Abstract
The main membrane proteins of caveolae (caveolin-1, -2 and -3) oligomerize within lipid rich domains to form regular invaginations of smooth muscle plasma membrane and participate in receptor internalization and desensitization independent of clathrin-coated vesicle endocytosis. We have previously shown that Gs-coupled VIP/PACAP receptors, VPAC2, predominantly expressed in smooth muscle cells of the gut, are exclusively phosphorylated by GRK2 leading to receptor internalization and desensitization. Herein, we characterized the role of caveolin-1 in VPAC2 receptor internalization and desensitization in gastric smooth muscle using three approaches: (i) methyl β-cyclodextrin (MβCD) to deplete cholesterol and disrupt caveolae in dispersed muscle cells, (ii) caveolin-1 siRNA to suppress caveolin-1 expression in cultured muscle cells, and (iii) caveolin-1 knockout mice (caveolin-1−/−). Pretreatment of gastric muscle cells with VIP stimulated tyrosine phosphorylation of caveolin-1, and induced VPAC2 receptor internalization (measured as decrease in 125I-VIP binding after pretreatment) and desensitization (measured as decrease in VIP-induced cAMP formation after pretreatment). Caveolin-1 phosphorylation, and VPAC2 receptor internalization and desensitization were blocked by disruption of caveolae with MβCD, suppression of caveolin-1 with caveolin-1 siRNA or inhibition of Src kinase activity by PP2. Pretreatment with VIP significantly inhibited adenylyl cyclase activity and muscle relaxation in response to subsequent addition of VIP in freshly dispersed muscle cells and in muscle strips isolated from wild type and caveolin-1−/−mice; however, the inhibition was significantly attenuated in caveolin-1−/− mice. These results suggest that caveolin-1 plays an important role in VPAC2 receptor internalization and desensitization.
Keywords: Caveolin-1, Endocytosis, G protein coupled receptor, GRK2
1. Introduction
The homologous peptides, vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP) are abundantly expressed in neurons of the enteric nervous system where they regulate secretion and motility [5,9,12]. The biological effects of VIP and PACAP are mediated by three types of Gs-coupled receptors that belong to the family of class II G protein-coupled receptors: VPAC1 and VPAC2 receptors, which possess equal affinity for VIP and PACAP, and PAC1 receptors, which possess high affinity for PACAP only [20,21,41]. Expression of VIP/PACAP receptors is tissue specific [41]. Gastrointestinal smooth muscle cells are shown to express predominantly VPAC2 receptors and their activation by VIP or PACAP causes stimulation of adenylyl cyclase activity, cAMP formation, cAMP-dependent protein kinase (PKA) activity, and muscle relaxation [27,32,40].
G protein-coupled receptors are rapidly attenuated or terminated by mechanisms that target receptors, G proteins, or effector enzymes. Regulation of VPAC2 receptors appears to be distinct from VPAC1 and PAC1 receptors and from other members of the class II G protein-coupled receptors such as secretin [10,18,30,33,37,38]. VPAC2 receptor internalization and desensitization of response are initiated upon receptor phosphorylation by G protein-coupled receptor kinase 2 (GRK2) [32]. Stimulation of cAMP-dependent protein kinase (PKA) by VPAC2 receptors accelerates this process via feedback phosphorylation of GRK2 at Ser685 [32].
Phosphorylation by GRKs and subsequent binding of the scaffolding protein β-arrestin to the phosphorylated receptor uncouples the receptor from the G protein as a prelude to receptor internalization [26,35]. The major internalization processes include the well-characterized clathrin-dependent pathway requiring dynamin for the scission and generation of endocytic vesicles, and less-well characterized clathrin-independent caveolae-dependent pathway [2,19,25]. Both clathrin-coated pits and caveolae act as microdomains to concentrate receptors, G-proteins and signaling molecules in the plasma membrane and coordinate intracellular signaling pathways [19,26].
Caveolae are caveolin-1-enriched invaginations. Caveolin-1−/− mice lack caveolae and expression of caveolin-1 is sufficient to generate caveolae in cells previously lacking caveolae [16,17]. Signaling by G protein-coupled receptors has been shown to be regulated by caveolin-1. Studies in PC12 cells have shown that translocation of PAC1 receptors into the caveolin-enriched microdomains enhances PAC1-mediated cAMP signaling and neurite outgrowth [42]. We have recently shown that VPAC2 receptor phosphorylation, internalization, and desensitization are exclusively mediated by GRK2 [32]. However, it is not known whether VPAC2 receptor signaling and internalization require caveolin-1 in gastrointestinal smooth muscle. In the present study, we examined whether VPAC2 receptors were internalized via caveolae using several complementary approaches. We herein report the VPAC2 receptor internalization and desensitization are significantly inhibited by disruption of caveolae, suppression of caveolin-1 or deletion of caveolin-1 gene, suggesting that VPAC2 receptor internalization is mediated via caveolae-dependent pathways.
2. Materials and methods
2.1. Preparation of dispersed gastric smooth muscle cells
Gastric smooth muscle cells were isolated from mouse stomach as previously described [27,32]. The Virginia Commonwealth University Institutional Animal Care and Use Committee approved the use of animals and protocol used in this study. Briefly, muscle strips were incubated at 31 °C for 20 min in HEPES medium with type II collagenase (0.1%) and soybean trypsin inhibitor (0.1%) (Worthington Biochemical (Freehold, NJ)). The partly digested strips were washed, muscle cells were allowed to disperse spontaneously for 30 min, and cells harvested by filtration though 500-μm Nitex and centrifuged twice at 350 × g for 10 min. Dispersed smooth muscle cells were cultured in DMEM containing 10% FBS until they attained confluence and were then passaged once for use in various studies [27,32]. In some experiments cells were treated with 10 mM methyl β cyclodextrin (MβCD) for 30 min to disrupt caveolae.
2.2. Transfection of caveolin-1 siRNA
The RNAi-Ready pSIREN-DNR-DsRed-Express Vector (BD Biosciences, Clontech) encoding caveolin-1 small-interfering RNA (Qiagen) was inserted between BamH1 and EcoR1 restriction sites and transfected into cultured gastric smooth muscle cells with lipofectamine™ 2000 reagent (Invitrogen) (Carlsbad, CA) according to the manufacturer’s recommendation and as described previously [39]. To check the specificity of the siRNA, empty vector without the siRNA sequence was used as control. Successful knockdown of caveolin-1 protein was verified by western blot and immunofluorescence microscopy.
2.3. VPAC2 receptor internalization
Binding of 125I-labeled VIP [125I-VIP] (NEN Life Sciences Products (Boston, MA) to freshly dispersed and cultured muscle cells was performed as previously described [27,32]. Cells were collected by centrifugation at 400 × g and resuspended in DMEM containing BSA (0.1%), amastatin (10 μM), and phosphoramidon (1 μM). Triplicate 0.5-ml (2 × 106 cell/ml) aliquots were incubated for 60 min at 4 °C with 50 pM 125I-VIP alone or in the presence of 10 μM VIP. Bound and free radioligands were separated by rapid filtration under reduced pressure through 5-μm polycarbonate nucleopore filters. Nonspecific binding (26 ± 5%) was calculated as the amount of radioactivity in the presence of 10 μM VIP.
Receptor internalization was determined by incubating muscle cells with VIP at 37 °C for different time periods; cells were then washed twice with phosphate buffered saline (PBS) at 4 °C to avoid further receptor internalization and recycling, and 125I-VIP binding to residual surface VPAC2 receptors was measured for 60 min at 4 °C and compared with control 125I-VIP binding in the absence of treatment with VIP.
2.4. Tyrosine phosphorylation of caveolin-1
Caveolin-1 phosphorylation was measured by immuno blot using phospho-caveolin-1 (Tyr14) (Cell Signaling; 3251) antibody. Control and MβCD treated muscle cells were incubated with VIP for 5 min in the presence or absence of a selective c-Src inhibitor, PP2 (1 μM), and solubilized on ice for 2 h in 20 mM Tris/HCl medium containing 1 mM DTT, 100 mM NaCl, 0.5% SDS, 1 mM PMSF, 10 μg/ml leupeptin, and 100 μg/ml aprotinin. The lysates were immunoprecipitated with VPAC2 receptor antibody (Santa Cruz, CA; SC-15960) and proteins were resolved by SDS-PAGE and transferred electrophoretically to PVDF membrane. The membranes were incubated for 12 h with phospho-caveolin-1 (Tyr14) antibody and then for 1 h with horseradish peroxidase-conjugated secondary antibody. The bands were identified by enhanced chemiluminescence [32].
2.5. Radioimmunoassay for cAMP
cAMP levels were measured by radioimmunoassay, as previously described [32]. Suspensions of smooth muscle cells (2 × 106 cells/ml) were stimulated for 1 min with VIP in the presence of 100 μM IBMX, and the reaction was terminated with 10% trichloroacetic acid. Samples were centrifuged, and the supernatant was extracted with diethyl ether and lyophilized. Samples were resuspended in sodium-acetate buffer (pH 6.2) followed by acetylation with triethylamine-acetic anhydride for 10 min. cAMP was measured in duplicates, and the results are expressed as picomoles per milligram of protein.
2.6. Measurement of relaxation in muscle strips
Muscle strips from mouse stomach were collected and rinsed immediately in Kreb’s solution containing 118 mM NaCl, 4.8 mM KCl, 1 mM MgSO4, 1.15 mM NaH2PO4, 15 mM NaHCO3, 10.5 mM glucose and 2.5 mM CaCl2. The stomach was emptied of its contents and the proximal part was used to prepare the muscle strips by cutting in the direction of circular muscle layer [14,15]. The strips were mounted between a glass support rod and an isometric FT03C transducer (Grass Technologies) connected to a computer recording system (Polyview). Preparations were allowed to equilibrate for 1 h at resting tension (1 g) before initiation of experiments and bath buffer solution was changed every 15 min during equilibration. To measure VIP-induced relaxation, the strips were precontracted with KCl (60 mM), and after obtaining stable sustained contraction VIP was added. At the end of each experiment, the strips were blotted dry and weighed (tissue wet weight). Contractile activity of muscle strips from stomach was calculated as maximum force generated in response to KCl and relaxation was calculated as percent decrease in maximum contraction.
2.7. Measurement of relaxation in isolated muscle cells
Cell aliquots of 0.4 ml (104 muscle cells/ml) in smooth muscle buffer (pH 7.4) were treated with VIP for 1 min followed by KCl (60 mM) for 30 s and the reactions were terminated with 1% acrolein. The mean length of 50 smooth muscle cells was measured by scanning micrometry as described previously [32,39]. The length of muscle cells treated with KCl in the presence or absence of VIP was compared with the length of untreated cells. Contraction was expressed as decrease in mean cell length from control, and relaxation was expressed as the percent decrease in muscle contraction [32,39].
Caveolin 1 knockout mice were purchased from Jackson Laboratories (Cav1tm1Mls/J, 004585) and maintained on standard laboratory chow and tap water ad libitum with 12-h:12-h light–dark cycles.
2.8. Statistical analysis
The results were expressed as means ± S.E. of n experiments and analysed for statistical significance using Student’s t-test for paired and unpaired values. Each experiment was done on cells obtained from different animals. A probability of p < 0.05 was considered significant.
3. Results
3.1. Caveolin-1-mediated VPAC2 receptor internalization
VPAC2 receptor internalization was assessed by the decrease in 125I-VIP binding to surface receptors after treatment of cells with different concentrations of VIP for 20 min or after treatment for different times with 1 μM VIP. The involvement of caveolae/caveolin-1 was examined in freshly dispersed muscle cells by pretreatment of cells with methyl β cyclodextrin (MβCD), which depletes cholesterol and disrupts caveolae or by suppression of caveolin-1 in cultured muscle cells. Specific binding of 125I-VIP was similar in freshly dispersed muscle cells (1897 ± 230 cpm/mg protein) and cultured muscle cells (2012 ± 302 cpm/mg protein). Neither total binding nor specific binding was affected by treatment of cells with MβCD or transfection of cells with caveolin-1 siRNA.
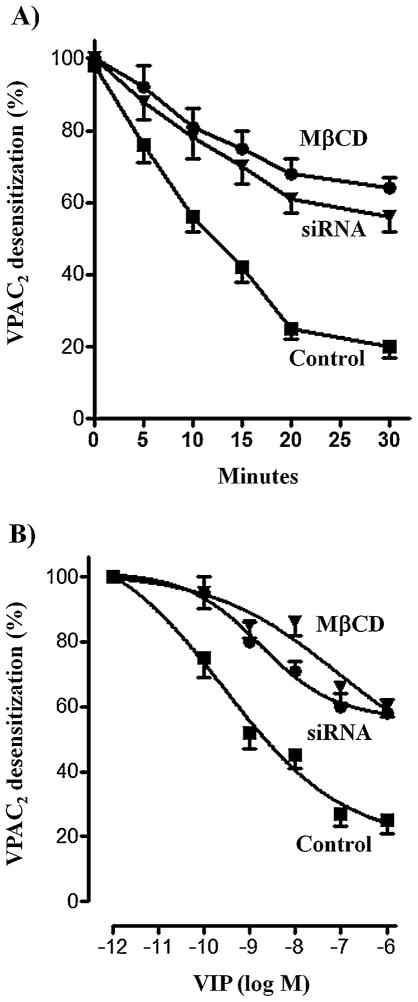
Pretreatment of freshly dispersed muscle cells or cultured smooth muscle cells with VIP caused a decrease in receptor binding to 125I-VIP reflecting VPAC2 receptor internalization. VPAC2 receptor internalization, measured as decrease in 125I-VIP binding after exposure to the ligand, was dependent on the time of exposure. VPAC2 receptor internalization was rapid [half-time (t0.5): ~7 min] and maximal after a 20-min exposure to VIP (69 ± 5% to 72 ± 5% decrease in binding) (Fig. 1A). The extent of internalization was similar in freshly dispersed muscle cells (72 ± 3% maximal decrease in binding) and cultured muscle cells (69 ± 5% maximal decrease in binding). In cells treated with MβCD or expressing caveolin-1 siRNA VPAC2 receptor internalization was slower [half-time (t0.5): ~ 12 min] compared to control cells (Fig. 1A). Maximal internalization of VPAC2 receptors was also significantly attenuated in freshly dispersed muscle cells treated with MβCD (24 ± 3% decrease in binding) or in cultured muscle cells transfected with caveolin-1 siRNA (30 ± 2% decrease in binding) (Fig. 1A).
Fig. 1.
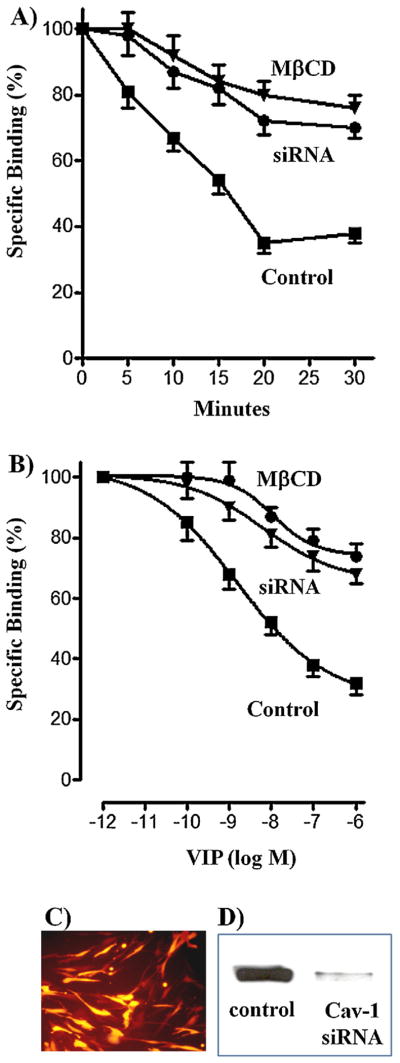
Effect of pretreatment with VIP on VPAC2 receptor internalization in cells treated with MβCD or in cells transfected with caveolin-1 siRNA. (A) Control muscle cells and cells treated with MβCD or expressing caveolin-1 siRNA were treated with VIP (1 μM) for various times up to 30 min, and 125I-VIP binding to surface receptors was measured. VPAC2 receptor internalization was assessed by the decrease in 125I-VIP binding to surface receptors after treatment with VIP. (B) Control muscle cells and cells treated with MβCD or expressing caveolin-1 siRNA were treated with various concentrations of VIP (1 pM–1 μM) for 30 min, and VPAC2 receptor internalization was assessed by the decrease in 125I-VIP binding to surface receptors after a 30-min treatment with VIP. Results are expressed as percent of specific binding in the absence of pretreatment and the specific binding was similar in control cells, cells treated with MβCD or in cells transfected with caveolin-1 siRNA. Internalization of VPAC2 receptors was dependent on the concentration of VIP and time of pretreatment. Internalization was significantly attenuated in cells treated with MβCD or in cells transfected with caveolin-1 siRNA. (C) Immunoflurescence of caveolin-1 siRNA transfection in cultured muscle cells. (D) Western blot of caveolin-1 expression in control cells and in cells transfected with caveolin-1 (Cav-1) siRNA. Values are expressed as means ± SE of 4 experiments.
VPAC2 receptor internalization was also dependent on the concentration of VIP of exposure (Fig. 1B). Maximum internalization was obtained with 1 μM VIP and was similar in freshly dispersed muscle cells (68 ± 5% maximal decrease in binding) and cultured muscle cells (71 ± 4% maximal decrease in binding). Maximal internalization of VPAC2 receptors was significantly attenuated in freshly dispersed muscle cells treated with MβCD (26 ± 3% decrease in binding) or in cultured muscle cells transfected with caveolin-1 siRNA (32 ± 4% decrease in binding) (Fig. 1B). These results suggest that VPAC2 receptor internalization was mediated by caveolin-1. Caveolin-1 transfection efficiency was analyzed by immunofluorescence and western blot (Fig. 1C and D).
3.2. Caveolin-1-mediated VPAC2 receptor desensitization
Previous studies in gastric smooth muscle cells have shown that VPAC2 receptors are coupled to activation of adenylyl cyclase via Gs [32]. VPAC2 receptor desensitization was assessed by the decrease in VIP-induced adenylyl cyclase activity after treatment with different concentrations of VIP for 30 min or after treatment for different times with 1 μM VIP. VIP (1 μM)-induced increase in cAMP formation was similar in freshly dispersed muscle cells (545 ± 65% increase above basal cAMP levels of 2.3 ± 0.3 pmol/mg protein) and cultured muscle cells (482 ± 56% increase above basal cAMP levels of 2.8 ± 0.3 pmol/mg protein). The increase in VIP-induced cAMP formation was not affected by treatment of cells with MβCD (524 ± 63% increase) or transfection of cells with caveolin-1 siRNA (462 ± 34% increase), suggesting that caveolin-1 had no effect on adenylyl cyclase activity.
Pretreatment of freshly dispersed muscle cells or cultured smooth muscle cells with VIP, however, caused a decrease in cAMP formation reflecting desensitization of VPAC2 receptors. VPAC2 receptor desensitization, measured after exposure to the ligand, was dependent on time of exposure. VPAC2 receptor desensitization was rapid [half-time (t0.5): ~8 min] and maximal after a 20-min exposure to VIP (Fig. 2A). Maximal desensitization was similar in freshly dispersed muscle cells (75 ± 6% decrease in cAMP formation) and cultured muscle cells (80 ± 2% decrease in cAMP formation). In cells treated with MβCD or expressing caveolin-1 siRNA VPAC2 receptor desensitization was slower [half-time (t0.5): ~11 min] compared to control cells (Fig. 2A). Maximal desensitization of VPAC2 receptors was also significantly attenuated in freshly dispersed muscle cells treated with MβCD (36 ± 3% decrease in cAMP formation) or in cultured muscle cells transfected with caveolin-1 siRNA (43 ± 4% in cAMP formation) (Fig. 2A).
Fig. 2.
Effect of pretreatment with VIP on VPAC2 receptor desensitization in cells treated with MβCD and in cells transfected with caveolin-1 siRNA. Adenylyl cyclase activity in response to VIP was measured as increase in cAMP formation in control cells, and in cells treated with MβCD or expressing caveolin-1 siRNA before (control) and after treatment of the cells with 1 μM VIP for various times up to 30 min (A) or different concentrations of VIP for 30 min (B). Desensitization of VPAC2 receptors was assessed by the decrease in cAMP response after treatment with VIP. Results are expressed as percent of control response to VIP before pretreatment. Basal levels (2.3 ± 0.3 to 2.8 ± 0.3 pmol/mg protein) and increase in response to VIP (15.2 ± 2.1 to 16.3 ± 2.5 pmol/mg protein) were not significantly different in control cells, and in cells treated with MβCD or transfected with caveolin-1 siRNA. Desensitization of VPAC2 receptors was dependent on the concentration of VIP and time of pretreatment in control cells, and in cells treated with MβCD or transfected with caveolin-1 siRNA. Desensitization of VPAC2 receptors, however, was significantly attenuated in cells treated with MβCD or transfected with caveolin-1 siRNA. Values are expressed as means ± SE of 4 experiments.
VPAC2 receptor desensitization was also dependent on the concentration of VIP of exposure (Fig. 2B). Maximal desensitization was obtained with 1 μM VIP and was similar in freshly dispersed muscle cells (78 ± 4% decrease in cAMP formation) and cultured muscle cells (82 ± 5% decrease in cAMP formation). Maximal desensitization of VPAC2 receptors was significantly attenuated in freshly dispersed muscle cells treated with MβCD (40 ± 5% decrease in cAMP formation) or in cultured muscle cells transfected with caveolin-1 siRNA (44 ± 3% decrease in cAMP formation) (Fig. 2B). These results suggest that VPAC2 receptor desensitization was mediated by caveolin-1 and was correlated with the VPAC2 receptor internalization.
To examine whether the decrease in cAMP response after exposure to ligand was due to internalization of receptors or inhibition of adenylyl cylase activity, effect of forskolin (10 μM) on cAMP formation was measured in control cells and in cells treated with VIP for 20 min. Forskolin-stimulated cAMP was similar in control cells (625 ± 15% increase above basal cAMP levels of 2.1 ± 0.2 pmol/mg protein) and in cells pretreated with VIP (592 ± 26% increase above basal cAMP levels of 2.3 ± 0.3 pmol/mg protein), suggesting that the decrease in VIP-induced cAMP formation after exposure to ligand was due to internalization of VPAC2 receptors.
3.3. Src kinase-mediated internalization and desensitization of VPAC2 receptors
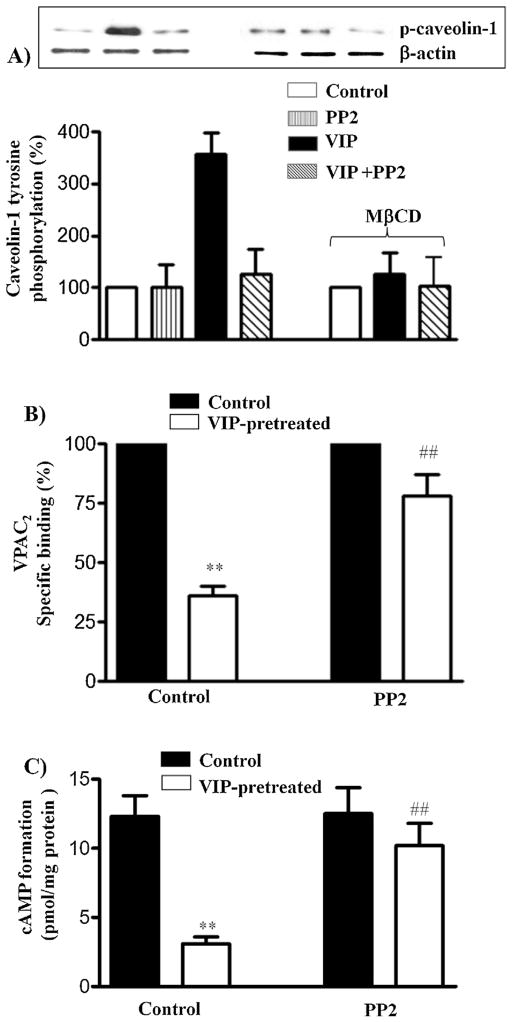
Previous studies have shown that tyrosine phosphorylation of caveolin-1 plays an important role in internalization of receptors via a caveolae-dependent pathway [3,7,24,34,36]. VIP-induced tyrosine phosphorylation of caveolin-1, in the presence or absence of Src kinase inhibitor PP2, was examined in VPAC2 immunoprecipitates using phosphotyrosine antibody. Treatment of cells with VIP induced tyrosine phosphorylation of caveolin-1 that was blocked by PP2 (1 μM) (Fig. 3A). Tyrosine phosphorylation of caveolin-1 was also blocked in cells treated with MβCD, suggesting that internalization of VPAC2 receptors is necessary for activation of Src kinase and tyrosine phosphorylation of caveolin-1.
Fig. 3.
Tyrosine phosphorylation of caveolin-1 and the effect of Src kinase inhibitor PP2 on VPAC2 receptor internalization and desensitization. (A) Control muscle cells and cells treated with MβCD were treated with VIP (1 μM) for 10 min in the presence or absence of Src kinase inhibitor PP2 (1 μM). Control cells were also treated with PP2 in the absence of VIP. Tyrosine phosphorylation of caveolin-1 was examined in caveolin-1 immunoprecipitates using phospho-specific antibody by western blot analysis. (B) Control muscle cells were treated with VIP (1 μM) for 30 min in the presence or absence of Src kinase inhibitor PP2 (1 μM) and 125I-VIP binding to surface receptors was measured. VPAC2 receptor internalization was assessed by the decrease in 125I-VIP binding to surface receptors after pretreatment with VIP. Results are expressed as percent of control specific binding in the absence of pretreatment and the specific binding was similar in control cells and in cells treated with PP2. Internalization of VPAC2 receptors, however, was significantly attenuated in cells treated with PP2. Values are expressed as means ± SE of 4 experiments. **p < 0.01 significant inhibition of specific binding compared to control cells. ##p < 0.01 significant attenuation of internalization in the presence of PP2 compared to cells in the absence of PP2. (C) Adenylyl cyclase activity in response to VIP was measured as increase in cAMP formation in control cells and after treatment of the cells with VIP for 30 min in the presence or absence of PP2 before VIP-induced increase in cAMP formation was measured by radioimmunoassay. Desensitization of VPAC2 receptors was assessed by the decrease in cAMP response after pretreatment with VIP. Results are expressed as percent of control response to VIP before pretreatment. Basal levels (2.1 ± 0.3 to 2.4 ± 0.2 pmol/mg protein) were not significantly different in control cells and in cells treated with PP2. Pretreatment of cell with VIP inhibited cAMP formation (desensitization) in response to subsequent addition of VIP (1 μM). Desensitization of VPAC2 receptors, however, was significantly attenuated in cells treated with PP2. Results are expressed as pmol/mg protein above basal levels. Values are expressed as means ± SE of 4 experiments. **p < 0.01 significant inhibition of cAMP formation compared to control cells. ##p < 0.01 significant attenuation of desensitization in the presence of PP2.
Internalization and desensitization of VPAC2 receptors after 20-min exposure to 1 μM VIP were also attenuated when the cells were treated with VIP in the presence of PP2 (Fig. 3B and C). These results suggest that internalization-induced activation of Src kinase and phosphorylation of caveolin-1 by Src kinase positively regulates caveolin-1-mediated internalization of VPAC2 receptors.
3.4. Desensitization of VPAC2-mediated cAMP formation and muscle relaxation via caveolin-1
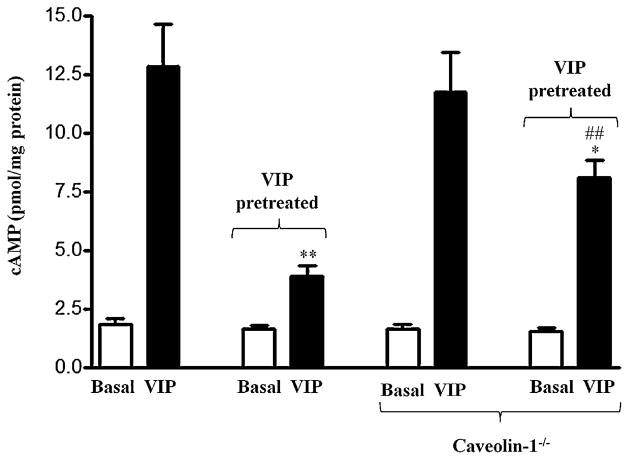
The role of caveolin-1 in desensitization of VPAC2-mediated cAMP formation was also measured in isolated muscle cells and muscle strips from wild type and caveolin-1−/− mice. VIP (1 μM)-induced increase in cAMP formation was similar in muscle cells from wild type (585 ± 24% increase above basal cAMP levels of 1.8 ± 0.2 pmol/mg protein) and caveolin-1−/− mice (616 ± 28% increase above cAMP levels of 1.6 ± 0.3 pmol/mg protein) (Fig. 4). These results are similar to that obtained with treatment of cells with MβCD or transfection of cells with caveolin-1 siRNA and suggest that caveolin-1 had no effect on VIP-induced adenylyl cyclase activity in gastric smooth muscle. Pretreatment of muscle cells with VIP, however, caused a decrease in cAMP formation in muscle cells from both wild type (74 ± 5% decrease in cAMP formation) and caveolin-1−/− mice (29 ± 2% decrease in cAMP formation) reflecting desensitization of VPAC2 receptors (Fig. 4). The decrease in cAMP formation, however, was significantly attenuated (59 ± 4% attenuation of decrease in cAMP formation) in muscle cells from caveolin-1−/− mice (Fig. 4).
Fig. 4.
Effect of pretreatment with VIP on desensitization of VPAC2-mediated adenylyl cyclase activity in gastric muscle from wild type mice and caveolin-1−/− mice. Adenylyl cyclase activity in response to VIP was measured as increase in cAMP formation in gastric muscle cells from wild type and caveolin-1−/− mice before and after treatment of the cells with VIP (1 μM) for 30 min. Desensitization of VPAC2 receptors was assessed by the decrease in cAMP response after treatment with VIP. Results are expressed as pmol of cAMP/mg protein. Pretreatment of cell with VIP inhibited cAMP formation (desensitization) in response to subsequent addition of VIP (1 μM). Desensitization of VPAC2 receptors was significantly attenuated in muscle cells from caveolin-1−/− mice. Values are expressed as means ± SE of 4 experiments.
In gastric smooth muscle, activation of VPAC2 receptors by VIP causes muscle relaxation [14,15,32]. The role of caveolin-1 in desensitization of muscle relaxation was measured in isolated muscle cells and muscle strips from wild type and caveolin-1−/− mice. Desensitization of response was measured as decrease in VIP-induced muscle relaxation after treatment with different concentrations of VIP for 20 min. Relaxation was measured as decrease in KCl-induced contraction. KCl-induced contraction was similar in muscle cells isolated from wild type mice (32 ± 3% decrease in cell length from basal cell length of 102 ± 3 μm) and caveolin-1−/− mice (29 ± 4% decrease in cell length from basal cell length of 98 ± 4 μm). Relaxation of KCl-induced contraction by 1 μM VIP was also similar in muscle cells from wild type mice (72 ± 5% relaxation) and caveolin-1−/− mice (67 ± 6% relaxation). These results suggest that caveolin-1 had no effect on VIP-induced muscle relaxation and this is consistent with the similar increase in cAMP formation in response to VIP in gastric muscle from wild type mice and caveolin-1 −/− mice.
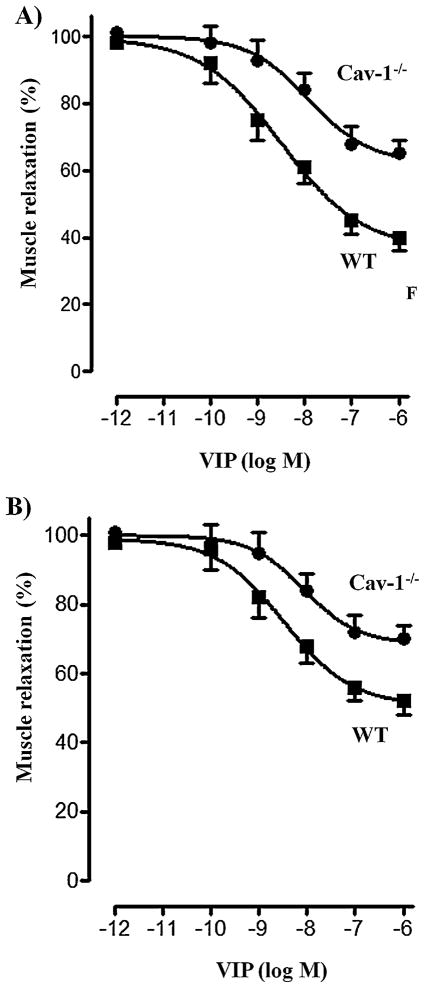
Pretreatment of freshly dispersed muscle cells with VIP caused a decrease in muscle relaxation in response to VIP reflecting desensitization of VPAC2 receptors. VPAC2 receptor desensitization, measured after a 30-min exposure to the ligand, was dependent on the concentration of VIP (Fig. 5A). Maximum desensitization obtained with 1 μM VIP was significantly attenuated in muscle cells from caveolin-1−/− mice (33 ± 3% decrease in relaxation) compared to wild type mice (59 ± 4% decrease in relaxation) (Fig. 5A). These results suggest that VPAC2 receptor desensitization was mediated by caveolin-1 and are correlated with the VPAC2 receptor internalization and desensitization of cAMP response.
Fig. 5.
Effect of pretreatment with VIP on VPAC2 receptor desensitization in gastric muscle from wild type mice and caveolin-1−/− mice. (A) Relaxation in response to VIP was measured as inhibition of KCl-induced contraction in gastric muscle cells from wild type and caveolin-1−/− mice before and after treatment of the cells with various concentrations of VIP for 30 min. Contraction was measures as decrease in cell length in response to KCl (60 mm) and relaxation was measured as decrease in contraction in the presence of VIP (1 μM). Desensitization of VPAC2 receptors was assessed by the decrease in relaxation response after treatment with VIP. Results are expressed as percent of control response to VIP before pretreatment. Control cell lengths (98 ± 4 to 102 ± 3 μm), KCl-induced contraction (29 ± 4% to 32 ± 3% decrease in control cell length) and VIP-induced relaxation (67 ± 6% to 72 ± 5% decrease in KCl-induced contraction) were not significantly different in wild type mice and caveolin-1−/− mice. Pretreatment of cells with VIP inhibited relaxation (desensitization) in response to subsequent addition of VIP (1 μM). Desensitization of VPAC2 receptors, however, was significantly attenuated in muscle cells from caveolin-1−/− mice. Values are expressed as means ± SE of 4 experiments. (B) Relaxation in response to VIP was measured as inhibition of KCl (60 mM)-induced contraction in gastric muscle strips from wild type and caveolin-1−/− mice before and after treatment of the cells with various concentrations of VIP for 30 min. Desensitization of VPAC2 receptors was assessed by the decrease in relaxation response after a 30-min treatment with VIP. Results are expressed as percent of control response to VIP before pretreatment. KCl-induced contraction (11.2 ± 2.8 to 12.5 ± 2.2 g) and VIP-induced relaxation (58 ± 5% to 61 ± 4% decrease in KCl-induced contraction) were not significantly different in wild type mice and caveolin-1−/− mice. Pretreatment of cells with VIP inhibited relaxation (desensitization) in response to subsequent addition of VIP (1 μM). Desensitization of VPAC2 receptors, however, was significantly attenuated in muscle cells from caveolin-1−/− mice. Values are expressed as means ± SE of 4 experiments.
To examine whether the decrease in muscle relaxation is due to internalization of receptors and internalization-dependent inhibition of adenylyl cylase activity, the effect of forskolin on muscle relaxation was measured in control cells and in cells treated with VIP for 20 min. Forskolin-induced muscle relaxation was similar in control cells (62 ± 3% relaxation) and in cells pretreated with VIP (58 ± 4% relaxation), suggesting that the decrease in VIP-induced relaxation after exposure to ligand is due to VPAC2 receptors internalization and desensitization of cAMP response.
Desensitization of VIP-induced relaxation was also examined in muscle strips from wild type and caveolin-1−/− mice in an organ bath experiment. Muscle strips from stomach were allowed to equilibrate to a passive tension of 1 g. KCl (60 mM) induced a sustained contraction (measured as increase in the amplitude of the tonic contraction) was similar in strips from wild type (12.5 ± 2.2 g) and caveolin-1−/− mice (11.2 ± 2.8 g). Similarly, the extent of VIP induced muscle relaxation (measured as decrease in KCl-induced sustained contraction) was similar in muscle strips from wild type (61 ± 4% relaxation) and caveolin-1−/− mice (58 ± 5% relaxation).
Pretreatment of muscle strips with VIP caused a decrease in muscle relaxation in response to VIP reflecting desensitization of VPAC2 receptors. VPAC2 receptor desensitization, measured after a 30-min exposure to the ligand, was dependent on the concentration of VIP (Fig. 5B). Maximum desensitization obtained with 1 μM VIP was significantly attenuated in muscle cells from caveolin-1−/− mice (48 ± 4% decrease in relaxation) compared to wild type mice (29 ± 3% decrease in relaxation) (Fig. 5B). The results confirmed the results obtained in isolated muscle cells.
4. Discussion
Although homologous desensitization of G protein-coupled receptors is initiated by GRK-mediated phosphorylation of the receptors, the pathways by which agonist-occupied receptors are internalized varies greatly among receptors. Our previous studies have shown that Gs-coupled VPAC2 receptors are exclusively phosphorylated by GRK2 and that feedback phosphorylation of GRK2 at Ser685 by PKA leads to an increase in GRK2 activity, GRK2 mediated receptor phosphorylation, internalization, and desensitization of the functional response [32]. In the present study we show that VPAC2 receptor internalization and desensitization are mediated via the caveolin-1-dependent pathway (Fig. 6). The essential role of caveolin-1 in VPAC2 receptor internalization and desensitization was reflected in measurements of VIP-induced VPAC2 receptor internalization, and desensitization of cAMP response and muscle relaxation by several complementary approaches. VIP-induced internalization, determined from the decrease in 125I-VIP binding to VPAC2 receptors, was greatly attenuated by disruption of caveolae in freshly dispersed muscle cells or selective suppression of caveolin-1 in cultured muscle cells. Desensitization of the functional response (adenylyl cyclase activity) also followed the pattern of internalization either by disruption of caveolae in freshly dispersed muscle cells or selective suppression of caveolin-1 in cultured muscle cells. Both approaches had similar effects on the extent and rate of internalization, suggesting that effect of MβCD is not due to non-specific effects of cholesterol depletion. The effect of MβCD or caveolin-1 siRNA on VPAC2 receptor internalization, and desensitization was mimicked by the selective blockade of Src kinase activity, implying an essential role of Src kinase phosphorylation of caveolin-1 in VPAC2 receptor internalization and desensitization. This conclusion is supported by measurement of caveolin-1 phosphorylation. VIP caused tyrosine phosphorylation of caveolin-1 and the phosphorylation was blocked by disruption of caveolae suggesting internalization-dependent stimulation of Src kinase activity. Previous studies have demonstrated tyrosine phosphorylation of caveolin at Y14 and regulation of caveolar endocytic vesicles by reversible phosphorylation [3,7,24,34,36].
Fig. 6.
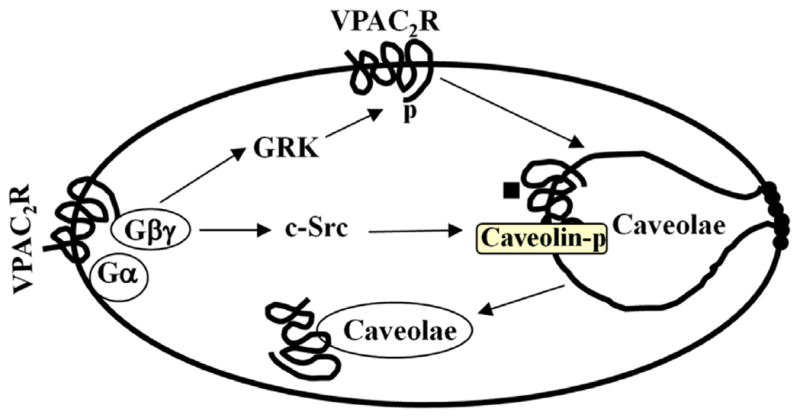
Desensitization of VPAC2 receptors via GRK2-dependent phosphorylation of receptors and Src kinase-dependent phosphorylation of caveolin. Treatment of cells with VIP causes VPAC2 receptor phosphorylation via Gβγ-dependent GRK2 [30] and caveolin-1 phosphorylation via Src kinase. Both GRK2- and caveolin-1-dependent pathways mediate VPAC2 receptor internalization and homologous desensitization.
The role of caveolin-1 in the functional desensitization of VPAC2 receptor was further corroborated by measurement of VIP-induced desensitization of cAMP response and muscle relaxation in gastric muscle from wild type and caveolin-1−/− mice. VIP-induced desensitization of cAMP response was greatly attenuated in gastric muscle from caveolin-1−/− mice compared to wild type mice. Desensitization of muscle relaxation, measured in muscle strips and isolated muscle cells, also followed the pattern of cAMP response by decreasing in gastric muscle from caveolin-1−/− mice.
G protein coupled receptors use several mechanisms for receptor internalization. These include: clathrin-mediated pathway that is dependent on both β-arrestin and dynamin; caveolae-mediated pathway that is dependent on dynamin, but independent of β-arrestin; and a non-caveolae-mediated pathway that is dependent on dynamin, but independent of β-arrestin and clathrin [26]. VPAC2 receptors belong to class II family of GPCRs and other receptors in this family include receptors for peptides such as PACAP, secretin, glucagon, glucagon-like peptide-1 (GLP-1), GLP-2, parathyroid hormone (PTH), gastric inhibitory polypeptide, calcitonin, calcitonin gene-related peptide (CGRP) and corticotropin-releasing factor [20,21,41]. The mechanism and receptor domain(s) involved in rapid desensitization of class II receptors have been documented in some studies. The use of ectopic expression of receptors and dominant-negative mutants has demonstrated the involvement of GRKs in the desensitization of response. Secretin receptor desensitization in HEK293 cells involves GRK2- and GRK5-mediated phosphorylation [37], whereas in NG108-15, GRK6 regulates the endogenous secretin receptor response [13]. Calcitonin receptor desensitization in HEK293 involves exclusively GRK6-mediated phosphorylation [1]. The parathyroid hormone receptor in HEK293 cells is phosphorylated by GRK2, but the receptor endocytosis is independent of phosphorylation [28]. Although both VPAC1 and VPAC2 receptors are rapidly internalized they differ in their trafficking pattern. Internalization of VPAC2 receptors, but not VPAC1 receptors, leads to rapid recycling (within 2 h) to cell surface [22,23]. VPAC1 receptor internalization involves GRK-mediated phosphorylation, β-arrestin translocation, and dynamin-dependent mechanisms. Co-transfection of GRK2, -3, -5 and -6, but not GRK4 enhanced VPAC1 receptor phosphorylation and desensitization [38].
Studies by Bokaei et al. [4] have identified a five-transmembrane isoforms of VPAC2 receptors in human tissues. This variant lacks the ability to stimulate Gs proteins and adenylyl cyclase activity, but has the ability to stimulate tyrosine kinase activity leading to phosphorylation of distinct set of proteins compared to activation of seven-transmembrane VPAC2 receptors. Two distinct splice variants of VPAC2 receptors lacking 14 amino acids in the seventh transmembrane domain and 114 amino acids in the third cytoplasmic loop were identified [31]. These isoforms are shown to bind VIP, albeit with different affinities, and activate adenylyl cyclase. Neither the expression of these splice variant in gastric smooth muscle nor the regulation of their internalization is known.
Site directed mutagenesis studies in CHO cells demonstrated the importance of several Ser and Thr sites in C-terminus and one Ser250 in intracellular domain 2 as potential candidates for VIP-induced VPAC1 receptor phosphorylation and phosphorylation-dependent VPAC1 receptor internalization [22]. Phosphorylation of Ser447 in the C-terminal tail, one of the potential GRK phosphorylation sites, is crucial for short-term VIP-induced desensitization, but not for down-regulation and internalization of human VPAC1 receptors [29]. These studies clearly demonstrate that the mechanism of receptor internalization and desensitization is receptor-specific. In smooth muscle, VPAC2 receptor internalization and desensitization involve GRK2-mediated phosphorylation, however, the involvement of β-arrestin and dynamin in the caveolae-mediated internalization was not explored in the present study. Previous studies have shown that caveolin-1 can also directly interact with GRK2 and regulate its activity [6]. Thus, it is possible that inhibition of VPAC2 receptor internalization and desensitization in cells lacking caveolin-1 could be due to inhibition of GRK2 activity. However, muscarinic m3 receptor internalization, which is also dependent on GRK2 in gastric smooth muscle, was not affected by suppression of caveolin-1 excluding the possible role of caveolin-1-induced inhibition of GRK2 in the suppression of VPAC2 receptor internalization and desensitization (data not shown).
There is a growing appreciation for the role of internalization-dependent signaling pathways in the regulation of physiological function. The functional significance of distinct pathways of internalization is emerging. Recent studies have shown that transforming growth factor β (TGFβ) receptors are internalized via both clathrin-dependent and clathrin-independent caveolae-dependent mechanisms [8]. Internalization via clathrin-dependent pathway promotes TGFβ signaling, whereas internalization via caveolae-dependent pathway promotes receptor turnover [11]. In the present study, we show that caveolin-1 regulates homologous desensitization of VPAC2 receptors but not the initial response to VIP. Disruption of caveolae, suppression of caveolin-1 or caveolin-1−/− gene deletion had no effect on VIP-induced cAMP formation and muscle relaxation.
In summary, herein we define a function for caveolin-1 in VPAC2 receptor regulation in smooth muscle cells. We provided compelling evidence that endogenous caveolin-1 is essential for VPAC2 receptor internalization and desensitization of response. This regulatory mechanism may play an important role in VPAC2 receptor function, and thus, smooth muscle tone. The molecular mechanisms that specify the targeting of VPAC2 receptors to caveolae is not known. Genetic deletion of caveolin-1 has been associated with various cardiovascular and pulmonary complications, and hence, understanding the role of caveolin-1 in mediating the desensitization of response to VIP following VPAC2 receptors activation may represent a new avenue for the treatment of gastrointestinal motility disorders.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant to K.S.M. (DK28300).
References
- 1.Aiyar N, Disa J, Dang K, Pronin AN, Benovic JL, Nambi P. Involvement of G protein-coupled receptor kinase-6 in desensitization of CGRP receptors. Eur J Pharmacol. 2000;403(1/2):1–7. doi: 10.1016/s0014-2999(00)00419-2. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 3.Aoki T, Nomura R, Fujimoto T. Tyrosine phosphorylation of caveolin-1 in the endothelium. Exp Cell Res. 1999;253(2):629–36. doi: 10.1006/excr.1999.4652. [DOI] [PubMed] [Google Scholar]
- 4.Bokaei PB, Ma XZ, Byczynski B, Keller J, Sakac D, Fahim S, et al. Identification and characterization of five-transmembrane isoforms of human vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors. Genomics. 2006;88:791–800. doi: 10.1016/j.ygeno.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Bornstein JC, Costa M, Grider JR. Enteric motor and interneuronal circuits controlling motility. Neurogastroenterol Motil. 2004;16(1):34–8. doi: 10.1111/j.1743-3150.2004.00472.x. [DOI] [PubMed] [Google Scholar]
- 6.Carman CV, Lisanti MP, Benovic JL. Regulation of G protein-coupled receptor kinases by caveolin. J Biol Chem. 1999;274(13):8858–64. doi: 10.1074/jbc.274.13.8858. [DOI] [PubMed] [Google Scholar]
- 7.Caselli A, Taddei ML, Manao G, Camici G, Ramponi G. Tyrosine-phosphorylated caveolin is a physiological substrate of the low M(r) protein-tyrosine phosphatase. J Biol Chem. 2001;276(22):18849–54. doi: 10.1074/jbc.M100705200. [DOI] [PubMed] [Google Scholar]
- 8.Chen YG. Endocytic regulation of TGF-beta signaling. Cell Res. 2009;19(1):58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- 9.Cooke HJ. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann N Y Acad Sci. 2000;915:77–80. doi: 10.1111/j.1749-6632.2000.tb05225.x. [DOI] [PubMed] [Google Scholar]
- 10.Couvineau A, Ceraudo E, Tan YV, Nicole P, Laburthe M. The VPAC1 receptor: structure and function of a class B GPCR prototype. Front Endocrinol (Lausanne) 2012;3:139. doi: 10.3389/fendo.2012.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol. 2003;5(5):410–21. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 12.Furness JB, Costa M. Projections of intestinal neurons showing immunoreactivity for vasoactive intestinal polypeptide are consistent with these neurons being the enteric inhibitory neurons. Neurosci Lett. 1979;15(2/3):199–204. doi: 10.1016/0304-3940(79)96113-5. [DOI] [PubMed] [Google Scholar]
- 13.Ghadessy RS, Willets JM, Kelly E. G protein-coupled receptor kinase 6 (GRK6) selectively regulates endogenous secretin receptor responsiveness in NG108-15 cells. Br J Pharmacol. 2003;138(4):660–70. doi: 10.1038/sj.bjp.0705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grider JR, Cable MB, Bitar KN, Said SI, Makhlouf GM. Vasoactive intestinal peptide. Relaxant neurotransmitter in tenia coli of the guinea pig. Gastroenterology. 1985;89(1):36–42. [PubMed] [Google Scholar]
- 15.Grider JR, Rivier JR. Vasoactive intestinal peptide (VIP) as transmitter of inhibitory motor neurons of the gut: evidence from the use of selective VIP antagonists and VIP antiserum. J Pharmacol Exp Ther. 1990;253(2):738–42. [PubMed] [Google Scholar]
- 16.Hardin CD, Vallejo J. Caveolins in vascular smooth muscle: form organizing function. Cardiovasc Res. 2006;69(4):808–15. doi: 10.1016/j.cardiores.2005.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hnasko R, Lisanti MP. The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol Interv. 2003;3(8):445–64. doi: 10.1124/mi.3.8.445. [DOI] [PubMed] [Google Scholar]
- 18.Holtmann MH, Roettger BF, Pinon DI, Miller LJ. Role of receptor phosphorylation in desensitization and internalization of the secretin receptor. J Biol Chem. 1996;271(38):23566–71. doi: 10.1074/jbc.271.38.23566. [DOI] [PubMed] [Google Scholar]
- 19.Insel PA, Head BP, Ostrom RS, Patel HH, Swaney JS, Tang CM, et al. Caveolae and lipid rafts: G protein-coupled receptor signaling microdomains in cardiac myocytes. Ann N Y Acad Sci. 2005;1047:166–72. doi: 10.1196/annals.1341.015. [DOI] [PubMed] [Google Scholar]
- 20.Laburthe M, Couvineau A. Molecular pharmacology and structure of VPAC receptors for VIP and PACAP. Regul Pept. 2002;108(2/3):165–73. doi: 10.1016/s0167-0115(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 21.Langer I, Robberecht P. Molecular mechanisms involved in vasoactive intestinal peptide receptor activation and regulation: current knowledge, similarities to and differences from the A family of G-protein-coupled receptors. Biochem Soc Trans. 2007;35(Pt 4):724–8. doi: 10.1042/BST0350724. [DOI] [PubMed] [Google Scholar]
- 22.Langlet C, Langer I, Vertongen P, Gaspard N, Vanderwinden JM, Robberecht P. Contribution of the carboxyl terminus of the VPAC1 receptor to agonist-induced receptor phosphorylation, internalization, and recycling. J Biol Chem. 2005;280(30):28034–43. doi: 10.1074/jbc.M500449200. [DOI] [PubMed] [Google Scholar]
- 23.Langlet C, Gaspard N, Nachtergael I, Robberecht P, Langer I. Comparative efficacy of VIP and analogs on activation and internalization of the recombinant VPAC2 receptor expressed in CHO cells. Peptides. 2004;25(12):2079–86. doi: 10.1016/j.peptides.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by Src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by c-Src in vivo. J Biol Chem. 1996;271(7):3863–8. [PubMed] [Google Scholar]
- 25.Lin FT, Krueger KM, Kendall HE, Daaka Y, Fredericks ZL, Pitcher JA, et al. Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J Biol Chem. 1997;272(49):31051–7. doi: 10.1074/jbc.272.49.31051. [DOI] [PubMed] [Google Scholar]
- 26.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115(Pt 3):455–65. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 27.Mahavadi S, Huang J, Sriwai W, Rao KR, Murthy KS. Cross-regulation of VPAC2 receptor internalization by m2 receptors via c-Src-mediated phosphorylation of GRK2. Regul Pept. 2007;139(1–3):109–14. doi: 10.1016/j.regpep.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malecz N, Bambino T, Bencsik M, Nissenson RA. Identification of phosphor-ylation sites in the G protein-coupled receptor for parathyroid hormone, receptor phosphorylation is not required for agonist-induced internalization. Mol Endocrinol. 1998;12(12):1846–56. doi: 10.1210/mend.12.12.0203. [DOI] [PubMed] [Google Scholar]
- 29.Marie JC, Rouyer-Fessard C, Couvineau A, Nicole P, Devaud H, El Benna J, et al. Serine 447 in the carboxyl tail of human VPAC1 receptor is crucial for agonist-induced desensitization but not internalization of the receptor. Mol Pharmacol. 2003;64(6):1565–74. doi: 10.1124/mol.64.6.1565. [DOI] [PubMed] [Google Scholar]
- 30.McDonald TP, Dinnis DM, Morrison CF, Harmar AJ. Desensitization of the human vasoactive intestinal peptide receptor (hVIP2/PACAP R): evidence for agonist-induced receptor phosphorylation and internalization. Ann N Y Acad Sci. 1998;865:64–72. doi: 10.1111/j.1749-6632.1998.tb11164.x. [DOI] [PubMed] [Google Scholar]
- 31.Miller AL, Verma D, Grinninger C, Huang MC, Goetzl EJ. Functional splice variants of the type II G protein-coupled receptor (VPAC2) for vasoactive intestinal peptide in mouse and human lymphocytes. Ann N Y Acad Sci. 2006;1070:422–6. doi: 10.1196/annals.1317.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murthy KS, Mahavadi S, Huang J, Zhou H, Sriwai W. Phosphorylation of GRK2 by PKA augments GRK2-mediated phosphorylation, internalization, and desensitization of VPAC2 receptors in smooth muscle. Am J Physiol Cell Physiol. 2008;294(2):C477–87. doi: 10.1152/ajpcell.00229.2007. [DOI] [PubMed] [Google Scholar]
- 33.Nachtergael I, Gaspard N, Langlet C, Robberecht P, Langer I. Asn229 in the third helix of VPAC1 receptor is essential for receptor activation but not for receptor phosphorylation and internalization: comparison with Asn216 in VPAC2 receptor. Cell Signal. 2006;18(12):2121–30. doi: 10.1016/j.cellsig.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127(5):1199–215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–62. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 36.Shajahan AN, Tiruppathi C, Smrcka AV, Malik AB, Minshall RD. Gβγ activation of Src induces caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279(46):48055–62. doi: 10.1074/jbc.M405837200. [DOI] [PubMed] [Google Scholar]
- 37.Shetzline MA, Premont RT, Walker JK, Vigna SR, Caron MG. A role for receptor kinases in the regulation of class II G protein-coupled receptors. Phosphorylation and desensitization of the secretin receptor. J Biol Chem. 1998;273(12):6756–62. doi: 10.1074/jbc.273.12.6756. [DOI] [PubMed] [Google Scholar]
- 38.Shetzline MA, Walker JK, Valenzano KJ, Premont RT. Vasoactive intestinal polypeptide type-1 receptor regulation. Desensitization, phosphorylation, and sequestration. J Biol Chem. 2002;277(28):25519–26. doi: 10.1074/jbc.M201815200. [DOI] [PubMed] [Google Scholar]
- 39.Sriwai W, Zhou H, Murthy KS. G(q)-dependent signalling by the lysophosphatidic acid receptor LPA(3) in gastric smooth muscle: reciprocal regulation of MYPT1 phosphorylation by rho kinase and cAMP-independent PKA. Biochem J. 2008;411(3):543–51. doi: 10.1042/bj20071299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teng B, Murthy KS, Kuemmerle JF, Grider JR, Makhlouf GM. Selective expression of vasoactive intestinal peptide (VIP)2/pituitary adenylate cyclase-activating polypeptide (PACAP)3 receptors in rabbit and guinea pig gastric and tenia coli smooth muscle cells. Regul Pept. 1998;77(1–3):127–34. doi: 10.1016/s0167-0115(98)00112-8. [DOI] [PubMed] [Google Scholar]
- 41.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61(3):283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Duan W, Cheung NS, Huang Z, Shao K, Li QT. Pituitary adenylate cyclase-activating polypeptide induces translocation of its G-protein-coupled receptor into caveolin-enriched membrane microdomains, leading to enhanced cyclic AMP generation and neurite outgrowth in PC12 cells. J Neurochem. 2007;103(3):1157–67. doi: 10.1111/j.1471-4159.2007.04813.x. [DOI] [PubMed] [Google Scholar]