Abstract
Aims: The collagen-stimulated generation of reactive oxygen species (ROS) regulates signal transduction in platelets, although the mechanism is unclear. The major targets of ROS include protein tyrosine phosphatases (PTPs). ROS-mediated oxidation of the active cysteine site in PTPs abrogates the PTP catalytic activity. The aim of this study was to elucidate whether collagen-induced ROS generation leads to PTP oxidation, which promotes platelet stimulation. Results: SH2 domain-containing PTP-2 (SHP-2) is oxidized in platelets by ROS produced upon collagen stimulation. The oxidative inactivation of SHP-2 leads to the enhanced tyrosine phosphorylation of spleen tyrosine kinase (Syk), Vav1, and Bruton's tyrosine kinase (Btk) in the linker for the activation of T cells signaling complex, which promotes the tyrosine phosphorylation-mediated activation of phospholipase Cγ2 (PLCγ2). Moreover, we found that, relative to wild-type platelets, platelets derived from glutathione peroxidase 1 (GPx1)/catalase double-deficient mice showed enhanced cellular ROS levels, oxidative inactivation of SHP-2, and tyrosine phosphorylation of Syk, Vav1, Btk, and PLCγ2 in response to collagen, which subsequently led to increased intracellular calcium levels, degranulation, and integrin αIIbβ3 activation. Consistent with these findings, GPx1/catalase double-deficiency accelerated the thrombotic response in FeCl3-injured carotid arteries. Innovation: The present study is the first to demonstrate that SHP-2 is targeted by ROS produced in collagen-stimulated platelets and suggests that a novel mechanism for the regulation of platelet activation by ROS is due to oxidative inactivation of SHP-2. Conclusion: We conclude that collagen-induced ROS production leads to SHP-2 oxidation, which promotes platelet activation by upregulating tyrosine phosphorylation-based signal transduction. Antioxid. Redox Signal. 20, 2528–2540.
Introduction
The adhesion of platelets to collagen that is exposed at blood vessel injury sites initiates platelet activation and platelet-mediated thrombus formation. Several platelet collagen receptors have been identified. The glycoprotein VI (GPVI) (56)/Fc receptor γ-chain (FcRγ) complex is the primary collagen receptor for activating platelets (43). The GPVI signaling cascade is initiated by Src family kinase-mediated phosphorylation within the FcRγ immunoreceptor tyrosine-based activation motif, resulting in the recruitment and activation of spleen tyrosine kinase (Syk) and tyrosine phosphorylation of the linker for the activation of T cells (LAT). LAT assembles a signaling complex that culminates in tyrosine phosphorylation-based activation of phospholipase C gamma 2 (PLCγ2), which mobilizes calcium and activates protein kinase C, thereby leading to granule release, αIIbβ3-integrin activation, and ultimately platelet aggregation, as reviewed previously (56). Therefore, protein tyrosine phosphorylation has been widely accepted as playing a central role in GPVI signaling in platelets. Because tyrosine phosphorylation has been thought to be controlled by the coordinated action of protein–tyrosine kinases and protein–tyrosine phosphatases (PTPs) (55), PTPs have long been thought to be important regulators of signal transduction in GPVI-stimulated platelets (25).
Innovation.
Previous studies have demonstrated that reactive oxygen species (ROS), which are produced after glycoprotein VI (GPVI) stimulation, are responsible for a series of platelet-activating events. However, the mechanism by which ROS positively regulate the GPVI signaling cascade remains to be established.
The present study is the first to demonstrate that SH2 domain-containing PTP-2 (SHP-2) is targeted by ROS produced in collagen-stimulated platelets and suggests that a novel mechanism for the regulation of platelet activation by ROS is due to oxidative inactivation of SHP-2, which promotes tyrosine phosphorylation-based signal transduction.
Previous studies have demonstrated that reactive oxygen species (ROS), which are produced after GPVI stimulation (4, 7, 30, 49, 59), are responsible for a series of platelet-activating events, including PLCγ2 activation (49), cytosolic calcium elevation (49), αIIbβ3-integrin activation (7), granule release (4, 30), aggregate formation (7, 49), and thrombus formation (7). However, the mechanism by which ROS positively regulate the GPVI signaling cascade remains to be established.
One redox-dependent regulatory mechanism is via PTPs, which have a cysteine in the catalytic site that can be reversibly oxidized by ROS (8, 40, 50, 51). ROS are actively produced by cells stimulated with growth factors (34, 38), insulin (36), and B-cell or T-cell ligands (32, 52), and their production is considered to be important for efficiently propagating the tyrosine phosphorylation-dependent signal transduction. The role of PTPs in regulating platelet activation is not as well understood as the role of protein–tyrosine kinases. Accumulating data indicate that several PTPs, including LMW-PTP, SHIP-1, PTEN, and SH2 domain-containing phosphatase-2 (SHP-2), negatively affect GPVI-mediated platelet activation (10, 37, 58). It has been suggested that SHP-2 is activated by an interaction with platelet–endothelial cell adhesion molecule-1 (PECAM-1) and then plays a negative regulatory role in GPVI-mediated platelet activation by dephosphorylating important signaling effectors and adaptor molecules (28, 39, 46). Hyperglycemia-induced ROS production causes oxidative inactivation of PTPs and enhanced tyrosine phosphorylation of Syk in platelets exposed to collagen (59), but currently, there is no evidence that a specific PTP is regulated by ROS in collagen-stimulated platelets.
The current study sought to elucidate the redox-dependent control of the GPVI signaling cascade by intracellular ROS production in platelets. Employing biochemical and genetic approaches, we show that ROS produced upon GPVI stimulation lead to the oxidative inactivation of SHP-2, which potentially promotes the phosphorylation of tyrosine residues on signaling molecules, culminating in PLCγ2 activation and platelet aggregation. Our results suggest that collagen-induced ROS generation initiates a positive feedback regulatory loop involving SHP-2 that, at least in part, controls the rate and extent of platelet activation.
Results
Collagen-induced ROS generation enhances a cascade of tyrosine phosphorylation events
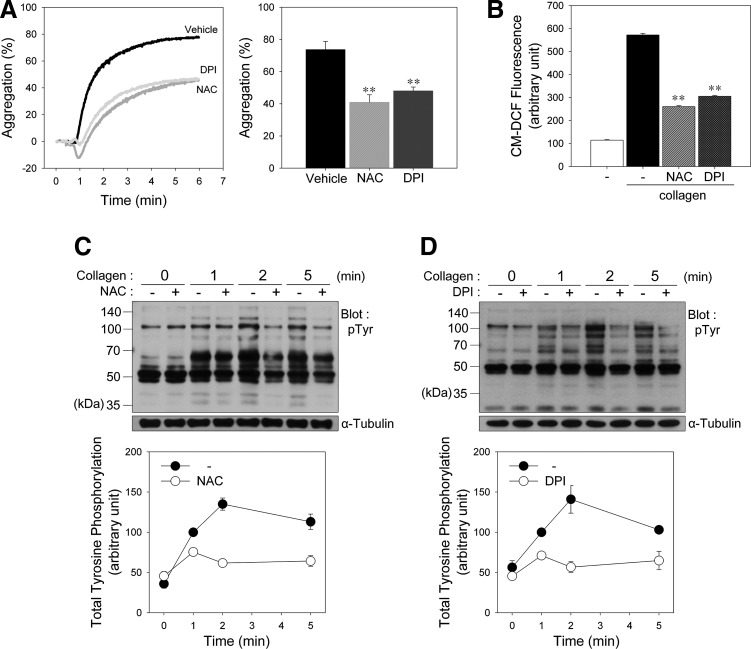
To determine whether collagen-induced ROS affect platelet activation, we stimulated platelets with collagen after pretreatment with N-acetyl-l-cysteine (NAC), a general ROS scavenger. Collagen-induced aggregation was inhibited by NAC, coinciding with a decrease in the intracellular ROS level (Fig. 1A, B). To confirm that NADPH oxidase (Nox) is responsible for ROS generation following collagen stimulation, we pretreated platelets with diphenyl iodonium (DPI), a Nox inhibitor, before the collagen stimulation.
FIG. 1.
Reactive oxygen species (ROS) produced upon collagen stimulation promote platelet aggregation and tyrosine phosphorylation. (A) Washed human platelets were preincubated for 5 min in the presence of vehicle, N-acetyl-l-cysteine (NAC) (1 mM), or diphenyl iodonium (DPI) (50 μM) as indicated and then stimulated in an aggregometer with collagen (10 μg/ml) under constant stirring for 5 min. Aggregation was assessed turbidimetrically and expressed as the percent change in light transmission, which for the buffer control was defined as 100%. The representative aggregation curves from three independent experiments are shown in each left panel. (B) CM-H2DCFDA-loaded platelets in fluoro-cuvettes were treated as described in the legend of (A), and CM-DCF fluorescence was monitored using a spectrofluorophotometer. In (A) and (B), the quantitative data are the means±S.D. (n=4; **p<0.01 versus vehicle). (C, D) Washed human platelets were preincubated for 5 min with (+) or without (−) NAC (1 mM; C) or DPI (50 μM; D) and then stimulated with collagen (10 μg/ml) for the indicated times. Whole cell lysates were subjected to immunoblot analysis of protein tyrosine phosphorylation using antiphosphotyrosine antibody (pTyr). The results shown are representative immunoblots from five independent experiments. The quantitative data are presented as mean±S.D. of triplicates.
To compare the effects of platelet agonists on aggregation and ROS production, platelets were also stimulated with collagen, thrombin, TXA2 analogue U46619, or adenosine diphosphate (ADP) (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/ars). Although platelet aggregation was induced by all agonists, the intracellular level of ROS was not significantly increased except collagen (Supplementary Fig. S1B). Thrombin-induced aggregation was not significantly inhibited by NAC or DPI at the same concentration showing inhibitory effects on collagen-induced aggregation (Supplementary Fig. S2), indicating that the inhibitory effect of NAC and DPI is not off-target but redox-dependent effects.
We next examined the effect of ROS on collagen-induced tyrosine phosphorylation in platelets. Incubation of platelets with collagen caused increased tyrosine phosphorylation of numerous proteins. In contrast, platelets pretreated with NAC (Fig. 1C) or DPI (Fig. 1D) showed a marked reduction in tyrosine phosphorylation, indicating that ROS generated after collagen stimulation are needed to induce an optimal tyrosine phosphorylation response for platelet activation through PTP oxidation.
Collagen-induced ROS generation leads to SHP-2 oxidation
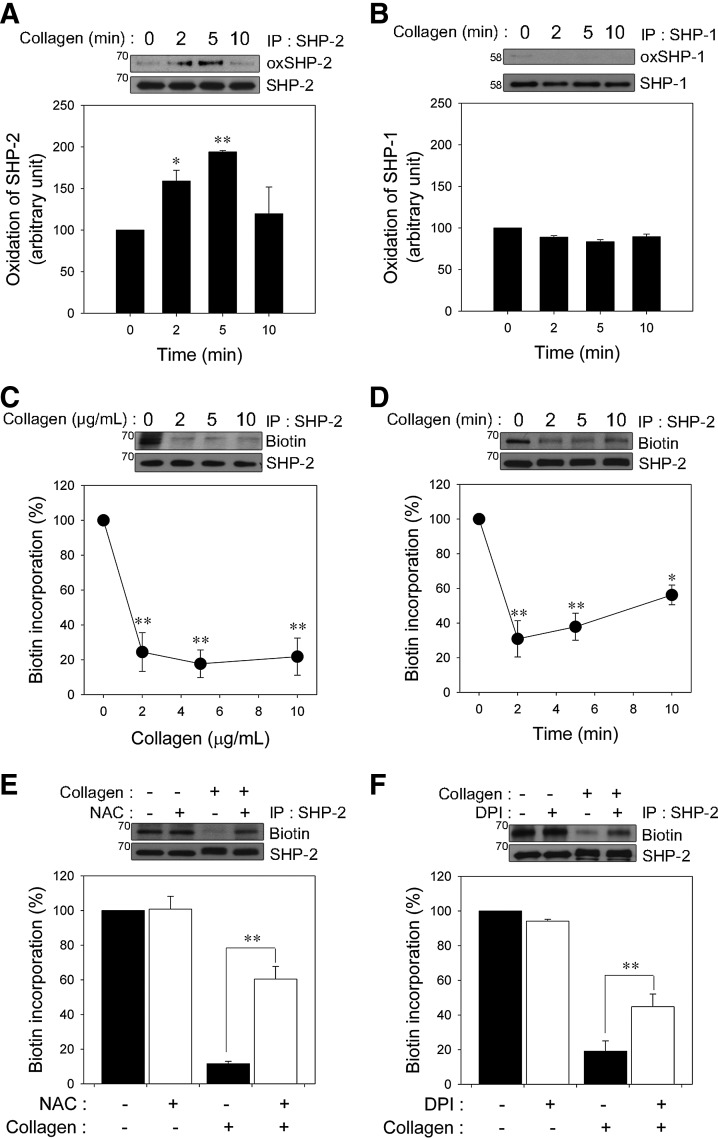
Because SHP-2 is recruited to the signaling complex containing a GPVI/FcRγ chain and LAT and plays a negative regulatory role in GPVI-mediated platelet activation (33, 39, 41), we assessed SHP-2 oxidation in platelets after collagen stimulation. To specifically detect the oxidation of the catalytic cysteine on SHP-2, we used a monoclonal anti-oxPTP antibody that was specific for the conserved active site of classical PTPs oxidized to sulfonic acid (VHCSO3HSAG) (48). The signature motif is conserved in SHP-2; therefore, the anti-oxPTP antibody has previously been used to monitor SHP-2 oxidation (29, 48, 57). Oxidized SHP-2 had an increased signal after cysteine oxidation in collagen-stimulated platelets (Fig. 2A). However, the oxidation was transient, indicating that collagen-stimulated SHP-2 oxidation is reversible.
FIG. 2.
Collagen-induced ROS generation leads to SH2 domain-containing PTP-2 (SHP-2) oxidation in platelets. (A, B) After collagen (10 μg/ml) stimulation for the indicated times, human platelets were lysed and immunoprecipitated with antibody specific for SHP-2 (A) or SHP-1 (B). Immunoprecipitates were reduced with 10 mM dithiothreitol and sequentially hyperoxidized with 100 μM pervanadate. Hyperoxidation of SHP-2 and SHP-1 was examined using immunoblot analysis with anti-oxPTP antibody. All of the immunoblots shown are representative of three independent experiments. The quantitative data are the mean±S.D. n=4; *p<0.05; **p<0.01 versus unstimulated control for an arbitrary amount of oxidized SHP-2 and SHP-1 after being normalized to the total amount of SHP-2 and SHP-1. (C, D) The platelets were stimulated with the indicated concentrations of collagen for 2 min (C) or with collagen (10 μg/ml) for the indicated times (D). (E, F) After preincubation with (+) or without (−) NAC (1 mM; E) or DPI (50 μM; F) for 5 min, the platelets were stimulated with collagen (10 μg/ml) for 2 min. In (C–F), the cell lysates were labeled with PEO-iodoacetyl biotin. SHP-2 was immunoprecipitated, and its biotinylation was analyzed by immunoblot analysis using horseradish peroxidase-conjugated streptavidin. The immunoblots shown are representative of three independent experiments. The quantitative data are represented by the percent change in biotin incorporation versus an unstimulated control, after being normalized to the amount of SHP-2. The data are the mean±S.D. (n=3). *p<0.05; **p<0.01 versus unstimulated control in C and D, and stimulated control in (E, F).
SHP-1 and SHP-2 are structurally homologous PTPs that possess tandem SH2 domains and have been thought to inhibit GPVI-regulated signal transduction (45). Thus, we examined whether SHP-1 is also oxidized. Under the same conditions in which reversible oxidation of SHP-2 was observed, SHP-1 oxidation was barely detected (Fig. 2B), although SHP-1 was oxidized by pervanadate as positive control (Supplementary Fig. S3). Similarly to previous studies in other cells (29, 32), our results also suggested that SHP-2 might be more redox sensitive than SHP-1 in collagen-stimulated platelets.
Next, we used the (+)-biotinyl-iodoacetamidyl-3,6-dioxaoctanediamine (PEO-iodoacetyl-biotin) reagent, a biotinylated thiol reactive probe, to confirm SHP-2 oxidation by selective direct labeling of the reduced thiols on the catalytic cysteine of SHP-2. Biotin incorporation into immunoprecipitated SHP-2 readily detected in unstimulated platelets was rapidly decreased in a dose-dependent manner (Fig. 2C), and the loss of biotin incorporation was recovered slowly during the follow-up period (Fig. 2D), indicating that the majority of the iodoacetamide labeling of SHP-2 was at the active cysteine site, and this oxidation was reversible. Consistent with a previous study (57), we found that SHP-2 oxidation detection using the PEO-iodoacetyl-biotin method, in comparison, was more sensitive than the anti-oxPTP antibody protocol. Thus, the former method was subsequently used to analyze SHP-2 oxidation in this study. To show that ROS generation leads to SHP-2 oxidation after specific stimulation of GPVI, platelets were stimulated with collagen-related peptide (CRP). As shown in the Supplementary Figure S4, the GPVI-specific agonist CRP also induced SHP-2 oxidation.
To further confirm the causal role of ROS in SHP-2 oxidation, the platelets were pretreated with NAC (Fig. 2E), Nox inhibitors including DPI, apocynin, 2-acetylphenothiazine, and VAS2870 (Fig. 2F and Supplementary Fig. S5) before the collagen stimulation. As expected, the loss of SHP-2 labeling was inhibited by ROS scavengers or Nox inhibitors, implying that ROS produced upon collagen stimulation are responsible for SHP-2 oxidation and inactivation in platelets.
SHP-2 associates with multiple proteins in the LAT signaling complex upon stimulation
After stimulation, SHP-2 directly or indirectly associates with signaling molecules, such as PECAM-1, Syk, LAT, Vav1, and SH2 domain-containing leukocyte protein of 76 kDa (SLP-76), in platelets and other cell systems (17, 20, 32, 39, 41); therefore, we examined the association between SHP-2 and other proteins using co-immunoprecipitation studies. SHP-2 immunoprecipitation from collagen-stimulated platelets showed an increased association of multiple proteins (Fig. 3A). As previously demonstrated (39), the association between PECAM-1 and SHP-2 was increased upon GPVI stimulation. Similar to previous results for the association of Syk with SHP-1 in GPVI-stimulated platelets (45), the association between Syk and SHP-2 also distinctly increased following collagen stimulation. Upon GPVI stimulation, LAT forms a platform for the assembly of a signaling complex that includes SLP-76, Grb2-like adapter downstream of Shc (Gads), Vav1, Bruton's tyrosine kinase (Btk), and PLCγ2, which culminates in PLCγ2 activation (56). In addition, LAT deficiency reduces the phosphorylation of Syk and PLCγ in GPVI-mediated platelet activation (24). LAT, SLP-76, Vav1, Btk, Gads, and PLCγ2 were detected in SHP-2 immunoprecipitates from stimulated platelets but were barely detected in resting platelets. However, there were no apparent changes in the level of the adhesion- and degranulation-promoting adapter protein (ADAP) or Grb2-associated binder 2 co-immunoprecipitated with SHP-2, implying a selective effect. Next, to confirm the recruitment of SHP-2 to the LAT signaling complex, LAT immunoprecipitates were examined (Fig. 3B). A low level of SHP-2 was present in the LAT immunoprecipitates from unstimulated platelets, and this association increased notably following collagen stimulation. Increased binding of SLP-76, Gads, Vav1, Btk, and PLCγ2 to immunoprecipitated LAT in stimulated platelets was also observed. These results and previous reports support the direct or indirect participation of SHP-2 in the LAT signaling complex in collagen-stimulated platelets.
FIG. 3.
Oxidative inactivation of SHP-2 is related to tyrosine phosphorylation of the signaling molecules in collagen-stimulated platelets. (A, B) Human washed platelets were stimulated with collagen (10 μg/ml) for 2 min. The cell lysates were immunoprecipitated with anti-SHP-2 antibody (A) or anti-linker for the activation of T cells (LAT) antibody (B). The immunoprecipitates were immunoblotted with antibodies specific for the indicated proteins. Representative data from three independent experiments are shown. In (C–F), human washed platelets were preincubated for 5 min in the presence of vehicle (−) or 1 mM NAC (+; C, E) or 50 μM DPI (+; D, F) and then stimulated with collagen (10 μg/ml) for 2 min. (C, D) SHP-2 immunoprecipitates from cell lysates were immunoblotted with anti-Syk-pY525/526, anti-phosphotyrosine (4G10), anti-Vav-pY174, anti-Btk-pY551, or anti-PLCγ2-pY753 antibody. The stripped blots were reprobed with the antibodies specific for the indicated proteins. The immunoblots shown are representative of three independent experiments. (E, F) Whole lysates were immunoblotted with anti-phosphorylation antibodies against the tyrosine residue as described in the legend of (C). Tyrosine phosphorylation of the LAT was immunoblotted using anti-phosphotyrosine (4G10) antibody after immunoprecipitation with anti-LAT antibody. Immunoblots of Syk, LAT, Vav, Btk, and phospholipase C gamma 2 (PLCγ2) for normalization were reprobed after stripping the antibodies. Representative data from three independent experiments are shown.
Effects of collagen-induced ROS generation on LAT-mediated PLCγ2 activation
The loss of the PTP activity caused by the oxidation or mutation of the catalytic cysteine leads to increased binding affinity for phosphorylated substrates (8, 16). Among the proteins present in the SHP-2 immunoprecipitates from the collagen-stimulated platelets (Fig. 3A), Syk, Btk, and Vav1 have already been proposed as potential substrates for SHP-2 or SHP-1 (14, 32, 35, 45, 59), suggesting a possible association between SHP-2 and these phosphorylated proteins that changes depending on ROS-mediated SHP-2 oxidation. To investigate this possibility, we analyzed SHP-2 immunoprecipitates with phospho-specific antibodies for Tyr525/Tyr526 on Syk, Tyr174 on Vav1, and Tyr551 on Btk. These tyrosine phosphorylations are necessary for the activation of these signaling molecules and can be used as markers of the activity of these molecules. The collagen-induced association of SHP-2 with phosphorylated Syk, Vav1, or Btk was apparently decreased by NAC or DPI (Fig. 3C, D). Upon collagen stimulation, Syk phosphorylates multiple tyrosine residues on LAT (44), and Btk seems to be responsible for phosphorylation of Tyr753 and Tyr759 at PLCγ2 (3, 54). Concomitant with the major changes in phosphorylated Syk and Btk, the amount of phosphorylated LAT and PLCγ2 present in SHP-2 immunoprecipitates was decreased in the NAC- or DPI-treated platelets. These results suggest that Syk, Btk, and Vav1 are potential substrates of SHP-2, and their activity can be regulated by SHP-2 in a redox-dependent manner in collagen-stimulated platelets.
Because SHP-2 oxidation was inhibited by ROS quenching, an antioxidant should decrease tyrosine phosphorylation in SHP-2 substrates. Thus, the effects of NAC or DPI on specific tyrosine phosphorylation on Syk, Vav1, and Btk were analyzed in total cell lysates (Fig. 3E, F). NAC or DPI inhibited collagen-stimulated Syk phosphorylation at Tyr525/Tyr526, which is critical for activating kinase function (18, 19). The inhibitory effects of NAC or DPI on the tyrosine phosphorylation of LAT might have resulted from the downregulation of the Syk activity. The data support a model in which SHP-2 is brought in proximity to the LAT signaling complex and downregulates Syk phosphorylation, which inhibits the ability of Syk to phosphorylate LAT and to induce assembly of the LAT signaling complex. Phosphorylation of Tyr174 on Vav1 and Tyr551 on Btk was also inhibited by a ROS scavenger, implying that a decrease in their activation can diminish PLCγ2 activity (3, 47, 54). Collagen-stimulated tyrosine phosphorylation of PLCγ2 at Tyr753, a phospho-site associated with an increased activity, was also inhibited by NAC or DPI treatment. The data suggest that ROS control tyrosine phosphorylation of Vav1 and Btk in the LAT signaling complex and subsequent PLCγ2 activation.
Glutathione peroxidase 1/catalase double deficiency leads to enhanced activation of PLCγ2 via oxidative inactivation of SHP-2 in collagen-stimulated platelets
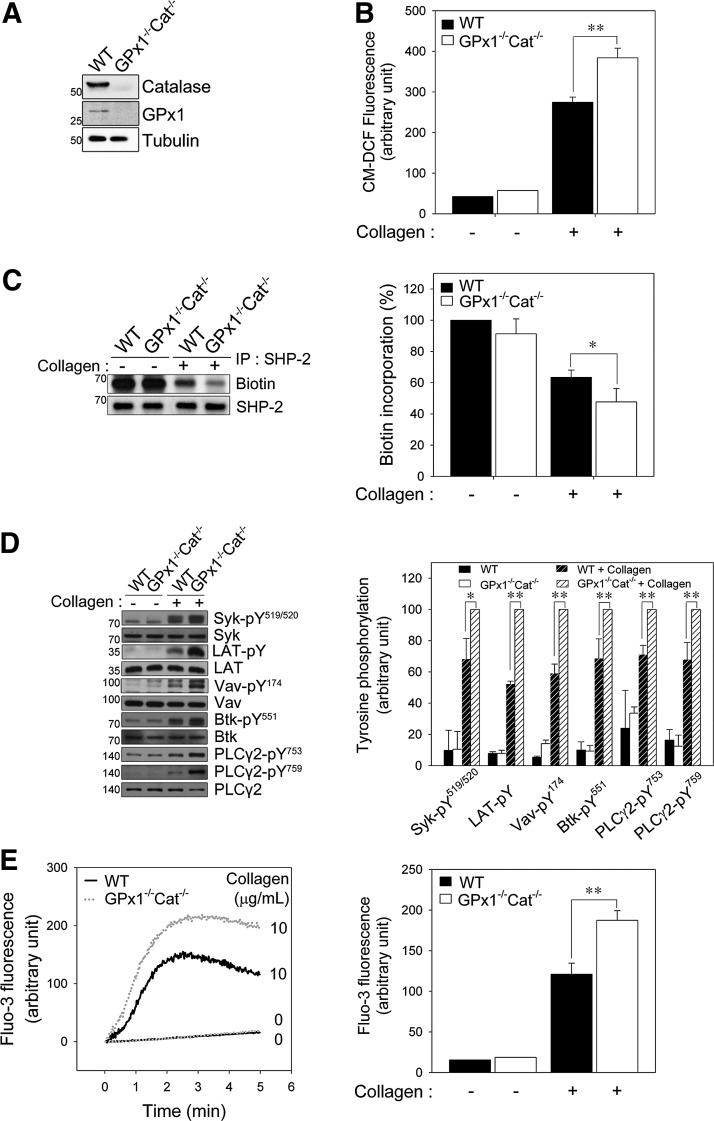
Among ROS, H2O2 is readily produced and removed following a physiological stimulus and can freely diffuse across cellular membranes; thus, H2O2 exhibits properties that are ideal for regulating signaling (12, 53). Glutathione peroxidase 1 (GPx1) and catalase are the principle enzymes responsible for eliminating H2O2. To establish a role for H2O2 in redox regulation of SHP-2, platelets from GPx1−/−Cat−/− and wild-type (WT) mice were studied (Fig. 4A). To exclude a possible change in GPVI expression level in GPx1−/−Cat−/− platelets, we measure the expression level. GPx1−/−Cat−/− platelets expressed similar level of GPVI as WT platelets (Supplementary Fig. S6).
FIG. 4.
GPx1/catalase double deficiency promotes PLCγ2 activation and oxidative inactivation of SHP-2 in collagen-stimulated platelets. (A) Washed platelets from wild-type (WT) and GPx1/catalase double-deficient (GPx1−/−Cat−/−) mice were lysed and immunoblotted with antibodies specific for GPx1 and catalase. (B) CM-H2DCFDA-loaded WT and GPx1−/−Cat−/− platelets in fluoro-cuvettes were stimulated with collagen (10 μg/ml) under constant stirring for 2 min, and fluorescence was monitored using a spectrofluorophotometer. The quantitative data are the mean±S.D. n=4; **p<0.01 of the CM-DCF fluorescence. (C, D) WT and GPx1−/−Cat−/− platelets were stimulated with collagen (10 μg/ml) for 2 min. In (C), biotin incorporation into SHP-2 was assessed as described in Figure 3C. In (D), immunoblot analysis of tyrosine phosphorylation on the indicated proteins was performed as described in Figure 3D. The immunoblots shown are representative of three independent experiments. The data are the mean±S.D. (n=3; *p<0.05, **p<0.01). (E) Fluo-3 AM-loaded WT and GPx1−/−Cat−/− platelets in fluoro-cuvettes were incubated with or without collagen under constant stirring and in the presence of 1 mM CaCl2. Fluorescence was monitored for the indicated times using a spectrofluorophotometer. The representative fluorescence tracings from three independent experiments are shown. The quantitative data are the mean±S.D. n=4; **p<0.01 for Fluo-3 fluorescence at 2 min after stimulation.
The intracellular ROS level of GPx1 or catalase single knockout platelets was higher than that of the WT platelets but is not increased as much as that of double knockout platelets (Supplementary Fig. S7). To more effectively regulate the level of ROS, we used GPx1/catalase double-deficient mice in this study. The intracellular ROS level was significantly higher in the GPx1−/−Cat−/− platelets after collagen stimulation than the WT platelets (Fig. 4B), indicating that the increase in intracellular ROS is limited by H2O2-eliminating enzymes. To more precisely define the impact of GPx1/catalase double deficiency on collagen-dependent signaling, we first examined SHP-2 oxidation. Biotin incorporation demonstrated that the SHP-2 oxidation level was greater in the GPx1−/−Cat−/− platelets than the WT platelets (Fig. 4C), indicating that the H2O2 produced after collagen stimulation is responsible for SHP-2 oxidation and inactivation in platelets.
We have demonstrated that SHP-2 can regulate GPVI signaling pathways in a redox-dependent manner, and Syk, Btk, and Vav1 are potential SHP-2 substrates. Therefore, we analyzed the activation of downstream signaling molecules using phospho-specific antibodies (Fig. 4D). The phosphorylation of Syk at Tyr519/520 (Tyr525/Tyr526 in the human protein), of Vav1 at Tyr174, and of Btk at Tyr551 was elevated in the GPx1−/−Cat−/− platelets compared to the WT platelets after collagen stimulation. Subsequently, collagen-stimulated PLCγ2 phosphorylation at Tyr753/759 was also increased in the GPx1−/−Cat−/− platelets compared to the WT platelets. Given that the release of calcium from intracellular stores was controlled by PLCγ2-mediated inositol 1,4,5-triphosphate production, we examined the effect of GPx1/catalase double deficiency on the rise in cytosolic calcium. Concomitant with the change in ROS-dependent PLCγ2 activation, the collagen-induced increase in cytosolic calcium was significantly greater in the GPx1−/−Cat−/− platelets than the WT platelets (Fig. 4E). These results support a model in which the H2O2 produced upon collagen stimulation leads to SHP-2 oxidation and promotes tyrosine phosphorylation of its substrates, which causes the eventual phosphorylation and activation of PLCγ2 and release of calcium from intracellular stores.
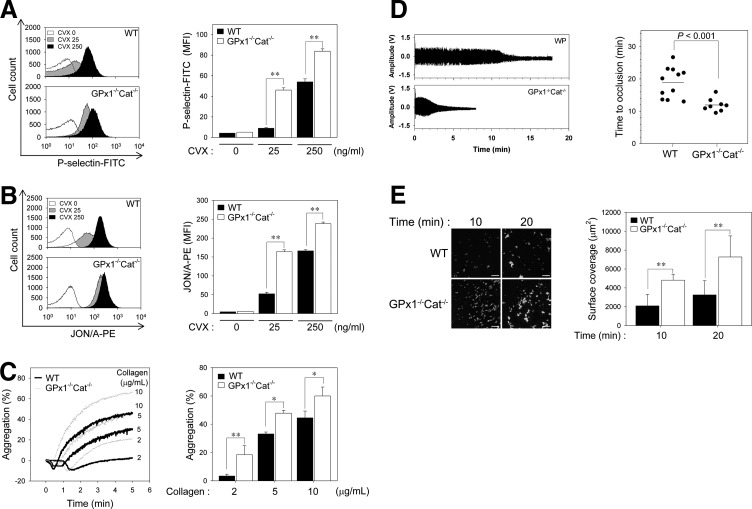
GPx1/catalase double deficiency enhances platelet activation and aggregation in a redox-dependent manner
To further examine the redox-dependent platelet activation events, we monitored the surface expression of P-selectin, a marker of degranulation, and integrin αIIbβ3 activation, in response to convulxin. Given that collagen can cause problems for flow cytometry by crosslinking with itself and aggregating platelets (21), we used convulxin, a GPVI activator (26). Convulxin-induced surface expression of P-selectin from α granules was greater in platelets from GPx1−/−Cat−/− mice than WT mice (Fig. 5A). Integrin αIIbβ3 activation by convulxin was also greater in the GPx1−/−Cat−/− platelets than the WT platelets (Fig. 5B). These results suggest that GPVI-induced H2O2 production promotes degranulation and inside–out activation of integrin αIIbβ3 by the increased cytosolic calcium resulting from PLCγ2 activation following oxidative inactivation of SHP-2.
FIG. 5.
GPx1/Cat double deficiency facilitates platelet aggregation and thrombus formation in a redox-dependent manner. (A, B) Washed platelets from the WT and GPx1−/−Cat−/− mice were incubated with (+) or without (−) convulxin (CVX, 25 or 250 ng/ml) for 5 min in the presence of 0.5 mg/ml FITC-conjugated anti-P-selectin (P-selectin-FITC in A) or 0.5 mg/ml PE-conjugated anti-active-integrin αIIbβ3 (JON/A-PE in B). Binding of anti-P-selectin (A) and active-integrin αIIbβ3 to platelets was analyzed using flow cytometry. The histograms are representative of at least three different experiments and three different mice. The quantitative data are the mean±S.D. (n=3; **p<0.01) for the mean fluorescence intensity. (C) Washed WT and GPx1−/−Cat−/− platelets were stimulated with the indicated concentrations of collagen for 5 min. Platelet aggregation was assessed and expressed as described in Figure 1A. The representative aggregation peaks from three independent experiments are shown. The quantitative data are the mean±S.D. (n=3; *p<0.05; **p<0.01). (D) The left carotid artery of WT and GPx1−/−Cat−/− mice was injured by the topical application of 20% ferric chloride. The blood volume changes in the carotid artery downstream of the site of injury were measured by the photoplethysmography method using a minimized OxiPulse probe in transmission mode. On the left panel, representative photoplethysmography waveforms from independent experiments are shown. The amplitudes, representing the differences between the peaks and valleys of each waveform, were averaged to obtain the mean blood volume changes in the carotid artery. The time to thrombotic occlusion was defined as the time required for >90% loss of the initial blood volume. The quantitative data are the mean±S.D. (n=8–11, p<0.001). (E) DiOC6-labeled platelets from WT and GPx1−/−Cat−/− mice were perfused through a collagen-coated coverslip placed in a parallel plate flow chamber at a shear rate of 150 s−1 for the indicated times. Chamber surface coverage by the thrombi (fluorescence positive) was quantified. Representative images of platelet adhesion from three independent experiments are shown. Scale bar represents 50 μm. The quantitative data are the means±S.D. (n=3; **p<0.01) for DiOC6 fluorescence.
To assess the functional consequences of GPx1/catalase double deficiency, we measured aggregation in response to graded concentrations of collagen (Fig. 5C). The concentrations of collagen that elicited low, intermediate, and high levels of responsiveness from the WT platelets elicited a significantly greater response from the GPx1−/−Cat−/− platelets, indicating that H2O2 functions as a stimulatory molecule for collagen-induced platelet aggregation through oxidative inactivation of SHP-2.
GPx1/catalase double deficiency accelerates thrombotic response in injured carotid arteries
To investigate the consequences of GPx1/catalase double deficiency on arterial thrombus formation, we induced carotid artery thrombi with FeCl3, and the time to arterial occlusion was monitored. Carotid occlusion in the WT mice occurred at a mean of 19.4 min, whereas in GPx1−/−Cat−/− mice, the mean was shortened to 11.9 min (p<0.001, Fig. 5D). To exclude the effects of other components in hemostasis/thrombosis, we analyzed thrombus formation using the ex vivo flow chamber assay (Fig. 5E). Compared to WT platelets, we found a marked increase in GPx1−/−/Cat−/− platelets adhesion to a collagen-coated surface under shear-flow conditions. These results further suggest that H2O2 promotes platelet activation through SHP-2 oxidation-mediated upregulation of tyrosine phosphorylation-based signal transduction, which accelerates arterial thrombus formation.
Discussion
Tyrosine phosphorylation is required for appropriate cell signaling and activation, and this phosphorylation is coordinated by the balanced and specific action of protein–tyrosine kinases and PTPs. Receptor-mediated ROS production has emerged as an important regulator of phosphorylation-dependent signal transduction (8, 50). Collagen-induced platelet activation is specifically caused by the binding of collagen to the GPVI receptor (43), signaling through which requires tyrosine phosphorylation of various signaling molecules (43, 56). Although the tyrosine phosphorylation response to a variety of physiological stimuli can be enhanced by ROS-dependent oxidation and PTP inactivation (8, 40, 50, 51), specific PTP regulation by ROS in collagen-stimulated platelets has not been understood. This study demonstrated that the PTP SHP-2 is transiently oxidized in platelets by ROS produced by collagen stimulation. Furthermore, we identified Syk, Vav1, and Btk as putative substrates of SHP-2 in collagen-stimulated platelets. The data suggest that oxidative inactivation of SHP-2 promotes collagen-induced phosphorylation of Syk, Vav1, Btk, and PLCγ2 in the LAT signaling complex and that these changes enhance granule secretion, integrin αIIbβ3 activation, platelet aggregation, and thrombosis.
SHP-1 and SHP-2 are structurally homologous PTPs possessing tandem SH2 domains. Similar to previous studies reporting that the ROS generation induced by activating platelet-derived growth factor or the T-cell receptor leads to SHP-2 but not SHP-1 oxidation (32, 38), our results also demonstrated that SHP-2 is selectively sensitive to ROS-mediated oxidation of the catalytic cysteine compared to SHP-1 in platelets. Although the intrinsic oxidation susceptibility of SHP-1 and SHP-2 has not yet been established, higher oxidation susceptibility for SHP-2 compared to SHP-1 in human T-cell blasts and NIH3T3 mouse fibroblasts treated with different H2O2 concentrations has recently been demonstrated (29, 32). Structural analyses of both PTPs have revealed that in the basal state the N-terminal SH2 domain folds into the catalytic domain and simultaneously hinders access to the substrates (5). Regarding SH2 domain-mediated regulation, the SH2 domain-mediated occlusion of the active site might obstruct the access for the ROS and thereby decrease oxidation susceptibility and play a significant protective role against oxidation in SHP-1's SH2 domain (57).
The current data suggest that after GPVI stimulation, SHP-2 associates with multiple proteins in the LAT signaling complex in which the oxidized SHP-2 is localized. The association of oxidized SHP-2 with proteins may be caused by prolonged binding to phosphorylated substrates (17, 32, 38). In platelets pretreated with an antioxidant, anti-SHP-2 immunoprecipitates showed decreased LAT association. Similarly, SHP-2 immunoprecipitation also revealed a limited association of SHP-2 with tyrosine-phosphorylated proteins primarily involved in a LAT signaling complex. Notably, our study suggests that Syk, Vav1, and Btk are SHP-2 substrates in collagen-stimulated platelets. However, we still cannot completely exclude the possibility that their tyrosine phosphorylations can be indirectly regulated by SHP-2 and that other PTPs are also involved. Accumulating data show that several PTPs, including LMW-PTP, SHIP-1, and PTEN, play a negative role in platelet activation (10, 37, 58) by dephosphorylation of multiple substrates. Although PTP-1B has been reported as a positive regulator of platelet signaling (2, 31), the possibility that PTP-1B oxidation promotes platelet activation by upregulating tyrosine phosphorylation-based signal transduction can be proposed.
Collagen-induced GPVI stimulation results in platelet aggregation, which is associated with subsequent ROS production by Nox. Previous studies with Nox inhibitors have indicated that Nox is responsible for the ROS production in GPVI-stimulated platelets (4, 7).
Because many mammalian cell types produce H2O2 for intracellular signaling in response to stimulation through various cell surface receptors (12, 50), in platelets after GPVI stimulation, the electrons generated by Nox, cyclooxygenase, or the mitochondrial electron transport chain appear to mediate the reduction of molecular oxygen to a superoxide anion (O2•−), which then undergoes spontaneous or enzyme-catalyzed dismutation to H2O2 (12, 50). H2O2 is a nonpolar nonradical molecule and can diffuse freely across membranes. This characteristic allows H2O2 to function as an efficient signaling molecule and thereby an intracellular mechanism for eliminating H2O2, which is an important factor in redox-dependent cell signaling (12, 50, 53). In mammalian cells, GPx and catalase are the major enzymes responsible for eliminating H2O2. Collagen-induced platelet aggregation has been shown to be closely associated with H2O2 production, and catalase has been demonstrated to inhibit H2O2 formation and platelet aggregation (13, 49). Deficiency of H2O2 eliminating systems would be expected to promote H2O2 accumulation by reducing the cellular capacity to remove the H2O2 generated in GPVI-stimulated platelets. In support of this notion, we found that after collagen stimulation, platelets isolated from the GPx1−/−Cat−/− mice had more ROS compared to WT mice. Although we did not directly detect the level of intracellular H2O2 in the GPx1−/−Cat−/− platelets, the higher level of intracellular ROS seems to be mainly due to more amount of H2O2. In addition, subsequent SHP-2 oxidation in the GPx1−/−Cat−/− platelets was greater than the WT platelets, implying the important functions of intracellular H2O2 in platelet activation via redox regulation of SHP-2. On the other hand, it can be possible that superoxide is more efficient ROS than H2O2 for inactivating SHP-2 as in the case of PTP1B described by Barrett et al. (6). Further analysis of the GPx1−/−Cat−/− platelets demonstrated enhanced tyrosine phosphorylation of Syk, LAT, Vav1, Btk, and PLCγ2 compared to the WT platelets, strongly suggesting that augmented intracellular H2O2 may be critical for modulating tyrosine phosphorylation-induced platelet activation.
Our data suggest that H2O2 modulates platelet α-granule release in activated platelets. Increased intracellular H2O2 resulting from the inhibition of GPx1 and catalase also promoted P-selectin surface expression in GPVI-stimulated platelets. Similarly, the GPx mimetic ebselen or the general antioxidant NAC inhibited P-selectin upregulation in convulxin-stimulated platelets (4). Taken together, the data support a model in which platelet-derived P-selectin surface expression is redox regulated in response to GPVI stimulation. Our present study demonstrates that depleting GPx1/catalase augments the inside–out activation of integrin αIIbβ3 in response to GPVI stimulation, which is consistent with a previous study that showed integrin αIIbβ3 activation is accompanied by a concomitant increase in ROS production in convulxin-stimulated platelets (4, 7). Thus, the data suggest that intracellular H2O2 regulates integrin αIIbβ3 activation in GPVI-stimulated platelets. It is unclear whether the molecular mechanism of α-granule secretion and integrin activation is directly involved in SHP-2 oxidation following ROS production; however, our results strongly suggest the involvement of intracellular ROS in regulating P-selectin and integrin. Given that intracellular calcium contributes to the reorganization of the actin cytoskeleton, which is necessary for degranulation and integrin αIIbβ3 activation (43, 56), the mechanism via SHP-2 oxidation is partly explained by the increased intracellular calcium resulting from PLCγ2 activation following oxidative inactivation of SHP-2.
A study illustrating the link between antioxidant proteins and platelet aggregation reported that the platelet GPx and catalase activities in patients with coronary heart disease were significantly lower than those in healthy subjects, suggesting that GPx and catalase in platelets may affect thrombotic processes in patients (9). Our loss-of-function studies also revealed that GPx1 and catalase are physiological regulators of endogenous H2O2-mediated signaling in platelets. Furthermore, GPx1/catalase double deficiency accelerated the thrombotic response in injured carotid arteries, indicating that H2O2 promotes platelet activation through a redox-dependent pathway and that GPx1 and catalase play a preventive role in platelet-mediated arterial thrombosis.
In conclusion, the present study has identified SHP-2 as a biological target of collagen-induced ROS in platelets. Furthermore, the loss-of-function study with GPx1−/−Cat−/−platelets suggests that the redox regulation of SHP-2 modulates collagen-induced aggregation and oxidant-stimulated arterial thrombosis; these results imply that increased ROS levels during inflammation or vessel damage may promote platelet aggregation and vascular thrombosis. Further studies on the signaling pathways regulated by ROS and antioxidant systems will help to develop a strategy for preventing platelet hyperactivation under pathological oxidative conditions.
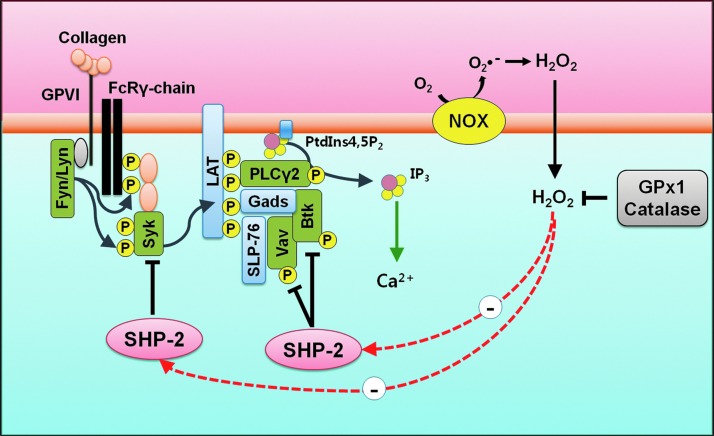
In summary, SHP-2 is oxidized in platelets by ROS produced upon collagen stimulation. The oxidative inactivation of SHP-2 leads to the enhanced tyrosine phosphorylation of Syk, Vav1, and Btk in the LAT signaling complex, which promotes the tyrosine phosphorylation-mediated activation of PLCγ2, which subsequently leads to increase in cytosolic calcium levels, platelet aggregation, and thrombosis. Antioxidants, GPx1 and catalase, decrease cellular H2O2 levels and SHP-2 oxidation in response to collagen. Finally, it leads to attenuation in platelet aggregation and thrombosis through decrease of PLCγ2 activity and intracellular calcium levels (Fig. 6).
FIG. 6.
Model of ROS and SHP-2 oxidation in platelet activation. Collagen-induced ROS generation oxidizes SHP-2 present in the LAT signaling complex. Oxidation of SHP-2 promotes phosphorylation of Syk, Vav1, and Btk, which culminate in PLCγ2 activation and increase in cytosolic calcium. This leads to platelet aggregation and thrombosis. In the presence of antioxidants, GPx1 or catalase, the effects of ROS are attenuated and SHP-2 remains more active. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Materials and Methods
Antibodies and reagents
CM-H2DCFDA and Fluo-3AM were from Molecular Probes. Monoclonal antibodies to SHP-2 and anti-CD62P-FITC were from BD-Biosciences. JON/A-PE was from Emfret Analytics. Polyclonal antibodies to GPx1 and catalase were from LabFrontier. Polyclonal antibodies to SHP-2, Btk, phospho-Vav (Tyr174), Syk, and monoclonal antibodies to α-tubulin were from Santa Cruz Biotechnology. Monoclonal anti-oxPTP antibody specific to the conserved active site of classical PTPs oxidized to the sulfonic acid (VHCSO3HSAG) (48) was from R&D Systems. Polyclonal antibody to phospho-Btk (Tyr551) was from Biosource. Polyclonal antibody to phospho-Syk (Tyr525/526) was from Cell Signaling Technology. Monoclonal antibodies to phosphotyrosine antibody (4G10) and Vav1, and polyclonal antibodies to ADAP, LAT, and Gads were from Upstate Biotechnology, Inc. Polyclonal antibodies to PLCγ2, phospho-PLCγ2 (Tyr753), and phospho-PLCγ2 (Tyr759) were kindly gifted by Dr. S.G Rhee (Ewha Womans University). Horseradish peroxidase (HRP)-conjugated streptavidin was from Pierce. Convulxin was from Alexis Biochemicals.
Experimental animals
C57BL/6 WT, glutathione peroxidase 1-deficient (GPx1−/−), catalase-deficient (Cat−/−), and GPx1/catalase double-deficient (GPx1−/−Cat−/−, Fig. 4A) mice on the C57BL/6 background (22, 23, 27) were housed under specific pathogen-free conditions at the Ewha Womans University. The animal handling and experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee. The animals were used from 6 to 8 weeks of age.
Human platelet preparation
Human blood was obtained from healthy drug-free volunteers and anticoagulated with acid/citrate/dextrose (22.0 g sodium citrate, 24.5 g dextrose, 7.3 g citric acid per 1 L). Platelet-rich plasma, which was obtained by centrifugation for 15 min at 150g, was further centrifuged for 10 min at 300g to concentrate the platelets. The platelet pellet was then suspended in a washing buffer containing Tyrode's buffer (10 mM HEPES [pH 7.4], 129 mM NaCl, 0.8 mM KH2PO4, 8.9 mM NaHCO3, 2.8 mM KCl, 0.8 mM MgCl2, and 5.6 mM glucose), 2 mM EDTA, 10% acid citrate dextrose solution, and 1 μM PGE1 and was centrifuged again. The platelets were finally resuspended at a concentration of 3×108 platelets/ml in Tyrode's buffer. Purity of the isolated human platelets was assessed by flow cytometry as shown in Supplementary Figure S8A. All of the studies using human platelets were approved by the Institute Faculty Ethics Committee at the Ewha Womans University.
Mouse platelet preparation
Mouse blood was collected from the abdominal aorta using a syringe containing 1 volume of acid/citrate/dextrose for 10 volume of blood under isofluran anesthesia. The blood was diluted with an equal volume of washing buffer. Platelet-rich plasma, which was obtained by centrifugation for 15 min at 50g, was further centrifuged for 10 min at 300g to concentrate the platelets. The platelet pellet was then suspended in washing buffer and spun once more. Platelets were finally resuspended at a concentration of 3×108 platelets/ml in Tyrode's buffer. Purity of the isolated mouse platelets was assessed by flow cytometry as shown in Supplementary Figure S8B.
Aggregation study
Washed platelets in Tyrode's buffer containing 0.35% bovine serum albumin were preincubated with 1 mM CaCl2 for 2 min before adding collagen, thrombin (Chrono-Log), U46619 (Calbiochem), or ADP (Sigma-Aldrich). Platelet aggregation was measured in a siliconized glass cuvette under continuous stirring at 1000 rpm at 37°C using a four-channel aggregometer (Chrono-Log).
Analysis of intracellular ROS and cytosolic calcium
Washed platelets suspended in phosphate-buffered saline (PBS) were incubated with 5 μM CM-H2DCFDA or 1 μM Fluo-3 AM for 15 min at 37°C in the dark. Then, the excess dye was removed, and the platelets were resuspended in Tyrode's buffer containing 1 mM CaCl2. After the dye-loaded platelets were stimulated with collagen, thrombin, U46619, or ADP under continuous stirring at 1000 rpm at 37°C, the intracellular ROS level at 495 nm excitation and 525 nm emission and the intracellular calcium level at 488 nm excitation and 525 nm emission were measured using a spectrofluorophotometer (Shimadzu).
Immunoblotting and immunoprecipitation
After stimulation, the platelets were lysed in cell extraction buffer (20 mM HEPES [pH 7.0], 150 mM NaCl, 1% Triton X-100, 10% glycerol, 1 mM EDTA, 2 mM EGTA, 20 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mM AEBSF). The cell debris was removed by centrifugation at 12,500g for 10 min. For the western blot and immunoprecipitation analyses, equal amounts of cell lysates were subjected to analysis using specific antibodies, as indicated.
Detection of oxidized SHP-2 with anti-oxPTP antibodies
As previously described (29, 48, 57), platelets were lysed using an oxygen-free buffer (50 mM HEPES [pH 6.5], 1% NP-40, 150 mM NaCl, 10% glycerol, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mM AEBSF) with 100 mM iodoacetamide in an anoxic chamber (<1% oxygen; Thermo Forma). Cell debris was removed by centrifugation at 12,500g for 10 min. SHP-2 was immunoprecipitated with antibody specific for SHP-2 and protein G-Sepharose 4B (Amersham) at 4°C. The beads were washed three times in lysis buffer to remove excess iodoacetamide. Reversibly oxidized SHP-2 was reduced with 10 mM dithiothreitol for 30 min and then further washed three times in 20 mM HEPES (pH 7.4) at room temperature. To oxidize SHP-2 to sulfonic acid (-SO3H), the reduced SHP-2 was finally incubated with 100 μM pervanadate for 1 h at 4°C. Hyperoxidized SHP-2 (SHP-2-SO3H) was then detected by immunoblotting with anti-oxPTP antibody (R&D Systems).
Biotinylation to detect oxidized thiols in SHP-2
As previously described (32), platelets were lysed using the oxygen-free buffer in an anoxic chamber (<1% oxygen). Cell debris was removed by centrifugation. The lysates were incubated with 1 mM PEO-iodoacetyl-biotin (Pierce) for 3 h under continuous shaking at 800 rpm at room temperature in the dark. SHP-2 was immunoprecipitated with specific antibody and protein G-Sepharose 4B for 2 h at 4°C. Biotin incorporation into the immunoprecipitated proteins was detected by immunoblot analysis with (HRP)-conjugated streptavidin (Pierce).
Flow cytometry
After being stimulated with convulxin (25 ng/ml), the platelets were incubated with FITC-conjugated anti-P-selectin (CD62P-FITC, 0.5 mg/ml) or PE-conjugated anti-active-integrin αIIbβ3 (JON/A-PE, 0.5 mg/ml) for 5 min in the dark. The reaction was stopped by adding ice-cold PBS. A FACSCalibur flow cytometer (Becton Dickinson) was used for all of the analyses with a minimum of 5×104 cells per sample for each measurement. The surface expression of P-selectin and active-integrin αIIbβ3 on the platelets was measured at 530 (FL1) and 585 nm (FL2), respectively. The relative change in fluorescence was analyzed using WinMDI software.
Assessment of arterial thrombosis after ferric chloride exposure
Thrombosis was induced in mice using a previously described carotid artery injury model (15). After an intraperitoneal injection containing 1.0 ml/kg Zoletil (Virbac Animal Health Co.) and 0.7 ml/kg Rompun (Bayer Korea Co.) for anesthesia, the left common carotid artery was exposed. Vascular injury was induced by applying a filter paper (1×1 mm) that had been saturated with 20% FeCl3 proximal to the carotid artery. The blood volume changes in the carotid artery downstream of the injury site were measured by the photoplethysmography method using a minimized OxiPulse probe (Hurev, Inc.) in transmission mode (1). USB 6009 (National Instrument, Inc.) and LabVIEW 7.1 (National Instrument, Inc.) were used to acquire and analyze the changes in photoplethysmography waveforms. The amplitudes representing the differences between the peaks and valleys of each waveform were averaged to obtain the mean blood volume changes in the carotid artery. The time to thrombotic occlusion was defined as the time required for >90% loss of the initial blood volume.
Ex vivo flow chamber assay
Washed platelet (1×108/ml) was incubated with 1 μM of the fluorescent dye DiOC6 (Sigma-Aldrich) for 10 min at 37°C as described (42). Collagen-coated coverslip (Neuvitro) was mounted on custom-made flow chamber (Chamlide CF, from LCI Korea). The fluorescently labeled platelet was then perfused over a matrix of collagen at 150 s−1 using a syringe pump (Harvard Apparatus, Inc.) (11). Nonadherent platelets in the chamber were washed with PBS. Adherent platelets were fixed with cold 4% paraformaldehyde for 15 min and then washed with PBS. Thrombus formation was visualized with a 40×long working distance objective for confocal microscopy (Nikon A1R). Flow chamber surface coverage by the thrombi was calculated using ImageJ software.
Statistical analysis
For the statistical analysis, all of the experiments were repeated at least three times, and the data were analyzed using Student's t-test.
Supplementary Material
Abbreviations Used
- ADAP
adhesion- and degranulation-promoting adapter protein
- ACD
acid citrate dextrose solution
- ADP
adenosine diphosphate
- Btk
Bruton's tyrosine kinase
- CM-H2DCFDA
5-(and-6)-carboxy-2′,7′-dichlorofluorescein
- CRP
collagen-related peptide
- DPI
diphenyl iodonium
- FcRγ-chain
Fc receptor γ-chain
- Fluo-3 AM
Fluo-3 acetoxymethyl ester
- Gab2
Grb2-associated binder 2
- Gads
Grb2-like adapter downstream of Shc
- GPVI
glycoprotein protein VI
- GPx1
glutathione peroxidase 1
- HRP
horseradish peroxidase
- LAT
linker for the activation of T cells
- NAC
N-acetylcysteine
- Nox
NADPH oxidase
- PBS
phosphate-buffered saline
- PECAM-1
platelet-endothelial cell adhesion molecule-1
- PEO-iodoacetyl-biotinz
(+)-biotinyl-iodoacetamidyl-3,6-dioxaoctanediamine
- PLCγ2
phospholipase C gamma 2
- PTP
protein tyrosine phosphatases
- ROS
reactive oxygen species
- SHP-2
SH2 domain-containing PTP-2
- SLP-76
SH2 domain-containing leucocyte protein of 76 kDa
- Syk
spleen tyrosine kinase,
- WT
wild-type
Acknowledgments
We thank Dr. H.A. Woo for catalase knockout mice. This study was supported by Bio R&D Program (M10642040002-07N4204-00210 to T.-S.C.) and Basic Science Research Program (2007-0053827 to T.-S.C.) from the National Research Foundation (NRF) grant funded by the Korea government (Ministry of Education, Science and Technology [MEST]), the Brain Korea 21 Scholars Program (to J.Y.J., J.H.M., Y.H.C., J.Y.B., S.B.W., and S.J.P.) and the Brain Korea 21 Plus Program (to J.Y.J. and S.B.W.).
Author Disclosure Statement
The authors declare no competing financial interests exist.
References
- 1.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas 28: R1–R39, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Arias-Salgado EG, Haj F, Dubois C, Moran B, Kasirer-Friede A, Furie BC, Furie B, Neel BG, and Shattil SJ. PTP-1B is an essential positive regulator of platelet integrin signaling. J Cell Biol 170: 837–845, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson BT, Ellmeier W, and Watson SP. Tec regulates platelet activation by GPVI in the absence of Btk. Blood 102: 3592–3599, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bakdash N. and Williams MS. Spatially distinct production of reactive oxygen species regulates platelet activation. Free Radic Biol Med 45: 158–166, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Barford D. and Neel BG. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure 6: 249–254, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Barrett WC, DeGnore JP, Keng YF, Zhang ZY, Yim MB, and Chock PB. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J Biol Chem 274: 34543–34546, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Begonja AJ, Gambaryan S, Geiger J, Aktas B, Pozgajova M, Nieswandt B, and Walter U. Platelet NAD(P)H-oxidase-generated ROS production regulates alphaIIbbeta3-integrin activation independent of the NO/cGMP pathway. Blood 106: 2757–2760, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Blanchetot C, Chagnon M, Dube N, Halle M, and Tremblay ML. Substrate-trapping techniques in the identification of cellular PTP targets. Methods 35: 44–53, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Buczynski A, Wachowicz B, Kedziora-Kornatowska K, Tkaczewski W, and Kedziora J. Changes in antioxidant enzymes activities, aggregability and malonyldialdehyde concentration in blood platelets from patients with coronary heart disease. Atherosclerosis 100: 223–228, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Chari R, Kim S, Murugappan S, Sanjay A, Daniel JL, and Kunapuli SP. Lyn, PKC-delta, SHIP-1 interactions regulate GPVI-mediated platelet-dense granule secretion. Blood 114: 3056–3063, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Diacovo TG, Grenache DG, Santoro SA, and Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol 161: 337–344, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Autreaux B. and Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8: 813–824, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Del Principe D, Menichelli A, De Matteis W, Di Corpo ML, Di Giulio S, and Finazzi-Agro A. Hydrogen peroxide has a role in the aggregation of human platelets. FEBS Lett 185: 142–146, 1985 [DOI] [PubMed] [Google Scholar]
- 14.Dustin LB, Plas DR, Wong J, Hu YT, Soto C, Chan AC, and Thomas ML. Expression of dominant-negative src-homology domain 2-containing protein tyrosine phosphatase-1 results in increased Syk tyrosine kinase activity and B cell activation. J Immunol 162: 2717–2724, 1999 [PubMed] [Google Scholar]
- 15.Farrehi PM, Ozaki CK, Carmeliet P, and Fay WP. Regulation of arterial thrombolysis by plasminogen activator inhibitor-1 in mice. Circulation 97: 1002–1008, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Flint AJ, Tiganis T, Barford D, and Tonks NK. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc Natl Acad Sci U S A 94: 1680–1685, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frearson JA. and Alexander DR. The phosphotyrosine phosphatase SHP-2 participates in a multimeric signaling complex and regulates T cell receptor (TCR) coupling to the Ras/mitogen-activated protein kinase (MAPK) pathway in Jurkat T cells. J Exp Med 187: 1417–1426, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii C, Yanagi S, Sada K, Nagai K, Taniguchi T, and Yamamura H. Involvement of protein-tyrosine kinase p72syk in collagen-induced signal transduction in platelets. Eur J Biochem 226: 243–248, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Furlong MT, Mahrenholz AM, Kim KH, Ashendel CL, Harrison ML, and Geahlen RL. Identification of the major sites of autophosphorylation of the murine protein-tyrosine kinase Syk. Biochim Biophys Acta 1355: 177–190, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Ganju RK, Brubaker SA, Chernock RD, Avraham S, and Groopman JE. Beta-chemokine receptor CCR5 signals through SHP1, SHP2, and Syk. J Biol Chem 275: 17263–17268, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Goodall AH. and Appleby J. Flow-cytometry analysis of platelet-membrane glycoprotein expression and platelet activation. In: Platelets and Megakaryocytes. edited by Gibbins JM. and Mahaut-Smith MP. Totowa, NJ: Humana Press, 2004, p. 233 [Google Scholar]
- 22.Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, and Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem 272: 16644–16651, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Ho YS, Xiong Y, Ma W, Spector A, and Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem 279: 32804–32812, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Hughes CE, Auger JM, McGlade J, Eble JA, Pearce AC, and Watson SP. Differential roles for the adapters Gads and LAT in platelet activation by GPVI and CLEC-2. J Thromb Haemost 6: 2152–2159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson SP, Schoenwaelder SM, Yuan Y, Salem HH, and Cooray P. Non-receptor protein tyrosine kinases and phosphatases in human platelets. Thromb Haemost 76: 640–650, 1996 [PubMed] [Google Scholar]
- 26.Jandrot-Perrus M, Lagrue AH, Okuma M, and Bon C. Adhesion and activation of human platelets induced by convulxin involve glycoprotein VI and integrin alpha2beta1. J Biol Chem 272: 27035–27041, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Johnson RM, Ho YS, Yu DY, Kuypers FA, Ravindranath Y, and Goyette GW. The effects of disruption of genes for peroxiredoxin-2, glutathione peroxidase-1, and catalase on erythrocyte oxidative metabolism. Free Radic Biol Med 48: 519–525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones KL, Hughan SC, Dopheide SM, Farndale RW, Jackson SP, and Jackson DE. Platelet endothelial cell adhesion molecule-1 is a negative regulator of platelet-collagen interactions. Blood 98: 1456–1463, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Karisch R, Fernandez M, Taylor P, Virtanen C, St-Germain JR, Jin LL, Harris IS, Mori J, Mak TW, Senis YA, Ostman A, Moran MF, and Neel BG. Global proteomic assessment of the classical protein-tyrosine phosphatome and “Redoxome”. Cell 146: 826–840, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krotz F, Sohn HY, Gloe T, Zahler S, Riexinger T, Schiele TM, Becker BF, Theisen K, Klauss V, and Pohl U. NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood 100: 917–924, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Kuchay SM, Kim N, Grunz EA, Fay WP, and Chishti AH. Double knockouts reveal that protein tyrosine phosphatase 1B is a physiological target of calpain-1 in platelets. Mol Cell Biol 27: 6038–6052, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon J, Qu CK, Maeng JS, Falahati R, Lee C, and Williams MS. Receptor-stimulated oxidation of SHP-2 promotes T-cell adhesion through SLP-76-ADAP. EMBO J 24: 2331–2341, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee FA, van Lier M, Relou IA, Foley L, Akkerman JW, Heijnen HF, and Farndale RW. Lipid rafts facilitate the interaction of PECAM-1 with the glycoprotein VI-FcR gamma-chain complex in human platelets. J Biol Chem 281: 39330–39338, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Lee SR, Kwon KS, Kim SR, and Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem 273: 15366–15372, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Maeda A, Scharenberg AM, Tsukada S, Bolen JB, Kinet JP, and Kurosaki T. Paired immunoglobulin-like receptor B (PIR-B) inhibits BCR-induced activation of Syk and Btk by SHP-1. Oncogene 18: 2291–2297, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Mahadev K, Zilbering A, Zhu L, and Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem 276: 21938–21942, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Mancini F, Rigacci S, Berti A, Balduini C, and Torti M. The low-molecular-weight phosphotyrosine phosphatase is a negative regulator of FcgammaRIIA-mediated cell activation. Blood 110: 1871–1878, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Meng TC, Fukada T, and Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell 9: 387–399, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Ming Z, Hu Y, Xiang J, Polewski P, Newman PJ, and Newman DK. Lyn and PECAM-1 function as interdependent inhibitors of platelet aggregation. Blood 117: 3903–3906, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monteiro HP, Arai RJ, and Travassos LR. Protein tyrosine phosphorylation and protein tyrosine nitration in redox signaling. Antioxid Redox Signal 10: 843–889, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Moraes LA, Barrett NE, Jones CI, Holbrook LM, Spyridon M, Sage T, Newman DK, and Gibbins JM. Platelet endothelial cell adhesion molecule-1 regulates collagen-stimulated platelet function by modulating the association of phosphatidylinositol 3-kinase with Grb-2-associated binding protein-1 and linker for activation of T cells. J Thromb Haemost 8: 2530–2541, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy AJ, Bijl N, Yvan-Charvet L, Welch CB, Bhagwat N, Reheman A, Wang Y, Shaw JA, Levine RL, Ni H, Tall AR, and Wang N. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nat Med 19: 586–594, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nieswandt B. and Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood 102: 449–461, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Pasquet JM, Gross B, Quek L, Asazuma N, Zhang W, Sommers CL, Schweighoffer E, Tybulewicz V, Judd B, Lee JR, Koretzky G, Love PE, Samelson LE, and Watson SP. LAT is required for tyrosine phosphorylation of phospholipase cgamma2 and platelet activation by the collagen receptor GPVI. Mol Cell Biol 19: 8326–8334, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasquet JM, Quek L, Pasquet S, Poole A, Matthews JR, Lowell C, and Watson SP. Evidence of a role for SHP-1 in platelet activation by the collagen receptor glycoprotein VI. J Biol Chem 275: 28526–28531, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Patil S, Newman DK, and Newman PJ. Platelet endothelial cell adhesion molecule-1 serves as an inhibitory receptor that modulates platelet responses to collagen. Blood 97: 1727–1732, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Pearce AC, Wilde JI, Doody GM, Best D, Inoue O, Vigorito E, Tybulewicz VL, Turner M, and Watson SP. Vav1, but not Vav2, contributes to platelet aggregation by CRP and thrombin, but neither is required for regulation of phospholipase C. Blood 100: 3561–3569, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Persson C, Sjoblom T, Groen A, Kappert K, Engstrom U, Hellman U, Heldin CH, den Hertog J, and Ostman A. Preferential oxidation of the second phosphatase domain of receptor-like PTP-alpha revealed by an antibody against oxidized protein tyrosine phosphatases. Proc Natl Acad Sci U S A 101: 1886–1891, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, and Violi F. Hydrogen peroxide is involved in collagen-induced platelet activation. Blood 91: 484–490, 1998 [PubMed] [Google Scholar]
- 50.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science 312: 1882–1883, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Salmeen A. and Barford D. Functions and mechanisms of redox regulation of cysteine-based phosphatases. Antioxid Redox Signal 7: 560–577, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, and Rao KV. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell 121: 281–293, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Stone JR. and Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal 8: 243–270, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Suzuki-Inoue K, Wilde JI, Andrews RK, Auger JM, Siraganian RP, Sekiya F, Rhee SG, and Watson SP. Glycoproteins VI and Ib-IX-V stimulate tyrosine phosphorylation of tyrosine kinase Syk and phospholipase Cgamma2 at distinct sites. Biochem J 378: 1023–1029, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tonks NK. and Neel BG. From form to function: signaling by protein tyrosine phosphatases. Cell 87: 365–368, 1996 [DOI] [PubMed] [Google Scholar]
- 56.Watson SP, Auger JM, McCarty OJ, and Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J Thromb Haemost 3: 1752–1762, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Weibrecht I, Böhmer S-A, Dagnell M, Kappert K, Östman A, and Böhmer F-D. Oxidation sensitivity of the catalytic cysteine of the protein-tyrosine phosphatases SHP-1 and SHP-2. Free Radic Biol Med 43: 100–110, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Weng Z, Li D, Zhang L, Chen J, Ruan C, Chen G, Gartner TK, and Liu J. PTEN regulates collagen-induced platelet activation. Blood 116: 2579–2581, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Yamagishi SI, Edelstein D, Du XL, and Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes 50: 1491–1494, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.