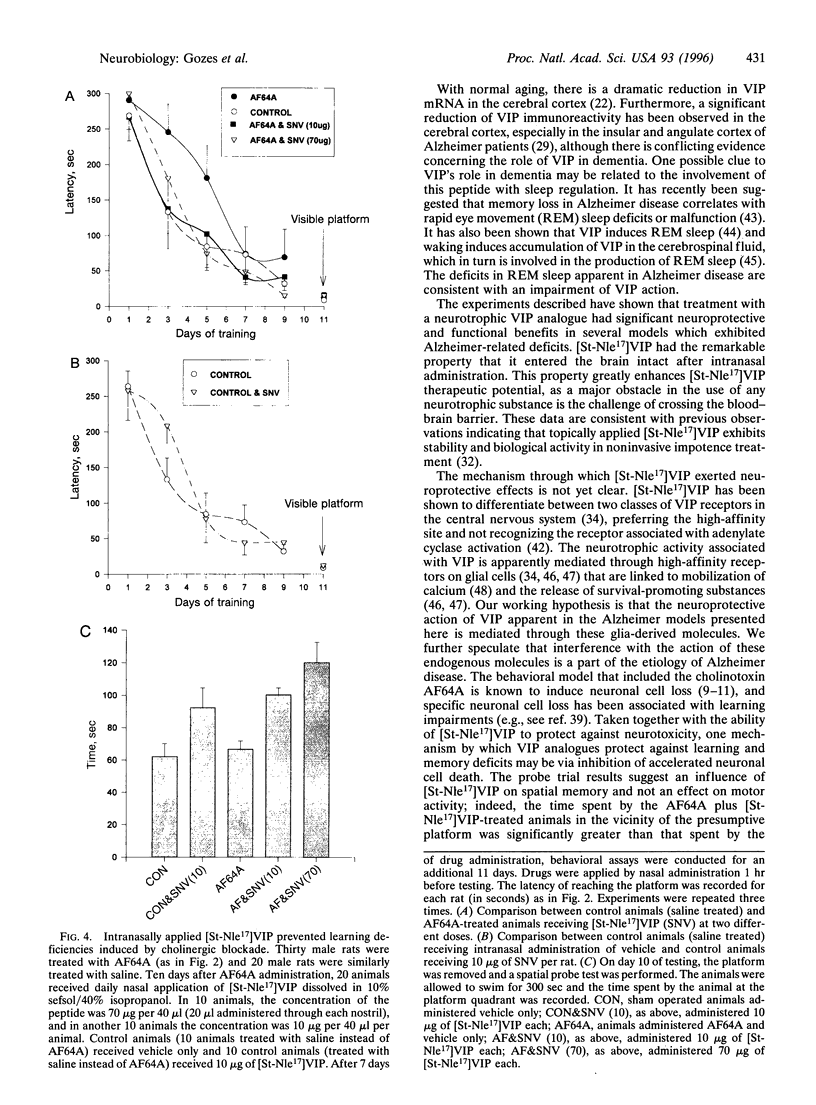
Neurodegenerative diseases, in which neuronal cell disintegrate, bring about deteriorations in cognitive functions as is evidenced in millions of Alzheimer patients. A major neuropeptide, vasoactive intestinal peptide (VIP), has been shown to be neuroprotective and to play an important role in the acquisition of learning and memory. A potent lipophilic analogue to VIP now has been synthesized, [stearyl-norleucine17]VIP ([St-Nle17]VIP), that exhibited neuroprotection in model systems related to Alzheimer disease. The beta-amyloid peptide is a major component of the cerebral amyloid plaque in Alzheimer disease and has been shown to be neurotoxic. We have found a 70% loss in the number of neurons in rat cerebral cortical cultures treated with the beta-amyloid peptide (amino acids 25-35) in comparison to controls. This cell death was completely prevented by cotreatment with 0.1 pM [St-Nle17]VIP. Furthermore, characteristic deficiencies in Alzheimer disease result from death of cholinergic neurons. Rats treated with a cholinergic blocker (ethylcholine aziridium) have been used as a model for cholinergic deficits. St-Nle-VIP injected intracerebroventricularly or delivered intranasally prevented impairments in spatial learning and memory associated with cholinergic blockade. These studies suggest both an unusual therapeutic strategy for treatment of Alzheimer deficiencies and a means for noninvasive peptide administration to the brain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Moroji T., Kosaka K. Somatostatin and vasoactive intestinal polypeptide in postmortem brains from patients with Alzheimer-type dementia. Neurosci Lett. 1984 Nov 23;52(1-2):73–78. doi: 10.1016/0304-3940(84)90353-7. [DOI] [PubMed] [Google Scholar]
- Baldino F., Jr, Fitzpatrick-McElligott S., Gozes I., Card J. P. Localization of VIP and PHI-27 messenger RNA in rat thalamic and cortical neurons. J Mol Neurosci. 1989;1(4):199–207. [PubMed] [Google Scholar]
- Brenneman D. E., Eiden L. E. Vasoactive intestinal peptide and electrical activity influence neuronal survival. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1159–1162. doi: 10.1073/pnas.83.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman D. E., Neale E. A., Foster G. A., d'Autremont S. W., Westbrook G. L. Nonneuronal cells mediate neurotrophic action of vasoactive intestinal peptide. J Cell Biol. 1987 Jun;104(6):1603–1610. doi: 10.1083/jcb.104.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman D. E., Nicol T., Warren D., Bowers L. M. Vasoactive intestinal peptide: a neurotrophic releasing agent and an astroglial mitogen. J Neurosci Res. 1990 Mar;25(3):386–394. doi: 10.1002/jnr.490250316. [DOI] [PubMed] [Google Scholar]
- Brenneman D. E., Westbrook G. L., Fitzgerald S. P., Ennist D. L., Elkins K. L., Ruff M. R., Pert C. B. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988 Oct 13;335(6191):639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Brumback R. A., Leech R. W. Alzheimer's disease: pathophysiology and the hope for therapy. J Okla State Med Assoc. 1994 Mar;87(3):103–111. [PubMed] [Google Scholar]
- Christos G. A. Is Alzheimer's disease related to a deficit or malfunction of rapid eye movement (REM) sleep? Med Hypotheses. 1993 Nov;41(5):435–439. doi: 10.1016/0306-9877(93)90121-6. [DOI] [PubMed] [Google Scholar]
- Dong X. W., Hanin I., Lorens S. A. AF64A affects septal choline acetyltransferase but not parvalbumin immunoreactive cells. Brain Res Bull. 1994;35(3):217–220. doi: 10.1016/0361-9230(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Dussaillant M., Sarrieau A., Gozes I., Berod A., Rostene W. Distribution of cells expressing vasoactive intestinal peptide/peptide histidine isoleucine-amide precursor messenger RNA in the rat brain. Neuroscience. 1992 Oct;50(3):519–530. doi: 10.1016/0306-4522(92)90444-7. [DOI] [PubMed] [Google Scholar]
- Eckenstein F., Baughman R. W. Two types of cholinergic innervation in cortex, one co-localized with vasoactive intestinal polypeptide. Nature. 1984 May 10;309(5964):153–155. doi: 10.1038/309153a0. [DOI] [PubMed] [Google Scholar]
- Fatatis A., Holtzclaw L. A., Avidor R., Brenneman D. E., Russell J. T. Vasoactive intestinal peptide increases intracellular calcium in astroglia: synergism with alpha-adrenergic receptors. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2036–2040. doi: 10.1073/pnas.91.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A., Brandeis R., Pittel Z., Karton I., Sapir M., Dachir S., Levy A., Heldman E. (+-)-cis-2-methyl-spiro(1,3-oxathiolane-5,3') quinuclidine (AF102B): a new M1 agonist attenuates cognitive dysfunctions in AF64A-treated rats. Neurosci Lett. 1989 Jul 31;102(2-3):325–331. doi: 10.1016/0304-3940(89)90100-6. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowa J. R., Panlilio L. V., Brenneman D. E., Gozes I., Fridkin M., Hill J. M. Learning impairment following intracerebral administration of the HIV envelope protein gp120 or a VIP antagonist. Brain Res. 1992 Jan 20;570(1-2):49–53. doi: 10.1016/0006-8993(92)90562-n. [DOI] [PubMed] [Google Scholar]
- Gozes I., Brenneman D. E. Neuropeptides as growth and differentiation factors in general and VIP in particular. J Mol Neurosci. 1993 Spring;4(1):1–9. doi: 10.1007/BF02736685. [DOI] [PubMed] [Google Scholar]
- Gozes I., Brenneman D. E. VIP: molecular biology and neurobiological function. Mol Neurobiol. 1989 Winter;3(4):201–236. doi: 10.1007/BF02740606. [DOI] [PubMed] [Google Scholar]
- Gozes I., Fridkin M. A fatty neuropeptide. Potential drug for noninvasive impotence treatment in a rat model. J Clin Invest. 1992 Sep;90(3):810–814. doi: 10.1172/JCI115955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I., Glowa J., Brenneman D. E., McCune S. K., Lee E., Westphal H. Learning and sexual deficiencies in transgenic mice carrying a chimeric vasoactive intestinal peptide gene. J Mol Neurosci. 1993 Fall;4(3):185–193. doi: 10.1007/BF02782501. [DOI] [PubMed] [Google Scholar]
- Gozes I., Lilling G., Glazer R., Ticher A., Ashkenazi I. E., Davidson A., Rubinraut S., Fridkin M., Brenneman D. E. Superactive lipophilic peptides discriminate multiple vasoactive intestinal peptide receptors. J Pharmacol Exp Ther. 1995 Apr;273(1):161–167. [PubMed] [Google Scholar]
- Gozes I., McCune S. K., Jacobson L., Warren D., Moody T. W., Fridkin M., Brenneman D. E. An antagonist to vasoactive intestinal peptide affects cellular functions in the central nervous system. J Pharmacol Exp Ther. 1991 Jun;257(3):959–966. [PubMed] [Google Scholar]
- Gozes I., Reshef A., Salah D., Rubinraut S., Fridkin M. Stearyl-norleucine-vasoactive intestinal peptide (VIP): a novel VIP analog for noninvasive impotence treatment. Endocrinology. 1994 May;134(5):2121–2125. doi: 10.1210/endo.134.5.8156912. [DOI] [PubMed] [Google Scholar]
- Gozes I., Schächter P., Shani Y., Giladi E. Vasoactive intestinal peptide gene expression from embryos to aging rats. Neuroendocrinology. 1988 Jan;47(1):27–31. doi: 10.1159/000124886. [DOI] [PubMed] [Google Scholar]
- Gozes I., Shani Y., Rostène W. H. Developmental expression of the VIP-gene in brain and intestine. Brain Res. 1987 Jul;388(2):137–148. doi: 10.1016/s0006-8993(87)80007-0. [DOI] [PubMed] [Google Scholar]
- Gáspár E., Heeringa M., Markel E., Luiten P. G., Nyakas C. Behavioral and biochemical effects of early postnatal cholinergic lesion in the hippocampus. Brain Res Bull. 1992 Jan;28(1):65–71. doi: 10.1016/0361-9230(92)90232-m. [DOI] [PubMed] [Google Scholar]
- Hedlund B., Abens J., Bartfai T. Vasoactive intestinal polypeptide and muscarinic receptors: supersensitivity induced by long-term atropine treatment. Science. 1983 Apr 29;220(4596):519–521. doi: 10.1126/science.6132446. [DOI] [PubMed] [Google Scholar]
- Jiménez-Anguiano A., Báez-Saldaña A., Drucker-Colín R. Cerebrospinal fluid (CSF) extracted immediately after REM sleep deprivation prevents REM rebound and contains vasoactive intestinal peptide (VIP). Brain Res. 1993 Dec 24;631(2):345–348. doi: 10.1016/0006-8993(93)91556-8. [DOI] [PubMed] [Google Scholar]
- Koliatsos V. E., Price D. L., Clatterbuck R. E., Markowska A. L., Olton D. S., Wilcox B. J. Neurotrophic strategies for treating Alzheimer's disease: lessons from basic neurobiology and animal models. Ann N Y Acad Sci. 1993 Sep 24;695:292–299. doi: 10.1111/j.1749-6632.1993.tb23069.x. [DOI] [PubMed] [Google Scholar]
- Kowall N. W., Beal M. F., Busciglio J., Duffy L. K., Yankner B. A. An in vivo model for the neurodegenerative effects of beta amyloid and protection by substance P. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7247–7251. doi: 10.1073/pnas.88.16.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Hedlund B., Bartfai T. Vasoactive intestinal polypeptide enhances muscarinic ligand binding in cat submandibular salivary gland. Nature. 1982 Jan 14;295(5845):147–149. doi: 10.1038/295147a0. [DOI] [PubMed] [Google Scholar]
- Masuo Y., Matsumoto Y., Tokito F., Tsuda M., Fujino M. Effects of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) on the spontaneous release of acetylcholine from the rat hippocampus by brain microdialysis. Brain Res. 1993 May 21;611(2):207–215. doi: 10.1016/0006-8993(93)90504-g. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. 1986 Feb 27-Mar 5Nature. 319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Garrud P., Rawlins J. N., O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982 Jun 24;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser E., Moser M. B., Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993 Sep;13(9):3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutt V. Vasoactive intestinal polypeptide and related peptides. Isolation and chemistry. Ann N Y Acad Sci. 1988;527:1–19. doi: 10.1111/j.1749-6632.1988.tb26968.x. [DOI] [PubMed] [Google Scholar]
- Nitsch R. M., Slack B. E., Wurtman R. J., Growdon J. H. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992 Oct 9;258(5080):304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- Nordberg A. Effect of long-term treatment with tacrine (THA) in Alzheimer's disease as visualized by PET. Acta Neurol Scand Suppl. 1993;149:62–65. doi: 10.1111/j.1600-0404.1993.tb04259.x. [DOI] [PubMed] [Google Scholar]
- Nordberg A., Lilja A., Lundqvist H., Hartvig P., Amberla K., Viitanen M., Warpman U., Johansson M., Hellström-Lindahl E., Bjurling P. Tacrine restores cholinergic nicotinic receptors and glucose metabolism in Alzheimer patients as visualized by positron emission tomography. Neurobiol Aging. 1992 Nov-Dec;13(6):747–758. doi: 10.1016/0197-4580(92)90099-j. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Burdick D., Walencewicz A. J., Glabe C. G., Cotman C. W. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993 Apr;13(4):1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. L., Koliatsos V. E., Sisodia S. S., Koo E. H., Martin L. J., Walker L. C., Appelgate M. D., Cork L. C. Amyloid-related proteins and nerve growth factor in Alzheimer's disease and animal models. Clin Neuropharmacol. 1991;14 (Suppl 1):S9–14. doi: 10.1097/00002826-199114001-00003. [DOI] [PubMed] [Google Scholar]
- Prospero-García O., Jiménez-Anguiano A., Drucker-Colín R. The combination of VIP and atropine induces REM sleep in cats rendered insomniac by PCPA. Neuropsychopharmacology. 1993 Jun;8(4):387–390. doi: 10.1038/npp.1993.80. [DOI] [PubMed] [Google Scholar]
- Roses A. D., Strittmatter W. J., Pericak-Vance M. A., Corder E. H., Saunders A. M., Schmechel D. E. Clinical application of apolipoprotein E genotyping to Alzheimer's disease. Lancet. 1994 Jun 18;343(8912):1564–1565. doi: 10.1016/s0140-6736(94)92960-2. [DOI] [PubMed] [Google Scholar]
- Schmechel D. E., Saunders A. M., Strittmatter W. J., Crain B. J., Hulette C. M., Joo S. H., Pericak-Vance M. A., Goldgaber D., Roses A. D. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J. Physiological production of the beta-amyloid protein and the mechanism of Alzheimer's disease. Trends Neurosci. 1993 Oct;16(10):403–409. doi: 10.1016/0166-2236(93)90008-a. [DOI] [PubMed] [Google Scholar]
- Shapira J. Research trends in Alzheimer's disease. J Gerontol Nurs. 1994 Apr;20(4):4–9. doi: 10.3928/0098-9134-19940401-04. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Duffy L. K., Kirschner D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990 Oct 12;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]