Abstract
Significance: Reactive oxygen species (ROS) promote genomic instability, altered signal transduction, and an environment that can sustain tumor formation and growth. The NOX family of NADPH oxidases, membrane-bound epithelial superoxide and hydrogen peroxide producers, plays a critical role in the maintenance of immune function, cell growth, and apoptosis. The impact of NOX enzymes in carcinogenesis is currently being defined and may directly link chronic inflammation and NOX ROS-mediated tumor formation. Recent Advances: Increased interest in the function of NOX enzymes in tumor biology has spurred a surge of investigative effort to understand the variability of NOX expression levels in tumors and the effect of NOX activity on tumor cell proliferation. These initial efforts have demonstrated a wide variance in NOX distribution and expression levels across numerous cancers as well as in common tumor cell lines, suggesting that much remains to be discovered about the unique role of NOX-related ROS production within each system. Progression from in vitro cell line studies toward in vivo tumor tissue screening and xenograft models has begun to provide evidence supporting the importance of NOX expression in carcinogenesis. Critical Issues: A lack of universally available, isoform-specific antibodies and animal tumor models of inducible knockout or over-expression of NOX isoforms has hindered progress toward the completion of in vivo studies. Future Directions: In vivo validation experiments and the use of large, existing gene expression data sets should help define the best model systems for studying the NOX homologues in the context of cancer. Antioxid. Redox Signal. 20, 2873–2889.
Introduction
Angiogenesis, proliferation, and metastasis are hallmarks of cellular transformation and tumor growth that have been associated with oxidative stress and genomic instability (14, 18, 27, 105, 121, 126, 130). Redox imbalance in tumors may originate from a variety of sources, including mitochondrial or NADPH oxidase-derived reactive oxygen species (ROS). Although a role for ROS in tumor biology was postulated well before the discovery of the NOX enzymatic family, the functions of these oxidases are redefining the role of superoxide and hydrogen peroxide (H2O2) in tumor cell homeostasis (100).
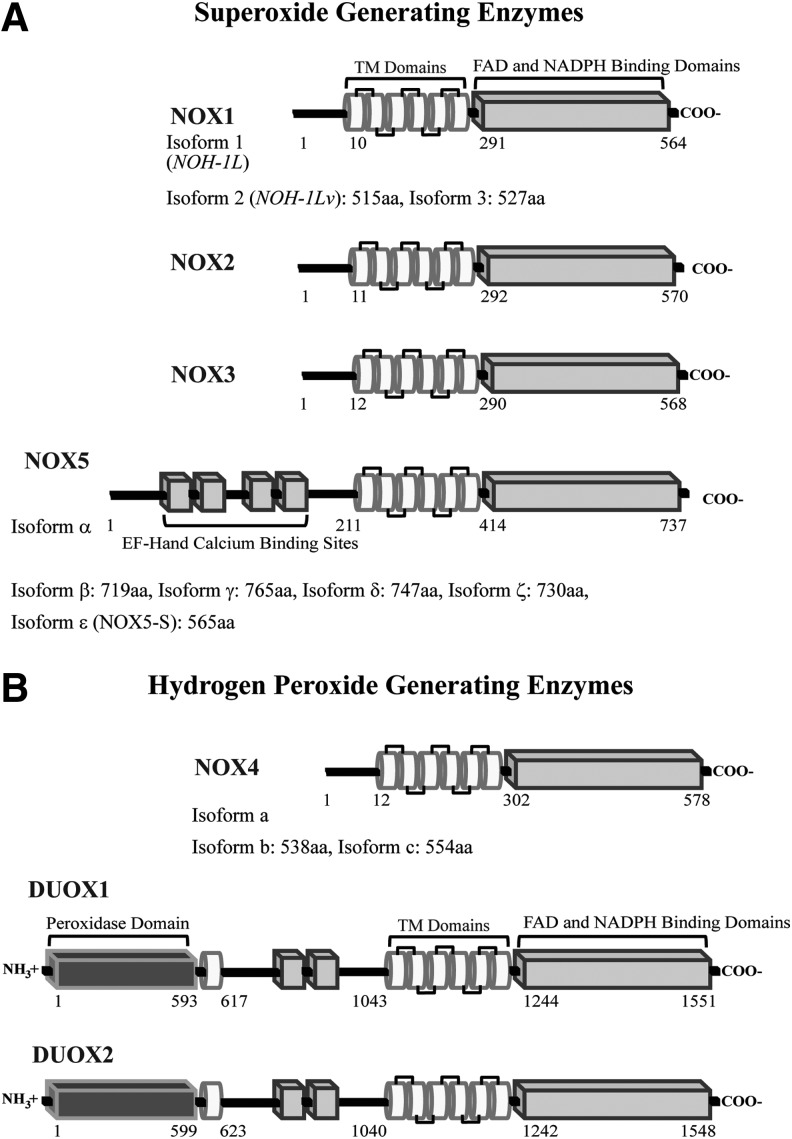
The NOX family, comprising seven enzymatic isoforms, produces ROS by the NADPH-dependent one-electron reduction of oxygen to superoxide. Isoform-specific production of H2O2 is unique to NOX4 and the dual oxidase (DUOX) enzymes (31, 87) (Fig. 1); controversy surrounds this ROS categorization with some researchers noting superoxide production by the NOX4 isoform. Variability in the specific ROS reported to be produced by NOX4 has been attributed to the interpretation of fluorescent microscopy studies and/or diverse cellular localization (112, 122). Structurally, each NOX/DUOX protein is anchored to the plasma membrane through six transmembrane helices, which bind two heme cofactors and allow for NADPH oxidation through a C-terminal FAD/NADPH binding domain (Fig. 2); other features vary between isoforms, such as cytosolic amino-terminal EF-calcium binding regions present in NOX5 and DUOX1/2, and a unique DUOX structural extension composed of a transmembrane helix and extracellular peroxidase-like domain, hence the DUOX nomenclature. Studies of the DUOX peroxidase-like domain suggest that it neither functions as a peroxidase, nor a superoxide dismutase (SOD), to account for the production of H2O2; as such, the role of the peroxidase-like domain and the complete mechanism of DUOX ROS production remains elusive (91, 92). The first NADPH oxidase discovered, the phagosomal membrane-associated NOX2 (originally gp91phox), shares sequence identity with the other family members as follows: 56% (NOX1), 58% (NOX3), 39% (NOX4), and 27% (NOX5). DUOX isoforms, DUOX1 and 2 are 83% homologous; they share 57% and 43% sequence similarity with NOX2, respectively (9, 23, 42, 127). The structural homologies among the NOX family have hindered both targeted therapeutic intervention and antibody development (3, 32, 72).
FIG. 1.
Classification of the NADPH oxidase family members by ROS generation. Illustration of each NOX/DUOX protein depicts important structural motifs and glycosylation sites; each isoform is categorized by function: (A) superoxide generating enzymes and (B) hydrogen peroxide generating enzymes. The known number of variants and the amino acids predicted to comprise each structural domain are listed (amino acid assignments were generated by Jpred3 secondary structure and TMHMM 2.0 servers). Putative TM domains (white tubes), which bind two heme molecules, and cytosolic EF-hand and FAD/NADPH binding domains (light gray rectangles) are displayed; each DUOX protein contains an N-terminal extracellular peroxidase-like domain (dark gray rectangles). DUOX, dual oxidase; ROS, reactive oxygen species.
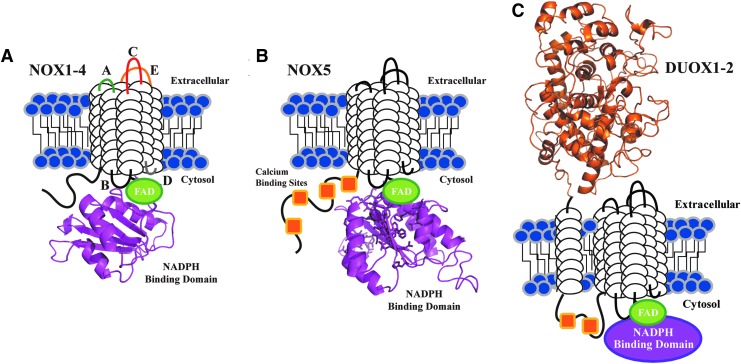
FIG. 2.
Schematic view of the conserved structural features of the NADPH oxidase family. Each NOX/DUOX isoform contains six putative TM domains (white cylindrical loops), with C-terminal FAD (green) and NADPH binding domains (purple). The NADPH binding domain structural models were created by the SWISS-MODEL program server with hNOX2 (PDB: 3A1F) as the template. A model of the peroxidase-like domain of DUOX2 is also highlighted and was created as previously reported (92); all models were visualized by Pymol software. (A) NOX1-4 isoforms are depicted with loop regions labeled based on established designations: extracellular loops A (green), C (red), E (yellow) and intracellular loops B (black) and D (gray). (B) Each NOX5 isoform contains four N-terminal calcium binding sites (orange squares); the hNOX2 and hNOX5 NADPH binding domains share 36% amino acid sequence identity. Amino acids displayed as sticks represent the predicted NADPH binding site residues. (C) DUOX1-2 are unique to the NADPH oxidase family, as both isoforms contain an extracellular N-terminal peroxidase homology domain (orange) tethered to a TM domain, and two cytosolic calcium binding sites.
ROS production by NOX members is regulated in response to interactions with a series of cytoplasmic and membrane-associated proteins (Table 1), as well as chemical stimuli such as calcium or phorbol 12-myristate 13-acetate. Phosphorylation events trigger the formation of an activated NOX enzymatic complex for isoforms 1–3 (19, 98). With the exception of NOX5, which to date has no known interaction partner essential for activity, all remaining NOX homologues (NOX1–4) form a heterodimer with p22phox, a small membrane-bound association factor required for complex formation and stability (78, 151). NOX1, NOX2, and NOX3 organize through recruitment of further cytosolic regulatory subunits p47phox (or the related NOX organizer 1 or NOXO1), p67phox (or the related NOX activator 1 or NOXA1), and the GTPase Rac isoforms (predominantly Rac1 associates with NOX1 and NOX3, Rac2 with the NOX2 enzyme) (1, 7, 65, 131, 133). NOX4 apparently has no other activation requirements, aside from interaction with p22phox, and is therefore constitutively active. The NOX proteins may further enhance their structural stability and/or activity through colocalization with other proteins such as p40phox, tyrosine kinase substrate 4 or 5 (Tks4/5), protein disulfide isomerases (ERp72), Hsp90, c-Abl, or Poldip2 (21, 22, 29, 33, 44, 57, 84, 143). DUOX proteins have a unique protein association with a maturation factor (DUOXA) required to afford trafficking from the endoplasmic reticulum (ER) to the plasma membrane, where a functional membrane complex is formed (46). This association is known to be preferential for DUOX1–DUOXA1 and DUOX2–DUOXA2; DUOXA can cross-function in supporting DUOX membrane transport, however, mismatched associations promote cell line-dependent variability in the level and specificity of membrane targeting and ROS production (superoxide vs. H2O2) (83, 95). Complex formation, organization, and characterization of individual association partners, briefly outlined above, have previously been extensively reviewed (13, 77).
Table 1.
Characteristics of the NADPH Oxidase Family
| Enzyme | Interaction partner(s)a | Cellular localization | Major tissue distributionb |
|---|---|---|---|
| NOX1 | p22phox, NOXA1/p67phox NOXO1/p47phox, Rac, Tks4/5, PDI (ERp72), Hsp90 | Plasma membrane caveoli, lipid rafts | Colon epithelium |
| NOX2 | p22phox, p67phox, p47phox, Rac, p40phox, Hsp90 | Plasma membrane | Phagocytes |
| NOX3 | p22phox, NOXA1/p67phox, NOXO1/p47phox, Rac | Plasma membrane | Inner ear |
| NOX4 | p22phox, Poldip2, Tks4/5, PDI | Plasma membrane, ER, mitochondrial and nuclear membranes | Kidney, ovary, brain |
| NOX5 | Hsp90, c-Abl | Plasma membrane, ER, nuclear membrane | Spleen, testis, lymph node |
| DUOX1 | DUOXA1, TPO, EFP1, NOXA1 | Plasma membrane | Thyroid, lung, prostate, testis |
| DUOX2 | DUOXA2, TPO, EFP1, NOXA1 | Plasma membrane, ER, vesicles | Thyroid, salivary gland, colon, pancreas |
The epithelial NOX/DUOX family members are recognized to be widely distributed from the kidney, colon, and thyroid tissues to the brain and inner ear (68) (Table 1). Many cell lines have been found to express more than a single NOX/DUOX protein, complicating research efforts. In vascular smooth muscle cells, NOX1 and NOX4 are the predominant members expressed (73). NOX2 and NOX4 play a role in regulating kidney function; both DUOX isoforms are highly expressed in the thyroid (28, 31, 118).
Whereas tissue distribution varies greatly, cellular localization is somewhat less diverse. All NOX proteins exist as transmembrane species and have been found, to varying degrees, at the plasma membrane surface. Intracellular localization has been characterized for NOX1, NOX4, and NOX5 in such structures as trafficking vesicles, the ER, mitochondria, or nuclear membranes (40, 45, 51, 63). The effect of the specific ROS, superoxide (NOX1/5), or H2O2 (NOX4/DUOX), on the intracellular environment, including the potential for genomic instability, will be discussed in the framework of each enzyme and its relevant tumor biology.
To date, the role of the NOX/DUOX family in the context of cancer biology has primarily been examined using human tumor cell lines. These cell lines, however, are known to differ significantly in gene expression levels from patient tumors, because they rapidly adapt to cell culture in vitro by upregulating genes involved in cellular proliferation (107, 115). Decades of usage, as well as contamination, have promoted loss of many of their initial gene expression characteristics (41, 67, 89, 117, 153). Sandberg and Ernberg have created a tissue similarity index to help guide investigators toward the best cell lines for experimentation within 10 important cancer cell types, by direct comparison of gene expression levels in existing cancer cell lines to those in both normal and tumor tissues (116). This study demonstrated what many had already reported, that while cell lines are useful cell biology tools, they have many limitations. Their application should be accompanied by the perspective that substantial differences exist between cell lines and tumors originating from the same tissues. Progress in understanding the role of the NOX family in tumor biology awaits movement toward in vivo tissue/tumor studies and animal models. However, difficulties in developing well-characterized, NOX-specific antibodies have also hindered the conduct of in vivo investigations. The recent development of NOX5 and DUOX monoclonal antibodies should broaden the scope of NOX/DUOX research (3, 145).
This review will focus on the evolution of investigative efforts in the field to study the role of the NOXs and DUOXs in cancer, profiling progress from cell line experiments to in vivo tumor studies. This direction is important to define the relevance of these enzymes and ROS in cancer biology and to evaluate how the development of novel therapeutic agents targeting this enzyme family could benefit cancer patients. Of the known epithelial proteins to be addressed, discussion will focus on the NOX1, NOX4, NOX5, and DUOX1/2 isoforms. The utility of bioinformatics approaches to direct investigative efforts with existing platforms of tumor tissue gene expression and cell line data in the context of the NOX proteins will also be reviewed.
NADPH oxidases: from cell lines to tumors
NOX1 (colon cancer)
Originally discovered during investigation of the human colon cancer cell line Caco2, NOX1 is expressed throughout the colonic epithelium (127). NOX1 protein levels vary according to anatomic localization along the gastrointestinal tract, with a general increase in enzymatic expression from small intestine to transverse, descending, and sigmoid colon (74). The functional role of NOX1-related superoxide production at the epithelial surface of the colon may extend beyond acknowledged host defense activities (43, 62, 76), through development of a mitogenic environment from intracellular ROS exposure, especially in cases of chronic inflammation.
Intracellular ROS production is known to promote base oxidation, cross-linking between DNA and proteins, and induction of DNA strand breaks. Oxidation of guanine (G) is commonly observed, due to its low nucleotide redox potential. These alterations are among the most prominent somatic mutations in lung, breast, ovarian, gastric, and colorectal cancers (135). The potential of human NOX1 to contribute to genomic instability was first characterized using active overexpression model systems in both HeLa cells and mouse embryo fibroblasts. Chronic oxidative stress by NOX1-induced ROS was shown to promote significant DNA damage (formation of 8-oxoGua); interestingly, no changes in the steady-state levels of DNA single-strand breaks were detected. The mechanism(s) underlying base damage remain to be clarified (24).
Beyond genomic instability, a novel connection to the proapoptotic tumor suppressor p53 has been described for the NOX1 family member. HIPK2, a transcriptional corepressor, which controls p53 function through phosphorylation and acetylation events, was shown to upregulate NOX1. In turn, NOX1 inhibited p53 Lys382 acetylation, a target of the deacetylase sirtuin 1 (SIRT1), responsible for deacetylation and inactivation of p53. This consequently impaired transcriptional induction of proapoptotic genes (110, 136). SIRT1 deacetylates a wide range of protein substrates, and has been implicated in chemotherapeutic resistance; therefore, the link to NOX1 could be important to many cancer-related biological outcomes (102).
Despite these investigations, debate continues regarding the role of NOX1 in colon carcinogenesis. One comparative study of NOX1 expression in adenomas and colonic adenocarcinomas demonstrated no statistical difference in expression levels between normal and malignant tissues (129). Subsequent studies, however, have detected significant NOX1 overexpression in precancerous tubular and villous adenomas as well as in moderate and well-differentiated adenocarcinomas (39, 58). In support of a role for NOX1 in colon carcinogenesis, activating mutations in the proto-oncogene K-Ras (codons 12 and 13) were discovered in concert with NOX1 overexpression in human colon cancers. Eighty percent of tumors with these mutations demonstrated a twofold or greater elevation in NOX1 mRNA versus normal tissues (74). Still, it remains unclear whether NOX1 is required for carcinogenesis, and whether NOX1 expression levels change during the transition from premalignant to advanced stage cancer (129).
Understanding the importance of NOX1 overexpression during tumorigenesis continues to be an area of active investigation. It has been suggested that NOX1-related ROS contribute to the development of inflammation-associated colonic malignancies. Nuclear factor-κB (NF-κB), a critical regulator of inflammation and cancer development, has been proposed as the link between NOX1 and the development of colon cancer (34, 85). Increased NOX1, and p50 and p65 (members of the NF-κB family) expression in colonic mucosa from rats exposed to a heterocyclic amine carcinogen, before formation of frank tumors, suggests a role for NOX1 in the initiation phase of PhIp-induced cancer (6, 39, 139). These experiments support the hypothesis that enhanced production of ROS, through increased NOX1 expression, activates NF-κB signaling, which could contribute to tumor initiation.
Beyond cancer initiation, the presence of an active NOX1 complex in advanced cancers suggests that NOX1-dependent ROS could support signaling pathways that promote colon cancer proliferation. Consistent with a role in growth, ROS production in colon tumor-derived cells expressing NOX1 is higher in subconfluent than in confluent (arrested) cells. ROS generation in this situation was suggested to be NOX derived by the use of a flavoprotein inhibitor, diphenylene iodonium (DPI), which blocked the NOX activity (106). NOX1-mediated ROS production has also proven essential for activation of the Wnt-β-catenin pathway involved in cell proliferation through nucleoredoxin oxidation and subsequent disruption of the nucleoredoxin dishevelled complex (60).
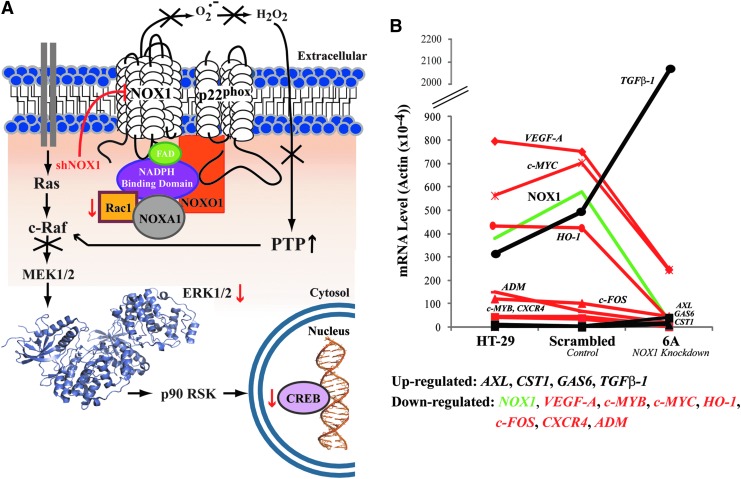
Further support for a functional NOX1 role in colon carcinogenesis was found by stable shRNA knockdown of NOX1 in HT-29 human colon cancer cells (59). Diminished constitutive ROS production was achieved as a result of significantly decreased NOX1 expression (80–90%). An accompanying G1/S block responsible for a two- to threefold increase in tumor cell doubling time, without increased apoptosis and changes in tumor cell morphology, was also observed. This block in cell cycle transition was related to a steady-state decrease in the phosphorylation of other mitogen-activated protein kinase (MAPK) signaling pathway enzymes (Rac1, ERK1/2, CREB), and concomitant downregulation of cyclin D1. With the activities of protein tyrosine and serine/threonine phosphatases enhanced by a decrease of intracellular oxidant, a known phenotype (66, 103), the observed MAP kinase pathway changes may be the downstream result of c-Raf inactivation through phosphatase binding (unpublished results, Fig. 3). No changes in the AKT or p38MAPK pathways were noted; microarray analysis found that NOX1 silencing was also associated with downregulation of several oncogenes, chemokines, and angiogenic factors. Corroborative in vivo studies provided direct comparison of gene expression levels, tumor growth, and angiogenesis for HT-29 xenografts from parental HT-29 cells versus knockdown cell xenografts. A significant decrease in tumor growth and angiogenesis was observed when NOX1 expression was attenuated; concomitant alterations in the expression of genes essential for proliferation were also observed in vivo following NOX1 knockdown (Fig. 3) (59). Interestingly, decreased NOX1 expression, and accordingly intracellular ROS, enhanced the expression of the growth inhibitor transforming growth factor β-1 (TGFβ-1) fourfold above scrambled control, consistent with a previous investigation (144).
FIG. 3.
Insights from in vitro and in vivo studies of NOX1 in HT-29 cells. (A) Schematic representation of the active NOX1 enzymatic complex and MAP kinase components affected by NOX1 expression levels. Stable shRNA knockdown of NOX1 in HT-29 cells results in a block in G1 phase of the cell cycle related to a decrease in the phosphorylation of Rac 1, ERK 1/2 (PDB: 2ZOQ, ERK 1), and CREB (red arrows). A subsequent decrease in ROS production and increase in the level of PTP was also observed; immunoprecipitation demonstrated phosphatase binding to c-Raf, providing a potential mechanism of MAP kinase inhibition. (B) mRNA expression levels of genes related to cell proliferation, angiogenesis, and invasion identified in tumor xenografts from HT-29 cells, cells stably transfected with scrambled shRNA, and NOX1 knockdown cells. Genes identified as either upregulated (black) or downregulated (red) in response to the expression of NOX1, as measured by real-time reverse transcriptase-polymerase chain reaction, are plotted [accession number GSE4561 (59)]. PTP, protein tyrosine and serine/threonine phosphatases; TGFβ-1, transforming growth factor β-1.
Whereas studies of NOX1 have mainly addressed its role in colon cancer, a role for NOX1 has also been suggested in gastric, prostate, and breast carcinomas, as well as melanoma (25, 79, 81, 110, 132). The significance of NOX1 expression in these tumors is speculative; further development of specific NOX1 antibodies may clarify the prevalence of NOX1 in these diseases.
NOX4 (glioblastoma, melanoma, renal, and ovarian cancer)
As the only H2O2-liberating NOX enzyme, NOX4 is distinguished not only by its ROS generation, but also by its lack of known protein partners beyond p22phox or required calcium stimulus for functionality. Constitutive activity, coupled with a pattern of both intracellular and plasma membrane associations, has been used to support the potential for NOX4 to produce genomic instability (45, 108). There has been considerable interest in the relationship of NOX4 to carcinogenesis over the past 5 years (142). Increased NOX4 expression has been found prominently in tumors of the brain, breast, ovary, pancreas, and kidney, as well as melanoma. However, in depth investigations have been hindered by the lack of a widely available, specific NOX4 monoclonal antibody and transgenic animal models for investigations of the role of NOX4 in tumor biology (45, 86, 94, 124, 141, 148, 149). Three different transgenic NOX4 overexpression mouse models have been published; one endothelial-specific system and two cardiomyocyte-specific systems (2, 112, 152). Interestingly, the endothelial tgNOX4 mouse had a lower systemic blood pressure compared to wild-type litter mates. This may be due to a bystander effect on p22phox, or perhaps, nonphysiologically overexpressed NOX4 localizes differently than endogenous proteins. Whereas overexpression of NOX4 in this mouse model was not noted to promote carcinogenesis, the development of more tumor-specific animal models is required to afford a proper insight into the role of NOX4 in cancer biology.
The initial suggestion that NOX4-derived ROS had an impact on the development of the most aggressive form of brain tumor, glioblastoma multiforme (GBM), originated from the demonstration of increased NOX4 expression levels in cultured glioma cells. NOX4-mediated ROS inhibition has also been shown to decrease the formation of invadopodia (phosphotyrosine-rich structures involved in glioma motility) (29). The in vivo relevance of NOX4 in GBM was validated by detection of high levels of NOX4 expression in both GBM cell lines (U87, KNS81, and KNS42), and in surgically resected human gliomas (124). Targeted knockdown of NOX4 by dsRNA in KNS81 and KNS42 cells promoted morphological changes within 48 h of transfection, including changes that were not similarly observed after DPI treatment. Retardation of cell growth and increased vulnerability to apoptosis from the chemotherapeutic agent cisplatin were also observed following NOX4 downregulation, through an unknown pathway. Immunohistochemical analyses, performed with a polyclonal antibody raised against the C-terminus of NOX4 (amino acids 559–578), suggest that NOX4 expression is increased in high-grade tumors. Reverse transcriptase-polymerase chain reaction (RT-PCR) evaluation demonstrated an increase in the expression of NOX4 with increasing tumor stage; grade IV GBM tumors demonstrated significantly higher NOX4 levels than either grade III (anaplastic astrocytoma) or grade II (diffuse astrocytoma) cancers. NOX4, therefore, may serve as a marker of adverse prognosis for patients with brain tumors.
Regulation of growth in melanoma cells by NOX4 has been suggested since 2002 (17). Yamaura et al. found by RT-PCR that 13 melanoma cell lines demonstrated significant NOX4 gene expression. Of these, MM-BP cells were chosen for focused experiments because no significant expression of other NOX isoforms could be demonstrated (149). DPI exposure and siRNA knockdown produced growth inhibition. This was accompanied by a decrease in intracellular ROS and anchorage-independent growth, indicating that NOX4-generated ROS were required to sustain melanoma development. MM-BP cells transfected with scrambled or NOX4-specific siRNAs produced tumors within 2 weeks of subcutaneous injection in athymic mice; however, significantly decreased tumor volumes were observed in the xenografts produced from NOX4 knockdown cells. Flow cytometry of melanoma cells depleted of ROS by DPI exposure, catalase treatment, or NOX4 siRNA knockdown resulted in a G2/M cell cycle arrest, suggesting that NOX4-derived ROS promote progression across the G2/M boundary. Dephosphorylation of CDK1 (cdc2) kinase is required for the transition from G2 to M phase. NOX4-mediated ROS production appears to increase the phosphorylation of CDK1, contributing to the G2 block. Further investigation is needed to determine the molecular signals connecting NOX4-induced ROS production and CDK1 phosphorylation.
NOX4 was first isolated from human fetal kidney cDNA; immunostaining of the human kidney cortex has established that abundant NOX4 expression occurs in distal renal tubular cells (23, 123). NOX1 and NOX4 are both expressed in renal cell carcinomas (RCCs); however, NOX4, rather than NOX1, appears to be upregulated in RCC lines when compared to normal cells of the kidney (15). RCC has been associated with inflammation, through observed upregulation of hypoxia inducible factor 1-alpha (HIF-1α), a component of the heterodimeric HIF-1 complex (HIF-1α and HIF-1β). Biallelic inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene is also linked to RCC formation through a hypoxic response. Mutations in VHL occur in ∼80% of RCCs; VHL-mediated degradation of HIF is significantly inhibited in the case of VHL mutation, producing increased levels of HIF proteins and their downstream targets (69). A link between NADPH oxidases and HIF proteins in the context of RCC was noted when VHL-deficient cells (786-O cell line) were found to downregulate HIF-2α expression after DPI treatment or stable NOX4 knockdown when compared to a normal kidney (15). Furthermore, NOX4 expression, under control of glycolytic intermediates, may be related to the production of the proinflammatory cytokines interleukin (IL)-6 and IL-8 in kidney cancer cells during hypoxia (30, 36).
Similar to renal cancer, the NOX4 isoform is the predominant NOX found to have increased expression in ovarian cancer cell lines; markedly higher NOX4 mRNA levels have been found in both OVCAR-3 and A2780 cells relative to immortalized normal ovarian cells (148). To determine whether NOX4-mediated ROS production plays a role in ovarian cancer-related angiogenesis through HIF-1 expression, OVCAR-3 cells were subjected to hypoxia, with a resultant upregulation of HIF-1α; DPI inhibited the upregulation of HIF-1α expression, without changing HIF-1β levels. Identification of the specific ROS, which affect HIF-1α expression, was determined using specific ROS scavengers (SOD, catalase, and sodium formate). Only exposure to catalase decreased HIF-1α expression, suggesting that H2O2 was responsible for enhancing HIF-1α levels. In vivo, DPI treatment decreased the growth of OVCAR-3 cell xenografts; adenoviral transduction of OVCAR-3 cells with catalase abolished H2O2 production and reduced tumor angiogenesis by 80%. Importantly, evaluation of ovarian tumor tissues has provided support for the relevance of NOX4 expression. Immunohistochemical staining of human ovarian tumors found that 63% expressed NOX4; however, no correlation could be demonstrated between NOX4 expression and tumor grade (45).
Recently, a role has been suggested for NOX4-related ROS in ovarian cancer metastasis and invasion. BLT-2, a low-affinity leukotriene B4 receptor, plays a critical role in the metastasis of ovarian cancer cells through STAT3 activation and related upregulation of matrix metalloproteinase 2 (MMP2). BLT-2 is highly expressed in the ovarian cancer cell lines OVCAR-3 and SKOV-3; when NOX4 siRNAs were transfected into these cells, STAT3 activation and MMP2 expression were diminished, decreasing the invasive properties of the cells. In SKOV-3 xenografts, treatment with a BLT-2 inhibitor significantly reduced the number of metastatic nodules by 60% (120).
NOX5 (prostate cancer, hairy cell leukemia, and esophageal cancer)
Discovery of a unique, calcium-regulated NOX protein was first reported in 2001 from mRNA transcripts in the human spleen, testis, and kidney (9, 23). Currently, there are six identified human splice variants of the NOX5 enzyme (α, β, γ, δ, ζ, and a truncated variant NOX5-S or ɛ); isoforms α and β are the most abundantly expressed in cells, and have been established as functional superoxide generating enzymes. Interestingly, in esophageal cancer cells, NOX5-S is the dominant isoform. Reports of the activity level of this N-terminal truncation, devoid of calcium stimulatory ability, have varied between nonfunctionality and basal level ROS production (9, 38, 104, 125). For the remaining NOX isoforms, γ, δ, and ζ activity has not yet been reported (104).
Studies on the relevance of NOX5 in tumor biology have been limited, in part, because of the absence of NOX5 in the genome of rodents and a lack of reliable immunological tools. The first report of the possible involvement of NOX5 in cancer was in 2003, where Brar et al. implicated NOX5 in the regulation of the growth and apoptosis of DU145 prostate cancer cells (16). However, three independent studies report the absence of detectable NOX5 mRNA in DU145 cells; thus, questions arise regarding the relevance of NOX5 in the proliferation of this cell line (10, 56, 58). In contrast, significant levels of NOX5 have been reported in PC-3 human prostate cancer cells. Detection of NOX5 in PC-3 cells coupled with the observation that NOX5 expression is upregulated by sterol regulatory element-binding protein-1 both in vitro and in vivo in the context of prostate cancer progression, does implicate a proliferative role for NOX5 in prostate cancer (54). There have been two other detailed studies of NOX5 in the context of tumor biology; in 2005, the expression of NOX5 in hairy cell leukemia was reported to regulate constitutive phosphorylation signals mediating proliferation, and in 2006, acid-induced NOX5-S expression was reported to contribute to increased cell proliferation and decreased apoptosis in Barrett's esophageal adenocarcinoma cells (38, 61).
Although there is a growing base of information regarding NOX5 regulation, signaling, and various biological functions, the role of NOX5-generated ROS in tumor biology is still largely unexplored (12). Recently, advances have been made in the development of a validated mouse monoclonal antibody against NOX5 and the characterization of NOX5 expression in human tumors and tumor cell lines (4). Raised against a truncated recombinant NOX5 protein (amino acids 600–746), sequence conservation over the antigenic C-terminal region provided a novel antibody that detects all isoforms of the NOX5 protein. Analysis of human tumor microarrays by immunohistochemistry with this tool has revealed high levels of NOX5 expression, with a frequency of intermediate to strong expression of 57–70% for several human tumors evaluated, including prostate and ovarian cancers. Although the relevance of NOX5 in tumors still remains unclear, the detection of elevated expression of NOX5 in tumor specimens provides an impetus for further exploration of the functional significance of NOX5 in the context of cancer development and progression.
DUOX1/2 (thyroid, lung, and pancreatic cancer)
H2O2-generating enzymes, DUOX1 and DUOX2, coexist in thyroid tissue, where they were first discovered to provide ROS for oxidation of iodine (by way of thyroid peroxidase [TPO]). Oxidized iodine is, in turn, covalently linked to available tyrosine residues in thyroglobulin, subsequently crosslinking tyrosines to form the thyroid hormone (both T3 and T4) (99). Analysis of DUOX expression in the thyroid has demonstrated that localization occurs at the apical membrane, with positive staining for DUOX in 40–60% of thyrocytes (20).
Studies of the role of DUOXs in carcinogenesis began when isolated thyroid carcinomas were first analyzed by RT-PCR for variation in DUOX gene expression by Lacroix et al. in 2001 (71). Analysis of both DUOX isoforms demonstrated no distinct trend toward gene upregulation or downregulation versus normal tissue. TPO, on the other hand, was observed to be downregulated in nine of ten cancers compared to matched normal tissues. Analysis at the protein level was more compelling; immunohistochemical staining of 86 primary carcinomas revealed positive DUOX expression in 67 (78%). In 29 of the samples with a high number of positive follicular cells, DUOX immunostaining was found at both the apical membrane and in the cytoplasm. This shift in protein distribution implies improper targeting or destabilization of the DUOX enzyme. The DUOX protein was found in 43 of 50 primary tumors with metastases (86%), and radioiodine uptake was observed for 37 of these 43 DUOX-positive tumors. H2O2 exposure from DUOX expression in thyroid cancer cells may enhance DNA damage, contributing to genetic instability in thyroid cancer (26).
The two DUOX isoforms demonstrate 83% homology in amino acid sequence; this has contributed to the inability to generate a monoclonal isoform-specific antibody. DUOX polyclonal isoform-specific antibodies were first reported in 2009 (83). Thus, the aforementioned immunohistochemical studies measured both DUOX1 and DUOX2, considering that both isoforms are expressed at significant levels in the normal thyroid. Evidence suggests that DUOX2 may provide most thyrocyte oxidase activity, because DUOX2 mono- or biallelic inactivating mutation(s) are linked to congenital hypothyroidism, a phenotype not rescued by DUOX1 (96, 101, 109, 138). However, DUOX1 is the main H2O2 source in the rat thyroid line PCCl3, suggesting that factors such as differential cellular localization or alternate activation mechanisms may prohibit DUOX1 substituting for DUOX2 in the human thyroid (113). Indeed, in vitro studies have shown that DUOX isoforms are differentially regulated by separate phosphorylation pathways; DUOX1 is activated by protein kinase A (Gs-PKA pathway), whereas DUOX2 is stimulated by protein kinase C (Gq-phospholipase C pathway) (114). Recent insights into DUOX transcriptional regulation have also favored DUOX2 through upregulation under inflammatory conditions. Raad et al. demonstrated that both DUOX2 and DUOXA2 genes were upregulated upon thyrocyte exposure to T helper (Th) 2 cytokines IL-4/IL-13, an effect that correlated with an increase in protein and extracellular H2O2 production (111). No effect on DUOX1/DUOXA1 was found. Therefore, while the Lacroix study was limited to demonstrating a relationship between DUOX expression and thyroid cancer, it seems likely that human cancer-related effects from ROS production may relate predominantly to DUOX2, although a contribution from DUOX1 cannot be ruled out.
As the dominant isoform expressed in the airway epithelium, DUOX1 has been linked to lung development, differentiation, mucus production, and host defense. DUOX2 is also present in lung epithelial cells, but at a significantly lower level of mRNA expression than DUOX1 (37, 134). These expression levels were perturbed upon treatment with Th1 (interferon-gamma [IFN-γ]) and Th2 (IL-4/IL-13) cytokines, with modest DUOX1 (Th2 related) and significant DUOX2 (Th1 related) mRNA increases, validated at the protein level by H2O2 production assay (50). DUOXs localize to the leading edge of migrating cells as functional calcium-activated complexes with a distinct distribution on the apical side of a differentiated lung epithelium. Overexpression of DUOX1-DUOXA1 in DUOX-deficient NCI-H661 lung cancer cells revealed that DUOX1 is present at the edge of plasma membrane protrusions (colocalized with DUOXA1). Expression of DUOX2 and its partner maturation factor DUOXA2 in the same cells led to enzymatic localization at the ER and intracellular structures (trafficking vesicles), with infrequent presentation at the plasma membrane (83).
Investigation of lung cancer cell lines and primary tumors demonstrated a consistent loss of DUOX gene expression. Whereas abundant in normal or immortalized lung epithelial cells, DUOX1 expression was lost in 75% of lung cancer cell lines screened, including A549, NCI-H661, NCI-H157, NCI-H441, and SHP-77 cells (82). DUOX2, DUOXA1, and DUOXA2 expression levels were also decreased from those in normal cells; DUOX2 and DUOXA2 are, however, routinely expressed at significantly lower levels in the lung than DUOX1. Recovery of DUOX1 expression was increased by treatment with the DNA methyltransferase inhibitor 5′-aza-2′-deoxycytidine in several lung cancer cell lines, suggesting that DUOX gene silencing is regulated through aberrant promoter hypermethylation. This observation is consistent with the presence of CpG islands, sites for potential methylation, in the promoters of both the DUOX1 and DUOX2 genes. Methylation was confirmed through bisulfate sequencing, at levels ranging from dense DUOX1 promoter hypermethylation in A549 and NCI-H661 cells, to 30–35% coverage of CpG sites in NCI-H157 cells; normal primary lung epithelial cells demonstrated insignificant methylation. Modest methylation was also noted for the DUOX2 promoter and was highest in A549 cells. These results were translated to primary tumors derived from non-small-cell lung cancer patients. Real-time PCR with DUOX-specific primers revealed a statistically significant reduction of DUOX1 expression in tumor tissue versus normal lung in 9 of 11 patients. Of these tumors, five that demonstrated significant DUOX1 downregulation were chosen for further study; promoter hypermethylation was detected in all five cases. Stable expression of the DUOX1 and DUOXA1 mRNAs in A549 and NCI-H157 lung cancer cells permitted calcium-dependent H2O2 production, which promoted greater cell migration, but showed no effect on proliferation. Because DUOX1 has been linked to airway host defense and wound healing, silencing of this gene could increase inflammation-related pre-neoplastic tissue injury (often triggered by inhalation of foreign substances) (140).
Recent studies have suggested that DUOX enzymes may be involved in the etiology of pancreatic cancer. Pancreatic inflammation plays a critical role in the development and progression of pancreatic tumors; experimentally, pancreatic cancer cells generate higher concentrations of ROS than respective normal cells (35, 48, 75, 88, 150). IFN-γ, a soluble proinflammatory cytokine, stimulates ROS production in human neutrophils and macrophages by upregulation of gp91phox and p67phox NADPH oxidase components (70). IFN-γ dramatically increased both DUOX2 and DUOXA2 mRNA expression in BxPC-3 and AsPC-1 pancreatic cancer cell lines without altering the expression of other NADPH oxidase family members. A newly characterized DUOX monoclonal antibody, developed against a truncated peroxidase-like domain antigen, confirmed that this increase in gene expression translated to the protein level (145). Both intra- and extracellular calcium-dependent ROS production followed IFN-γ-related increases in DUOX2-DUOXA2 expression, suggesting that a fully functional DUOX2-DUOXA2 enzymatic complex was formed following IFN-γ exposure. Enhanced DUOX2 expression was demonstrated to be the result of Stat-1 binding to a putative Stat recognition site in the DUOX2 promoter.
The role of Toll-like receptor 4 (TLR4), a known target of bacterial lipopolysaccharide (LPS), in the upregulation of DUOX2 in pancreatic cancer cells was evaluated in a subsequent study (147). Exposure to both IFN-γ and LPS synergistically upregulated the expression of DUOX2 and DUOXA2 in BxPC-3 cells, and increased intra- and extracellular H2O2 production by way of TLR4. DUOX2 expression was also markedly upregulated in BxPC-3 cell xenografts in the absence of cytokine treatment, presumably as a result of the proinflammatory milieu of the tumor microenvironment. Patients with chronic pancreatitis were also found to have increased DUOX expression adjacent to areas of inflammatory cell infiltrates by immunohistochemistry, further supporting a role for inflammatory stimuli in the regulation of DUOX2 expression (147).
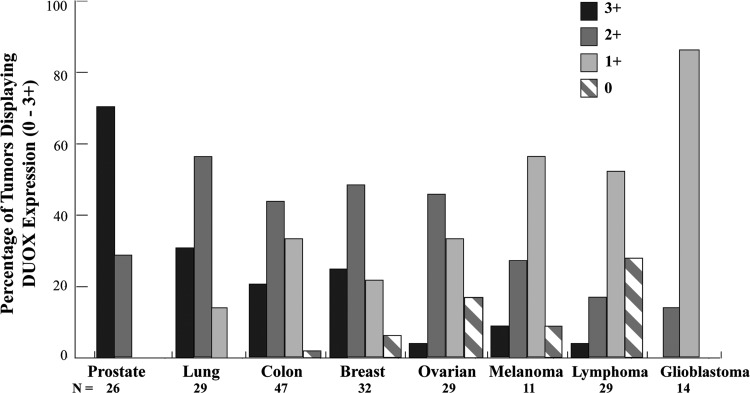
Immunohistochemical examination of a human tumor panel in related studies has found expression of DUOX to be highest in prostate adenocarcinomas; greater than 60% of breast and colon cancers also demonstrated high level DUOX expression (strong+intermediate staining, Fig. 4) (145). Expression of DUOX2 was observed to be significantly increased in human colon biopsies and isolated intestinal epithelial cells from patients with chronic inflammatory conditions that are predisposed to cancer, Crohn's disease, and ulcerative colitis, compared to adjacent normal colon mucosa (80). DUOX2 is also upregulated 10-fold in premalignant adenomatous polyps versus adjacent mucosa by RNA microarray analysis as well as in some surgically resected colon cancers (58, 64). These recent observations in human tumors suggest that further study of DUOX2 in colon adenocarcinoma is warranted.
FIG. 4.
Distribution of DUOX expression levels in tumors from a multitumor tissue microarray (TARP MTA-3). DUOX expression was studied by immunohistochemistry in 217 tumor samples as well as negative control tissues. Staining of DUOX protein occurred in a cytoplasmic pattern and was scored on a scale from 0 to 3 as follows: negative stain (0, stripes), weak staining (1+, light gray), intermediate staining (2+, dark gray), and strong staining (3+, black). Bar graphs depict the staining percentage achieved for each tumor type at each expression level, with the total number of samples (N) evaluated for each cancer listed. DUOX expression was weakly positive in normal bone marrow, pancreas, and stomach tissues, while negative in other normal tissues evaluated, including the brain, liver, lung, small bowel, and testis. Distribution of positive staining was statistically different between tumor types, p<0.01.
Bioinformatics tools for the study of NOX family members in cancer
In the context of cancer biology, where prohibitive cost may preclude many laboratories from pursuing in vivo studies, bioinformatics tools provide access to gene expression profiles and patient-specific tumor data that would otherwise be unavailable (49, 93, 119, 137). Large-scale clinical data sets, coupled with high-throughput analytical techniques, can also afford a perspective focusing on gene networks or pathways rather than individual genes (97). Evaluation of difficult biological systems, where lack of specific antibodies, subtle tumor versus normal tissue gene expression variations, and broad tissue distribution are hindrances, may greatly benefit from large-scale screening and predictive tools to define in vivo relevance. Hence, current bioinformatics platforms may advance NOX/DUOX research through insights drawn regarding gene expression in both cancer cell lines and human tumors. Outlined below are databases that have provided some initial insights regarding NOX/DUOX enzymes in oncology.
The Cancer Cell Line Encyclopedia (www.broadinstitute.org/ccle)
A large-scale genomic dataset for 1047 human cancer cell lines from 36 tumor types, together with pharmacologic profiling of tumor cell line sensitivity for 24 anticancer compounds, was produced by the Broad Institute in collaboration with the Novartis Institutes for Biomedical Research (10). As an ongoing project, the collection of gene expression, chromosomal copy number, and parallel sequencing data is constantly growing.
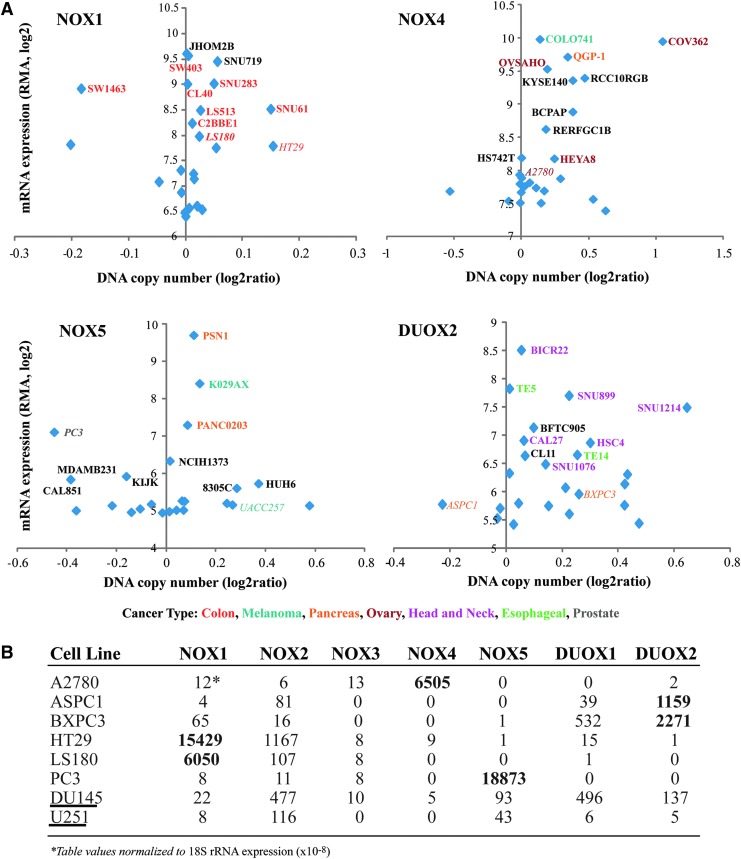
Analysis of this database for the NOX enzymes resulted in profiles for NOX1, NOX4, NOX5, and DUOX2, relating mRNA expression levels to specific cancer cell lines (Fig. 5). The top 25 cell lines with the highest gene expression for each protein were graphed; the names for the top 10 cell lines, plus literature relevant examples, are displayed. Color association by tumor type helps to illustrate correlations to specific NOX family members; for NOX1, 8 of 10 cancer cell lines with the highest expression levels are from the colon, while 6 of the top DUOX2 cell lines are head and neck cancers. Correlation of several cell lines to published expression values is also demonstrated; HT-29 cells have significant NOX1 mRNA, and have been studied extensively at the protein level and in xenograft models, while A2780 cells possess high NOX4 mRNA expression levels relative to normal ovarian cells and demonstrate increased ROS production (59, 148). These investigative observations aid in validating the information available from this database; however, no direct estimate of the translation of mRNA to protein can be drawn. This must be evaluated on a case by case basis. Interestingly, two cell lines of questionable oxidase-specific cancer relevance, DU145 (prostate cancer and NOX5) and U251 (GBM and NOX4) were not found by the Cancer Cell Line Encyclopedia (CCLE) screen to have significant levels of either NOX protein (Fig. 5) (16, 53).
FIG. 5.
Comparison of mRNA expression levels for NOX/DUOX enzymes in cell lines acquired and analyzed by the Broad Institute CCLE. (A) The top 25 cell lines showing the highest expression levels of NOX1, NOX2, NOX5, and DUOX2 were graphically presented based on mRNA values, with the names of the top 10 cell lines for each enzyme displayed (bold). The cell names are further highlighted by a color code to portray the pattern of cancer types associated with each enzyme. Cancer cell lines listed in italics were evaluated by quantitative polymerase chain reaction for the mRNA levels of NOX and DUOX proteins (B) and/or have been listed as a recommended cell line based on precedent literature (Table 2), demonstrating consistency with the CCLE obtained data [(58) and unpublished results]. U251 and DU145 human tumor lines are highlighted in (B) (underline), demonstrating a correlation with the CCLE results and disparity with the literature, as negligible levels of NOX4 and NOX5 RNA were measured. CCLE, Cancer Cell Line Encyclopedia.
Oncomine (www.oncomine.org)
Originally conceived by scientists at the University of Michigan, Oncomine was first released in 2003, with 40 microarray data sets. As a cancer microarray database, the latest version, Oncomine 4.4.3, provides registered users from the academic and nonprofit cancer research communities free access to data from over 600 gene expression datasets (99, 100). This resource acts as a hub for cell line data from the CCLE (917 cell lines), and the Sanger Institute (www.sanger.ac.uk/, 732 cell lines), and provides the ability to survey over 10,000 gene expression and DNA copy number sets from The Cancer Genome Atlas project. Differential expression analyses comparing many major cancer types to respective normal tissues, as well as clinical outcomes, pathology subtype (cancer stage), and drug sensitivity data are available for exploration. Data can be queried and visualized for a specific gene or multiple genes across a variety of selected analyses. This bioinformatics tool has been successfully used to produce data on numerous cancers, including colon, breast, and prostate adenocarcinomas (11, 47, 90, 128).
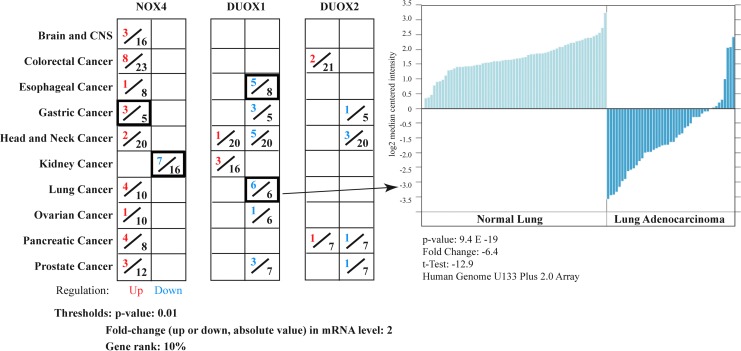
To illustrate the value of the Oncomine database for the study of NADPH oxidases in cancer, differential expression analyses for the H2O2 producing members of the NOX/DUOX family, NOX4, DUOX1 and DUOX2, were performed for cancer versus normal tissues from microarray datasets of primary tumors (Fig. 6). NOX4 was found to be upregulated in nine of the ten cancer types profiled. Interestingly, NOX4 was significantly upregulated in three of the five gastric cancer analyses, a tumor type that has not been previously associated with this NOX isoform; NOX4 was downregulated in 7 of 16 renal adenocarcinoma analyses, whereas RCC cell line-based studies have demonstrated upregulation (15). This suggests that critical differences may exist between the in vitro cell line and in vivo tissue studies in the context of RCC. A trend for DUOX1 downregulation across analyzed tumor types is supportive of the initial epigenetic study of DUOX in lung cancer (82); significant downregulation in both esophageal and lung cancers was noted. One of the six sets of analyses for lung cancer is highlighted in Figure 6, representative of the data available from individual analyses of each tumor type (52). No significant datasets or distinct trend in regulation was found relating DUOX2 expression to cancer under the chosen thresholds.
FIG. 6.
Comparison of analyses of mRNA expression values for cancer versus normal samples of NOX4, DUOX1 and DUOX2 obtained from the Oncomine database. The hydrogen peroxide converting members of the NOX/DUOX family are profiled, with the number of analyses of cancer versus normal tissue demonstrating significant gene upregulation (red) or downregulation (blue) out of total analyses (black) that met the threshold criteria shown across 10 cancer types. One analysis set for DUOX1 expression in lung adenocarcinoma tissue is highlighted [215800_at, (52)], which demonstrates the downregulation of DUOX1 in lung adenocarcinoma.
Conclusions
Research into the biochemical role of NADPH oxidases in the initiation and progression of human cancers is in its earliest stages. Hindered by a lack of available, well-qualified antibodies and in vivo models, as well as crystal structures for targeted inhibitor development, in vitro studies have dominated the field. Definition of in vivo relevance should be the focus of future research efforts, coupled with the refinement of reliable in vitro systems (Table 2). Recent studies of the tumor microenvironment have implicated oxidative stress-related chronic inflammation as a mediator of tumorgenesis, linking cancer to the upregulation of several NADPH oxidases. Further validation of the NOX/inflammation/cancer connection through investigation of DNA damage and studies of gene networks should be pursued, aided by bioinformatics strategies.
Table 2.
Suggested In Vitro and In Vivo Models for Study of hNOX Isoforms in Tumor Biology
| Tumor | Cell line(s) | Xenograft(s) | NOX/DUOX proteins | References |
|---|---|---|---|---|
| Glioblastoma multiforme | U87 | NOX4 | (124) | |
| KNS81 | ||||
| KNS42 | ||||
| Colon cancer | HT-29a | HT-29 | NOX1, DUOX2 | (145–147) |
| LS174Ta | LS-174T | |||
| Caco-2a | ||||
| Lung cancer | NCI-H661 | DUOX1, DUOX2 | (82) | |
| NCI-H157 | ||||
| Melanoma | UACC257a | MM-BP | NOX4, NOX5 | (4, 55, 149) |
| MM-BP | A375 | |||
| Ovarian cancer | OVCAR-3b | OVCAR-3 | NOX2, NOX4 | (45, 120, 148) |
| A2780b | ||||
| SKOV-3 | ||||
| Pancreatic cancer | BxPC-3a | BxPC-3 | DUOX2 | (146, 147) |
| AsPC-1a | AsPC-1 | |||
| CFPAC-1a | ||||
| Renal cell carcinoma | 786-O | 786-O | NOX2, NOX4 | (15, 36, 123) |
| RCC4 | ||||
| KPK13 |
Validated by our laboratory at the RNA and protein levels.
Validated by our laboratory at the RNA level; validated at the protein level in the literature.
PDI, protein disulfide isomerase; RCC, renal cell carcinoma.
Abbreviations Used
- CCLE
Cancer Cell Line Encyclopedia
- DPI
diphenylene iodonium
- DUOX
dual oxidase
- H2O2
hydrogen peroxide
- HIF-1α
hypoxia inducible factor 1-alpha
- IFN-gamma
interferon-γ
- IL
interleukin
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MMP2
matrix metalloproteinase 2
- NF-κB
nuclear factor-κB
- PDI
protein disulfide isomerase
- RCC
renal cell carcinoma
- ROS
reactive oxygen species
- SIRT1
sirtuin 1
- SOD
superoxide dismutase
- TGFβ-1
transforming growth factor β-1
- Th
T-helper
- Tks
tyrosine kinase substrate
- TLR4
Toll-like receptor 4
- TPO
thyroid peroxidase
- VHL
von Hippel-Lindau
Acknowledgments
Thanks are extended to William C. Reinhold and Sudhir Varma for insights into the use of bioinformatics databases. This work was supported by federal funds from the Center for Cancer Research and the Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services.
References
- 1.Abo A, Pick E, Hall A, Totty N, Teahan CG, and Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353: 668–670, 1991 [DOI] [PubMed] [Google Scholar]
- 2.Ago T, Kuroda J, Pain J, Fu C, Li H, and Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106: 1253–1264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, and Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci 69: 2327–2343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antony S, Wu Y, Hewitt SM, Anver MR, Butcher D, Jiang G, Meitzler JL, Liu H, Juhasz A, Lu J, Roy KK, and Doroshow JH. Characterization of NADPH oxidase 5 expression in human tumors and tumor cell lines with a novel mouse monoclonal antibody. Free Radic Biol Med 65C: 497–508, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babior BM, Lambeth JD, and Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys 397: 342–344, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest 107: 241–246, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banfi B, Clark RA, Steger K, and Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem 278: 3510–3513, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, and Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem 279: 46065–46072, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, and Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem 276: 37594–37601, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, Reddy A, Liu M, Murray L, Berger MF, Monahan JE, Morais P, Meltzer J, Korejwa A, Jane-Valbuena J, Mapa FA, Thibault J, Bric-Furlong E, Raman P, Shipway A, Engels IH, Cheng J, Yu GK, Yu J, Aspesi P, Jr., de Silva M, Jagtap K, Jones MD, Wang L, Hatton C, Palescandolo E, Gupta S, Mahan S, Sougnez C, Onofrio RC, Liefeld T, MacConaill L, Winckler W, Reich M, Li N, Mesirov JP, Gabriel SB, Getz G, Ardlie K, Chan V, Myer VE, Weber BL, Porter J, Warmuth M, Finan P, Harris JL, Meyerson M, Golub TR, Morrissey MP, Sellers WR, Schlegel R, and Garraway LA. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483: 603–607, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu A, Rojas H, Banerjee H, Cabrera IB, Perez KY, De Leon M, and Casiano CA. Expression of the stress response oncoprotein LEDGF/p75 in human cancer: a study of 21 tumor types. PLoS One 7: e30132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedard K, Jaquet V, and Krause KH. NOX5: from basic biology to signaling and disease. Free Radic Biol Med 52: 725–734, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Block K. and Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat Rev Cancer 12: 627–637, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Block K, Gorin Y, Hoover P, Williams P, Chelmicki T, Clark RA, Yoneda T, and Abboud HE. NAD(P)H oxidases regulate HIF-2alpha protein expression. J Biol Chem 282: 8019–8026, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP, Quinn MT, Krenitsky K, Ardie KG, Lambeth JD, and Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol 285: C353–C369, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Whorton AR, and Hoidal JR. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am J Physiol Cell Physiol 282: C1212–C1224, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med 18: 775–794, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Burnham DN, Uhlinger DJ, and Lambeth JD. Diradylglycerol synergizes with an anionic amphiphile to activate superoxide generation and phosphorylation of p47phox in a cell-free system from human neutrophils. J Biol Chem 265: 17550–17559, 1990 [PubMed] [Google Scholar]
- 20.Caillou B, Dupuy C, Lacroix L, Nocera M, Talbot M, Ohayon R, Deme D, Bidart JM, Schlumberger M, and Virion A. Expression of reduced nicotinamide adenine dinucleotide phosphate oxidase (ThoX, LNOX, Duox) genes and proteins in human thyroid tissues. J Clin Endocrinol Metab 86: 3351–3358, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Chen F, Pandey D, Chadli A, Catravas JD, Chen T, and Fulton DJ. Hsp90 regulates NADPH oxidase activity and is necessary for superoxide but not hydrogen peroxide production. Antioxid Redox Signal 14: 2107–2119, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W, Shang WH, Adachi Y, Hirose K, Ferrari DM, and Kamata T. A possible biochemical link between NADPH oxidase (Nox) 1 redox-signalling and ERp72. Biochem J 416: 55–63, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Cheng G, Cao Z, Xu X, van Meir EG, and Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Chiera F, Meccia E, Degan P, Aquilina G, Pietraforte D, Minetti M, Lambeth D, and Bignami M. Overexpression of human NOX1 complex induces genome instability in mammalian cells. Free Radic Biol Med 44: 332–342, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Choi JA, Lee JW, Kim H, Kim EY, Seo JM, Ko J, and Kim JH. Pro-survival of estrogen receptor- negative breast cancer cells is regulated by a BLT2-reactive oxygen species-linked signaling pathway. Carcinogenesis 31: 543–551, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Coclet J, Foureau F, Ketelbant P, Galand P, and Dumont JE. Cell population kinetics in dog and human adult thyroid. Clin Endocrinol (Oxf) 31: 655–665, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Cui X. Reactive oxygen species: the achilles' heel of cancer cells? Antioxid Redox Signal 16: 1212–1214, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, and Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 275: 23227–23233, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, and Courtneidge SA. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal 2: ra53, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dosquet C, Schaetz A, Faucher C, Lepage E, Wautier JL, Richard F, and Cabane J. Tumour necrosis factor-alpha, interleukin-1 beta and interleukin-6 in patients with renal cell carcinoma. Eur J Cancer 30A: 162–167, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, and Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem 274: 37265–37269, 1999 [DOI] [PubMed] [Google Scholar]
- 32.El-Benna J, Dang PM, and Perianin A. Towards specific NADPH oxidase inhibition by small synthetic peptides. Cell Mol Life Sci 69: 2307–2314, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Jamali A, Valente AJ, Lechleiter JD, Gamez MJ, Pearson DW, Nauseef WM, and Clark RA. Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl. Free Radic Biol Med 44: 868–881, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fantini MC. and Pallone F. Cytokines: from gut inflammation to colorectal cancer. Curr Drug Targets 9: 375–380, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Farrow B. and Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol 10: 153–169, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Fitzgerald JP, Nayak B, Shanmugasundaram K, Friedrichs W, Sudarshan S, Eid AA, DeNapoli T, Parekh DJ, Gorin Y, and Block K. Nox4 mediates renal cell carcinoma cell invasion through hypoxia-induced interleukin 6- and 8-production. PLoS One 7: e30712, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forteza R, Salathe M, Miot F, and Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol 32: 462–469, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Fu X, Beer DG, Behar J, Wands J, Lambeth D, and Cao W. cAMP-response element-binding protein mediates acid-induced NADPH oxidase NOX5-S expression in Barrett esophageal adenocarcinoma cells. J Biol Chem 281: 20368–20382, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Fukuyama M, Rokutan K, Sano T, Miyake H, Shimada M, and Tashiro S. Overexpression of a novel superoxide-producing enzyme, NADPH oxidase 1, in adenoma and well differentiated adenocarcinoma of the human colon. Cancer Lett 221: 97–104, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Fulton DJ. Nox5 and the regulation of cellular function. Antioxid Redox Signal 11: 2443–2452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gazdar AF, Girard L, Lockwood WW, Lam WL, and Minna JD. Lung cancer cell lines as tools for biomedical discovery and research. J Natl Cancer Inst 102: 1310–1321, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geiszt M, Kopp JB, Varnai P, and Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A 97: 8010–8014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geiszt M, Lekstrom K, Brenner S, Hewitt SM, Dana R, Malech HL, and Leto TL. NAD(P)H oxidase 1, a product of differentiated colon epithelial cells, can partially replace glycoprotein 91phox in the regulated production of superoxide by phagocytes. J Immunol 171: 299–306, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Gianni D, Taulet N, DerMardirossian C, and Bokoch GM. c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol Biol Cell 21: 4287–4298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graham KA, Kulawiec M, Owens KM, Li X, Desouki MM, Chandra D, and Singh KK. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol Ther 10: 223–231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grasberger H. and Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem 281: 18269–18272, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, Asangani IA, Ateeq B, Chun SY, Siddiqui J, Sam L, Anstett M, Mehra R, Prensner JR, Palanisamy N, Ryslik GA, Vandin F, Raphael BJ, Kunju LP, Rhodes DR, Pienta KJ, Chinnaiyan AM, and Tomlins SA. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487: 239–243, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greer JB. and Whitcomb DC. Inflammation and pancreatic cancer: an evidence-based review. Curr Opin Pharmacol 9: 411–418, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Hanauer DA, Rhodes DR, Sinha-Kumar C, and Chinnaiyan AM. Bioinformatics approaches in the study of cancer. Curr Mol Med 7: 133–141, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, and Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett 579: 4911–4917, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Helmcke I, Heumuller S, Tikkanen R, Schroder K, and Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal 11: 1279–1287, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Hou J, Aerts J, den Hamer B, van Ijcken W, den Bakker M, Riegman P, van der Leest C, van der Spek P, Foekens JA, Hoogsteden HC, Grosveld F, and Philipsen S. Gene expression-based classification of non-small cell lung carcinomas and survival prediction. PLoS One 5: e10312, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsieh CH, Wu CP, Lee HT, Liang JA, Yu CY, and Lin YJ. NADPH oxidase subunit 4 mediates cycling hypoxia-promoted radiation resistance in glioblastoma multiforme. Free Radic Biol Med 53: 649–658, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Huang WC, Li X, Liu J, Lin J, and Chung LW. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol Cancer Res 10: 133–142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang X, Zhuang J, Teng X, Li L, Chen D, Yan X, and Tang F. The promotion of human malignant melanoma growth by mesoporous silica nanoparticles through decreased reactive oxygen species. Biomaterials 31: 6142–6153, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Itoh T, Terazawa R, Kojima K, Nakane K, Deguchi T, Ando M, Tsukamasa Y, Ito M, and Nozawa Y. Cisplatin induces production of reactive oxygen species via NADPH oxidase activation in human prostate cancer cells. Free Radic Res 45: 1033–1039, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Janiszewski M, Lopes LR, Carmo AO, Pedro MA, Brandes RP, Santos CX, and Laurindo FR. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J Biol Chem 280: 40813–40819, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Juhasz A, Ge Y, Markel S, Chiu A, Matsumoto L, van Balgooy J, Roy K, and Doroshow JH. Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free Radic Res 43: 523–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Juhasz AN, Markel S, Gaur S, Matsumoto L, Van Balgooy J, Metz M, and Doroshow JH. Inhibition of Nox1 gene expression with siRNA in human colon cancer cells decreases tumor growth and markers of angiogenesis in vivo. Proc Amer Assoc Cancer Res 46: 1436–1437, 2005 [Google Scholar]
- 60.Kajla S, Mondol AS, Nagasawa A, Zhang Y, Kato M, Matsuno K, Yabe-Nishimura C, and Kamata T. A crucial role for Nox 1 in redox-dependent regulation of Wnt-beta-catenin signaling. FASEB J 26: 2049–2059, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Kamiguti AS, Serrander L, Lin K, Harris RJ, Cawley JC, Allsup DJ, Slupsky JR, Krause KH, and Zuzel M. Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia. J Immunol 175: 8424–8430, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Kawahara T, Kuwano Y, Teshima-Kondo S, Takeya R, Sumimoto H, Kishi K, Tsunawaki S, Hirayama T, and Rokutan K. Role of nicotinamide adenine dinucleotide phosphate oxidase 1 in oxidative burst response to Toll-like receptor 5 signaling in large intestinal epithelial cells. J Immunol 172: 3051–3058, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Kawahara T. and Lambeth JD. Phosphatidylinositol (4,5)-bisphosphate modulates Nox5 localization via an N-terminal polybasic region. Mol Biol Cell 19: 4020–4031, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kita H, Hikichi Y, Hikami K, Tsuneyama K, Cui ZG, Osawa H, Ohnishi H, Mutoh H, Hoshino H, Bowlus CL, Yamamoto H, and Sugano K. Differential gene expression between flat adenoma and normal mucosa in the colon in a microarray analysis. J Gastroenterol 41: 1053–1063, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Knaus UG, Heyworth PG, Evans T, Curnutte JT, and Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science 254: 1512–1515, 1991 [DOI] [PubMed] [Google Scholar]
- 66.Knebel A, Rahmsdorf HJ, Ullrich A, and Herrlich P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J 15: 5314–5325, 1996 [PMC free article] [PubMed] [Google Scholar]
- 67.Korch C, Spillman MA, Jackson TA, Jacobsen BM, Murphy SK, Lessey BA, Jordan VC, and Bradford AP. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol Oncol 127: 241–248, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krause KH. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn J Infect Dis 57: S28–S29, 2004 [PubMed] [Google Scholar]
- 69.Krieg M, Haas R, Brauch H, Acker T, Flamme I, and Plate KH. Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene 19: 5435–5443, 2000 [DOI] [PubMed] [Google Scholar]
- 70.Kumatori A, Yang D, Suzuki S, and Nakamura M. Cooperation of STAT-1 and IRF-1 in interferon-gamma-induced transcription of the gp91(phox) gene. J Biol Chem 277: 9103–9111, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Lacroix L, Nocera M, Mian C, Caillou B, Virion A, Dupuy C, Filetti S, Bidart JM, and Schlumberger M. Expression of nicotinamide adenine dinucleotide phosphate oxidase flavoprotein DUOX genes and proteins in human papillary and follicular thyroid carcinomas. Thyroid 11: 1017–1023, 2001 [DOI] [PubMed] [Google Scholar]
- 72.Lambeth JD, Krause KH, and Clark RA. NOX enzymes as novel targets for drug development. Semin Immunopathol 30: 339–363, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, and Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 88: 888–894, 2001 [DOI] [PubMed] [Google Scholar]
- 74.Laurent E, McCoy JW, 3rd, Macina RA, Liu W, Cheng G, Robine S, Papkoff J, and Lambeth JD. Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int J Cancer 123: 100–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee JK, Edderkaoui M, Truong P, Ohno I, Jang KT, Berti A, Pandol SJ, and Gukovskaya AS. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology 133: 1637–1648, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Leto TL. and Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal 8: 1549–1561, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Leto TL, Morand S, Hurt D, and Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal 11: 2607–2619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leusen JH, Bolscher BG, Hilarius PM, Weening RS, Kaulfersch W, Seger RA, Roos D, and Verhoeven AJ. 156Pro→Gln substitution in the light chain of cytochrome b558 of the human NADPH oxidase (p22-phox) leads to defective translocation of the cytosolic proteins p47-phox and p67-phox. J Exp Med 180: 2329–2334, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim SD, Sun C, Lambeth JD, Marshall F, Amin M, Chung L, Petros JA, and Arnold RS. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate 62: 200–207, 2005 [DOI] [PubMed] [Google Scholar]
- 80.Lipinski S, Till A, Sina C, Arlt A, Grasberger H, Schreiber S, and Rosenstiel P. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J Cell Sci 122: 3522–3530, 2009 [DOI] [PubMed] [Google Scholar]
- 81.Liu F, Gomez Garcia AM, and Meyskens FL., Jr.NADPH oxidase 1 overexpression enhances invasion via matrix metalloproteinase-2 and epithelial-mesenchymal transition in melanoma cells. J Invest Dermatol 132: 2033–2041, 2012 [DOI] [PubMed] [Google Scholar]
- 82.Luxen S, Belinsky SA, and Knaus UG. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res 68: 1037–1045, 2008 [DOI] [PubMed] [Google Scholar]
- 83.Luxen S, Noack D, Frausto M, Davanture S, Torbett BE, and Knaus UG. Heterodimerization controls localization of Duox-DuoxA NADPH oxidases in airway cells. J Cell Sci 122: 1238–1247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, and Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maeda S, Chang L, Li ZW, Luo JL, Leffert H, and Karin M. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity 19: 725–737, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Maranchie JK. and Zhan Y. Nox4 is critical for hypoxia-inducible factor 2-alpha transcriptional activity in von Hippel-Lindau-deficient renal cell carcinoma. Cancer Res 65: 9190–9193, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, and Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 88.Masamune A, Watanabe T, Kikuta K, Satoh K, and Shimosegawa T. NADPH oxidase plays a crucial role in the activation of pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol 294: G99–G108, 2008 [DOI] [PubMed] [Google Scholar]
- 89.Masters JR. HeLa cells 50 years on: the good, the bad and the ugly. Nat Rev Cancer 2: 315–319, 2002 [DOI] [PubMed] [Google Scholar]
- 90.Medale-Giamarchi C, Lajoie-Mazenc I, Malissein E, Meunier E, Couderc B, Berge Y, Filleron T, Keller L, Marty C, Lacroix-Triki M, Dalenc F, Doisneau-Sixou SF, and Favre G. RhoB modifies estrogen responses in breast cancer cells by influencing expression of the estrogen receptor. Breast Cancer Res 15: R6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meitzler JL. and Ortiz de Montellano PR. Caenorhabditis elegans and human dual oxidase 1 (DUOX1) “peroxidase” domains: insights into heme binding and catalytic activity. J Biol Chem 284: 18634–18643, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meitzler JL. and Ortiz de Montellano PR. Structural stability and heme binding potential of the truncated human dual oxidase 2 (DUOX2) peroxidase domain. Arch Biochem Biophys 512: 197–203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mo Q, Wang S, Seshan VE, Olshen AB, Schultz N, Sander C, Powers RS, Ladanyi M, and Shen R. Pattern discovery and cancer gene identification in integrated cancer genomic data. Proc Natl Acad Sci U S A 110: 4245–4250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mochizuki T, Furuta S, Mitsushita J, Shang WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayama J, Konagai A, Hirose K, Kiyosawa K, and Kamata T. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene 25: 3699–3707, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Morand S, Ueyama T, Tsujibe S, Saito N, Korzeniowska A, and Leto TL. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J 23: 1205–1218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moreno JC, Bikker H, Kempers MJ, van Trotsenburg AS, Baas F, de Vijlder JJ, Vulsma T, and Ris-Stalpers C. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med 347: 95–102, 2002 [DOI] [PubMed] [Google Scholar]
- 97.Morris DS, Tomlins SA, Rhodes DR, Mehra R, Shah RB, and Chinnaiyan AM. Integrating biomedical knowledge to model pathways of prostate cancer progression. Cell Cycle 6: 1177–1187, 2007 [DOI] [PubMed] [Google Scholar]
- 98.Nauseef WM, McCormick S, Renee J, Leidal KG, and Clark RA. Functional domain in an arginine-rich carboxyl-terminal region of p47phox. J Biol Chem 268: 23646–23651, 1993 [PubMed] [Google Scholar]
- 99.Nunez J. and Pommier J. Formation of thyroid hormones. Vitam Horm 39: 175–229, 1982 [DOI] [PubMed] [Google Scholar]
- 100.Oberley LW, Oberley TD, and Buettner GR. Cell division in normal and transformed cells: the possible role of superoxide and hydrogen peroxide. Med Hypotheses 7: 21–42, 1981 [DOI] [PubMed] [Google Scholar]
- 101.Ohye H, Fukata S, Hishinuma A, Kudo T, Nishihara E, Ito M, Kubota S, Amino N, Ieiri T, Kuma K, and Miyauchi A. A novel homozygous missense mutation of the dual oxidase 2 (DUOX2) gene in an adult patient with large goiter. Thyroid 18: 561–566, 2008 [DOI] [PubMed] [Google Scholar]
- 102.Olmos Y, Brosens JJ, and Lam EW. Interplay between SIRT proteins and tumour suppressor transcription factors in chemotherapeutic resistance of cancer. Drug Resist Updat 14: 35–44, 2011 [DOI] [PubMed] [Google Scholar]
- 103.Ostman A, Hellberg C, and Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer 6: 307–320, 2006 [DOI] [PubMed] [Google Scholar]
- 104.Pandey D, Patel A, Patel V, Chen F, Qian J, Wang Y, Barman SA, Venema RC, Stepp DW, Rudic RD, and Fulton DJ. Expression and functional significance of NADPH oxidase 5 (Nox5) and its splice variants in human blood vessels. Am J Physiol Heart Circ Physiol 302: H1919–H1928, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pelicano H, Carney D, and Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 7: 97–110, 2004 [DOI] [PubMed] [Google Scholar]
- 106.Perner A, Andresen L, Pedersen G, and Rask-Madsen J. Superoxide production and expression of NAD(P)H oxidases by transformed and primary human colonic epithelial cells. Gut 52: 231–236, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perou CM, Jeffrey SS, van de Rijn M, Rees CA, Eisen MB, Ross DT, Pergamenschikov A, Williams CF, Zhu SX, Lee JC, Lashkari D, Shalon D, Brown PO, and Botstein D. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci U S A 96: 9212–9217, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, and Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 8: 1473–1484, 2006 [DOI] [PubMed] [Google Scholar]
- 109.Pfarr N, Korsch E, Kaspers S, Herbst A, Stach A, Zimmer C, and Pohlenz J. Congenital hypothyroidism caused by new mutations in the thyroid oxidase 2 (THOX2) gene. Clin Endocrinol (Oxf) 65: 810–815, 2006 [DOI] [PubMed] [Google Scholar]
- 110.Puca R, Nardinocchi L, Starace G, Rechavi G, Sacchi A, Givol D, and D'Orazi G. Nox1 is involved in p53 deacetylation and suppression of its transcriptional activity and apoptosis. Free Radic Biol Med 48: 1338–1346, 2010 [DOI] [PubMed] [Google Scholar]
- 111.Raad H, Eskalli Z, Corvilain B, Miot F, and De Deken X. Thyroid hydrogen peroxide production is enhanced by the Th2 cytokines, IL-4 and IL-13, through increased expression of the dual oxidase 2 and its maturation factor DUOXA2. Free Radic Biol Med 56: 216–225, 2013 [DOI] [PubMed] [Google Scholar]
- 112.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, and Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol 31: 1368–1376, 2011 [DOI] [PubMed] [Google Scholar]
- 113.Rigutto S, Hoste C, Dumont JE, Corvilain B, Miot F, and De Deken X. Duox1 is the main source of hydrogen peroxide in the rat thyroid cell line PCCl3. Exp Cell Res 313: 3892–3901, 2007 [DOI] [PubMed] [Google Scholar]
- 114.Rigutto S, Hoste C, Grasberger H, Milenkovic M, Communi D, Dumont JE, Corvilain B, Miot F, and De Deken X. Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation. J Biol Chem 284: 6725–6734, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, Pergamenschikov A, Lee JC, Lashkari D, Shalon D, Myers TG, Weinstein JN, Botstein D, and Brown PO. Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet 24: 227–235, 2000 [DOI] [PubMed] [Google Scholar]
- 116.Sandberg R. and Ernberg I. Assessment of tumor characteristic gene expression in cell lines using a tissue similarity index (TSI). Proc Natl Acad Sci U S A 102: 2052–2057, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland JA, Smallridge RC, and Haugen BR. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab 93: 4331–4341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sedeek M, Hebert RL, Kennedy CR, Burns KD, and Touyz RM. Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curr Opin Nephrol Hypertens 18: 122–127, 2009 [DOI] [PubMed] [Google Scholar]
- 119.Segal E, Friedman N, Koller D, and Regev A. A module map showing conditional activity of expression modules in cancer. Nat Genet 36: 1090–1098, 2004 [DOI] [PubMed] [Google Scholar]
- 120.Seo JM, Park S, and Kim JH. Leukotriene B4 receptor-2 promotes invasiveness and metastasis of ovarian cancer cells through signal transducer and activator of transcription 3 (STAT3)-dependent up-regulation of matrix metalloproteinase 2. J Biol Chem 287: 13840–13849, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Seril DN, Liao J, Yang GY, and Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis 24: 353–362, 2003 [DOI] [PubMed] [Google Scholar]
- 122.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, and Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, and Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem 276: 1417–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 124.Shono T, Yokoyama N, Uesaka T, Kuroda J, Takeya R, Yamasaki T, Amano T, Mizoguchi M, Suzuki SO, Niiro H, Miyamoto K, Akashi K, Iwaki T, Sumimoto H, and Sasaki T. Enhanced expression of NADPH oxidase Nox4 in human gliomas and its roles in cell proliferation and survival. Int J Cancer 123: 787–792, 2008 [DOI] [PubMed] [Google Scholar]
- 125.Si J, Fu X, Behar J, Wands J, Beer DG, Souza RF, Spechler SJ, Lambeth D, and Cao W. NADPH oxidase NOX5-S mediates acid-induced cyclooxygenase-2 expression via activation of NF- kappaB in Barrett's esophageal adenocarcinoma cells. J Biol Chem 282: 16244–16255, 2007 [DOI] [PubMed] [Google Scholar]
- 126.Storz P. Reactive oxygen species in tumor progression. Front Biosci 10: 1881–1896, 2005 [DOI] [PubMed] [Google Scholar]
- 127.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, and Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401: 79–82, 1999 [DOI] [PubMed] [Google Scholar]
- 128.Sun L, Pan J, Peng L, Fang L, Zhao X, Yang Z, and Ran Y. Combination of haptoglobin and osteopontin could predict colorectal cancer hepatic metastasis. Ann Surg Oncol 19: 2411–2419, 2012 [DOI] [PubMed] [Google Scholar]
- 129.Szanto I, Rubbia-Brandt L, Kiss P, Steger K, Banfi B, Kovari E, Herrmann F, Hadengue A, and Krause KH. Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J Pathol 207: 164–176, 2005 [DOI] [PubMed] [Google Scholar]
- 130.Szatrowski TP. and Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res 51: 794–798, 1991 [PubMed] [Google Scholar]
- 131.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, and Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem 278: 25234–25246, 2003 [DOI] [PubMed] [Google Scholar]
- 132.Tominaga K, Kawahara T, Sano T, Toida K, Kuwano Y, Sasaki H, Kawai T, Teshima-Kondo S, and Rokutan K. Evidence for cancer-associated expression of NADPH oxidase 1 (Nox1)-based oxidase system in the human stomach. Free Radic Biol Med 43: 1627–1638, 2007 [DOI] [PubMed] [Google Scholar]
- 133.Ueyama T, Geiszt M, and Leto TL. Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases. Mol Cell Biol 26: 2160–2174, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van der Vliet A. NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic Biol Med 44: 938–955, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van Loon B, Markkanen E, and Hubscher U. Oxygen as a friend and enemy: how to combat the mutational potential of 8-oxo-guanine. DNA Repair (Amst) 9: 604–616, 2010 [DOI] [PubMed] [Google Scholar]
- 136.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, and Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107: 149–159, 2001 [DOI] [PubMed] [Google Scholar]
- 137.Verhaak RG, Tamayo P, Yang JY, Hubbard D, Zhang H, Creighton CJ, Fereday S, Lawrence M, Carter SL, Mermel CH, Kostic AD, Etemadmoghadam D, Saksena G, Cibulskis K, Duraisamy S, Levanon K, Sougnez C, Tsherniak A, Gomez S, Onofrio R, Gabriel S, Chin L, Zhang N, Spellman PT, Zhang Y, Akbani R, Hoadley KA, Kahn A, Kobel M, Huntsman D, Soslow RA, Defazio A, Birrer MJ, Gray JW, Weinstein JN, Bowtell DD, Drapkin R, Mesirov JP, Getz G, Levine DA, and Meyerson M. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clin Invest 123: 517–525, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vigone MC, Fugazzola L, Zamproni I, Passoni A, Di Candia S, Chiumello G, Persani L, and Weber G. Persistent mild hypothyroidism associated with novel sequence variants of the DUOX2 gene in two siblings. Hum Mutat 26: 395, 2005 [DOI] [PubMed] [Google Scholar]
- 139.Wang R, Dashwood WM, Nian H, Lohr CV, Fischer KA, Tsuchiya N, Nakagama H, Ashktorab H, and Dashwood RH. NADPH oxidase overexpression in human colon cancers and rat colon tumors induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Int J Cancer 128: 2581–2590, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wesley UV, Bove PF, Hristova M, McCarthy S, and van der Vliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem 282: 3213–3220, 2007 [DOI] [PubMed] [Google Scholar]
- 141.Weyemi U, Caillou B, Talbot M, Ameziane-El-Hassani R, Lacroix L, Lagent-Chevallier O, Al Ghuzlan A, Roos D, Bidart JM, Virion A, Schlumberger M, and Dupuy C. Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr Relat Cancer 17: 27–37, 2010 [DOI] [PubMed] [Google Scholar]
- 142.Weyemi U, Redon CE, Parekh PR, Dupuy C, and Bonner WM. NADPH oxidases NOXs and DUOXs as putative targets for cancer therapy. Anticancer Agents Med Chem 13: 502–514, 2013 [PMC free article] [PubMed] [Google Scholar]
- 143.Wientjes FB, Hsuan JJ, Totty NF, and Segal AW. p40phox, a third cytosolic component of the activation complex of the NADPH oxidase to contain src homology 3 domains. Biochem J 296 (Pt 3): 557–561, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wolf G, Hannken T, Schroeder R, Zahner G, Ziyadeh FN, and Stahl RA. Antioxidant treatment induces transcription and expression of transforming growth factor beta in cultured renal proximal tubular cells. FEBS Lett 488: 154–159, 2001 [DOI] [PubMed] [Google Scholar]
- 145.Wu Y, Antony S, Hewitt SM, Jiang G, Yang SX, Meitzler JL, Juhasz A, Lu J, Liu H, Doroshow JH, and Roy K. Functional activity and tumor-specific expression of dual oxidase 2 in pancreatic cancer cells and human malignancies characterized with a novel monoclonal antibody. Int J Oncol 42: 1229–1238, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu Y, Antony S, Juhasz A, Lu J, Ge Y, Jiang G, Roy K, and Doroshow JH. Up-regulation and sustained activation of Stat1 are essential for interferon-gamma (IFN-gamma)-induced dual oxidase 2 (Duox2) and dual oxidase A2 (DuoxA2) expression in human pancreatic cancer cell lines. J Biol Chem 286: 12245–12256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu Y, Lu J, Antony S, Juhasz A, Liu H, Jiang G, Meitzler JL, Hollingshead M, Haines DC, Butcher D, Roy K, and Doroshow JH. Activation of TLR4 is required for the synergistic induction of dual oxidase 2 and dual oxidase A2 by IFN-gamma and lipopolysaccharide in human pancreatic cancer cell lines. J Immunol 190: 1859–1872, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, and Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res 67: 10823–10830, 2007 [DOI] [PubMed] [Google Scholar]
- 149.Yamaura M, Mitsushita J, Furuta S, Kiniwa Y, Ashida A, Goto Y, Shang WH, Kubodera M, Kato M, Takata M, Saida T, and Kamata T. NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res 69: 2647–2654, 2009 [DOI] [PubMed] [Google Scholar]
- 150.Yu JH, Lim JW, Kim H, and Kim KH. NADPH oxidase mediates interleukin-6 expression in cerulein-stimulated pancreatic acinar cells. Int J Biochem Cell Biol 37: 1458–1469, 2005 [DOI] [PubMed] [Google Scholar]
- 151.Yu L, Zhen L, and Dinauer MC. Biosynthesis of the phagocyte NADPH oxidase cytochrome b558. Role of heme incorporation and heterodimer formation in maturation and stability of gp91phox and p22phox subunits. J Biol Chem 272: 27288–27294, 1997 [DOI] [PubMed] [Google Scholar]
- 152.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, and Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A 107: 18121–18126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao M, Sano D, Pickering CR, Jasser SA, Henderson YC, Clayman GL, Sturgis EM, Ow TJ, Lotan R, Carey TE, Sacks PG, Grandis JR, Sidransky D, Heldin NE, and Myers JN. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clin Cancer Res 17: 7248–7264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]