Abstract
Modafinil, a wake-promoting agent used to treat sleep disorders, is thought to enhance cognition. Although modafinil has shown promise as a pharmacotherapy for the treatment of cocaine dependence, it is unknown to what extent cognitive effects may play a role in such treatment. We examined the effect of modafinil on the Balloon Analogue Risk Task (BART), a behavioral measure in which higher scores are purported to reflect a greater propensity for risk-taking. Thirty cocaine dependent individuals, enrolled in a randomized clinical trial of modafinil 400mg (n=12) versus placebo (n=18), were administered the BART during the second week of inpatient treatment for cocaine dependence. A comparison cohort of healthy participants (n=19) performed the BART under similar conditions. Modafinil treatment was associated with significantly higher BART scores (p=0.01), which were comparable to scores in healthy persons. BART scores in placebo treated participants were much lower than previously reported in healthy participants, and lower than those observed in the comparison cohort. As propensity toward risk taking is typically associated with higher BART scores as well as increased risk for substance use, our findings may reflect a novel aspect of cognitive impairment related to chronic cocaine use. Notably, the low BART scores reflect highly suboptimal performance on the task, and the observed effect of modafinil may indicate a normalization of this impairment and have implications for treatment outcome.
Keywords: cocaine dependence, modafinil, cognition, risk taking, Balloon Analogue Risk Task
1. INTRODUCTION
Chronic cocaine use is associated with long-term neurocognitive impairments (Jovanovski et al, 2005 and Bolla et al, 1998) that may predispose individuals to stimulant dependence (Ersche et al, 2012) and could be the result of long-term cocaine use (Beveridge et al, 2008). Cognitive impairment is associated with lower treatment retention and engagement (Aharonovich et al, 2006, 2008), so may prove to be an important target for pharmacotherapies developed to treat cocaine use disorders (Sofuoglu et al, 2013).
Modafinil is an agent with pro-cognitive effects (Minzenberg and Carter, 2008), and has shown promise in the treatment of cocaine dependence (Mariani and Levin, 2012) in laboratory self-administration studies (Hart et al, 2008) and in double-blind, placebo-controlled clinical trials (Dackis et al, 2005), although positive results have not been universal (Dackis et al, 2012) and may be limited to sub-populations of cocaine users such as persons without alcohol dependence (Anderson et al, 2009). A wake-promoting agent, modafinil is FDA approved for the treatment of several sleep disorders including narcolepsy. Modafinil has also been shown to benefit working memory and attention in in persons with cocaine dependence who were not alcohol dependent (Kalechstein et al, 2013), and when given in the morning has a normalizing effect on sleep architecture in this population (Morgan et al, 2007, 2010). These effects on treatment outcome, cognition and sleep may be interrelated: for instance, changes in sleep architecture during abstinence and laboratory cocaine use correspond with sleep-dependent cognitive performance (Morgan et al, 2006, 2008). However, the effect of modafinil on risk-taking behavior in cocaine dependent individuals is not known.
The Balloon Analogue Risk Task (BART) is a task developed to assess risk-taking tendency (Lejuez et al, 2002). Previous studies in healthy populations have consistently associated higher BART scores with greater self-reported risk taking, including social drinking and experimentation with illicit substances (Lejuez et al, 2002, 2007, and Aklin et al, 2005). However, fewer studies have examined BART performance in chronic substance users (e.g. Lejuez et al, 2004; Hopko et al, 2006; Tull et al, 2009), and it is not clear whether BART score differences are associated with chronic substance use. An early study found that smokers scored higher than nonsmokers (Lejuez et al, 2003), but other work has found no differences between non-substance-users and either smokers (Dean et al, 2011), cannabis users (Gonzalez et al, 2012), or recreational stimulant users (Bishara et al, 2009).
Given the potential effects of modafinil on both cognition and clinical outcomes in substance users, we sought to examine differences in BART performance in cocaine dependent individuals who were administered either modafinil or placebo in the context of a randomized clinical trial.
2. METHODS
2.1 Cocaine dependent participants
Participants were recruited as part of an ongoing clinical trial on the use of modafinil to treat cocaine dependence. The first 30 participants who completed the at least 10 days of the initial 2-week inpatient portion of this trial were administered the BART (18 placebo, 12 modafinil).
Participants were chronic cocaine users who met DSM-IV criteria and were seeking treatment for cocaine dependence. Potential participants were excluded if they reported lifetime dependence on substances other than cocaine and nicotine, or current alcohol use in excess of 350 grams (25 standard drinks) per week for the past month (to approximate the population that appears to respond best to modafinil; Anderson et al, 2009); if they had a medical condition that would render study participation unsafe, a known hypersensitivity to modafinil, or any non-substance related Axis I disorder; if they currently used any psychiatric medications, medications that affect sleep, or medications not safe to take with modafinil; or, if female and of childbearing potential, if they were pregnant, lactating, or unwilling to use contraceptives for the duration of the study.
Urn randomization was used to balance age, gender, and recent quantity of cocaine used between groups. All participants reviewed and signed a consent form, approved by the local institutional review board, before entering the study.
2.2 Healthy comparison participants
Nineteen healthy volunteers were recruited as a reference group for BART data and did not receive study medication or placebo. Eligibility criteria were similar to those used for cocaine dependent individuals, except these individuals were excluded if they had any current illicit substance use, or any history of substance abuse or dependence other than nicotine.
Healthy participants were screened by phone, and then reported to the laboratory for a single 1–2 hour session. Healthy participants were given a series of questionnaires including the Shipley Institute of Living Scale (Zachary, 1986 and Shipley, 1940) and the Southern Oaks Gambling Scale (SOGS) (Lesieur and Blume, 1987).
2.3 Study design
Cocaine dependent participants were admitted to an inpatient research unit for 12 days prior to 6 weeks of outpatient treatment. Placebo treatment for all participants began on Day 2 (at 7:30 AM), and modafinil up-titration began on Day 5 at 100mg, and increased by 100mg per day until 400mg daily was achieved on Day 8. No participant complained of side-effects that resulted in a change in the up-titration schedule.
2.4 Questionnaires
Demographic information was collected during the telephone screen. During the subsequent in-person screen, cocaine dependent participants completed the Shipley Institute of Living Scale, SOGS, Addiction Severity Index (ASI) (McLellan et al, 1980, 1992), and a 30-day timeline follow back of all substance use (Miller and Del Boca, 1994 and Sobell and Sobell, 1980).
2.5 Balloon Analogue Risk Task (BART)
A standard 30-trial form of the BART (Lejuez et al, 2002) was administered on Day 10 for cocaine dependent participants, and was administered to healthy participants during the single session. During this task, a balloon is displayed on the screen, along with a button that will pump up the balloon. Each pump inflates the balloon and deposits 5 cents in a temporary bank. At any point, the participant may cease pumping and click another button to collect the money accrued, transfer it into a permanent bank, and continue to the next balloon. However, if a balloon pops, no money is collected on that trial. At all times, the amount in the permanent bank and the number of pumps made on the current balloon are displayed on-screen.
The probability of the balloon popping was 1/128 on the first pump and increased with successive pumps such that the probability of a pop was 1/(129 - pump number). The maximum times a balloon could be inflated therefore was 128, and the optimal number of pumps (to maximize earnings) was 64 (Lejuez et al, 2002).
Participants were instructed in how to select the pump and collect buttons, and were told that the objective was to win as much money as possible, that there were 30 balloons, and that the balloons could pop at any point between the first pump to the time that it filled up the entire screen. Participants were not given additional compensation based on task performance.
2.6 Statistical analyses
Potential differences in demographics, other baseline information, and outcomes between modafinil and placebo groups were compared using unpaired two-tailed t-tests and Fisher’s exact tests. The main BART outcome measure was average adjusted pump count (i.e. including only trials in which the balloon did not pop; Lejuez et al, 2002; Pleskac et al 2008). Trials in which the balloon was not pumped at all (i.e. zero payment) were not included in the analysis. Such trials occurred rarely (1.2% of all trials across all sessions) and appeared to be inadvertent.
To investigate effects of feedback on performance, average number of pumps on collect trials following balloon pops (i.e. negative feedback) was compared to average number of pumps on collect trials following collect trials (i.e. positive feedback). Changes in performance within the single task session was also examined; the session was split into three blocks of 10 trials each (as in Lejuez et al, 2002, 2003) and repeated measures ANOVA was used to test for effect of block and for any interaction effects.
Potential relationships between BART score and demographic and other variables were assessed post-hoc with correlational analysis and t-tests.
Post hoc power analysis based on the main outcome measure and the number of cocaine dependent participants analyzed in this study indicate a power of 67.6% at α = 0.05.
3. RESULTS
Demographic variables of interest are provided in Table 1. No statistically significant differences were found between the modafinil and placebo groups in any demographic or baseline measure. Comparison participants appeared to be more educated and have higher estimated IQ in addition to lower SOGS scores and less alcohol and nicotine use than cocaine dependent participants.
Table 1.
| Placebo | Modafinil | Healthy | |
|---|---|---|---|
| n = | 18 | 12 | 19 |
| Demographics | Mean ± SD | ||
| Age (years) | 44 ± 7 | 42 ± 7 | 40 ± 6 |
| Gender (% male) | 83% | 83% | 74% |
| Race (% African American) | 67% | 67% | 42% |
| Years of education | 12.1 ± 1.4 | 12.1 ± 2.1 | 14.2 ± 1.9 |
| Shipley Institute of Living Scale (estimated IQ) | 89 ± 13 | 86 ± 16 | 101 ± 11 |
| Southern Oaks Gambling Scale | 2.2 ± 4.0 | 3.9 ± 4.4 | 0.8 ± 1.7 |
| Addiction Severity Index (mean of composite scores) | 0.21 ± 0.09 | 0.20 ± 0.07 | |
| Days abstinent at first BART test | 12 ± 4 | 13 ± 4 | |
| Substance use | Median (sIQR) | ||
| Cocaine frequency (days/month) | 14 (4) | 9 (5) | |
| Cocaine amount (grams/month) | 12 (13) | 9 (6) | |
| Years of cocaine use | 26 (4) | 25 (5) | |
| Alcohol (drinks/month) | 21 (49) | 16 (31) | 2 (3) |
| Nicotine (cigarettes/day) | 8 (6) | 6 (5) | 0 (0) |
| Marijuana (joints/month) | 0 (0) | 0 (0) | 0 (0) |
| Caffeine (drinks/week) | 9 (6) | 11 (8) | 7 (4) |
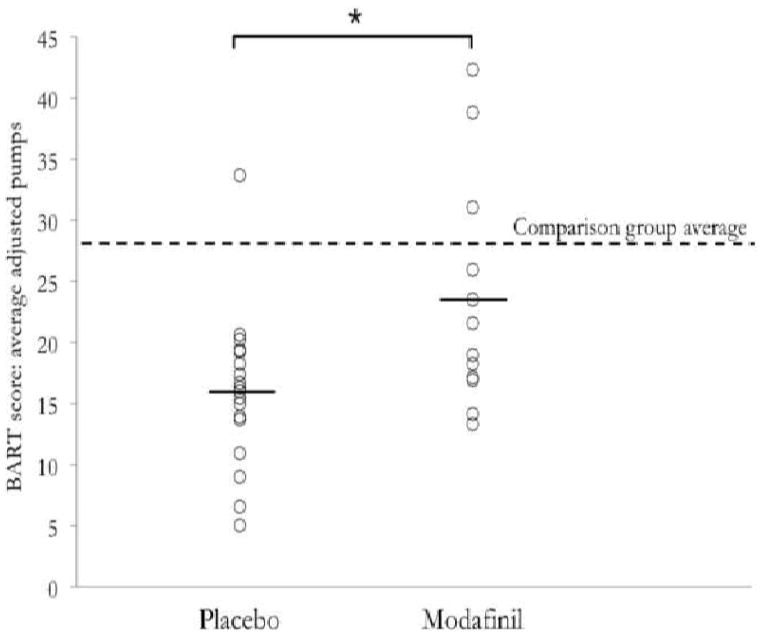
The placebo group exhibited significantly lower BART scores (average adjusted pumps = 16.0 ± 1.5 [SEM]) than the modafinil group (23.5 ± 2.7; p = 0.01; Figure 1). Similar differences were observed in unadjusted number of pumps and total money earned (all p < .05). BART score did not change across three blocks of 10 trials (F[2,89]=0.5, p >0.6) and there was no group by block interaction (F[2,89]=1.03, p >0.3). There were no differences in median response times between groups (all p > 0.1), or in the effect of popped balloon trials on the subsequent unpopped trial.
Figure 1.
No statistically significant correlations were found between the BART and any demographic variables examined.
4. DISCUSSION
To our knowledge, this work comprises the first effort to examine the effect of a medication on BART performance within a clinical population. Cocaine dependent individuals treated with modafinil exhibited overall higher scores on the BART than the placebo group, comparable to performance in an age-matched comparison group. The placebo group’s scores not only appeared lower than those treated with modafinil, but also fell below the range of typical performance in healthy subjects (i.e. 26–35 average adjusted pumps [Pleskac et al, 2008]) and the comparison participants run in the present study. Additionally, we found no associations between BART scores and several measures of risk-taking behavior, including lifetime years of cocaine use or current degree of cocaine use.
Although BART scores correlate with other risk-taking measures in healthy populations, almost all participants pump the balloon far fewer than 64 times (Lejuez et al, 2002), even though overall net reward increases up to that point. Therefore, higher BART scores in practice are associated with better performance on the task. This suggests that to some degree, advantageous decision-making and concepts such as “functional impulsivity” play a role in BART performance (Vigil-Colet, 2007 and Lee et al, 2009), and abnormally low scores may reflect impairment in these areas. Whether functional impulsivity will be associated with clinical outcomes remains unknown; however, we hypothesize that the expression of functional impulsivity may promote positive behavioral exploration and derivation of reward therefrom, and hence may benefit cocaine dependent patients both by reducing the threshold for engaging in behaviors and by reinforcing behaviors other than the established habit of cocaine use.
While most previous studies in substance using populations have linked higher BART scores to certain risk behaviors, none has reported direct evidence for a link between higher BART scores and chronic substance use (e.g. Lejuez et al, 2004, Hopko et al, 2006, Tull et al, 2009, and Bornovalova et al, 2005). Moreover, several recent studies have found an inverse relationship between BART scores and substance dependence, similar to the results reported here. Lower BART scores were associated with greater nicotine dependence in adolescent smokers (Ryan et al, 2013) and with severity of alcohol problems in problem drinkers (Courtney et al, 2012). Together with the current findings, it appears that chronic use of substances may be associated with decreased BART scores; our findings further suggest that a cognitive enhancing medication like modafinil may normalize performance in chronic cocaine users. Although it may seem paradoxical, this view does not necessarily conflict with previous findings in healthy persons. Rather, it opens up the possibility that the cognitive processes engaged by the BART are both relevant to pre-morbid risk taking behavior and are susceptible to impairment by chronic substance use.
A potential mechanism for the reduction of functional risk taking may be the downregulation of dopamine that occurs in cocaine dependent individuals, which is associated with decreased prefrontal activity and diminished sensitivity to reward (Volkow et al, 2009). For example, sleep deprivation, which downregulates dopamine receptor expression (Volkow et al, 2012), resulted in decreased BART scores in healthy volunteers, and dextroamphetamine (but not modafinil) increased scores in this sleep deprived group (Killgore et al, 2008). Similarly, carriers of the 9-repeat dopamine transporter allele, which is thought to reduce dopamine availability in the striatum, were found to score slightly lower on the BART than did non-9-repeat carriers, likely due to diminished reward sensitivity (Mata et al, 2012). Modafinil may therefore increases BART scores in cocaine dependent individuals by increasing dopamine signaling via dopamine transporter inhibition.
The main limitation of the present work is its modest sample size. Although small, the sample size was comparable to other studies of the BART in stimulant users (Bishara et al, 2009 and Bornovalova et al, 2005), and stringent exclusion criteria were applied that ruled out psychiatric co-morbidity and dependence on other substances. In addition, other measures of risk taking behavior such as risky sexual behavior, poor diet, or criminality, were not collected. Another potential limitation is that participants were not directly compensated based on BART performance. However, previous work in both healthy and substance using populations did not base payment on task performance and nonetheless obtained typical average scores (Gonzalez et al, 2012 and Fernie et al, 2010). Nevertheless, the possibility remains that the effect of modafinil might reflect a difference in motivation between groups. However, we observed no between-group differences in time spent on any part of the task, nor did we observe a difference in performance trajectory from beginning to end of the task. Furthermore, although modafinil has been shown to increase task enjoyment on a variety of cognitive tasks, it has been hypothesized to do so by improving the participant’s perceived ability to succeed on the tasks (Müller et al, 2013).
5. CONCLUSIONS
In summary, despite the likelihood that the BART reflects both adaptive and maladaptive risk-taking, in healthy populations the task appears to identify potentially harmful risky behavior. However, the present work suggests that below a certain threshold of performance, a more complex picture emerges wherein severely diminished BART scores reflect deficiencies in decision making, rather than increased risk-taking tendency. Consequently, in this population of cocaine dependent patients, an increase in BART scores could indicate a normalizing effect of treatment on cognitive function.
Highlights.
We used the Balloon Analogue Risk Task to assess risk taking in cocaine dependence.
Chronic cocaine users taking modafinil were compared to those taking placebo.
The modafinil group scored comparably to an age matched healthy comparison group.
The placebo group scored lower than both the modafinil group and healthy group.
This suggests an impairment of beneficial risk taking, normalized by modafinil.
Acknowledgments
Role of Funding Source
This research was funded by NIDA R01 DA011744. NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
This work was supported by NIDA R01 DA011744 (PTM) and the Department of Mental Health & Addiction Services of the state of Connecticut.
Footnotes
Clinical trial registration: ClinicalTrials.gov NCT01137396
Contributors
P.T.M. designed the study and wrote the protocol. S.V.C., E.L.F., and A.J.B. collected the data. S.V.C. and P.T.M. conducted the statistical analyses and wrote the manuscript, and all authors approved the final draft.
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sofija V. Canavan, Email: svcanavan@uchicago.edu.
Erica L. Forselius, Email: erica.forselius@yale.edu.
Andrew J. Bessette, Email: andrew.bessette@yale.edu.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81(3):313–22. doi: 10.1016/j.drugalcdep.2005.08.003. Epub 2005 Sep 19. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Amrhein PC, Bisaga A, Nunes EV, Hasin DS. Cognition, commitment language, and behavioral change among cocaine-dependent patients. Psychol Addict Behav. 2008;22(4):557–62. doi: 10.1037/a0012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aklin WM, Lejuez CW, Zvolensky MJ, Kahler CW, Gwadz M. Evaluation of behavioral measures of risk taking propensity with inner city adolescents. Behav Res Ther. 2005;43(2):215–28. doi: 10.1016/j.brat.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, 3rd, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104(1–2):133–9. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3257–66. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishara AJ, Pleskac TJ, Fridberg DJ, Yechiam E, Lucas J, Busemeyer JR, Finn PR, Stout JC. Similar Processes Despite Divergent Behavior in Two Commonly Used Measures of Risky Decision Making. J Behav Decis Mak. 2009;22(4):435–454. doi: 10.1002/bdm.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Cadet JL, London ED. The neuropsychiatry of chronic cocaine abuse. J Neuropsychiatry Clin Neurosci. 1998;10(3):280–9. doi: 10.1176/jnp.10.3.280. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, Lejuez CW. Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Exp Clin Psychopharmacol. 2005;13(4):311–8. doi: 10.1037/1064-1297.13.4.311. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Arellano R, Barkley-Levenson E, Gálvan A, Poldrack RA, Mackillop J, Jentsch JD, Ray LA. The relationship between measures of impulsivity and alcohol misuse: an integrative structural equation modeling approach. Alcohol Clin Exp Res. 2012;36(6):923–31. doi: 10.1111/j.1530-0277.2011.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Plebani JG, Pettinati HM, Sparkman T, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abuse Treat. 2012;43:363–312. doi: 10.1016/j.jsat.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, Sugar CA, Hellemann G, London ED. Is all risk bad? Young adult cigarette smokers fail to take adaptive risk in a laboratory decision-making test. Psychopharmacology (Berl) 2011;215(4):801–11. doi: 10.1007/s00213-011-2182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Chamberlain SR, Müller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am J Psychiatry. 2012:926–36. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie G, Cole JC, Goudie AJ, Field M. Risk-taking but not response inhibition or delay discounting predict alcohol consumption in social drinkers. Drug Alcohol Depend. 2010;112(1–2):54–61. doi: 10.1016/j.drugalcdep.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, Diviak KR. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. J Clin Exp Neuropsychol. 2012;34(9):962–76. doi: 10.1080/13803395.2012.703642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33(4):761–8. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Hopko DR, Lejuez CW, Daughters SB, Aklin WM, Osborne A, Simmons BL, Strong DR. Construct validity of the Balloon Analogue Risk Task (BART): relationship with MDMA use by inner-city drug users in residential treatment. J Psychopathol Behav Assess. 2006;28:95–101. [Google Scholar]
- Kalechstein AD, Mahoney JJ, 3rd , Yoon JH, Bennett R, De la Garza R., 2nd Modafinil, but not escitalopram, improves working memory and sustained attention in long-term, high-dose cocaine users. Neuropharmacology. 2013;64:472–8. doi: 10.1016/j.neuropharm.2012.06.064. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Grugle NL, Killgore DB, Leavitt BP, Watlington GI, McNair S, Balkin TJ. Restoration of risk-propensity during sleep deprivation: caffeine, dextroamphetamine, and modafinil. Aviat Space Environ Med. 2008;79(9):867–74. doi: 10.3357/asem.2259.2008. [DOI] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27(2):189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Lee TM, Guo LG, Shi HZ, Li YZ, Luo YJ, Sung CY, Chan CC, Lee TM. Neural correlates of traditional Chinese medicine induced advantageous risk-taking decision making. Brain Cogn. 2009;71(3):354–61. doi: 10.1016/j.bandc.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Jones HA, Richards JB, Strong DR, Kahler CW, Read JP. The Balloon Analogue Risk Task (BART) Differentiates Smokers and Nonsmokers. Exp Clin Psychopharmacol. 2003;11(1):26–33. doi: 10.1037//1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Simmons BL, Aklin WM, Daughters SB, Dvir S. Risk-taking propensity and risky sexual behavior of individuals in residential substance use treatment. Addict Behav. 2004;29(8):1643–7. doi: 10.1016/j.addbeh.2004.02.035. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin W, Daughters S, Zvolensky M, Kahler C, Gwadz M. Reliability and Validity of the Youth Version of the Balloon Analogue Risk Task (BART Y) in the Assessment of Risk-Taking Behavior Among Inner-City Adolescents. J Clin Child Adolesc Psychol. 2007;36(1):106–11. doi: 10.1080/15374410709336573. [DOI] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): a new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144(9):1184–8. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Levin FR. Psychostimulant treatment of cocaine dependence. Psychiatr Clin North Am. 2012 Jun;35(2):425–39. doi: 10.1016/j.psc.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata R, Hau R, Papassotiropoulos A, Hertwig R. DAT1 Polymorphism Is Associated with Risk Taking in the Balloon Analogue Risk Task (BART) PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0039135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl. 1994;12:112–8. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33(7):1477–502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: Evidence for occult insomnia. Drug Alcohol Depend. 2006;82(3):238–49. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Malison RT. Cocaine and sleep: early abstinence. ScientificWorldJournal. 2007;7:223–30. doi: 10.1100/tsw.2007.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep architecture, cocaine and visual learning. Addiction. 2008;103(8):1344–52. doi: 10.1111/j.1360-0443.2008.02233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott E, Pittman B, Stickgold R, Malison RT. Normalizing effects of modafinil on sleep in chronic cocaine users. Am J Psychiatry. 2010;167 (3):331–40. doi: 10.1176/appi.ajp.2009.09050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U, Rowe JB, Rittman T, Lewis C, Robbins TW, Sahakian BJ. Effects of modafinil on non-verbal cognition, task enjoyment and creative thinking in healthy volunteers. Neuropharmacology. 2013;64:490–5. doi: 10.1016/j.neuropharm.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleskac TJ, Wallsten TS, Wang P, Lejuez CW. Development of an automatic response mode to improve the clinical utility of sequential risk-taking tasks. Exp Clin Psychopharmacol. 2008;16(6):555–64. doi: 10.1037/a0014245. [DOI] [PubMed] [Google Scholar]
- Ryan KK, Mackillop J, Carpenter MJ. The relationship between impulsivity, risk-taking propensity and nicotine dependence among older adolescent smokers. Addict Behav. 2013;38(1):1431–4. doi: 10.1016/j.addbeh.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley WC. A self-administering scale for measuring intellectual impairment and deterioration. The Journal of Psychology. 1940;9:371–377. [Google Scholar]
- Sobell LC, Sobell MB. Convergent validity: an approach to increasing confidence in treatment outcome conclusions with alcohol and drug abusers. In: Sobell LC, Sobell MB, Ward E, editors. Evaluation alcohol and drug abuse treatment effectiveness: recent advances. Pergamon Press; Almsford, NY: 1980. [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropsychopharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tull MT, Trotman A, Duplinsky MS, Reynolds EK, Daughters SB, Potenza MN, Lejuez CW. The Effect of PTSD on Risk-Taking Propensity among Crack/Cocaine Users in Residential Substance Abuse Treatment. Depress Anxiety. 2009;26(12):1158–64. doi: 10.1002/da.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil-Colet A. Impulsivity and decision making in the balloon analogue risk-tasking task. Personality and Individual Differences. 2007;43(1):37–45. [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang FJ. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Logan J, Benveniste H, Kim R, Thanos PK, Ferré S. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J Neurosci. 2012;32(19):6711–7. doi: 10.1523/JNEUROSCI.0045-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale: Revised Manual. Los Angeles: Western Psychological Services; 1986. [Google Scholar]