Abstract
Numerous studies indicate that placebo analgesia can be established via conditioning procedures. However, these studies have exclusively involved conditioning under continuous reinforcement. Thus, it is currently unknown whether placebo analgesia can be established under partial reinforcement and how durable any such effect would be. We tested this possibility using electro-cutaneous pain in healthy volunteers. Sixty undergraduates received placebo treatment (activation of a sham electrode) under the guise of an analgesic trial. The participants were randomly allocated to different conditioning schedules, namely continuous reinforcement (CRF), partial reinforcement (PRF), or control (no conditioning). Conditioning was achieved by surreptitiously reducing pain intensity during training when the placebo was activated compared with when it was inactive. For the CRF group, the placebo was always followed by a surreptitious reduction in pain during training. For the PRF group, the placebo was followed by a reduction in pain stimulation on 62.5% of trials only. In the test phase, pain stimulation was equivalent across placebo and no placebo trials. Both continuous and partial reinforcement produced placebo analgesia, with the magnitude of initial analgesia being larger following continuous reinforcement. However, while the placebo analgesia established under continuous reinforcement extinguished during test phase, the placebo analgesia established under partial reinforcement did not. These findings indicate that partial reinforcement can induce placebo analgesia and that these effects are more resistant to extinction than those established via continuous reinforcement. Partial reinforcement may, therefore, reflect a novel way of enhancing clinical outcomes via the placebo effect.
A wealth of research indicates that placebo effects can ameliorate both experimental [5,7,12,13,15,16,22,34,36,38,41–44] and clinical pain [26,32,40]. Recent neuroimaging studies demonstrate that placebo analgesia is accompanied by modulation of brain activity in regions known to process pain [8,44]. Furthermore, some of the neurobiological mechanisms underlying placebo analgesia are beginning to be understood, particularly the importance of endogenous opioids in placebo analgesia established via instruction [5,6,32]. Despite these advances, significant gaps remain in our knowledge of the optimal conditions for producing and maintaining placebo analgesia.
Most modern accounts view the placebo effect as a learning phenomenon in which verbal instruction and prior experience combine to produce a placebo effect [14,28,29]. While numerous studies have confirmed that conditioning either alone or in combination with verbal suggestion can produce placebo effects [4,5,12,15,16,24,30,34,36,41–43], these studies have almost exclusively employed conditioning schedules in which presentation of the placebo is always followed by analgesia during acquisition, referred to as continuous reinforcement [10,19]. Thus, it is currently unknown whether placebo effects can be established with variable conditioning schedules in which the placebo is only followed by analgesia on some occasions, referred to as partial reinforcement [10,19]. This is particularly interesting, because, in practice, patients are likely to experience fluctuations in both the severity of their symptoms and the efficacy of their treatments.
In addition, few studies have investigated how long placebo effects last once established [12,15,34], which may well differ depending on how the effect is established. A number of animal studies indicate a partial reinforcement extinction effect, whereby partial reinforcement leads to more durable responding than continuous reinforcement [23,25,27,35]. Thus, partial reinforcement may be one way to increase the longevity of placebo analgesia. Understanding the durability of placebo analgesia and placebo effects more generally is essential for determining the extent to which placebo effects could be used to enhance outcomes in clinical practice.
The current study addressed these gaps by comparing the magnitude and durability of placebo analgesia following continuous and partial reinforcement schedules using experimentally-induced pain. In terms of magnitude, we hypothesised that both continuous and partial reinforcement would induce placebo analgesia, but that this effect would be stronger following continuous reinforcement. This is because partial reinforcement provides some experience of the placebo not working, which weakens the association between placebo and analgesia. In terms of extinction, consistent with the animal literature [23,25,27,35], we hypothesised that if partial reinforcement did induce placebo analgesia, then it would be more resistant to extinction than the placebo analgesia established under continuous reinforcement. This is because the lack of reinforcement during training may make it harder for the partial reinforcement to detect a shift from training to testing compared with the continuous reinforcement group. To our knowledge, this is the first attempt to establish a placebo analgesic effect using partial reinforcement in humans.
METHODS
Participants
Sixty-six (39 female; mean age=19.8, SD=3.82) healthy undergraduate psychology students from the University of Sydney participated to gain course credit. The study was advertised on an online system within the School of Psychology where the students could choose from a number of different studies. To be included in this study, participants had to be at least 18years old, fluent in English, and not have any current or previous heart problems. The study had approval from the University of Sydney’s Human Research Ethics Committee.
Design
The key variable in this study was the between-subjects manipulation of conditioning schedule as shown in Table 1. Participants were recruited under the guise of a trial investigating the analgesic properties of Transcutaneous Electrical Nerve Stimulation (TENS). They were then randomised to one of three groups: continuous reinforcement (CRF), partial reinforcement (PRF), or no conditioning (control). The two conditioning groups were told that they would receive a series of painful stimuli with (placebo) and without (no placebo) activation of the TENS machine. No TENS was actually delivered at any stage. Instead, a placebo device was used which involved an electrode being placed on the participants’ forearm, with ‘activation’ signalled by tactile and auditory cues. Conditioning was achieved by surreptitiously reducing the intensity of the painful stimuli on placebo relative to no placebo trials. The conditioning phase consisted of 32 trials in total: 16 with activation of the placebo device and 16 without activation of the placebo device presented in quasi-random order for each participant. In the CRF group, pain stimuli were reduced on all trials that the placebo was activated in the conditioning phase. In the partial reinforcement group, pain stimuli were reduced on only 62.5% of the trials that the placebo was activated in the conditioning phase. The test phase occurred immediately after the conditioning phase, with no break or signal that a new phase had begun. In this phase, the conditioning groups underwent a further 16 placebo and 16 no placebo trials with pain stimuli at full intensity on all trials, providing the test of placebo analgesia and whether or not it extinguishes after reinforcement has been withdrawn.
Table 1.
Summary of study design. In the CRF and PRF groups the participants were led to believe the placebo device was a TENS machine that would reduce their pain. In the control group, the participants were told that the same device was a method of measuring skin conductance, with no mention of any potential effects on pain. The device was active on half the trials and inactive on the other half. The active trials in the CRF and PRF groups constituted the placebo trials.
| Group | Instruction | Conditioning | Extinction |
|---|---|---|---|
| CRF (n=20) | Told receiving TENS to reduce pain | 16 placebo → 60% 16 no placebo → 100% |
16 placebo → 100% 16 no placebo → 100% |
| PRF (n=20) | Told receiving TENS to reduce pain | 10 placebo → 60% 6 placebo → 100% 16 no placebo → 100% |
16 placebo → 100% 16 no placebo → 100% |
| Control (n=20) | Told no treatment controls | 16 active + 16 inactive → 100% 16 active + 16 inactive → 60% |
|
The control group was told that they had been allocated to receive no treatment and would experience a series of pain stimuli without any TENS stimulation. To control for any possible analgesic effect of the activation of the device, participants were exposed to the device activated, but did not receive the placebo instructions and were merely told that the researchers were piloting a new way of assessing skin conductance. The control group received a total of 64 control stimulations: 32 with the device activated and 32 with the device inactive. Blocks with full and reduced pain stimuli were used such that the control group experienced high and low pain similarly to the CRF and PRF group, except that this was not contingent upon whether or not the device was activated. The dependent variable was pain report following each painful stimulus.
Materials
Verbal instructions
All participants were given an information sheet on arrival that described TENS only briefly as involving passing an electrical current through the skin, with no suggestion of how this might affect their pain. The two conditioning groups receive more substantial information on TENS as follows. Prior to the placebo device being attached, they received a one page handout including sections “What is TENS used for?”, “How does TENS work?”, and “What’s so good about TENS?”. The handout suggested that TENS was effective for reducing pain by “sending stimulation to block or reduce pain signals going to the brain” and was accompanied by references to journal articles on TENS for pain. The conditioning groups were also given oral instructions that supported this as the placebo device was being attached to their arm. These instructions were:
“This is the TENS electrode [researcher shows participant the placebo device]. TENS stands for transcutaneous electrical nerve stimulation. TENS can reduce pain by inhibiting the pain signals that travel up your arm and into your brain. The TENS itself is not painful, but you will feel a small sensation when it’s turned on. I’ll give you an example of what it feels like now.”
The control group were given no additional information about TENS other than the brief mention in the initial information sheet. They did not receive the TENS handout. They only received oral instructions suggesting that a device measuring skin conductance was being attached to their arm. The instructions were:
“You have been allocated to the control group, which means that you will not receive TENS. But, your skin conductance will still be measured. This is the electrode that measures skin conductance [researcher shows participant the device]. Skin conductance is a measure of autonomic arousal. You will feel a slight sensation when the skin conductance is being recorded, but it won’t be painful. Because the skin conductance electrode can interfere with other equipment, we will only turn it on half the time. I’ll give you an example of what it feels like now.”
Placebo device
No TENS was actually delivered to the participant. TENS was simply used as a cover story for the placebo effect. The placebo device was a stimulus isolator (Model FE180, ADInstruments) that generated tactile stimulation via direct currents. Two electrodes, 4cm apart from each other, were attached to the dorsal forearm of the non-dominant hand. Activation of the device involved the delivery of 16 consecutive square pulses with pulse width of 0.2ms and series of coinciding tones. Intensity of the stimulation was calibrated for each participant to a level he/she found just perceptible. This was done by giving all participants an example of the activation of the placebo device prior to testing and in the absence of any pain stimuli. The initial stimulation was originally set at 1mA and was increased by 1mA until it became noticeable to the participant, creating a very gentle vibration on the participants’ skin at the site of the electrode.
Pain stimuli
Pain was induced via electro-cutaneous stimulation similar to that used in other studies on placebo analgesia [12,15]. Each stimulus consisted of an electrical shock delivered to the back of the participant’s non-dominant hand via two silver chloride electrodes, each approximately 1cm apart. Stimuli were generated by a pain stimulator (Model SHK1, Contact Precision Instruments). The stimuli were square pulses with duration of 0.5 sec and frequency of 100 Hz.
The intensity of the pain stimuli was calibrated for each participant individually prior to testing. This was done by initially delivering stimuli at a very low and usually imperceptible level and then increasing the intensity of the stimuli in steps until participants reached a level that they felt was “definitely painful, but tolerable”. To ensure this was at least somewhat painful and to avoid potential floor effects, when this level was reached, the participant was asked to verbally report their pain out of ten. If their reported pain was less than 6 out of ten, then they were asked whether they felt comfortable trying a higher intensity, such that participants pain ratings at the end of calibration were at least 6 out of ten on a verbally reported scale. The level of intensity reached at the end of calibration was labelled as the 100% intensity for that particular participant. Intensity of each stimulus during the experiment was determined on the individual’s 100% intensity level, their experimental condition, and particular trial.
Pain ratings
Participants were asked to rate their pain following each painful stimulus on a 100 point computerised visual analogue scale (VAS). Three anchors were used, with 0 (No pain) and 100 (Very painful) on the left and right extremes respectively, and 50 (Moderately painful) in the middle.
Trial structure and conditioning manipulation
Each trial consisted of a single pain stimulus followed by a pain rating. Within a given trial, each pain stimulus was signalled by a 10 second countdown culminating in an “X” appearing on the computer screen, 0.5 seconds after which the stimulation was delivered. After each stimulus, participants rated the intensity of the pain on the computerised VAS. In between each trial, participants had a rest of 10–15 seconds. On placebo trials, the placebo device was activated for 8sec during the countdown. On no placebo trials, the placebo device remained inactive.
The conditioning phase included 16 placebo and 16 no placebo trials. The CRF received a surreptitious reduction in painful stimulation on all 16 placebo trials. This was achieved by lowering the pain intensity to 60% on placebo trials, a similar sized reduction to those previously used on conditioned placebo analgesia studies [20]. The PRF group received the same surreptitious reduction in painful stimulation, but only on 62.5% of placebo trials; that is, pain intensity was lowered in 10 out of the 16 placebo trials, as shown in Table 1. The placebo trials were intermixed with no placebo trials in quasi-randomised order within participants, such that there were no more than two placebo or no placebo trials in a row. This trial order was employed to ensure that the different trials were distributed across the test session both within and across participants. The test/extinction phase consisted of 32 trials (16 placebo and 16 no placebo) in which the intensity of painful stimuli was set at 100% on both placebo and no placebo trials.
For the control group, the conditioning and extinction phases were identical. Participants in this group received half of their trials at 100% pain intensity and the other half at 60% pain intensity, but this was unrelated to whether or not the device was activated. While control groups in most previous studies received the same 100% shock throughout the experiment (e.g.,[12,34]), we included the variation in pain intensity to ensure the control group had experience with the two levels of painful stimulation and using different levels of pain. This meant the control group experienced 16 active and 16 inactive trials with 100% painful stimulation and 16 active and 16 inactive trials with 60% painful stimuli. These trials were presented in eight blocks of 8 trials (4 with the device active and 4 with the device inactive) with four blocks being at 100% intensity and four at 60% intensity. The blocked design was intended to prevent potential superstitious conditioning, even though the two events were non-contingent.
Exit questionnaire
An exit questionnaire was used to test whether participants guessed the true nature of the study, as well as their knowledge of the placebo-shock reduction contingency across groups. The first question asked: “What do you think the study was about?” with an open response. The second and third questions assessed contingency knowledge for the first and second half of the experiment, respectively. The questions read “In the first [or second] half of the experiment, did you notice any reduction in pain when TENS was turned on compared with when it was not turned on?”. Participants had to make a forced choice response, either ‘yes’ or ‘no’ and then rate how consistent they thought this relationship was on a 10-point scale ranging from 1 (Not at all) to 10 (Very consistent). The same questions were asked to the control group, except they concerned the relationship between pain reduction and the supposed skin conductance measurements.
Procedure
Participants attended a single one-hour session and were tested individually in an isolated cubicle. Upon arrival, they were given an information sheet that described the study as a test of the acute effect of TENS on psychophysiological responses to pain. The two conditioning groups were then told that they had been allocated to receive TENS and were given the handout on TENS. The control group were told that they had been allocated to receive no treatment and simply rested for two minutes. The placebo device was then introduced and attached to the participant during which each group was given the relevant oral instructions.
The experimenter then explained the trial structure to the participant, left the room, and then initiated the computerised programme that controlled the delivery of the pain stimuli, activation of the ‘TENS’ device, and pain ratings. The conditioning phase was initiated and was followed immediately by the test/extinction phase without any notification to the participant. At the end of the test/extinction phase, participants completed the exit questionnaire assessing the beliefs about the study. A debrief statement was sent to all participants via email at the completion of the study.
Data handling and analysis
Participants (n=6) were excluded if their average pain rating on no placebo (CRF and PRF) or device in active trials (control group) at 100% pain intensity was less than 20 out of 100. ANOVA and Chi-square tests of independence tested for baseline differences in age and gender. We checked the normality of the pain ratings across each of the 64 trials within each group. Deviations from normality were rare (only 15 out of 192 tests, i.e. 8%) and consistent with the Type I error rate of .05 (5%) in these tests, suggesting acceptable levels of normality.
For the main analysis, conditioning and test phases were analysed separately. In each phase, the groups were compared by calculating difference scores between pain with and without the “TENS” device activated that were analysed via mixed ANCOVAs with group and trial as factors, controlling for age and gender. For the test phase, we followed this up with within-groups analysis of placebo analgesia using repeated measures ANCOVAs with treatment and trial as factors, again controlling for age and gender. The critical test of the magnitude of the placebo effect established under each conditioning schedule was the difference in pain ratings on the first placebo trial and no placebo trial in the test/extinction phase, i.e. Trial 17. We expected that the strongest placebo analgesic effect would occur immediately after the conditioning phase. This is because the first test trial occurs before any extinction has taken place. To explore changes over time and compare rates of extinction across the groups, we tested linear trends whenever trial was included as factor. To isolate the effects of the different conditioning schedules we conducted planned pairwise comparisons between each group. All analyses were conducted in IBM SPSS Statistics 20.0, covariates were mean centred to reduce multicollinearity, Greenhouse-Geisser corrections were made whenever the sphericity assumption was not met (unadjusted degrees of freedom are reported), and results were considered statistically significant when p<.05.
RESULTS
There were no differences in age or gender across the three groups, F2,57=.26, p=.775 and χ2 (df=2, N=60)=1.67, p=.435, respectively.
Training Phase
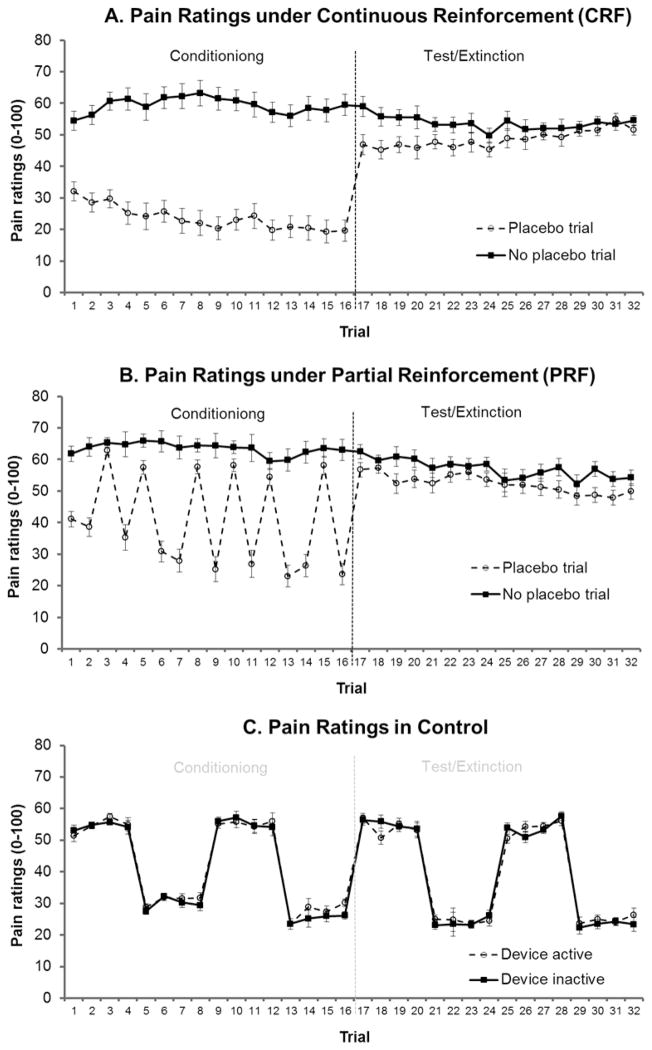
Pain ratings during training are shown Figure 1 (Trials 1–16). The training phase was separated intro trials on which the PRF group were reinforced in the presence of the placebo versus the non-reinforced placebo trials. For the reinforced trials, the two-way analysis with group and trial as factors revealed a significant main effect of group (F2,55=72.7, p<.001). Pairwise comparisons revealed that the differences in pain ratings on placebo versus no placebo trials was significantly greater in both the CRF group and the PRF group relative to the equivalent control trials (F1,55=114.5, p<.001 and F1,55=101.5, p<.001, respectively), confirming the conditioning manipulation effectively reduced pain on the relevant trials. Importantly, there was no difference in magnitude of the pain reduction on reinforced trials between the CRF and PRF groups (F<1). There was also a significant main effect of trial (F9,495=11.6, p<.001). A significant linear trend indicated that the differences in pain ratings on placebo versus no placebo trials increased over the training phase, averaged across groups (F1,55=31.8, p<.001). Interaction contrasts comparing linear trends between groups revealed significant interactions in linear trends for CRF versus control and for PRF versus control (F1,55=11.9, p=.001 and F1,55=20.2, p<.001, respectively). These results indicated that the magnitude of pain relief caused by the surreptitious reduction increased across trials. There was, importantly, no difference in linear trends between the CRF and PRF group (F1,55=1.12, p=.294). For the trials on which the PRF group were not reinforced, there was also a main effect of group (F 2,55=111.1, p<.001) with pairwise comparisons between groups revealing that the difference in pain ratings was significantly larger for the CRF group compared with both the PRF group and the control group (F1,55=198.9, p<.001 and F1,55=126.2, p<.001, respectively), which was unsurprising because the former’s pain intensity was at 60% whereas the latter’s were at 100%. The reduction in pain on placebo trials in the PRF group was larger equivalent trials in the control group even though these trials were all at 100% intensity (F1,55=7.10, p=.01), confirming evidence of a placebo effect during training. Neither the main effect nor the overall linear trend for trial reached statistical significant (F5,275=2.18, p=.07 and F1,55=1.60 p=.21, respectively). The trial by group interaction was also not significant (F2,55=2.05, p=.13) nor were any of the pairwise group by linear trend interactions, although the linear trends for CRF vs control bordered significance (F1,55=4.00, p=.05).
Figure 1.
Covariate (age, gender) adjusted mean (±SE of mean difference) pain ratings with the device active versus inactive for A. Continuous reinforcement group, who demonstrated a significant placebo effect that extinguished over the course of the test phase. B. Partial reinforcement group who also demonstrated a significant placebo effect, although weaker than continuous reinforcement, which did not appear to extinguish. C. Control group, who received no conditioning and no placebo instruction showed no difference between active and inactive trials.
Thus, the training data confirmed that the surreptitious reduction effectively reduced pain and, importantly, that the magnitude of this reduction was equivalent for the CRF and PRF groups.
Test Phase
Inter- group comparisons
In order to compare pain ratings across groups, we calculated difference scores between pain on trials when the “TENS” device was active compared with when it was inactive. This adjusts for the variability in shock intensities experienced over the course of the test phase among the experimental groups. These differences are shown in Figure 2.
Figure 2.
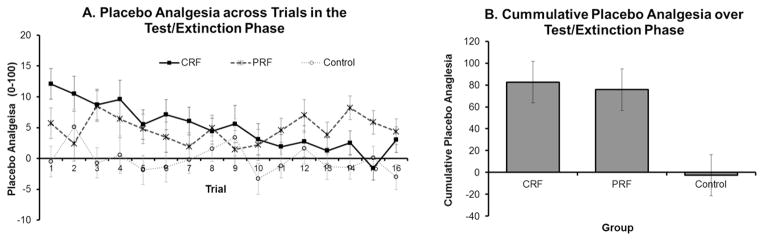
Covariate (age, gender) adjusted mean difference (±SE) in pain ratings with the device active and inactive during the test/extinction phase across groups for each individual trial (A) and cumulatively over the entire test/extinction phase (B). Higher scores indicate less pain with the device active. The Continuous Reinforcement Group (CRF) demonstrated a significant placebo effect of the first test trial that extinguished over the course of the test phase. The Partial Reinforcement Group (PRF) also demonstrated a significant placebo effect on the first test trial, although significantly weaker than CRF, but this did not appear to extinguish over the test phase.
On the initial test trial, there was a significant main effect of group (F2,55=4.00, p=.05). Pairwise comparisons revealed a placebo effect in the CRF group, with the pain reduction induced by the placebo treatment 15.8 points greater than control (F1,55=20.5, p<.001). There was also a placebo effect in the PRF group, with the pain reduction induced by the placebo treatment being 8.6 points greater than control (F1,55=5.96, p=.02). Further, these comparisons revealed that the placebo analgesia induced by CRF was 7.2 points larger than that induced by PRF (F1,55=4.16, p=.046).
The two-way group by trial analysis over the entire test phase revealed a significant main effect of group (F2,55=6.42, p=.003). Pairwise comparisons confirmed the placebo analgesia observed on the first test trial for CRF versus control and PRF versus control were evident when averaged across all test trials (F1,55=10.5, p=.002 and F1,55=8.50 p=.005, respectively). However, in this case, there was no difference in the magnitude of the placebo analgesia between CRF and PRF when averaged over all test trials (F<1). There was no a significant main effect of trial overall (F15,825=1.40, p=.18), but there was a significant linear trend indicating that the difference in pain ratings between placebo and no placebo trials decreased across the test phase (F1,55=13.6, p=.001). There was also a significant trial by group interaction (F30,825=1.61, p=.048). The pairwise comparisons revealed significant interactions of linear trends between the CRF group and the control group and between the CRF group and the PRF group (F1,55=17.4, p<.001 and F1,55=20.8, p<.001, respectively) indicating that the placebo analgesia in the CRF group decreased relative to both control and PRF, indicating extinction. There was, however, no significant interaction of linear trends between the PRF group and control (F<1), suggesting the placebo analgesia induced via PRF did not extinguish. Confirmation of these effects can be seen by analysing differences between placebo and no placebo trials within the CRF and PRF group, as per below.
CRF Group
In the CRF group, there was clear evidence placebo analgesia on the initial test trial, with pain rated as 12.3 (SD=14.2) lower in the presence of the placebo compared with no placebo, despite shock level being at 100% on both trials (F1,17=13.8, p=.002), as can be seen in Figure 1A. The two-way analysis of trial by treatment over the entire test phase revealed a significant main effect of treatment with pain on placebo trials being lower than that on no placebo trials (F1,17=11.4, p=.004). There was no main effect of trial, nor a significant linear trend in trials when averaged across treatments (both F<1). However, there was a significant overall trial by treatment interaction (F15,255=2.73, p=.02) which was accompanied by a significant interaction in linear trends, indicating that difference in pain ratings between placebo and no placebo trials gradually decreased over the test phase with extinction of the placebo analgesia (F1,17=17.1, p=.001).
PRF Group
As with the CRF group, there was evidence of placebo analgesia on the initial test trial in the PRF group, with pain rated as 5.5 (SD=10.1) lower in the presence of the placebo compared with no placebo, despite shock level being at 100% on both trials (F1,17=4.68, p=.045), as shown in Figure 1B. Two-way analysis of trial by treatment over the entire test phase revealed a significant main effect of treatment with pain rated as lower on placebo trials compared with no placebo trials (F1,17=10.9, p=.004). There was a main effect of trial (F15,255=3.53, p=.003), with a significant linear trend suggesting a decrease in pain across the test phase when averaged across treatment (F1,17=13.3, p=.002). Importantly, however, there was no overall interaction between trial and treatment, nor a significant interaction in linear trends (both F<1) suggesting that the placebo analgesia in the PRF group was maintained over the test phase.
Control Group
There was no difference between the training and test phase for this group. Therefore, the control group data were analysed over the entire study session, but separately for 60% and 100% shock intensity. At 60% intensity, there was a trend towards a significant main effect of treatment, suggesting that pain ratings in fact tended to be higher on device active trials compared with trials on which the device was inactive (F1,17=3.91, p=.065), as shown in Figure 1C. The main effect of trial was marginally non-significant (F15,255=2.76, p=.053) as was the linear trend (F1,17=3.79, p=.068), suggesting a tendency for pain ratings to decrease across trials when averaged over treatment. There was no interaction between trial and treatment (both F<1). At 100% intensity, there was no main effects of treatment or trial (both F<1) nor any significant interactions between the two (highest F15,255=1.30, p=.27). This indicated that there was no difference in pain between active and inactive trials and no change in the magnitude of pain across trials in the control group at 100% pain intensity. Overall, this indicated that activation of the device used as a placebo in the CRF and PRF groups had no inherent analgesic effects.
Exit questionnaire
Participants appeared to find the cover story credible. Sixty percent of participants reported that the aim of the study was to test the effect of TENS on pain, specifically. The remaining participants generally gave very brief responses that were consistent with the cover story, usually that the aim was to test pain tolerance, without mentioning TENS specifically (26.7%). Only one participant mentioned anything vaguely related to the placebo effect, which was that the study aimed to test the effect of a “pre-hint” on pain. There were no differences in beliefs about the cover story between the CRF and PRF groups.
DISCUSSION
The current study tested the effect of different reinforcement schedules on the magnitude and durability of placebo analgesia. Four novel and important findings emerged. The first is that placebo analgesia can be established under partial reinforcement. The second is that the placebo analgesia established under partial reinforcement was weaker than that established under continuous reinforcement. The third is that the placebo analgesia established under continuous reinforcement extinguished. The fourth, and perhaps most interesting, is that the placebo analgesia established under partial reinforcement appeared resistant to extinction. These findings have a number of important practical and theoretical implications.
The initial pain relief we observed in the test phase following continuous reinforcement, i.e. when placebo treatment was always followed by a surreptitious reduction in pain during conditioning, is consistent with numerous previous studies using conditioning to produce placebo analgesia [5,12,15,16,30,34,36,41–43]. However, we extended this previous research by demonstrating that placebo analgesia can also be induced following partial reinforcement, i.e. when placebo treatment was usually, but not always followed by a surreptitious reduction in pain during training, which has not previously been tested. It is important to emphasise, however, that the magnitude of the initial placebo analgesia established under partial reinforcement was weaker than the placebo analgesia established under continuous reinforcement. This is consistent with animal conditioning studies demonstrating weaker conditioned responding following partial reinforcement compared with continuous reinforcement [1,2,21]. Thus, while partial reinforcement can induce placebo analgesia, it appears that continuous reinforcement will produce stronger initial placebo analgesia.
Perhaps most interestingly, the durability of the placebo analgesia differed depending on the reinforcement schedule under which it was established. While the placebo effect established under continuous reinforcement extinguished relative to both controls and the PRF group over the test phase, the placebo effect established under partial reinforcement was maintained relative to both controls and the CRF group. This study, therefore, provides the first evidence of a partial reinforcement extinction effect for placebo analgesia. That is, placebo analgesia established under partial reinforcement appears more durable than placebo analgesia established under continuous reinforcement. While no previous studies have compared continuous versus partial reinforcement training schedules for placebo effects in humans, the current finding is consistent with animal studies demonstrating greater resistance to extinction following partial reinforcement [35].
There are a number of possibilities to explain such an effect. The most parsimonious is probably a Bayesian account in which the shift from training to extinction is harder to detect in the partial reinforcement group compared with the continuous reinforcement group because, in the former, the probability of reinforcement during training is lower during training is more similar to the test phase [17]. Other accounts make similar predictions based on learning phenomena such as generalisation decrement [35]. Generalisation decrement refers to when conditioned responding becomes weaker as the eliciting cue or context becomes more dissimilar from the originally trained cue or context. According to this account of the partial reinforcement extinction effect, the non-reinforced trials during training under partial reinforcement serve as contextual cues themselves, which are encoded as part of the context. This means that in test phase, when no trials are reinforced, the context is more similar to training for those who received partial reinforcement compared with those who received continuous reinforcement, because the latter have never previously experienced non-reinforced trials. This results in less generalisation decrement and hence slower extinction following partial reinforcement [35].
The extinction we observed in the continuous reinforcement group differs from other studies that have tested the durability of placebo analgesia and found no evidence of extinction [12,34]. One potential reason for evidence of extinction in the current study compared with the lack of evidence in Colloca and Benedetti’s [12] study, which also used continuous reinforcement to establish placebo analgesia, is the difference in the length of the test phases across these studies. In the current study, we tested extinction over 16 test trials whereas Colloca and Benedetti [12] tested it over six trials, the latter of which may not have been sufficient to detect extinction. The pattern of results in Figure 2A supports this possibility, with the placebo inducing analgesia relative to the control on the sixth test trial (i.e. trial 22), that had clearly diminished by the 16th test trial (i.e. trial 32). Thus, longer test phases may be required to demonstrate extinction. Montgomery and Kirsch [34] found no evidence of extinction in a group of participants given suggestion of placebo analgesia without conditioning. Their experimental design also differed in terms of the type of placebo (inert cream) and number of test trials (10). Moreover, it is plausible that placebo effects established via suggestion alone extinguish differently to those established via suggestion with conditioning, the latter of which was the focus here, which could explain the apparent differences in results. Furthermore, the current result is consistent with the broader learning literature in which there is a large body of evidence that demonstrates a gradual reduction in conditioned responding after reinforcement is withdrawn, i.e. extinction [9,33]
At this point, it is also worth noting that some authors have used data from double-blind placebo-controlled clinical trials in which placebo treated patients report ongoing improvement as evidence against extinction of placebo effects, including for rheumatoid arthritis [39] and pain due to irritable bowel syndrome [11]. There is also evidence of prolonged (50 days) placebo analgesia in a case study of a chronic pain patient [31]. However, such findings are limited in that double-blind placebo-controlled trials do not include natural history groups meaning that any improvement observed in the placebo group could be attributable to other factors, such as, regression to the mean or spontaneous recovery, and case studies of single patients may not generalise to other patients. In addition, the durability of a placebo effect may vary depending on the specific condition being treated and the psychological processes underlying the effect.
The differences in the magnitude and durability of placebo effects established under different reinforcement schedules have some important clinical implications. The current results suggest that continuous reinforcement is the most effective training schedule in order to induce a large one-off placebo effect. However, partial reinforcement appears the most effective in terms of inducing a more durable placebo effect. One interesting application of this finding is for drug dose reductions/discontinuation. It may be possible to use partial reinforcement schedules when delivering active drugs in which the active treatment is occasionally replaced by a placebo [3,18,37]. Although these findings need to be replicated in clinical pain with active treatments, pharmacological partial reinforcement schedules could lead to a reduction in the total amount of active painkillers required to maintain the same treatment effect whilst reducing tolerance and side effects. Furthermore, in the case of drugs that lead to discontinuation effects, such a schedule could produce more durable placebo effects that attenuate withdrawal effects during dose reduction.
There were some potential limitations to the current study worth noting. First, while placebo effect established under partial reinforcement appeared to be maintained over the course of the test phase, suggesting non-extinction, it could be the case that with further test trials, this effect would extinguish. Nonetheless, it is clear from the results that over the course of the 16 test trials the rate of extinction was much more rapid following continuous reinforcement compared with partial reinforcement, whether or not the latter would have eventually extinguished with more test trials. Second, there was an asymmetry between the continuous and partial reinforcement groups in terms of the total number of reinforced trials they each experienced during training. This was an intentional decision in order to match the total length of training across the two groups. However, it would be interesting for future studies to compare different reinforcement schedules on placebo effects matched on the number of reinforced trials compared with matching for training length. Third, extinction was tested within a single test phase consisting of 16 placebo and inactive trials, each, i.e. Trials 17–32. As such, it would be worth testing the extent to which the current findings generalise to a clinical setting in which both training and testing occur over a number of days, rather than in a single session. Finally, we did not assess participants’ expectancies over the course of the study. Assessing participant expectancies in future studies would provide interesting data on how different reinforcement schedules affect expectancies and the extent to which these mediate the differences in placebo analgesia observed across the schedules. Indeed, one potentially important difference between conditioning processes in humans compared with those in animals in so far as they relate to placebo analgesia, is how consciously accessible and verbalisable expectancies might affect conditioning procedures in humans.
In conclusion, the current study provides novel evidence that placebo analgesia can be established under partial reinforcement and that such effects, while weaker initially, are more durable than placebo analgesia established under continuous reinforcement. These findings support recent suggestions that partial reinforcement could be an interesting method of reducing total drug dosages whilst maintaining therapeutic benefit.
Summary.
This study provides the first evidence that placebo analgesia can be established via partial reinforcement and, importantly, that these effects are resistant to extinction.
Acknowledgments
The authors wish to thank Professor Justin Harris, University of Sydney for his useful advice on the current project. Dr Luan Colloca is supported by the Intramural Research Program of the National Institute of Mental Health and National Center for Complementary and Alternative Medicine.
Footnotes
Conflict of Interest
The authors have no conflict of interest in producing this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ader R. Conditioned immunopharmacological effects in animals: Implications for a conditioning model of pharmacotherapy. In: White L, Tursky B, Schwartz GE, editors. Placebo: Theory, research, and mechanisms. New York: Guilford Press; 1985. pp. 306–323. [Google Scholar]
- 2.Ader R, Cohen N. Behaviorally conditioned immunosuppression. Psychosom Med. 1975;37:333–340. doi: 10.1097/00006842-197507000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Ader R, Mercurio MG, Walton J, James D, Davis M, Ojha V, Kimball AB, Fiorentino D. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosom Med. 2010;72:192–197. doi: 10.1097/PSY.0b013e3181cbd38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albring A, Wendt L, Benson S, Witzke O, Kribben A, Engler H, Schedlowski M. Placebo Effects on the Immune Response in Humans: The Role of Learning and Expectation. PLoS ONE. 2012;7:e49477. doi: 10.1371/journal.pone.0049477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: Expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benedetti F. The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. Pain. 1996;64:535–543. doi: 10.1016/0304-3959(95)00179-4. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta J-K. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25:10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The Effect of Treatment Expectation on Drug Efficacy: Imaging the Analgesic Benefit of the Opioid Remifentanil. Science Translational Medicine. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 9.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 10.Bouton ME. Learning and Behavior: A Contemporary Synthesis. Sunderland, MA: Sinauer Associates; 2007. [Google Scholar]
- 11.Chey WD, Chey WY, Heath AT, Dukes GE, Carter EG, Northcutt A, Ameen VZ. Long-term safety and efficacy of alosetron in women with severe diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2004;99:2195–2203. doi: 10.1111/j.1572-0241.2004.30509.x. [DOI] [PubMed] [Google Scholar]
- 12.Colloca L, Benedetti F. How prior experience shapes placebo analgesia. Pain. 2006;124:126–133. doi: 10.1016/j.pain.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Colloca L, Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Curr Opin Anaesthesiol. 2007;20:435–439. doi: 10.1097/ACO.0b013e3282b972fb. [DOI] [PubMed] [Google Scholar]
- 14.Colloca L, Miller FG. Harnessing the placebo effect: the need for translational research. Philos Trans R Soc Lond B Biol Sci. 2011;366:1922–1930. doi: 10.1098/rstb.2010.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. How the number of learning trials affects placebo and nocebo responses. Pain. 2010;151:430–439. doi: 10.1016/j.pain.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colloca L, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. Pain. 2008;136:211–218. doi: 10.1016/j.pain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Courville AC, Daw ND, Touretzky DS. Bayesian theories of conditioning in a changing world. Trends Cogn Sci. 2006;10:294–300. doi: 10.1016/j.tics.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Doering BK, Rief W. Utilizing placebo mechanisms for dose reduction in pharmacotherapy. Trends Pharmacol Sci. 2012;33:165–172. doi: 10.1016/j.tips.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Domjan M. The Principles of Learning and Behavior: Active Learning Edition. Belmont, CA: Thomson Wadsworth; 2006. [Google Scholar]
- 20.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the Opioidergic Descending Pain Control System Underlies Placebo Analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald RD. Effects of partial reinforcement with acid on the classically conditioned salivary response in dogs. J Comp Physiol Psychol. 1963;56:1056–1060. doi: 10.1037/h0048204. [DOI] [PubMed] [Google Scholar]
- 22.Geers AL, Wellman JA, Fowler SL, Helfer SG, France CR. Dispositional Optimism Predicts Placebo Analgesia. The journal of pain : official journal of the American Pain Society. 2010;11:1165–1171. doi: 10.1016/j.jpain.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haselgrove M, Aydin A, Pearce JM. A Partial Reinforcement Extinction Effect Despite Equal Rates of Reinforcement During Pavlovian Conditioning. J Exp Psychol Anim Behav Process. 2004;30:240–250. doi: 10.1037/0097-7403.30.3.240. [DOI] [PubMed] [Google Scholar]
- 24.Herrnstein R. Placebo effect in the rat. Science. 1962;138:677–678. doi: 10.1126/science.138.3541.677. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins WO, Stanley JC. Partial reinforcement: a review and critique. Psychol Bull. 1950;47:193–234. doi: 10.1037/h0060772. [DOI] [PubMed] [Google Scholar]
- 26.Kaptchuk TJ, Stason WB, Davis RB, Legedza ART, Schnyer RN, Kerr CE, Stone DA, Nam BH, Kirsch I, Goldman RH. Sham device v inert pill: randomised controlled trial of two placebo treatments. Br Med J. 2006:391–397. doi: 10.1136/bmj.38726.603310.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimnle GA. Hilgard and Marquis’ conditioning and learning. New York: Appleton-Century-Crofts; 1961. [Google Scholar]
- 28.Kirsch I. Response expectancy as a determinant of experience and behaviour. Am Psychol. 1985;40:1189–1202. [Google Scholar]
- 29.Kirsch I. How Expectancies Shape Experience. Washington: American Psychological Association; 1999. [Google Scholar]
- 30.Klinger R, Soost S, Flor H, Worm M. Classical conditioning and expectancy in placebo hypoalgesia: A randomized controlled study in patients with atopic dermatitis and persons with healthy skin. Pain. 2007;128:31–39. doi: 10.1016/j.pain.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 31.Kupers R, Maeyaert J, Boly M, Faymonville M, Laureys S. Naloxone-insensitive epidural placebo analgesia in a chronic pain patient. Anesthesiology. 2007;106:1239–1242. doi: 10.1097/01.anes.0000265418.68005.8a. [DOI] [PubMed] [Google Scholar]
- 32.Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet. 1978;2:654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- 33.Lovibond PF. Cognitive Processes in Extinction. Learn Mem. 2004;11:495–500. doi: 10.1101/lm.79604. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72:107–113. doi: 10.1016/s0304-3959(97)00016-x. [DOI] [PubMed] [Google Scholar]
- 35.Pearce JM, Redhead ES, Aydin A. Partial Reinforcement in Appetitive Pavlovian Conditioning with Rats. The Quarterly Journal of Experimental Psychology Section B. 1997;50:273–294. doi: 10.1080/713932660. [DOI] [PubMed] [Google Scholar]
- 36.Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83:147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 37.Sandler AD, Glesne CE, Bodfish JW. Conditioned placebo dose reduction: a new treatment in attention-deficit hyperactivity disorder? J Dev Behav Pediatr. 2010;31:369–375. doi: 10.1097/DBP.0b013e3181e121ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang CW, Colagiuri B. Can an Educational Handout Enhance Placebo Analgesia for Experimentally-Induced Pain? PLoS ONE. 2013;8:e77544. doi: 10.1371/journal.pone.0077544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traut EF, Rassarelli EW. Placebo in the treatment of rheumatoid arthritis and other rheumatic conditions. Ann Rheum Dis. 1957;16:18–22. doi: 10.1136/ard.16.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vase L, Robinson ME, Verne G, Price DD. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115:338–347. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Voudouris NJ, Peck CL, Coleman G. Conditioned placebo responses. J Pers Soc Psychol. 1985;48:47–53. doi: 10.1037//0022-3514.48.1.47. [DOI] [PubMed] [Google Scholar]
- 42.Voudouris NJ, Peck CL, Coleman G. Conditioned response models of placebo phenomena: Further support. Pain. 1989;38:109–116. doi: 10.1016/0304-3959(89)90080-8. [DOI] [PubMed] [Google Scholar]
- 43.Voudouris NJ, Peck CL, Coleman G. The role of conditioning and verbal expectancy in the placebo response. Pain. 1990;43:121–128. doi: 10.1016/0304-3959(90)90057-K. [DOI] [PubMed] [Google Scholar]
- 44.Wager TD, Matre D, Casey KL. Placebo effects in laser-evoked pain potentials. Brain, Behavior, and Immunity. 2006;20:219–230. doi: 10.1016/j.bbi.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]