Abstract
Significance: The evolution of the lungs and circulatory systems in vertebrates ensured the availability of molecular oxygen (O2; dioxygen) for aerobic cellular metabolism of internal organs in large animals. O2 serves as the physiologic terminal acceptor of mitochondrial electron transfer and of the NADPH oxidase (Nox) family of oxidoreductases to generate primarily water and reactive oxygen species (ROS), respectively. Recent advances: The purposeful generation of ROS by Nox family enzymes suggests important roles in normal physiology and adaptation, most notably in host defense against invading pathogens and in cellular signaling. Critical issues: However, there is emerging evidence that, in the context of chronic stress and/or aging, Nox enzymes contribute to the pathogenesis of a number of lung diseases. Future Directions: Here, we review evolving functions of Nox enzymes in normal lung physiology and emerging pathophysiologic roles in lung disease. Antioxid. Redox Signal. 20, 2838–2853.
Introduction
The primary function of the lungs is to facilitate the diffusion of gases, primarily the exchange of carbon dioxide for oxygen (O2), across alveolar-capillary membranes. This is accomplished by ventilation, which brings the ambient air we breathe into close proximity with the systemic circulation. Adult human lungs exchange between 10,000 and 20,000 liters of air daily (19). This exposes the lungs to a variety of potentially injurious environmental agents, both infectious and noninfectious. Infectious agents are typically eradicated by host defense mechanisms involving a combination of epithelial barrier function, innate immune cell activation, and efficient mucociliary clearance. A large inoculum of pathogen or the inability to eradicate highly virulent strains may evoke a host fibrotic response to “wall off” and restrict the spread of pathogens. Noninfectious injury evokes similar host responses and, when chronic, may result in tissue remodeling responses that span a spectrum from pulmonary fibrosis to emphysema. A number of host factors, including genetic/epigenetic factors and age, may influence the susceptibility to infectious or noninfectious injury and its related complications that result in a number of clinical syndromes and phenotypes.
NADPH oxidase (Nox) and Dual oxidase (Duox) enzymes are an evolutionarily conserved family that has diversified to seven members in mammals (Nox1–5 and Duox1–2) (15, 80, 147). NOX enzymes typically catalyze the reduction of molecular oxygen (O2) to superoxide (O2•−), the primary product of the enzymatic reaction in most cases (14, 89). Depending on the microenvironment or cellular compartment in which it is produced, spontaneous or superoxide dismutase (SOD)-catalyzed reduction of O2•− to hydrogen peroxide (H2O2) may occur in association with the generation of other reactive oxygen species (ROS). ROS function as signaling molecules and regulators of cell function when they are generated in a compartmentalized and regulated manner (159). Here, we examine roles of these ROS-generating enzymes in normal cellular physiology of the lung and in the pathogenesis of selected lung diseases.
Biochemistry and Structure of Nox Enzymes
The Nox enzymes are encoded by seven genes in humans and six in mice (which lacks Nox5) (29, 89, 146). Nox1, Nox3, and Nox4 encode proteins that are similar in size and domain structure to Nox2. They consist of a C-terminal flavoprotein domain containing an NADPH-binding region and a flavin adenine dinucleotide binding region; the N-terminal hydrophobic domain consists of six transmembrane α helices that contain two heme-binding sites (90). Nox5 includes two major forms; the structure of the short form (Nox5-S) is similar to Nox1, Nox3, and Nox4, while the long form consists of the same domains along with an N-terminal extension containing a calcium-binding domain. Duox1 and Duox2 build on the Nox5 structure with an N-terminal extension consisting of a peroxidase homology region. Nox5, Duox1, and Duox2 are activated by calcium as predicted by their calcium-binding domains.
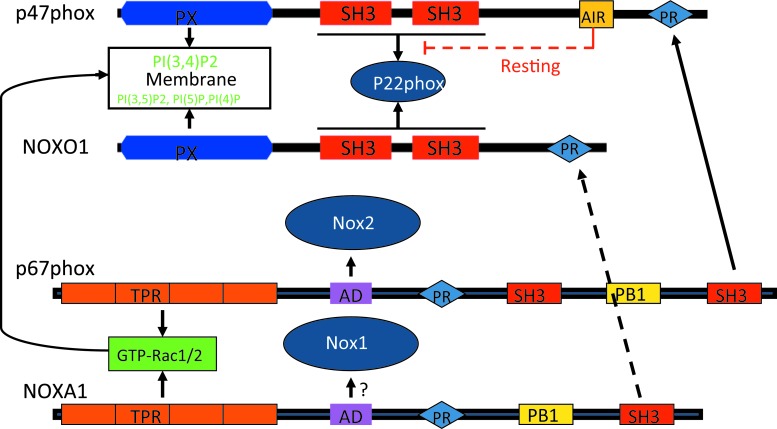
The activation of Nox2 by regulatory subunits has been extensively studied and has been reviewed elsewhere (14, 89). Nox1 is the first identified homolog of Nox2 (146). Nox1-dependent ROS generation can be reconstituted in cells by co-transfection with the regulatory subunits NoxO1 and NoxA1 (12, 30, 31, 152) (Fig. 1). Nox3 is primarily expressed in the kidney and inner ear (13, 29), although it may be induced in the lung (186). Similar to Nox1 and Nox2, it is also associated with p22phox in biological membranes and is regulated by regulatory subunits. However, Nox3 activation reveals more flexibility. For example, NoxO1 alone is sufficient to activate Nox3; p67phox further potentiates the effect of NoxO1 on Nox3 activation (32). The combination of NoxA1 and p47phox may also mediate Nox3 activation, suggesting variable mechanisms for activation of Nox3. Nox4 is more ubiquitously expressed. Similar to Nox1, 2, and 3, it functionally associates with p22phox and is generally considered a constitutively activated Nox enzyme. Interestingly, Nox4 is unique among the Nox1–5 isoforms in generating H2O2, not O2•− (106, 136, 170–172), although mechanisms for this and its biological significance remain to be elucidated.
FIG. 1.
Domain structures and interactions with regulatory subunits in the activation of Nox1 and Nox2. Solid lines represent the known interactions for activation; dashed lines represent inhibitory effects; question marks represent predicted interaction. PX domains of both p47phox and NoxO1 bind to membrane lipids. AD, activation domain; PR, proline-rich region; PX, phox homology domain; TPR, tetratricopeptide repeat; NOX, NADPH oxidase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Anatomic and Cellular Localization of Nox Enzymes
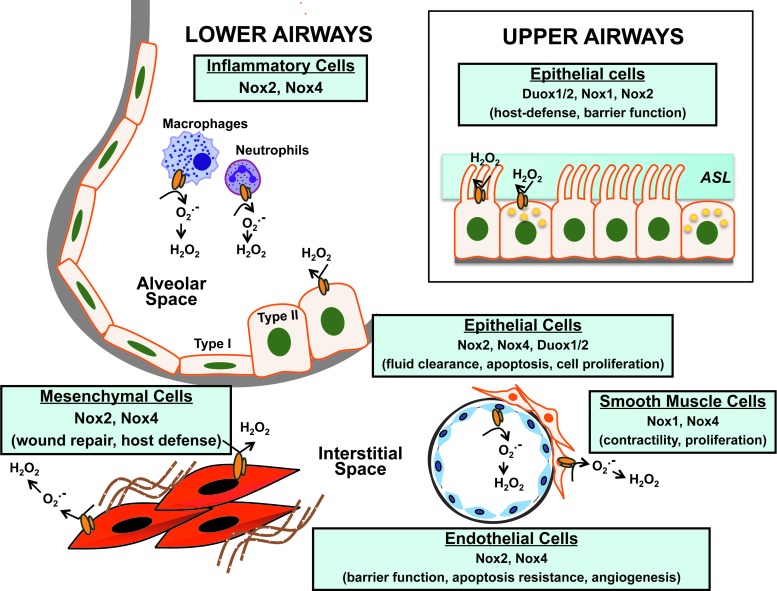
The lung is a complex organ and comprises more than forty cell types, including immune cells (18). It extends from the proximal conducting airways involving the trachea, bronchi, and bronchioles to the distal gas-exchanging alveolo-capillary units. Nox/Duox enzymes have been localized to various anatomic structures of the lungs and to specific cell types (Fig. 2).
FIG. 2.
Cellular localization of Nox enzymes in the lung. Nox/Duox isoforms are expressed in specific lung cells, where they mediate diverse functions in both normal physiologic and/or pathologic states. The catalytic subunits of Nox enzymes are schematically represented by an orange “duplex.” Refer to text for related references and details. ASL, airway surface liquid; Duox, dual oxidase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Nox expression and function in the upper respiratory tract
The combined action of resident immune cells and the secretory products of epithelial cells maintain the sterility of the airways. Nox/Duox enzymes participate in innate immunity and host defense of the lung (89). Duox1/2 localize to the surface of tracheal epithelial cells where they produce H2O2 and, in the presence of lumenal lactoperoxidase, generate antimicrobial hypothiocyanite (OSCN−) (128). A deficiency of this antimicrobial system has been implicated as an important host defense mechanism against Staphylococcus aureus and Pseudomonas aeruginosa infections in cystic fibrosis (115). This Duox-dependent antimicrobial system is induced by virulence factors such as flagellin (for Duox2), and the anti-inflammatory cytokines, interferon γ (INF-γ) (for Duox2), interleukin (IL)-4, and IL-13 (for Duox1) (68). In addition to microbicidal products such as OSCN−, the airway surface liquid (ASL) covering the upper airways contains complex polysaccharide mucins that trap pathogens and particulates from entering the lower airway. Secretion of Mucin-5 Subtype AC (MUC5AC) by human bronchial epithelial cells has been shown to be dependent on Duox1 activation by neutrophil elastase via PKCdelta/PKC (137); this supports Duox1 as a therapeutic target in chronic inflammatory airway diseases that are characterized by mucus hypersecretion. The ASL anti-microbial property depends partly on the maintenance of its pH. Secretion of H+ by tracheal epithelial cells has been shown to be mediated by an extracellular H2O2-dependent mechanism and is correlated with the expression of Duox1/2, p22phox, p40phox, p47phox, and p67phox (135). Viral infection of the airways triggers secretion of anti-inflammatory cytokines by immune cells and epithelial cells. Silencing of Nox2 expression/activity in human bronchial epithelial cells by small interfering RNA (siRNA) or the scavenging of ROS with antioxidants blocks the up-regulation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and cytokine production in response to the respiratory syncytial virus and the sendai-virus (51). In contrast, Nox1-derived ROS has been reported to contribute to barrier dysfunction and transmigration of rhinoviruses by disruption of the zona occludins of the apical tight junctions (33).
Nox expression and function in the lower airways
Two morphologically distinct cell populations constitute the alveolar epithelium: type I (which covers 95% of the epithelial surface) and type II cells. Efficient alveolar gas exchange is dependent on alveolar fluid clearance via the action of epithelial sodium channel (ENaC) that is expressed on the surface of type I and type II cells (46). Nox2/Rac1-dependent production of ROS has been shown to control the activity of ENaC in mouse and rat epithelial cells; lack (Nox2-deficient mice) or decreased Nox2 activity (pharmacologic blockade of Nox2 with Rac-1 inhibitor NSC23766) as well as ROS scavenging with TEMPO resulted in alveolar fluid retention in an in-vivo model of lipopolysaccharide-induced lung injury (63). Up-regulation of Nox2 and Rac-1 in the mouse alveolar epithelium after chronic exposure to alcohol has been linked to ENaC hyperactivity, suggesting a potential mechanism underlying the increased incidence of acute respiratory distress syndrome (ARDS) observed in the alcoholic population (47). In addition, Nox2 has been implicated in cell cycle control of alveolar epithelial cells; PPAR-γ activation through Nox2-derived ROS promotes cell-cycle progression from G0/G1 into S and G2/M phases (162). Nox4 expression is induced in alveolar type II cells in response to lung injury, and Nox4-derived ROS have been shown to induce apoptosis of alveolar epithelial cells and promote lung fibrosis (24). Duox1/2 are expressed by type II epithelial cells and Duox-generated H2O2 has been suggested to control acid release during lung development in mice (53, 54).
Nox expression by lung fibroblasts
The generation of ROS by NAD(P)H-like enzymes in fibroblasts was described well before the cloning and identification of the Nox gene family (108, 157, 158). The primary Nox isoform expressed in fibroblasts is Nox4, although p67phox and p47phox are also co-expressed in the absence of Nox2 (43). Nox4-derived ROS appears to mediate signaling events in cell that regulate IL-8 secretion (43), fibroblast migration (5), and myofibroblast differentiation (5, 37, 71). Deficiency or silencing of Nox4 protects against the development of experimental lung fibrosis in mice (24, 71), and this enzyme is also highly expressed in the lungs of patients with idiopathic pulmonary fibrosis (5, 71).
Nox expression in the pulmonary endothelium
The pulmonary endothelium serves as a tightly controlled barrier to prevent plasma exudation into the interstitium and alveolar space. The primary Nox isoforms expressed by vascular endothelial cells are Nox2 and Nox4. In P. aeruginosa lung infection, Nox4 and Nox2 play distinct roles in regulating lung inflammation, apoptosis, and permeability; Nox2 was critical in regulating inflammation, while Nox4 mediated apoptosis of endothelial cells and vascular permeability (58). Vascular cell adhesion molecule-1 signals activation of Nox2, which then mediates ROS-dependent activation of PKCα, protein tyrosine phosphatase 1B (PTP1B), and extracellular-signal-regulated kinases 1/2 (ERK1/2) and initiation of leucocyte migration (1, 34). Nox4-dependent ROS regulates endothelial cell motility and angiogenesis; RNAi-mediated silencing of Nox4 or pretreatment with N-acetylcysteine attenuates hyperoxia-induced endothelial cell migration and capillary tube formation (124). Hyperoxia has been shown to induce Nox4 expression via nuclear factor (erythroid-derived 2)-like 2 (Nrf2) binding to antioxidant response elements on the Nox4 promoter (125). Hyperoxia has also been shown to stimulate phosphorylation of myosin light chain (MLC) and to recruit phosphorylated and nonphosphorylated cortactin, MLC, Src, and p47phox to caveolin-enriched microdomains (CEMs), which are essential for Nox activation (165). This process involves c-Abl-mediated dynamin 2 phosphorylation that is required for the recruitment of p47phox to CEMs (139). Nox2-mediated ROS in pulmonary artery endothelial cells has been implicated in the induction of autophagy, which contributes to impaired angiogenesis in persistent pulmonary hypertension in fetal lambs (154).
Nox expression by pulmonary smooth muscle cells
Smooth muscles cells (SMCs) are found in the medial layer of the vasculature, where their primary function is to control pulmonary perfusion. SMCs are also found underlying the tracheal and bronchial epithelium, where their contraction is stimulated in response to inflammation. Several reports point to a key role of Nox4 in the contractility and proliferation of airway/vascular SMCs induced by pro-fibrotic cytokines or hypoxia (145, 148). Hypoxia induces Nox4 expression in SMCs via a hypoxia-inducible transcription factor HIF-1α (44, 112). Hypoxia-induced mitochondrial ROS production has been shown to activate protein kinase C-ξ (PKCξ) and Nox, providing a positive feedback mechanism to further increase intracellular ROS, calcium-induced calcium-release, and SMC contraction (129, 175). The pro-fibrotic cytokine, transforming growth factor-β1 (TGF-β1), induces the expression of Nox4 in human pulmonary artery SMCs via an Smad2/3-dependent pathway, which mediates ROS-dependent ERK1/2 phosphorylation and cellular proliferation (145).
Nox expression by immune cells
The prototypical Nox isoform, Nox2, has been well characterized in phagocytic cells as a critical component of the innate immune response [reviewed in Nauseef (117)]. However, in addition to its conserved role in combating invading pathogens, Nox2 appears to mediate additional (paradoxical) roles in suppressing inflammation (62, 181, 188). Nox1 and Nox4 are also expressed in monocyte/macrophage populations (164, 182). Metabolic stress (low-density lipoprotein and high D-glucose) induces Nox4 expression, which mediates monocyte chemotaxis in response to monocyte chemoattractant protein (MCP)-1, thereby contributing to vascular injury (93, 164). Further studies are required to determine the function of nonclassical Nox isoforms in immune cells.
In summary, Nox isoforms are expressed in the different compartments of the human lung, where they regulate several critical functions. The production of H2O2 by Duox/Nox enzymes by epithelial cells of the upper and lower airways as well as by immune cells is of primary importance in the lung host defense system. Indeed, H2O2 serves as a substrate to produce OSCN−, regulates chemotaxis and the production of other host defense molecules. Nox-generated H2O2 also contributes to airway epithelial cells proliferation and differentiation by activating signaling cascades that modulate gene transcription. Transcription factors such as NF-kB, p53, and activator protein 1 (AP-1), which are redox sensitive, provide the link between oxidative stress and gene expression. Finally, there is an emerging role of Nox enzymes in lung remodeling (ECM production, angiogenesis) by controlling mechanisms such as cell differentiation, motility, and apoptosis.
Subcellular Compartmentalization and Function of Nox Isoforms
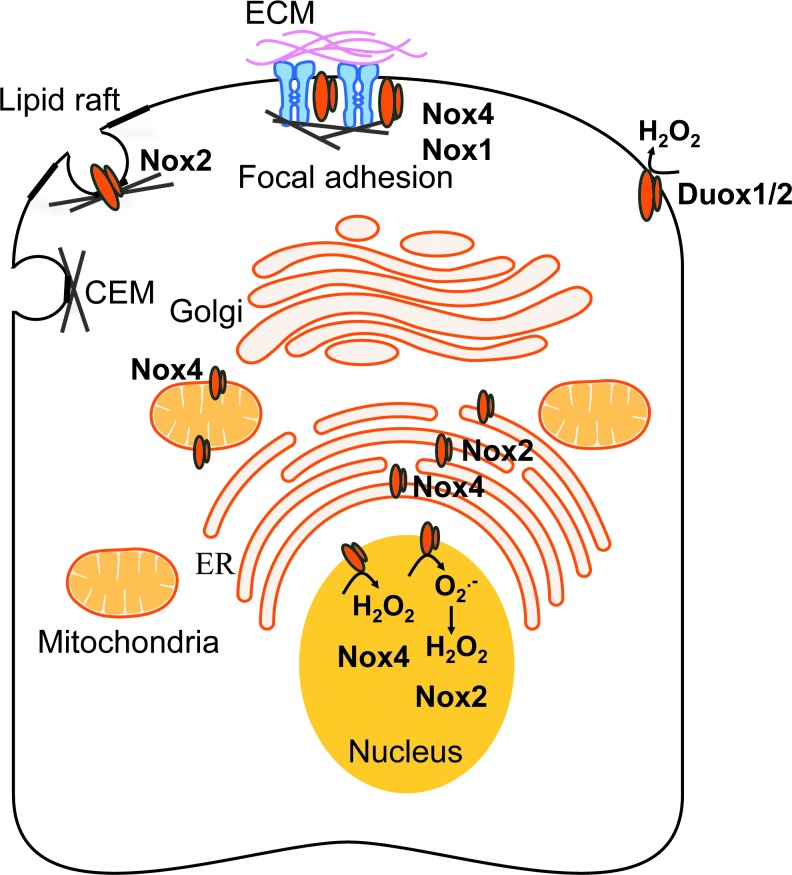
The signaling functions of Nox enzymes are likely to be controlled by their subcellular localization, which dictates signaling specificity, reactivity, and half lives of ROS (Fig. 3). The study of Nox subcellular localization remains challenging due to the lack of isoform-specific inhibitors. Chimera studies in which the N-terminus or the cytoplasmic tail of different Nox isoforms is interchanged indicate that the Nox amino-terminal tail likely determines Nox subcellular localization (73, 172).
FIG. 3.
Subcellular localization of Nox enzymes. Nox/Duox isoforms localize to specific subcellular compartments to mediate their cellular functions. The catalytic subunits of Nox enzymes are schematically represented by an orange “duplex.” CEM, caveolin-enriched microdomain; ECM, extracellular matrix; ER, endoplasmic reticulum; H2O2, hydrogen peroxide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Nox/Duox at the plasma membrane
Nox1, Nox2, and Nox4 have been localized to the plasma membrane. Their localization often associates with specific signaling domains, such as lipid rafts, caveolae, or focal adhesions, where they facilitate signaling of cellular proliferation, differentiation, and migration (166). For instance, lipid raft-generated ROS stimulate proliferation and migration of endothelial cells by activating vascular endothelial growth factor receptor-2 (VEGFR2) and downstream p38 mitogen-activated protein kinases (MAPK) activation (122). Nox2-generated ROS in caveolae regulates blood–brain barrier function through the modulation of occludin expression via an ERK1/2-dependent signaling cascade (123). Nox4 has been co-localized with vinculin at the site of focal adhesions in vascular SMCs; in association with polymerase delta-interacting protein 2 (Poldip2), Nox4 participates in stress fiber formation (102). Nox1-dependent ROS have been reported to regulate stress fiber assembly at the site of focal adhesions in Ras-transformed-Swiss3T3 fibroblasts (138).
The Duox isoform was first identified at the apical membrane of thyrocytes, where they generate H2O2, the substrate for follicular thyroperoxidase that catalyzes oxidization of iodide and formation of thyroxine (65, 114). Duox2 is mainly expressed by thyrocytes, while the apical surface of the airway epithelial cells is the primary expression site of Duox1 (54, 56). Production of Duox-generated H2O2 is necessary to confer anti-microbial properties to the ASL (135). Functional Duox1/2 also localize at the leading edge of migrating lung cancer or epithelial cells, suggesting a key role of Duox-generated H2O2 in metastasis and wound repair (101, 177).
Nox in the endoplasmic reticulum
The first evidence of an endoplasmic reticulum (ER) localization of Nox to the ER came from studies using overexpression of tagged or chimeric Nox in HEK293 cells that are designed to study Nox-antibodies specificity or domains within the Nox sequence which are responsible for their localization (183). A signaling function for ER Nox-dependent ROS production has been reported in the acute myeloid leukemia (AML) cell line, MV4–11, on stimulation of the tyrosine-like kinase receptor, Fms-like tyrosine kinase 3 (FLT3) (180). Mutations of the FLT3 are associated with the development of AML. Inhibition of the FLT3 receptor or knocking down the expression of p22phox abolishes ER-generated H2O2, while preserving mitochondrial-ROS; ER-associated H2O2 production has been suggested to promote the synthesis of the proto-oncogene Pim-1 via signal transducer and activator of transcription 5 (STAT5) signaling, thus promoting the oncogenic process (180). In macrophages, the antimicrobial function of Nox2-depend ROS is executed, in part, by the translocation of Nox2 and p22phox from intracellular compartments (ER, endosomes) to the plasma membrane after stimulation with INF-γ (27).
Several lines of evidence link ER stress-induced apoptosis to increased cytosolic production of ROS, although further studies are warranted to determine the precise source(s) of ROS (36, 96). Another proposed function of Nox-dependent ROS in the ER relates to the control of protein trafficking. Through inactivation of PTP1B, Nox4-dependent ROS negatively regulates the trafficking of the endothelial growth factor (EGF) receptor to the plasma membrane, thus terminating EGF signaling in endothelial cells (28). Cytosolic ROS also contributes to regulation of muscle contraction in skeletal muscle cells. Nox2, Nox4, and p22phox have been reported to co-localize with transverse tubules and the sarcolemma in murine skeletal muscle cells; muscle contraction induces the recruitment of the activator and regulator subunits p67 and p40phox and activates ROS-production (131).
Nox in the nucleus
Nox2 and Nox4 have been localized to the nucleus/peri-nuclear region of certain cell types. Nuclear Nox2-dependent ROS has been implicated in apoptosis of endothelial cell death that is triggered by exposure to homocysteine (140). In this study, 3D-digital imaging showed the accumulation of Nox2 and p47phox to the nucleus of human umbilical vein endothelial cells on homocysteine in association with the formation of nitrotyrosine, suggesting O2•− production, and induced cleaved-caspase-3 activity (140). In contrast, another study indicated Nox4, but not Nox2, in the nucleus of human pulmonary artery endothelial cells in which it mediated hyperoxia-induced cell migration and capillary tube formation (124). In murine embryo fibroblasts (NIH3T3 cells), nuclear Nox4 mediates TGF-β1-induced plasminogen activator inhibitor-1 (PAI-1) gene expression, at least in part through oxidative modification and inhibition of MAPK phosphatase-1 (MKP-1), a nuclear phosphatase (99).
Nox in the mitochondria
So far, Nox4 is the only member of the Nox gene family to have been reported functionally expressed in the mitochondria. Mitochondrial Nox4-produced ROS have been implicated in the etiology of several pathologies through the modulation of senescence, apoptosis, and oncogenicity (17, 64). A 73-amino-acid long domain within Nox4 amino-terminal tail, identified by sequence analysis using the Mitoprot program combined with mutagenesis (64), targets Nox4 to the mitochondria. One of the first evidence for the functional expression of mitochondrial Nox4 came from a study performed in a rat model of diabetes (17). Using subcellular fractionation assays combined with microscopy and measurement of mitochondrial activity, this study showed that Nox4 up-regulation in the cortex of diabetic rat kidney was linked to glucose-induced mitochondrial ROS (17). More recently, angiotensin II has been shown to increase mitochondrial Nox4-dependent ROS (O2•− and H2O2) via mitochondrial membrane depolarization in a model of kidney tubular cells; this increase in mitochondrial ROS activated the intrinsic pathway of apoptosis with release of cytochrome c and apoptosis-inducible factor (82).
Mitochondrial Nox4 has been reported as a major source of O2•− in the failing heart (3, 86); these studies employed cardiac-specific Nox4 conditional knockout mice (86) or cardiac Nox4 over-expressing mice (2). Up-regulation of Nox4-dependent mitochondrial O2•− mediates cardiac myocyte apoptosis, fibrosis, and heart failure after pressure overload (86). Breast cancer has also been linked to oxidative stress; although Nox1, Nox4, and Nox5 are expressed in breast tissue, mitochondrial Nox4-dependent H2O2 production appears critical in the tumorigenic process (64). It remains to be determined whether Nox4 and potentially other Nox isoforms are localized to the mitochondria of specific lung cells; their functional roles in such cells will also need to be elucidated.
In summary, Nox/Duox isoforms are known to be localized to the plasma membrane in specialized structures such as lipid rafts, caveolae, or focal adhesions. The cell surface localization of Nox enzymes, while essential in host defense and phagocytosis, is critical for their emerging roles in mediating intracellular signaling, endocytosis, cellular adhesion, and migration. Nox enzymes are also found in biological membranes of intracellular organelles such as the ER, nucleus, and mitochondria. Although much work needs to be done on the functional roles of Nox enzyme in specific organelles, there is a growing recognition of their participation in the mediating stress responses in each of these subcellular compartments.
Nox Enzymes in Lung Health
The evolutionary conservation and diversification of Nox enzymes suggest essential and adaptive roles of Nox-generated ROS in human physiology. Physiological roles of ROS include signal transduction (8, 146), angiogenesis (7), and innate immunity (61). Nox2 generates high levels of ROS in neutrophils as a central mechanism of host defense against microbial infection, including infection of airways and lung, and has been reviewed elsewhere (49, 84, 169). A functional role of Nox2 role in innate immunity is well exemplified by its loss-of-function mutations in chronic granulomatous disease (CGD). CGD is caused by an inherited mutation of Nox2 or its subunits. Patients with CGD suffer recurrent infections of multiple organs, and pulmonary infection is the leading cause of death (9, 143).
Other Nox/Duox isoforms have been proposed to function in host defense and innate immunity. Studies in gastric mucosal cells support a role for Nox1 in antimicrobial host defense (81, 155), although a similar role for NOX1 in the lung is yet to be demonstrated. Duox1 and Duox2 in upper airway epithelium generate H2O2 to mediate lactoperoxidase-catalyzed generation of microbicidal oxidants (61, 115, 179). The expression of Duox1 and Duox2 in salivary, tracheal, and bronchial epithelium supports a broad role of these isoforms in host defense functions at these epithelial surfaces (56, 61, 115, 128, 135).
Nonphagocytic Nox isoforms generate ROS at lower levels and in a regulated manner, participate in signaling of immune responses and other cellular functions (89). Nox enzymes may be induced by a variety of bacteria and viruses and participate in host inflammatory responses. For example, airway instillation of P. aeruginosa significantly increases the expression of Nox2 and Nox4 in lung microvascular endothelial cells, where they mediate distinct signaling functions (58). Nonimmune mediated functions of Nox-derived ROS include, but are not limited to cell growth, proliferation, angiogenesis, apoptosis, and autophagy (22, 48, 52, 89, 133).
Nox Enzymes in Lung Diseases
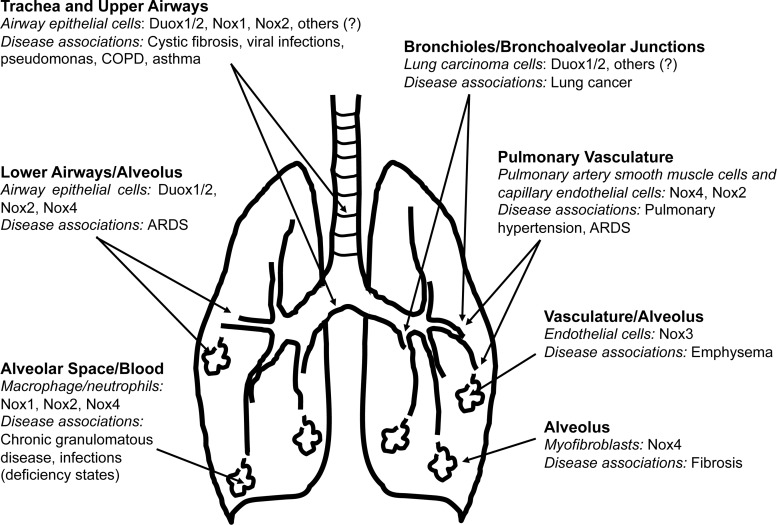
Nox/Duox enzymes contribute to a variety of lung diseases, either due to a loss of function or due to heightened expression/activity of specific isoforms (Fig. 4). In this section, we consider a select group of lung diseases in which Nox enzymes have been implicated in pathogenesis.
FIG. 4.
Anatomic localization of Nox enzymes in the lung and associated diseases. Nox/Duox isoforms are expressed in multiple cell types and anatomic locations, extending from the proximal trachea and large airways to terminal bronchioles and alveoli. Proposed associations of Nox/Duox isoforms with specific lung diseases are indicated. ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease. “?” indicates non-identified Nox and/or nox subunits.
Pulmonary infections and inflammatory diseases
A central theme with many of the Nox family genes is their participation in various aspects of host defense against pathogens. Indeed, Nox2 is well established as an archaic antimicrobial defense mechanism that is conserved across multiple species (45, 117). In addition to the predicable susceptibility to infections with loss of function of Nox2, there is also evidence for a homeostatic role of Nox2 in controlling inflammation.
CGD, characterized by susceptibility to recurrent pyogenic infections, is the prototypical example of a human disease that is associated with inherited loss-of-function of genes encoding components of the Nox2 enzymatic complex. Initially characterized as a fatal granulomatous disease of childhood, the clinical course of CGD is marked by recurrent, suppurative infections (21). CGD can be associated with a defect in any of the subunits of the multicomponent Nox2 enzyme (45, 95, 118, 130, 153). Emerging data suggest a shift in the most common infecting organisms from staphylococci and enteric bacteria to other pathogens, including Aspergillus pneumonia and Burkholderia cepacia (78).
An unexpected role for Nox2 in suppressing neutrophilic inflammation has also been suggested (35). Similar findings of a putative “anti-inflammatory” role for p47phox/Nox2 are reported in mice challenged with intra-peritoneal live Escherichia coli to induce sepsis (60), and in murine models of emphysema (181), pneumococcal pneumonia (105), influenza pneumonia (141), and disseminated Cryptococcus neoformans infection (142). A deficiency in neutrophil cytosolic factor-1, required for activation of Nox2, protects from virus-induced acute lung injury (ALI) (76). These studies support a homeostatic role Nox2 (and potentially other p47phox- and/or p22phox- requiring Nox enzymes) in modulating the host inflammatory responses.
Asthma
Systemic and airway-associated oxidative stress is increased in asthmatic patients compared with healthy individuals; this has been correlated to the degree of airflow obstruction and airway hyperresponsiveness (AHR) (41, 121, 148). Serum levels of damaged lipid and carbonylated proteins are increased in asthmatic children compared with controls (121). Airway SMCs of asthmatics demonstrate an increase in stress-induced DNA damage and ROS production; compounds such as apocynin diphenylene iodonium (DPI) that are known to inhibit Nox activity, albeit not specific, decreased agonist-induced airway smooth muscle contraction, which underlines AHR (148). Apocynin has also been shown to inhibit the production of anti-inflammatory cytokines (tumor necrosis factor α [TNF-α], IL-1β, and IL-6) by the airway mucosa, as well as the migration of macrophages and eosinophils (83). The effect of apocynin on cytokine synthesis is likely due to the regulation of gene expression by oxidative stress-responsive sensitive transcription factors (159).
The activity of Nox2 and Nox4 isoforms are primarily implicated in the increased oxidative stress that is associated with asthmatic airways, both in humans and in experimental murine models. Studies using Nox2 knockout mice sensitized with ovalbumin suggest a role of Nox2 in the cross-talk between T-lymphocytes and macrophages to limit the inflammatory response and to restrain acute allergic reactions (11). Accordingly, Nox2 deficiency resulted in enhanced recruitment of inflammatory cells to the airways and cytokine production, which worsens the asthmatic phenotype compared with wild-type (WT) mice (10). Alternatively, Nox2 has also been suggested to promote asthmatic airway inflammation, primarily by enhancing recruitment of eosinophils (1, 34). While Nox2 modulates inflammatory responses in asthma, Nox4-generated ROS have been suggested to underlie AHR by mediating airway smooth muscle hypercontractility. Indeed, Nox4 expression is enhanced in primary airway smooth muscle derived from asthmatic patients, and the silencing of its expression abrogates agonist-induced contraction of airway smooth muscle (148). Therefore, targeting of Nox2 and Nox4 may offer therapeutic benefits in specific phenotypes of asthma.
Acute lung injury
ALI and the ARDS represent clinical syndromes of varying severity and diverse causes that present with a set of defined clinical-physiologic-radiologic criteria (55). The common pathophysiologic feature involves disruption of the alveolo-capillary membrane, resulting in diffuse bilateral infiltrates on chest radiographs and severe arterial hypoxemia (176). The generation of ROS by enzymatic and nonenzymatic mechanisms, including activation of NOX enzymes, may contribute to the pathobiology of ALI/ARDS (26).
Nox1 is an important contributor to ROS production, epithelial cell death, and disruption of the alveolo-capillary barrier during hyperoxia; this oxidative stress-induced ALI involves activation of JNK and ERK pathways (25). Vascular endothelial cells function is also critical in maintaining the alveolo-capillary barrier function. Both Nox2 and Nox4 are expressed in pulmonary vascular ECs and contribute to hyperoxia-induced ROS generation (124). Hyperoxia induces pulmonary edema and neutrophil influx into the alveolar space of WT mice, effects that are attenuated in Nox2-deficient mice. The observed protection is incomplete, suggesting the potential involvement of other Nox isoforms, including Nox4, in alveolo-capillary barrier dysfunction (124). Interestingly, baseline levels of Nox4 mRNA expression are increased in Nox2-deficient mice compared with WT mice, suggesting the existence of a compensatory mechanism for ROS production. Nox2 promotes NF-κB-dependent acute inflammatory responses, neutrophil influx, and tissue injury specifically in the lungs, but not other organs, in response to systemic TNF-α administration (185). In response to acid aspiration, however, Nox2 appears to reduce neutrophil accumulation, while Nrf2 decreases ALI without affecting neutrophil influx (39); these observations suggest distinct functions of Nox2 and Nrf2 in modulating inflammation and injury in the lung.
Pulmonary arterial hypertension
Chronic hypoxia is the most common risk factor for pulmonary arterial hypertension (PAH), characterized by vascular remodeling and enhanced vasoreactivity. Specific Nox isoforms, in particular Nox2 and Nox4, have been implicated in hypoxia-induced pulmonary hypertension (50, 57, 98, 112). Nox2 has been implicated in hypoxia-induced endothelial dysfunction (57). Hypoxia-induced PAH in mice has been linked to increased Nox4 expression in pulmonary artery (SMCs) (112), suggesting a key role for Nox4 in vascular remodeling that is associated with hypoxia-induced PAH. TGF-β-induced Nox4 expression and ROS production have been shown to mediate proliferation of human pulmonary artery SMCs (77, 145). Targeting Nox1/4 with a pharmacological inhibitor, GKT137831, attenuates hypoxia-induced pulmonary SMC proliferation, vascular remodeling, and the development of PAH (66).
Nox-derived ROS may directly cause extracellular Ca2+ influx by inhibiting voltage-dependent K+ (KV) channels and the opening of store-operated Ca2+ channels, as well as intracellular Ca2+ release by activating ryanodine receptors, leading to an increase in intracellular Ca2+ concentration and associated SMC contraction (175). The Nox inhibitor and ROS scavenger, apocynin, as well as Nox4 siRNA reverses the hypoxia-induced decrease in Kv current density, whereas the protein levels of the channels remain unaffected by Nox4 silencing; this effect appears to be related to, at least in part, direct effects of Nox4-derived ROS in cysteine oxidation of the Kv1.5 channel (111). Finally, in addition to the intima and media, remodeling of the vascular adventitia may contribute to hypoxia-induced PAH. For example, hypoxia has also been shown to up-regulate Nox4 expression in pulmonary artery adventitial fibroblasts in association with increased ROS levels and increased cellular proliferation, effects that are abrogated by siRNA silencing of Nox4 (97).
Emphysema
Emphysema is the most common cause of chronic obstructive pulmonary disease (COPD), and it is primarily related to cigarette smoking. Human subjects with COPD and cigarette smokers exhibit differential Duox1 and Duox2 depending on smoking status and type of lung epithelium sampled; for example, airway epithelium of current smokers express decreased Duox1 and increased Duox2 compared with never smokers, whereas former smokers with COPD demonstrate reduced levels of both Duox isoforms (116, 127). In contrast, alveolar epithelial Duox1 and Duox2 were expressed at low levels and were unchanged regardless of smoking or COPD status (116). The precise role of Duox enzymes in COPD pathogenesis requires further studies.
The role of Nox2 in emphysema has been studied in knockout mouse models. Mice deficient in p47phox or Nox2 (gp91phox−/−) exhibit increased cigarette smoke (CS)-induced lung inflammation and emphysema despite decreased ROS production; this was associated with increased production of pro-inflammatory cytokines/chemokines via a toll-like receptor 4 (TLR4)-NFκB pathway, suggesting that Nox2 may mediate anti-inflammatory functions by restraining TLR4 activation (181). It is interesting to note that aging gp91phox−/− mice (>6 months) exhibit spontaneous emphysema (79); in this study, basal levels of oxidative stress markers were not altered in p47phox or Nox2-deficient mice, indicating stress-mediated responses. Interestingly, CS-exposed p47phox or Nox2-deficient mice, despite a compensatory increase in Nox4 expression, demonstrate reduced CS-induced release of ROS, lipid peroxidation, and DNA damage. In contrast, a pro-inflammatory role for p47phox-containing Nox enzyme(s) was suggested as p47phox null mice develop less inflammation with lower levels of IL-6, keratinocyte-derived chemokine (KC/CXCL1) and monocyte chemoattractant protein-1 (MCP1/CCL2) in lung lavage specimens after CS exposure in comparison to wild-type mice (87). Gene profiling studies in lung tissues from CS-exposed mice recently revealed up-regulation of NoxO1, which primarily regulates Nox1 activation, indicating that specific roles of other Nox isoforms in emphysema remain to be determined (109).
An unexpected role for Nox3 and TLR4 regulation in emphysema has been elucidated (186). Mice deficient in TLR4 develop age-dependent emphysema, a phenotype that is ameliorated with chemical Nox inhibitors or Nox3 siRNA, suggesting a role for Nox3-generated ROS in emphysema (186). Lung endothelial cells from TLR4-deficient mice were identified as the primary source of increased ROS production, which potentiates matrix degrading enzymatic activity (186). It is possible that Nox3, due to its potential pro-oxidant effects in the lung, requires tight suppression (by TLR4) under homeostatic conditions; however, pathologic states of TLR4 deficiency may allow for unrestrained Nox3 activity and pro-oxidant effects that contribute to emphysema. Indeed, aging and CS exposure are associated with depressed TLR4 function in human subjects (103, 167), supporting the theory of a disrupted TLR-Nox3 axis in human emphysema.
Pulmonary fibrosis
Pulmonary fibrosis is a chronic “scarring” disease of the lung that may result from known causes (e.g., environmental exposures, drugs, and connective tissue diseases) or unknown etiology (i.e., idiopathic). In almost all cases, the fibrotic reponse is characterized by the accumulation of activated myofibroblasts and the deposition of extracellular matrix (ECM) (161). Myofibroblast differentiation is mediated by soluble factors, primarily TGF-β1 (42, 160), and by ECM factors, primarily tissue stiffness (75, 187). In addition to its multiple fibrogenic actions, myofibroblasts generate ROS in response to TGF-β1 (37, 158, 173). Although the cellular localization/compartmentalization of Nox4 has not been clarified in myofibroblasts, a unique feature of NOX4 activity is its capacity for constitutive generation of extracellular H2O2 (106, 136, 170–172). Extracellular generation of H2O2 by lung myofibroblasts may mediate additional fibrogenic effects by inducing apoptosis of adjacent lung epithelial cells (173), or by inducing matrix cross-linking reactions (91), potentially contributing to tissue stiffness.
A pro-fibrogenic role for Nox4 in a number of organs systems has been proposed, including kidney fibrosis (16, 120, 151, 178), vascular remodeling/fibrosis associated with chronic hypertension (4), cardiac fibrosis (74, 86, 144, 174), pancreatic fibrosis (107), liver cirrhosis (6, 40, 132), and lung fibrosis (24, 71). Interestingly, recent studies also suggest a protective effect of Nox4, primarily in the kidney (119) and cardiovascular system (23, 134, 184).
In addition to Nox4, other Nox isoforms have been implicated in lung fibrosis. A p47phox-requiring Nox isoform is required for the development of fibrosis in a murine lung injury model; this bleomycin injury model is inflammation dependent and the observed protection in p47phox−/− mice may be related to modulation of neutrophilic inflammation and/or matrix metalloproteinase-9 activity in the bronchoaveolar lavage of these deficient animals (104). Studies of the gelatinase activities of lung fibroblasts from p47phox−/− mice will provide further insights into the extent by which p47phox-dependent ROS function in fibrotic lung remodeling. Nox1 has not been implicated in lung fibrosis, although there is evidence for a role of this isoform in liver fibrosis (38). Importantly, a pharmacologic inhibitor against Nox1/4 appears to be effective as an anti-fibrotic agent in preclinical models (59, 88).
Lung cancer
Nox enzymes may participate in several of the key events in the multi-step development of human cancers (67, 69). Studies more than two decades ago indicated the generation of ROS by an Nox-like flavoenzyme in several different cancer cells, although the identity of the enzymatic source(s) was not known at that time (150). Since then, specific Nox isoforms have been identified in a variety of human malignancies, including colon (92, 126, 149), gastric (163), pancreatic (94, 113, 168), and prostate (20) cancers. The tumorigenic potential of Nox1 was demonstrated by showing that Nox1-transfected cells produce phenotypically aggressive tumors in athymic mice (8, 146). Nox1 silencing in K-Ras transformed cells abrogates anchorage-independent cell growth and capacity for tumor formation in vivo (110). In fact, Nox1 was originally referred to as the “mitogenic oxidase” (8, 146); however, its effects on specific cell types are likely contextual and include other cellular functions. A number of reports support a role for Nox1 in angiogenesis (7). Vascular endothelial growth factor (VEGF) functions as a key mediator of neovascularization within tumors. Ras-induced VEGF transcription is mediated by an NOX1/Ras/ERK-MAPK pathway (85).
Resistance to apoptosis is another hallmark of cancer cells (67). Nox4 promotes apoptosis resistance in pancreatic cancer cells (168). Nox5 has been reported to mediate cell proliferation and resistance to apoptosis in prostate cancer cells (20). A potential mechanism by which the Nox isoforms promote apoptosis resistance may involve ROS-mediated inactivation of PTPs (94); alternatively or in parallel, activation of the phosphoinositide-3-phosphate (PI3K)/AKT and apoptosis signal-regulating kinase 1 pathway may contribute to Nox4-induced pro-survival signaling (113). Specific roles for Nox4 in lung cancer are yet to be determined. However, Duox1 and Duox2 and their maturation factors are reported to be down-regulated by promoter methylation in primary lung carcinomas (100). Restoration of functional Duox1 reverses the phenotype of the lung cancer cells lines, supporting epigenetic mechanisms involving Duox1 in lung carcinogenesis (100). Further studies are required to clearly define the precise roles of Nox enzymes in various aspects of cancer development and progression.
Conclusion
Nox family enzymes serve many homeostatic and host defense functions in the lung. Our current understanding of the physiological roles of Nox/Duox enzymes in the lung is still in its very early stages. A clearer understanding of these roles and their aberrant function in disease pathogenesis will expedite the eventual development and testing of Nox inhibitors in specific lung diseases (70, 156). All of the Nox enzymes are likely to be contextual in their actions and, thus, defining their “disease context” is critical in designing informative preclinical and successful clinical studies. Animal models of most chronic lung diseases have significant limitations, and this highlights the importance of validating the expression and localization of Nox isoforms in lung cells/tissues derived from patients with specific lung disorders. The utility of Nox enzymes as biomarkers in disease expression and progression deserves further studies.
Abbreviations Used
- AHR
airway hyperresponsiveness
- AKT
protein kinase B
- ALI
acute lung injury
- AML
acute myeloid leukemia
- AP-1
activator protein 1
- ARDS
acute respiratory distress syndrome
- ASL
airway surface liquid
- CEM
caveolin-enriched microdomain
- CGD
chronic granulomatous disease
- COPD
chronic obstructive pulmonary disease
- CS
cigarette smoke
- CXCL1
Chemokine (C-X-C motif) ligand 1
- DPI
diphenylene iodonium
- Duox
dual oxidase
- ECM
extracellular matrix
- EGF
endothelial growth factor
- ENaC
epithelial sodium channel
- ER
endoplasmic reticulum
- ERK
extracellular-signal-regulated kinases
- FLT3
Fms-like tyrosine kinase 3
- H2O2
hydrogen peroxide
- IL
interleukin
- INF-γ
interferon γ
- MAPK
mitogen-activated protein kinases
- MCP
monocyte chemoattractant protein
- MKP-1
MAPK phosphatase-1
- MLC
myosin light chain
- MUC5AC
Mucin-5 Subtype AC
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- NOX
NADPH oxidase
- O2
oxygen
- O2−•
superoxide anion
- OSCN−
hypothiocyanite
- p53
tumor protein 53
- PAH
pulmonary arterial hypertension
- PAI-1
plasminogen activator inhibitor-1
- PI3K
phosphoinositide-3-phosphate
- PKC
protein kinase C
- Poldip2
polymerase delta-interacting protein 2
- PPAR-γ
peroxisome proliferator-activated receptor- γ
- PTP1B
protein tyrosine phosphatase 1B
- Rac1
Ras-related C3 botulinum toxin substrate 1
- Ras
Rat sarcoma
- ROS
reactive oxygen species
- SMC
smooth muscle cell
- SOD
superoxide dismutase
- STAT5
signal transducer and activator of transcription 5
- TGF-β
transforming growth factor-β
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor α
- VEGFR2
vascular endothelial growth factor receptor-2
- WT
wild-type
Acknowledgments
This work is supported in part by Veterans Administration Health System grant, 1IK2BX001477 (to L.H.); American Heart Association grant, 12GRNT12040409 (to G.C.); National Institutes of Health grants, K08 HL094666 (to T.R.L.); R01 HL086836 and R21 AI101642 (to G.C.); and R01 HL067967, R01 HL094230, and P50 HL107181 (to V.J.T.).
References
- 1.Abdala-Valencia H, Earwood J, Bansal S, Jansen M, Babcock G, Garvy B, Wills-Karp M, and Cook-Mills JM. Nonhematopoietic NADPH oxidase regulation of lung eosinophilia and airway hyperresponsiveness in experimentally induced asthma. Am J Physiol Lung Cell Mol Physiol 292: L1111–L1125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ago T, Kuroda J, Pain J, Fu C, Li H, and Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106: 1253–1264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ago T, Matsushima S, Kuroda J, Zablocki D, Kitazono T, and Sadoshima J. The NADPH oxidase Nox4 and aging in the heart. Aging (Albany NY) 2: 1012–1016, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akasaki T, Ohya Y, Kuroda J, Eto K, Abe I, Sumimoto H, and Iida M. Increased expression of gp91phox homologues of NAD(P)H oxidase in the aortic media during chronic hypertension: involvement of the renin-angiotensin system. Hypertens Res 29: 813–820, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Amara N, Goven D, Prost F, Muloway R, Crestani B, and Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax 65: 733–738, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, and Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56: 2316–2327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, and Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci U S A 99: 715–720, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold RS, Shi J, Murad E, Whalen AM, Sun CQ, Polavarapu R, Parthasarathy S, Petros JA, and Lambeth JD. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci U S A 98: 5550–5555, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baehner RL. and Nathan DG. Leukocyte oxidase: defective activity in chronic granulomatous disease. Science 155: 835–836, 1967 [DOI] [PubMed] [Google Scholar]
- 10.Banerjee ER. and Henderson WR, Jr., Defining the molecular role of gp91phox in the immune manifestation of acute allergic asthma using a preclinical murine model. Clin Mol Allergy 10: 2, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee ER. and Henderson WR, Jr., Role of T cells in a gp91phox knockout murine model of acute allergic asthma. Allergy Asthma Clin Immunol 9: 6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banfi B, Clark RA, Steger K, and Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem 278: 3510–3513, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, and Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem 279: 46065–46072, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Bedard K, Lardy B, and Krause KH. NOX family NADPH oxidases: not just in mammals. Biochimie 89: 1107–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Block K, Eid A, Griendling KK, Lee DY, Wittrant Y, and Gorin Y. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J Biol Chem 283: 24061–24076, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Block K, Gorin Y, and Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A 106: 14385–14390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borok Z, Whitsett JA, Bitterman PB, Thannickal VJ, Kotton DN, Reynolds SD, Krasnow MA, Bianchi DW, Morrisey EE, Hogan BL, Kurie JM, Walker DC, Radisky DC, Nishimura SL, Violette SM, Noble PW, Shapiro SD, Blaisdell CJ, Chapman HA, Kiley J, Gail D, and Hoshizaki D. Cell plasticity in lung injury and repair: report from an NHLBI workshop, April 19–20, 2010. Proc Am Thorac Soc 8: 215–222, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brain JD. The respiratory tract and the environment. Environ Health Perspect 20: 113–126, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP, Quinn MT, Krenitsky K, Ardie KG, Lambeth JD, and Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol 285: C353–C369, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Bridges RA, Berendes H, and Good RA. A fatal granulomatous disease of childhood; the clinical, pathological, and laboratory features of a new syndrome. AMA J Dis Child 97: 387–408, 1959 [PubMed] [Google Scholar]
- 22.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med 18: 775–794, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, and Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res 93: 802–805, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, Guichard C, Arbiser JL, Banfi B, Pache JC, Barazzone-Argiroffo C, and Krause KH. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal 15: 607–619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carnesecchi S, Deffert C, Pagano A, Garrido-Urbani S, Metrailler-Ruchonnet I, Schappi M, Donati Y, Matthay MA, Krause KH, and Barazzone-Argiroffo C. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am J Respir Crit Care Med 180: 972–981, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carnesecchi S, Pache JC, and Barazzone-Argiroffo C. NOX enzymes: potential target for the treatment of acute lung injury. Cell Mol Life Sci 69: 2373–2385, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casbon AJ, Long ME, Dunn KW, Allen LA, and Dinauer MC. Effects of IFN-gamma on intracellular trafficking and activity of macrophage NADPH oxidase flavocytochrome b558. J Leukoc Biol 92: 869–882, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K, Kirber MT, Xiao H, Yang Y, and Keaney JF, Jr., Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181: 1129–1139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng G, Cao Z, Xu X, van Meir EG, and Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Cheng G, Diebold BA, Hughes Y, and Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem 281: 17718–17726, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Cheng G. and Lambeth JD. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem 279: 4737–4742, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Cheng G, Ritsick D, and Lambeth JD. Nox3 regulation by NOXO1, p47phox, and p67phox. J Biol Chem 279: 34250–34255, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Comstock AT, Ganesan S, Chattoraj A, Faris AN, Margolis BL, Hershenson MB, and Sajjan US. Rhinovirus-induced barrier dysfunction in polarized airway epithelial cells is mediated by NADPH oxidase 1. J Virol 85: 6795–6808, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook-Mills JM, Marchese ME, and Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal 15: 1607–1638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper AM, Segal BH, Frank AA, Holland SM, and Orme IM. Transient loss of resistance to pulmonary tuberculosis in p47(phox−/−) mice. Infect Immun 68: 1231–1234, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa RO, Lacor PN, Ferreira IL, Resende R, Auberson YP, Klein WL, Oliveira CR, Rego AC, and Pereira CM. Endoplasmic reticulum stress occurs downstream of GluN2B subunit of N-methyl-d-aspartate receptor in mature hippocampal cultures treated with amyloid-beta oligomers. Aging Cell 11: 823–833, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, and Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97: 900–907, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Cui W, Matsuno K, Iwata K, Ibi M, Matsumoto M, Zhang J, Zhu K, Katsuyama M, Torok NJ, and Yabe-Nishimura C. NOX1/nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation. Hepatology 54: 949–958, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Davidson BA, Vethanayagam RR, Grimm MJ, Mullan BA, Raghavendran K, Blackwell TS, Freeman ML, Ayyasamy V, Singh KK, Sporn MB, Itagaki K, Hauser CJ, Knight PR, and Segal BH. NADPH oxidase and Nrf2 regulate gastric aspiration-induced inflammation and acute lung injury. J Immunol 190: 1714–1724, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Minicis S, Seki E, Paik YH, Osterreicher CH, Kodama Y, Kluwe J, Torozzi L, Miyai K, Benedetti A, Schwabe RF, and Brenner DA. Role and cellular source of nicotinamide adenine dinucleotide phosphate oxidase in hepatic fibrosis. Hepatology 52: 1420–1430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deshane J, Zmijewski JW, Luther R, Gaggar A, Deshane R, Lai JF, Xu X, Spell M, Estell K, Weaver CT, Abraham E, Schwiebert LM, and Chaplin DD. Free radical-producing myeloid-derived regulatory cells: potent activators and suppressors of lung inflammation and airway hyperresponsiveness. Mucosal Immunol 4: 503–518, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desmouliere A, Geinoz A, Gabbiani F, and Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122: 103–111, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhaunsi GS, Paintlia MK, Kaur J, and Turner RB. NADPH oxidase in human lung fibroblasts. J Biomed Sci 11: 617–622, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Diebold I, Petry A, Hess J, and Gorlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell 21: 2087–2096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinauer MC. The respiratory burst oxidase and the molecular genetics of chronic granulomatous disease. Crit Rev Clin Lab Sci 30: 329–369, 1993 [DOI] [PubMed] [Google Scholar]
- 46.Downs CA, Kriener LH, Yu L, Eaton DC, Jain L, and Helms MN. beta-Adrenergic agonists differentially regulate highly selective and nonselective epithelial sodium channels to promote alveolar fluid clearance in vivo. Am J Physiol Lung Cell Mol Physiol 302: L1167–L1178, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Downs CA, Trac D, Brewer EM, Brown LA, and Helms MN. Chronic alcohol ingestion changes the landscape of the alveolar epithelium. Biomed Res Int 2013: 470217, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Faurschou M, and Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect 5: 1317–1327, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Fike CD, Slaughter JC, Kaplowitz MR, Zhang Y, and Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L881–L888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fink K, Duval A, Martel A, Soucy-Faulkner A, and Grandvaux N. Dual role of NOX2 in respiratory syncytial virus- and sendai virus-induced activation of NF-kappaB in airway epithelial cells. J Immunol 180: 6911–6922, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Finkel T. Reactive oxygen species and signal transduction. IUBMB Life 52: 3–6, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Fischer H. Mechanisms and function of DUOX in epithelia of the lung. Antioxid Redox Signal 11: 2453–2465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fischer H, Gonzales LK, Kolla V, Schwarzer C, Miot F, Illek B, and Ballard PL. Developmental regulation of DUOX1 expression and function in human fetal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 292: L1506–L1514, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, and Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA 307: 2526–2533, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Forteza R, Salathe M, Miot F, Forteza R, and Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol 32: 462–469, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Fresquet F, Pourageaud F, Leblais V, Brandes RP, Savineau JP, Marthan R, and Muller B. Role of reactive oxygen species and gp91phox in endothelial dysfunction of pulmonary arteries induced by chronic hypoxia. Br J Pharmacol 148: 714–723, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu P, Mohan V, Mansoor S, Tiruppathi C, Sadikot RT, and Natarajan V. Role of nicotinamide adenine dinucleotide phosphate-reduced oxidase proteins in Pseudomonas aeruginosa-induced lung inflammation and permeability. Am J Respir Cell Mol Biol 48: 477–488, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaggini F, Laleu B, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, and Page P. Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual Nox4/Nox1 inhibitors. Bioorg Med Chem 19: 6989–6999, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, Liu QH, and Malik AB. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J Immunol 168: 3974–3982, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Geiszt M, Witta J, Baffi J, Lekstrom K, and Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J 17: 1502–1504, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez A, Hung CY, and Cole GT. Absence of phagocyte NADPH oxidase 2 leads to severe inflammatory response in lungs of mice infected with Coccidioides. Microb Pathog 51: 432–441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodson P, Kumar A, Jain L, Kundu K, Murthy N, Koval M, and Helms MN. Nadph oxidase regulates alveolar epithelial sodium channel activity and lung fluid balance in vivo via O(−)(2) signaling. Am J Physiol Lung Cell Mol Physiol 302: L410–L419, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Graham KA, Kulawiec M, Owens KM, Li X, Desouki MM, Chandra D, and Singh KK. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol Ther 10: 223–231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grasberger H, De Deken X, Miot F, Pohlenz J, and Refetoff S. Missense mutations of dual oxidase 2 (DUOX2) implicated in congenital hypothyroidism have impaired trafficking in cells reconstituted with DUOX2 maturation factor. Mol Endocrinol 21: 1408–1421, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, and Hart CM. The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am J Respir Cell Mol Biol 47: 718–726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanahan D. and Weinberg RA. Hallmarks of cancer: the next generation. Cell 144: 646–674, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, and Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett 579: 4911–4917, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Hayes P. and Knaus UG. Balancing reactive oxygen species in the epigenome: NADPH oxidases as target and perpetrator. Antioxid Redox Signal 18: 1937–1945, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Hecker L, Cheng J, and Thannickal VJ. Targeting NOX enzymes in pulmonary fibrosis. Cell Mol Life Sci 69: 2365–2371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, and Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.This reference has been deleted.
- 73.Helmcke I, Heumuller S, Tikkanen R, Schroder K, and Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal 11: 1279–1287, 2009 [DOI] [PubMed] [Google Scholar]
- 74.Henderson BC, Tyagi N, Ovechkin A, Kartha GK, Moshal KS, and Tyagi SC. Oxidative remodeling in pressure overload induced chronic heart failure. Eur J Heart Fail 9: 450–457, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, and Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 47: 340–348, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, and Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133: 235–249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, and Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 296: L489–L499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnston RB., Jr.Clinical aspects of chronic granulomatous disease. Curr Opin Hematol 8: 17–22, 2001 [DOI] [PubMed] [Google Scholar]
- 79.Kassim SY, Fu X, Liles WC, Shapiro SD, Parks WC, and Heinecke JW. NADPH oxidase restrains the matrix metalloproteinase activity of macrophages. J Biol Chem 280: 30201–30205, 2005 [DOI] [PubMed] [Google Scholar]
- 80.Kawahara T, Quinn MT, and Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol 7: 109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawahara T, Teshima S, Oka A, Sugiyama T, Kishi K, and Rokutan K. Type I Helicobacter pylori lipopolysaccharide stimulates toll-like receptor 4 and activates mitogen oxidase 1 in gastric pit cells. Infect Immun 69: 4382–4389, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim SM, Kim YG, Jeong KH, Lee SH, Lee TW, Ihm CG, and Moon JY. Angiotensin II-induced mitochondrial Nox4 is a major endogenous source of oxidative stress in kidney tubular cells. PLoS One 7: e39739, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim SY, Moon KA, Jo HY, Jeong S, Seon SH, Jung E, Cho YS, Chun E, and Lee KY. Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunol Cell Biol 90: 441–448, 2012 [DOI] [PubMed] [Google Scholar]
- 84.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol 77: 598–625, 2005 [DOI] [PubMed] [Google Scholar]
- 85.Komatsu D, Kato M, Nakayama J, Miyagawa S, and Kamata T. NADPH oxidase 1 plays a critical mediating role in oncogenic Ras-induced vascular endothelial growth factor expression. Oncogene 27: 4724–4732, 2008 [DOI] [PubMed] [Google Scholar]
- 86.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, and Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A 107: 15565–15570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lagente V, Planquois JM, Leclerc O, Schmidlin F, and Bertrand CP. Oxidative stress is an important component of airway inflammation in mice exposed to cigarette smoke or lipopolysaccharide. Clin Exp Pharmacol Physiol 35: 601–605, 2008 [DOI] [PubMed] [Google Scholar]
- 88.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, and Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem 53: 7715–7730, 2010 [DOI] [PubMed] [Google Scholar]
- 89.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 90.Lambeth JD, Cheng G, Arnold RS, and Edens WA. Novel homologs of gp91phox. Trends Biochem Sci 25: 459–461, 2000 [DOI] [PubMed] [Google Scholar]
- 91.Larios JM, Budhiraja R, Fanburg BL, and Thannickal VJ. Oxidative protein cross-linking reactions involving L-tyrosine in transforming growth factor-beta1-stimulated fibroblasts. J Biol Chem 276: 17437–17441, 2001 [DOI] [PubMed] [Google Scholar]
- 92.Laurent E, McCoy JW, 3rd, Macina RA, Liu W, Cheng G, Robine S, Papkoff J, and Lambeth JD. Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int J Cancer 123: 100–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee CF, Qiao M, Schroder K, Zhao Q, and Asmis R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ Res 106: 1489–1497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee JK, Edderkaoui M, Truong P, Ohno I, Jang KT, Berti A, Pandol SJ, and Gukovskaya AS. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology 133: 1637–1648, 2007 [DOI] [PubMed] [Google Scholar]
- 95.Leusen JH, de Boer M, Bolscher BG, Hilarius PM, Weening RS, Ochs HD, Roos D, and Verhoeven AJ. A point mutation in gp91-phox of cytochrome b558 of the human NADPH oxidase leading to defective translocation of the cytosolic proteins p47-phox and p67-phox. J Clin Invest 93: 2120–2126, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li G, Scull C, Ozcan L, and Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol 191: 1113–1125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, and Hanze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal 10: 1687–1698, 2008 [DOI] [PubMed] [Google Scholar]
- 98.Liu JQ, Zelko IN, Erbynn EM, Sham JS, and Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006 [DOI] [PubMed] [Google Scholar]
- 99.Liu RM, Choi J, Wu JH, Gaston Pravia KA, Lewis KM, Brand JD, Mochel NS, Krzywanski DM, Lambeth JD, Hagood JS, Forman HJ, Thannickal VJ, and Postlethwait EM. Oxidative modification of nuclear mitogen-activated protein kinase phosphatase 1 is involved in transforming growth factor beta1-induced expression of plasminogen activator inhibitor 1 in fibroblasts. J Biol Chem 285: 16239–16247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Luxen S, Belinsky SA, and Knaus UG. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res 68: 1037–1045, 2008 [DOI] [PubMed] [Google Scholar]
- 101.Luxen S, Noack D, Frausto M, Davanture S, Torbett BE, and Knaus UG. Heterodimerization controls localization of Duox-DuoxA NADPH oxidases in airway cells. J Cell Sci 122: 1238–1247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, and Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.MacRedmond RE, Greene CM, Dorscheid DR, McElvaney NG, and O'Neill SJ. Epithelial expression of TLR4 is modulated in COPD and by steroids, salmeterol and cigarette smoke. Respir Res 8: 84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, Bertrand CP, and Lagente V. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res 6: 11, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marriott HM, Jackson LE, Wilkinson TS, Simpson AJ, Mitchell TJ, Buttle DJ, Cross SS, Ince PG, Hellewell PG, Whyte MK, and Dockrell DH. Reactive oxygen species regulate neutrophil recruitment and survival in pneumococcal pneumonia. Am J Respir Crit Care Med 177: 887–895, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, and Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 107.Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, and Shimosegawa T. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol 295: G709–G717, 2008 [DOI] [PubMed] [Google Scholar]
- 108.Meier B, Cross AR, Hancock JT, Kaup FJ, and Jones OT. Identification of a superoxide-generating NADPH oxidase system in human fibroblasts. Biochem J 275 (Pt 1): 241–245, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meng QR, Gideon KM, Harbo SJ, Renne RA, Lee MK, Brys AM, and Jones R. Gene expression profiling in lung tissues from mice exposed to cigarette smoke, lipopolysaccharide, or smoke plus lipopolysaccharide by inhalation. Inhal Toxicol 18: 555–568, 2006 [DOI] [PubMed] [Google Scholar]
- 110.Mitsushita J, Lambeth JD, and Kamata T. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res 64: 3580–3585, 2004 [DOI] [PubMed] [Google Scholar]
- 111.Mittal M, Gu XQ, Pak O, Pamenter ME, Haag D, Fuchs DB, Schermuly RT, Ghofrani HA, Brandes RP, Seeger W, Grimminger F, Haddad GG, and Weissmann N. Hypoxia induces Kv channel current inhibition by increased NADPH oxidase-derived reactive oxygen species. Free Radic Biol Med 52: 1033–1042, 2012 [DOI] [PubMed] [Google Scholar]
- 112.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, and Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007 [DOI] [PubMed] [Google Scholar]
- 113.Mochizuki T, Furuta S, Mitsushita J, Shang WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayama J, Konagai A, Hirose K, Kiyosawa K, and Kamata T. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene 25: 3699–3707, 2006 [DOI] [PubMed] [Google Scholar]
- 114.Moreno JC, Bikker H, Kempers MJ, van Trotsenburg AS, Baas F, de Vijlder JJ, Vulsma T, and Ris-Stalpers C. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med 347: 95–102, 2002 [DOI] [PubMed] [Google Scholar]
- 115.Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr., Nauseef WM, Dupuy C, and Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med 175: 174–183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nagai K, Betsuyaku T, Suzuki M, Nasuhara Y, Kaga K, Kondo S, and Nishimura M. Dual oxidase 1 and 2 expression in airway epithelium of smokers and patients with mild/moderate chronic obstructive pulmonary disease. Antioxid Redox Signal 10: 705–714, 2008 [DOI] [PubMed] [Google Scholar]
- 117.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev 219: 88–102, 2007 [DOI] [PubMed] [Google Scholar]
- 118.Newburger PE, Skalnik DG, Hopkins PJ, Eklund EA, and Curnutte JT. Mutations in the promoter region of the gene for gp91-phox in X-linked chronic granulomatous disease with decreased expression of cytochrome b558. J Clin Invest 94: 1205–1211, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nlandu Khodo S, Dizin E, Sossauer G, Szanto I, Martin PY, Feraille E, Krause KH, and de Seigneux S. NADPH-oxidase 4 protects against kidney fibrosis during chronic renal injury. J Am Soc Nephrol 23: 1967–1976, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ohshiro Y, Ma RC, Yasuda Y, Hiraoka-Yamamoto J, Clermont AC, Isshiki K, Yagi K, Arikawa E, Kern TS, and King GL. Reduction of diabetes-induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase Cbeta-null mice. Diabetes 55: 3112–3120, 2006 [DOI] [PubMed] [Google Scholar]
- 121.Okros Z, Endreffy E, Novak Z, Maroti Z, Monostori P, Varga IS, Kiraly A, and Turi S. Changes in NADPH oxidase mRNA level can be detected in blood at inhaled corticosteroid treated asthmatic children. Life Sci 91: 907–911, 2012 [DOI] [PubMed] [Google Scholar]
- 122.Oshikawa J, Kim SJ, Furuta E, Caliceti C, Chen GF, McKinney RD, Kuhr F, Levitan I, Fukai T, and Ushio-Fukai M. Novel role of p66Shc in ROS-dependent VEGF signaling and angiogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 302: H724–H732, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park M, Hennig B, and Toborek M. Methamphetamine alters occludin expression via NADPH oxidase-induced oxidative insult and intact caveolae. J Cell Mol Med 16: 362–375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, and Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal 11: 747–764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pendyala S, Moitra J, Kalari S, Kleeberger SR, Zhao Y, Reddy SP, Garcia JG, and Natarajan V. Nrf2 regulates hyperoxia-induced Nox4 expression in human lung endothelium: identification of functional antioxidant response elements on the Nox4 promoter. Free Radic Biol Med 50: 1749–1759, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Perner A, Andresen L, Pedersen G, and Rask-Madsen J. Superoxide production and expression of NAD(P)H oxidases by transformed and primary human colonic epithelial cells. Gut 52: 231–236, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pierrou S, Broberg P, O'Donnell RA, Pawlowski K, Virtala R, Lindqvist E, Richter A, Wilson SJ, Angco G, Moller S, Bergstrand H, Koopmann W, Wieslander E, Stromstedt PE, Holgate ST, Davies DE, Lund J, and Djukanovic R. Expression of genes involved in oxidative stress responses in airway epithelial cells of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 175: 577–586, 2007 [DOI] [PubMed] [Google Scholar]
- 128.Rada B. and Leto TL. Characterization of hydrogen peroxide production by Duox in bronchial epithelial cells exposed to Pseudomonas aeruginosa. FEBS Lett 584: 917–922, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, and Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 45: 1223–1231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Royer-Pokora B, Kunkel LM, Monaco AP, Goff SC, Newburger PE, Baehner RL, Cole FS, Curnutte JT, and Orkin SH. Cloning the gene for an inherited human disorder—chronic granulomatous disease—on the basis of its chromosomal location. Nature 322: 32–38, 1986 [DOI] [PubMed] [Google Scholar]
- 131.Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A, and Jackson MJ. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal 18: 603–621, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sancho P, Mainez J, Crosas-Molist E, Roncero C, Fernandez-Rodriguez CM, Pinedo F, Huber H, Eferl R, Mikulits W, and Fabregat I. NADPH oxidase NOX4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS One 7: e45285, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, and Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 26: 1749–1760, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, and Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012 [DOI] [PubMed] [Google Scholar]
- 135.Schwarzer C, Machen TE, Illek B, and Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem 279: 36454–36461, 2004 [DOI] [PubMed] [Google Scholar]
- 136.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, and Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shao MX. and Nadel JA. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-alpha-converting enzyme. J Immunol 175: 4009–4016, 2005 [DOI] [PubMed] [Google Scholar]
- 138.Shinohara M, Shang WH, Kubodera M, Harada S, Mitsushita J, Kato M, Miyazaki H, Sumimoto H, and Kamata T. Nox1 redox signaling mediates oncogenic Ras-induced disruption of stress fibers and focal adhesions by down-regulating Rho. J Biol Chem 282: 17640–17648, 2007 [DOI] [PubMed] [Google Scholar]
- 139.Singleton PA, Pendyala S, Gorshkova IA, Mambetsariev N, Moitra J, Garcia JG, and Natarajan V. Dynamin 2 and c-Abl are novel regulators of hyperoxia-mediated NADPH oxidase activation and reactive oxygen species production in caveolin-enriched microdomains of the endothelium. J Biol Chem 284: 34964–34975, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sipkens JA, Hahn N, van den Brand CS, Meischl C, Cillessen SA, Smith DE, Juffermans LJ, Musters RJ, Roos D, Jakobs C, Blom HJ, Smulders YM, Krijnen PA, Stehouwer CD, Rauwerda JA, van Hinsbergh VW, and Niessen HW. Homocysteine-induced apoptosis in endothelial cells coincides with nuclear NOX2 and peri-nuclear NOX4 activity. Cell Biochem Biophys 67: 341–352, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Snelgrove RJ, Edwards L, Rae AJ, and Hussell T. An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur J Immunol 36: 1364–1373, 2006 [DOI] [PubMed] [Google Scholar]
- 142.Snelgrove RJ, Edwards L, Williams AE, Rae AJ, and Hussell T. In the absence of reactive oxygen species, T cells default to a Th1 phenotype and mediate protection against pulmonary Cryptococcus neoformans infection. J Immunol 177: 5509–5516, 2006 [DOI] [PubMed] [Google Scholar]
- 143.Soler-Palacin P, Margareto C, Llobet P, Asensio O, Hernandez M, Caragol I, and Espanol T. Chronic granulomatous disease in pediatric patients: 25 years of experience. Allergol Immunopathol (Madr) 35: 83–89, 2007 [DOI] [PubMed] [Google Scholar]
- 144.Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, and Sowers JR. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology 148: 3773–3780, 2007 [DOI] [PubMed] [Google Scholar]
- 145.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, and Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L661–L673, 2006 [DOI] [PubMed] [Google Scholar]
- 146.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, and Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401: 79–82, 1999 [DOI] [PubMed] [Google Scholar]
- 147.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J 275: 3249–3277, 2008 [DOI] [PubMed] [Google Scholar]
- 148.Sutcliffe A, Hollins F, Gomez E, Saunders R, Doe C, Cooke M, Challiss RA, and Brightling CE. Increased nicotinamide adenine dinucleotide phosphate oxidase 4 expression mediates intrinsic airway smooth muscle hypercontractility in asthma. Am J Respir Crit Care Med 185: 267–274, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Szanto I, Rubbia-Brandt L, Kiss P, Steger K, Banfi B, Kovari E, Herrmann F, Hadengue A, and Krause KH. Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J Pathol 207: 164–176, 2005 [DOI] [PubMed] [Google Scholar]
- 150.Szatrowski TP. and Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res 51: 794–798, 1991 [PubMed] [Google Scholar]
- 151.Taira M, Toba H, Murakami M, Iga I, Serizawa R, Murata S, Kobara M, and Nakata T. Spironolactone exhibits direct renoprotective effects and inhibits renal renin-angiotensin-aldosterone system in diabetic rats. Eur J Pharmacol 589: 264–271, 2008 [DOI] [PubMed] [Google Scholar]
- 152.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, and Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem 278: 25234–25246, 2003 [DOI] [PubMed] [Google Scholar]
- 153.Teimourian S, Zomorodian E, Badalzadeh M, Pouya A, Kannengiesser C, Mansouri D, Cheraghi T, and Parvaneh N. Characterization of six novel mutations in CYBA: the gene causing autosomal recessive chronic granulomatous disease. Br J Haematol 141: 848–851, 2008 [DOI] [PubMed] [Google Scholar]
- 154.Teng RJ, Du J, Welak S, Guan T, Eis A, Shi Y, and Konduri GG. Cross talk between NADPH oxidase and autophagy in pulmonary artery endothelial cells with intrauterine persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302: L651–L663, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Teshima S, Tsunawaki S, and Rokutan K. Helicobacter pylori lipopolysaccharide enhances the expression of NADPH oxidase components in cultured guinea pig gastric mucosal cells. FEBS Lett 452: 243–246, 1999 [DOI] [PubMed] [Google Scholar]
- 156.Thannickal VJ. Oxygen in the evolution of complex life and the price we pay. Am J Respir Cell Mol Biol 40: 507–510, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thannickal VJ, Aldweib KD, and Fanburg BL. Tyrosine phosphorylation regulates H2O2 production in lung fibroblasts stimulated by transforming growth factor beta1. J Biol Chem 273: 23611–23615, 1998 [DOI] [PubMed] [Google Scholar]
- 158.Thannickal VJ. and Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem 270: 30334–30338, 1995 [DOI] [PubMed] [Google Scholar]
- 159.Thannickal VJ. and Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000 [DOI] [PubMed] [Google Scholar]
- 160.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, and Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem 278: 12384–12389, 2003 [DOI] [PubMed] [Google Scholar]
- 161.Thannickal VJ, Toews GB, White ES, Lynch JP, 3rd, and Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 55: 395–417, 2004 [DOI] [PubMed] [Google Scholar]
- 162.Tickner J, Fan LM, Du J, Meijles D, and Li JM. Nox2-derived ROS in PPARgamma signaling and cell-cycle progression of lung alveolar epithelial cells. Free Radic Biol Med 51: 763–772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tominaga K, Kawahara T, Sano T, Toida K, Kuwano Y, Sasaki H, Kawai T, Teshima-Kondo S, and Rokutan K. Evidence for cancer-associated expression of NADPH oxidase 1 (Nox1)-based oxidase system in the human stomach. Free Radic Biol Med 43: 1627–1638, 2007 [DOI] [PubMed] [Google Scholar]
- 164.Ullevig S, Zhao Q, Lee CF, Seok Kim H, Zamora D, and Asmis R. NADPH oxidase 4 mediates monocyte priming and accelerated chemotaxis induced by metabolic stress. Arterioscler Thromb Vasc Biol 32: 415–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Usatyuk PV, Singleton PA, Pendyala S, Kalari SK, He D, Gorshkova IA, Camp SM, Moitra J, Dudek SM, Garcia JG, and Natarajan V. Novel role for non-muscle myosin light chain kinase (MLCK) in hyperoxia-induced recruitment of cytoskeletal proteins, NADPH oxidase activation, and reactive oxygen species generation in lung endothelium. J Biol Chem 287: 9360–9375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 11: 1289–1299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.van Duin D, Allore HG, Mohanty S, Ginter S, Newman FK, Belshe RB, Medzhitov R, and Shaw AC. Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J Infect Dis 195: 1590–1597, 2007 [DOI] [PubMed] [Google Scholar]
- 168.Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, and Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem 279: 34643–34654, 2004 [DOI] [PubMed] [Google Scholar]
- 169.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci 59: 1428–1459, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.von Lohneysen K, Noack D, Hayes P, Friedman JS, and Knaus UG. Constitutive NADPH oxidase 4 activity resides in the composition of the B-loop and the penultimate C terminus. J Biol Chem 287: 8737–8745, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.von Lohneysen K, Noack D, Jesaitis AJ, Dinauer MC, and Knaus UG. Mutational analysis reveals distinct features of the Nox4-p22 phox complex. J Biol Chem 283: 35273–35282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.von Lohneysen K, Noack D, Wood MR, Friedman JS, and Knaus UG. Structural insights into Nox4 and Nox2: motifs involved in function and cellular localization. Mol Cell Biol 30: 961–975, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, Toews GB, and Thannickal VJ. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J 19: 854–856, 2005 [DOI] [PubMed] [Google Scholar]
- 174.Wang P, Tang F, Li R, Zhang H, Chen S, Liu P, and Huang H. Contribution of different Nox homologues to cardiac remodeling in two-kidney two-clip renovascular hypertensive rats: effect of valsartan. Pharmacol Res 55: 408–417, 2007 [DOI] [PubMed] [Google Scholar]
- 175.Wang YX. and Zheng YM. ROS-dependent signaling mechanisms for hypoxic Ca(2+) responses in pulmonary artery myocytes. Antioxid Redox Signal 12: 611–623, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ware LB. and Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 177.Weyemi U, Caillou B, Talbot M, Ameziane-El-Hassani R, Lacroix L, Lagent-Chevallier O, Al Ghuzlan A, Roos D, Bidart JM, Virion A, Schlumberger M, and Dupuy C. Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr Relat Cancer 17: 27–37, 2010 [DOI] [PubMed] [Google Scholar]
- 178.Whaley-Connell A, Habibi J, Nistala R, Cooper SA, Karuparthi PR, Hayden MR, Rehmer N, DeMarco VG, Andresen BT, Wei Y, Ferrario C, and Sowers JR. Attenuation of NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension 51: 474–480, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wijkstrom-Frei C, El-Chemaly S, Ali-Rachedi R, Gerson C, Cobas MA, Forteza R, Salathe M, and Conner GE. Lactoperoxidase and human airway host defense. Am J Respir Cell Mol Biol 29: 206–212, 2003 [DOI] [PubMed] [Google Scholar]
- 180.Woolley JF, Naughton R, Stanicka J, Gough DR, Bhatt L, Dickinson BC, Chang CJ, and Cotter TG. H2O2 production downstream of FLT3 is mediated by p22phox in the endoplasmic reticulum and is required for STAT5 signalling. PLoS One 7: e34050, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yao H, Edirisinghe I, Yang SR, Rajendrasozhan S, Kode A, Caito S, Adenuga D, and Rahman I. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am J Pathol 172: 1222–1237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yeligar SM, Harris FL, Hart CM, and Brown LA. Ethanol induces oxidative stress in alveolar macrophages via upregulation of NADPH oxidases. J Immunol 188: 3648–3657, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang L, Nguyen MV, Lardy B, Jesaitis AJ, Grichine A, Rousset F, Talbot M, Paclet MH, Qian G, and Morel F. New insight into the Nox4 subcellular localization in HEK293 cells: first monoclonal antibodies against Nox4. Biochimie 93: 457–468, 2011 [DOI] [PubMed] [Google Scholar]
- 184.Zhang M, Perino A, Ghigo A, Hirsch E, and Shah AM. NADPH oxidases in heart failure: poachers or gamekeepers? Antioxid Redox Signal 18: 1024–1041, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang WJ, Wei H, Tien YT, and Frei B. Genetic ablation of phagocytic NADPH oxidase in mice limits TNFalpha-induced inflammation in the lungs but not other tissues. Free Radic Biol Med 50: 1517–1525, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang X, Shan P, Jiang G, Cohn L, and Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest 116: 3050–3059, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, and Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest 123: 1096–1108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, and Abraham E. Antiinflammatory effects of hydrogen peroxide in neutrophil activation and acute lung injury. Am J Respir Crit Care Med 179: 694–704, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]