Abstract
A small library of arylthioamides 1–12 was easily synthesized, and their H2S-releasing properties were evaluated both in the absence or in the presence of an organic thiol such as l-cysteine. A number of arylthioamides (1–3 and 7) showed a slow and l-cysteine-dependent H2S-releasing mechanism, similar to that exhibited by the reference slow H2S-releasing agents, such as diallyl disulfide (DADS) and the phosphinodithioate derivative GYY 4137. Compound 1 strongly abolished the noradrenaline-induced vasoconstriction in isolated rat aortic rings and hyperpolarized the membranes of human vascular smooth muscle cells in a concentration-dependent fashion. Finally, a significant reduction of the systolic blood pressure of anesthetized normotensive rats was observed after its oral administration. Altogether these results highlighted the potential of arylthioamides 1–3 and 7 as H2S-donors for basic studies, and for the rational design/development of promising pharmacotherapeutic agents to treat cardiovascular diseases.
Keywords: Hydrogen sulphide, H2S-releasing drugs, cardiovascular system, hypertension, potassium channels, hybrid drugs, vascular smooth muscle, thioamide
Hydrogen sulfide (H2S) is emerging as a hot topic in the field of drug discovery. Indeed, it is recognized as an important physiological gasotransmitter in mammalian body. Particularly, it plays a key role in regulating the cardiovascular homeostasis by acting as a vasodilator; this effect is mainly due to the activation of vascular ATP-sensitive (KATP) and voltage-gated (Kv7) potassium channels.1−4 In mice models, blunted levels of H2S in blood, heart, and aorta are associated with increased blood pressure and decreased endothelium-mediated vasorelaxant effects.5 In addition, in experimental models of hypertension, it has been evidenced that exogenous H2S can effectively prevent the progression of the pathology6 and decrease the blood pressure values.3 Therefore, impaired production of endogenous H2S is likely to contribute to the pathogenesis of hypertension, highlighting the great potential usefulness of the pharmacological modulation of this gasotransmitter.7,8 As the administration of gaseous H2S is greatly limited by the risk of a poor posology control and overdose, the use of appropriate chemicals, behaving as H2S-releasing agents, appears to be safer. Thus, compounds exhibiting the pharmacodynamic profile of H2S-releasing agents may be viewed as both powerful tools for basic studies and new potential pharmacotherapeutic agents for treatment of many cardiovascular diseases.
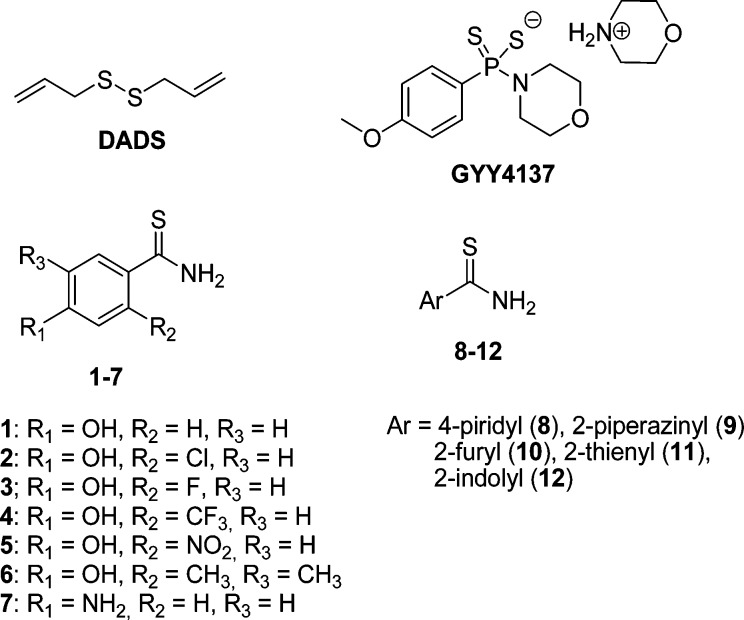
NaHS is the prototypical example of a H2S-generating agent: it is a rapid H2S donor and the most widely used H2S donor for experimental purposes. However, this salt is not suitable for clinical purposes, as the quick release of H2S may cause adverse effects, such as a rapid and excessive lowering of blood pressure.9 For a safer and effective pharmacological administration, ideal H2S donors should generate H2S with slower releasing rates.10,11 Organic polysulphides of garlic, such as diallyl disulfide (DADS, Chart 1), act as H2S-releasing compounds, with a relatively slow mechanism, requiring the presence of reduced glutathione.12 Other examples of original synthetic H2S-releasing agents have been described in the literature, including a number of aminothiol derivatives,13 and the phosphinodithioate derivative GYY4137 (morpholin-4-ium 4-methoxyphenylmorpholinophosphinodithioate, Chart 1).14 Furthermore, some H2S-releasing chemical moieties, such as the dithiolethione,15−18 the thioamide, and the isothiocyanate, have been used for synthesizing multifunctional drugs.19,20
Chart 1. Structures of Known and Newly Synthesized H2S-Releasing Agents 1–12.
In this work, a small library of arylthioamides (compounds 1–12, Chart 1) was easily prepared through suitable synthetic routes and evaluated for their H2S-releasing properties. The p-hydroxybenzothioamide 1(19,20) was selected as lead compound to build the library by inserting a number of modifications on the aromatic moiety: (i) introduction of different groups (Cl, F, CF3, NO2, and CH3) at the 2- and/or 5-position of the phenyl ring (2–6); (ii) replacement of the 4-hydroxy group with an amino moiety (7); and (iii) replacement of the phenyl ring with electron poor or electron rich heterocycles such as pyridine (8) and piperazine (9), or furane (10), thiophene (11), and indole (12), respectively (Chart 1). Finally, compound 1 was submitted to further experimental protocols aimed to evaluate the vasorelaxing effects on rat aortic rings, the membrane hyperpolarizing activity on human vascular smooth muscle (VSM) cells and the effects on the blood pressure of normotensive rats.
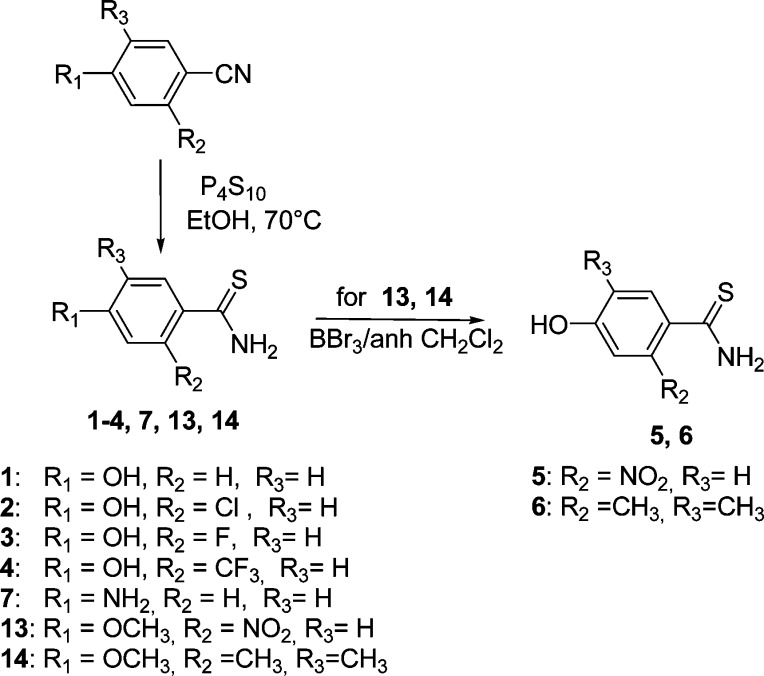
The target compounds 1–4 and 7 were easily obtained by reaction of the appropriate benzonitrile and P4S10 in ethanol at 70 °C for 10 h (Scheme 1). The same procedure allowed the preparation of the methoxy-substituted intermediates 13 and 14, which were demethylated with BBr3 in dry dichloromethane to yield derivatives 5 and 6 (Scheme 1).
Scheme 1. Synthesis of 1–7, 13, and 14.
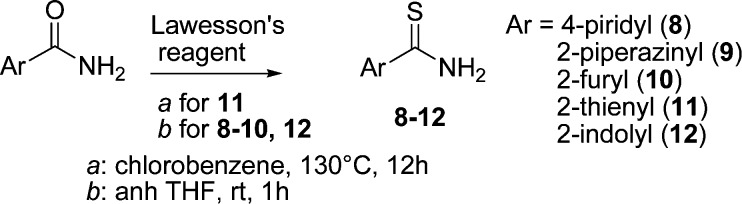
The heterocyclic compounds 8–12 were prepared from the corresponding amides by treatment with Lawesson’s reagent in chlorobenzene at 130 °C for 12 h and in dry THF at room temperature for 12 h, for 11 and 8–10, 12, respectively (Scheme 2). All the products were finally purified by recrystallization from toluene or flash chromatography (Supporting Information).
Scheme 2. Synthesis of 8–12.
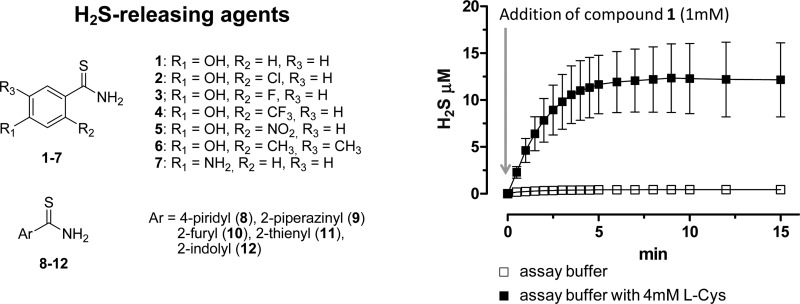
In order to characterize the potential H2S-releasing properties of arylthioamides 1–12, all the compounds were added at 1 mM concentration to the assay buffer (pH 7.4, 35–37 °C). The generation of H2S was evaluated by an amperometric approach, allowing to have a real-time determination of the H2S-release and thus to perform a qualitative/quantitative description of the process. In addition, since the release of H2S from DADS is reported to be a process, which requires the presence of organic thiols such as reduced glutathione,12 the experiments were also performed in the presence of 4 mM l-cysteine. NaHS, DADS, and GYY4137 were also assayed for comparison purposes. As expected, the addition of 1 mM NaHS at pH 7.4, both in the presence or in the absence of l-cysteine, was followed by an immediate formation of high concentration of H2S (about 200 μM). The concentration of H2S showed a rapid fall (by about 40%) in the first two minutes after the addition of NaHS, followed by a slower and apparently constant decline in the remaining time (data not shown).
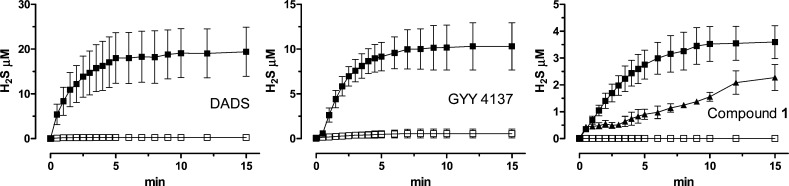
In order to obtain a satisfactory description of the kinetics of those compounds generating H2S with a slow and progressive process, the parameters of Cmax (maximal concentration of H2S at the steady state) and t1/2 (time required to reach a concentration = 50% of Cmax) were extrapolated from the curves H2S-release vs time. Figure 1 reports the curves H2S-release vs time for DADS, GYY4137, and 1, taken as representative of the thioamide series. The values of Cmax and t1/2 of the tested compounds are listed in Table 1.
Figure 1.
Curves describe the increase of H2S concentration, with respect to time, following the incubation of DADS, GYY 4137, and 1 in the assay buffer, in the absence (white squares) or in the presence of l-cysteine (black squares) or glutathion (black triangles). H2S was recorded by amperometry; the vertical bars indicate the SEM.
Table 1. Parameters of Cmax and t1/2 Relative to the H2S-Releasing Effects Exhibited by the Arylthioamides 1-12 and Reference Drugs, after Their Incubation in the Assay Buffer at Physiological pH and Temperature, in the Absence or in Presence (+l-Cysteine) of 4 mM l-Cysteine; nc = Not Calculated.
| assay buffer |
assay buffer + l-cysteine |
|||
|---|---|---|---|---|
| compound | Cmax (μM) | t1/2 (min) | Cmax (μM) | t1/2 (min) |
| 1 | <2 | nc | 3.6 ± 0.6 | 2.1 ± 0.2 |
| 2 | <2 | nc | 10.8 ± 2.6 | 2.6 ± 0.8 |
| 3 | <2 | nc | 15.2 ± 3.9 | 2.2 ± 0.7 |
| 4 | <2 | nc | <2 | nc |
| 5 | <2 | nc | <2 | nc |
| 6 | 6.1 ± 0.3 | 5.0 ± 0.3 | 11.6 ± 0.3 | 4.1 ± 0.4 |
| 7 | <2 | nc | 6.0 ± 2.8 | 4.3 ± 1.0 |
| 8 | 3.1 ± 1.1 | 3.1 ± 0.6 | 16.1 ± 5.0 | 3.1 ± 0.2 |
| 9 | 14.8 ± 0.5 | 13.7 ± 1.7 | 21.4 ± 1.0 | 6.4 ± 0.6 |
| 10 | 7.9 ± 0.7 | 7.5 ± 0.5 | 10.6 ± 0.7 | 5.8 ± 0.4 |
| 11 | 4.7 ± 0.4 | 1.8 ± 0.4 | 5.7 ± 0.5 | 1.5 ± 0.4 |
| 12 | 14.4 ± 0.6 | 11.7 ± 1.0 | 15.9 ± 1.6 | 9.2 ± 0.7 |
| DADS | <2 | nc | 19.4 ± 5.5 | 1.5 ± 0.3 |
| GYY4137 | <2 | nc | 10.3 ± 2.6 | 2.5 ± 0.8 |
The incubation of DADS and GYY4137 in the assay buffer led to a negligible formation of H2S, while in the presence of l-cysteine, a slow and significant release of H2S was observed. Analogously, in the assay buffer alone, p-hydroxybenzothioamide 1 did not show significant H2S-releasing properties. In contrast, 1 exhibited an l-cysteine-dependent release of H2S (Figure 1 and Table 1). In order to evaluate the possible influence of other organic thiols, the H2S-releasing properties of 1 were also tested in the presence of reduced glutathion (GHS). Like l-cysteine, even GSH triggered a significant, but slower, release of H2S from 1 (Figure 1 and Table 1).
As concerns the hydroxybenzothioamide derivatives 2–6 (Table 1), the incubation of 2 (2-Cl), 3 (2-F), and 6 [3,5-(CH3)2] led to a l-cysteine-dependent release of H2S. The Cmax parameters of 2, 3 and 6 were higher than that exhibited by 1 and were comparable to those of the reference drugs DADS and GYY4137. Moreover, it should be noted that in the absence of l-cysteine, the H2S-release from 2 and 3 was negligible, whereas the release from 6 was lower than that observed in the presence of the aminoacid, although it was still significant. Compounds 4 and 5, bearing strong electron-withdrawing groups, such as 2-CF3 and 2-NO2, respectively, showed l-cysteine-dependent H2S-releasing properties significantly lower with respect to the parent compound 1. Finally, the replacement of the 4-OH group of 1 with a primary amino moiety (see compound 7) led to a slight, albeit not significant, improvement of the H2S-release.
Mixed results are associated with the substitution of the phenyl ring with electron poor or electron rich heterocycles (8–12). In particular, the pyridylthioamide 8 (electron poor) and the thienylthioamide 11 (electron rich) both exhibited a modest but significant generation of H2S in the absence of l-cysteine; whereas, in the presence of the aminoacid, compound 8 showed a dramatically increased Cmax, a feature not possessed by compound 11. The heterocyclic derivatives 9 (electron poor), 10, and 12 (electron rich) exhibited high and l-cysteine-independent H2S-releasing effects. The profile of l-cysteine-independent H2S donor of deivatives 9, 10, and 12 is likely to be due to an easy and spontaneous hydrolysis of the H2S-releasing moiety.
This first phase of the experimental work led to identify a number of H2S-releasing arylthioamides (1–3 and 7) that showed an l-cysteine-dependent mechanism. Such a profile has been suggested to be convenient for the development of a potential drug, as this feature may provide a selective H2S-release in a biological environment (i.e., in the presence of endogenous organic thiols such as l-cysteine, reduced glutathion, etc.).
Compound 1 was selected for further pharmacological studies on the basis of the following issues: (i) 1 showed a thiol-dependent H2S-releasing profile; (ii) although it has been already reported as a H2S-releasing side-chain in naproxen analogues endowed with reduced toxicity,19,20 its H2S-mediated cardiovascular effects have not been yet specifically investigated.
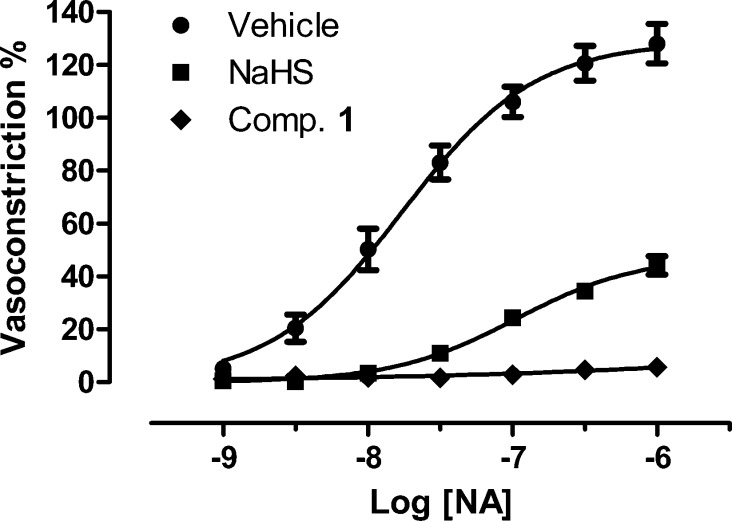
First, the influence of 1 (or the relative vehicle) on the vasoconstrictor effects of noradrenaline (NA) was tested in isolated rat aortic rings and compared with that of NaHS (Figure 2).11 NA produced a strong contractile response, with an Emax of 128 ± 7 and a potency value (pEC50) of 7.76 ± 0.08. In the presence of 1 mM NaHS, the vasoconstrictor effects of NA were strongly inhibited (Emax 44 ± 3; pEC50 6.98 ± 0.09). It should be highlighted that, at pH 7.4 and 37 °C, 1 mM NaHS is expected to readily produce an initial and transient concentration of H2S of about 200 μM, due to the protonation of the hydrosulphide anion.21 Such a theoretical hypothesis is well confirmed by the above experimental data. Interestingly, the pretreatment of the aortic rings with compound 1 (1 mM) almost completely abolished the ability of NA to evoke any vasoconstriction (Figure 2).
Figure 2.
Concentration–response curves to NA, obtained on endothelium-denuded rat aortic rings preincubated with NaHS (squares), 1 (diamonds), or the corresponding vehicle (spots). The effects are expressed as a % of the contractile responses previously induced by the administration of 60 mM KCl. The vertical bars indicate the SEM.
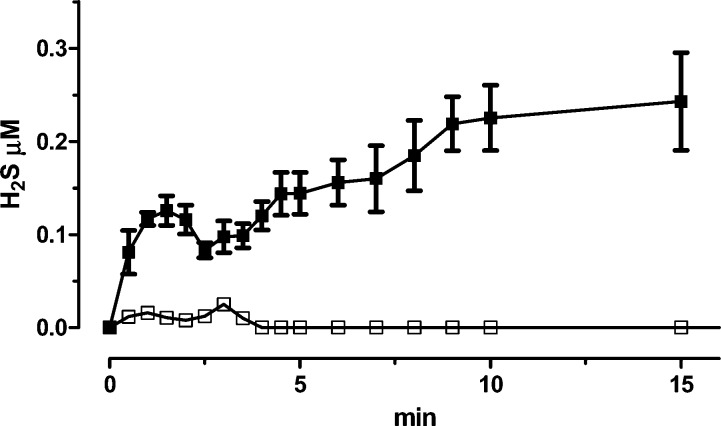
Noteworthy, the previous amperometric measurements indicated that 1 ensured a long-lasting release of relatively low concentrations of H2S, requiring the presence of l-cysteine or glutathion; however, in this experimental setup, exogenous l-cysteine or glutathion were not added. Therefore, the above experimental result suggests that (a) the amounts of organic thiols endogenously present in the biological sample (i.e., the aortic rings) can ensure the release of H2S from 1; (b) a prolonged presence of relatively low concentrations of H2S seems to be highly effective in modulating the activity of vasoconstrictor agents, such as NA. In order to confirm the above hypotheses, compound 1 was also incubated in Tyrode buffer (i.e., in the same conditions of the functional experiments), in the presence or in the absence of rat aortic tissue. Neither l-cysteine nor glutathion were exogenously added. The formation of H2S was continuously evaluated by amperometry. In the presence of the vascular tissue (rat aortic rings), the addition of compound 1 led to a significant time-dependent formation of H2S. Fifteen minutes after the incubation, the concentration of H2S in the Tyrode buffer was 0.24 ± 0.05 μM (Figure 3). This evidence indicates that compound 1 is effectively transformed into H2S, by means of biomolecules (probably, organic thiols) endogenously present in the vascular tissue; in particular, effective H2S concentrations are likely to be produced in the intracellular compartment (i.e., where the levels of endogenous thiols are highly available). In fact, in the absence of the vascular tissue, no significant production of H2S was detected (Figure 3).
Figure 3.
Curves describe the increase of H2S concentration, with respect to time, following the incubation of 1 in Tyrode buffer, in the absence (white squares) or in the presence of aortic tissue (black squares). H2S was recorded by amperometry; the vertical bars indicate the SEM.
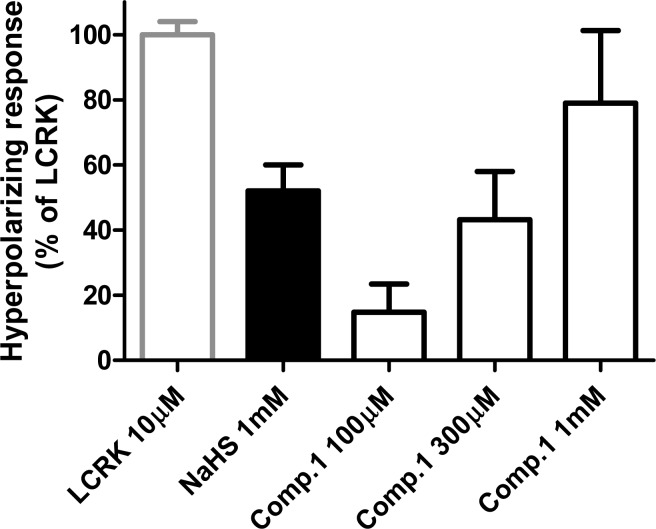
As the vasodilator effects of H2S are largely mediated by the activation of KATP channels and consequent membrane hyperpolarization of VSM cells,2−4 it was thought interesting to evaluate the effects of 1 and NaHS on the membrane potential of cultured human VSM cells (HASMCs). Furthermore, the KATP-activator levcromakalim (LCRK) was selected as reference hyperpolarizing agent. As shown in Figure 4, the administration of NaHS caused a significant membrane hyperpolarization of HASMCs; the maximal hyperpolarization induced by 1 mM NaHS was 52 ± 8% of that induced by LCRK (10 μM). Compound 1 hyperpolarized the membranes of VSM cells in a concentration-dependent fashion (Figures 3 and 4), showing a 79 ± 22% hyperpolarizing effect (expressed as a % of the effect produced by LCRK at 10 μM) at the highest concentration (1 mM). As already observed in rat aortic rings, these results indicate that the release of H2S from 1 can be promoted by the organic thiols endogenously present in the HASMCs and that the long-lasting presence of relatively low concentrations of H2S is more effective than a high and transient peak concentration.
Figure 4.
Hyperpolarizing effects induced by LCRK, NaHS, and increasing concentrations of 1 in HASMCs. Data are calculated as changes in relative fluorescence and then expressed as a % of the maximal effect induced by LCRK. The vertical bars indicate the SEM.
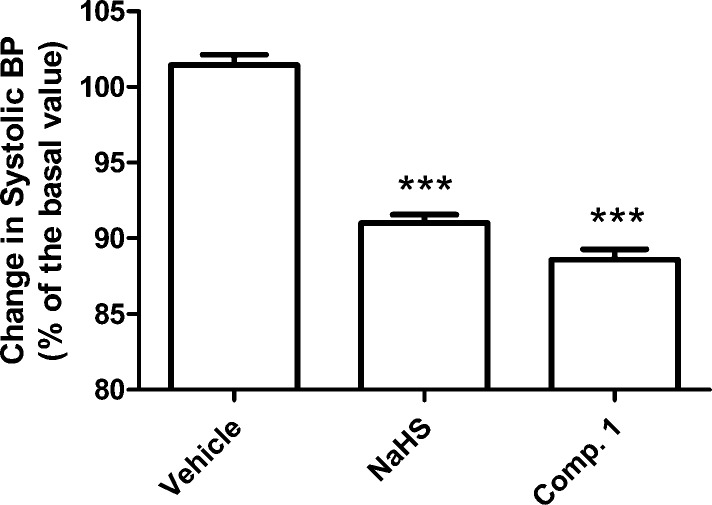
Finally, the influence of 1 and NaHS on the blood pressure of normotensive rats was tested. The anesthetized normotensive rats showed a basal level of systolic pressure of 134 ± 2 mmHg. After oral administration of the vehicle, no significant change of the systolic blood pressure was observed (102 ± 1% of the basal levels), whereas the systolic blood pressure of the animals was significantly reduced after oral administration of 0.1 mg/kg NaHS (91 ± 1% of the basal levels) and equimolar doses of 1 (89 ± 1% of the basal levels); Figure 5.
Figure 5.
Histograms represent the mean levels of systolic pressure, recorded in normotensive rats, 20 min after the oral administration of NaHS (0.1 mg/kg), compound 1 (equimolar dose), or their vehicle. Data are calculated as % of the basal systolic blood pressure, recorded before the administration. The vertical bars indicate the SEM; *** = significantly different (P < 0.001) from vehicle.
In conclusion, this work furnished a small library of thioamides showing different H2S-releasing rates. Some derivatives (1–3 and 7) exhibited smart H2S-releasing properties triggered by the presence of organic thiols, while other derivatives (6 and 8–12) were thiol-independent H2S donors. The p-hydroxybenzothioamide 1 produced typical vascular effects of H2S, both in in vitro and in vivo experiments. Specifically, compound 1, which was selected as representative for investigating the potential vascular effects of all the analogues of this series, inhibited the NA-induced vasoconstriction in isolated rat aortic rings, hyperpolarized the membranes of human HASMCs, and reduced the systolic blood pressure after oral administration. These thioamide derivatives might represent useful tools for the rational development of promising H2S-releasing agents addressed to the therapeutic treatment of cardiovascular diseases.
Acknowledgments
This article has been supported by the ‘‘Regional Health Research Program 2009’’ of Regione Toscana, Italy.
Glossary
Abbreviations
- DADS
diallyl disulfide
- GYY4137
morpholin-4-ium 4-methoxyphenyl-morpholino-phosphinodithioate
- NA
noradrenaline
- SEM
standard error of mean
- VSM
vascular smooth muscle
Supporting Information Available
Synthesis, characterization, and pharmacological studies of compounds 1–12. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- Hosoki R.; Matsuki N.; Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Ndisang J. F.; Tang G.; Cao K.; Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am. J. Physiol.: Heart Circ. Physiol. 2004, 287, H2316–2323. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Zhang J.; Lu Y.; Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli A.; Testai L.; Breschi M. C.; Lawson K.; McKay N. G.; Miceli F.; Taglialatela M.; Calderone V. Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacol. Res. 2013, 70, 27–34. [DOI] [PubMed] [Google Scholar]
- Yang G.; Wu L.; Jiang B.; Yang W.; Qi J.; Cao K.; Meng Q.; Mustafa A. K.; Mu W.; Zhang S.; Snyder S. H.; Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G.; Chen F.; Cheng Y.; Tang C.; Du J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J. Hypertens. 2003, 21, 1879–1885. [DOI] [PubMed] [Google Scholar]
- Martelli A.; Testai L.; Breschi M. C.; Blandizzi C.; Virdis A.; Taddei S.; Calderone V. Hydrogen sulphide: novel opportunity for drug discovery. Med. Res. Rev. 2012, 32, 1093–1130. [DOI] [PubMed] [Google Scholar]
- Pan L. L.; Liu X. H.; Gong Q. H.; Yang H. B.; Zhu Y. Z. Role of cystathionine γ-lyase/hydrogen sulfide pathway in cardiovascular disease: A novel therapeutic strategy?. Antioxid. Redox Signaling 2012, 17, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. F.; Xiao C. S.; Hui R. T. Calcium sulphide (CaS), a donor of hydrogen sulphide (H2S): a new antihypertensive drug?. Med. Hypotheses 2009, 73, 445–447. [DOI] [PubMed] [Google Scholar]
- Caliendo G.; Cirino G.; Santagada V.; Wallace J. L. Synthesis and biological effects of hydrogen sulfide (H2S): Development of H2S-releasing drugs as pharmaceuticals. J. Med. Chem. 2010, 53, 6275–6286. [DOI] [PubMed] [Google Scholar]
- Martelli A.; Testai L.; Marino A.; Breschi M. C.; Da Settimo F.; Calderone V. Hydrogen sulphide: biopharmacological roles in the cardiovascular system and pharmaceutical perspectives. Curr. Med. Chem. 2012, 19, 3325–3336. [DOI] [PubMed] [Google Scholar]
- Benavides G. A.; Squadrito G. L.; Mills R. W.; Patel H. D.; Isbell T. S.; Patel R. P.; Darley-Usmar V. M.; Doeller J. E.; Kraus D. W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 17977–17982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Wang H.; Xian M. Cysteine-activated hydrogen sulfide (H2S) donors. J. Am. Chem. Soc. 2011, 133, 15–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Whiteman M.; Guan Y. Y.; Neo K. L.; Cheng Y.; Lee S. W.; Zhao Y.; Baskar R.; Tan C. H.; Moore P. K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [DOI] [PubMed] [Google Scholar]
- Isenberg J. S.; Jia Y.; Field L.; Ridnour L. A.; Sparatore A.; Del Soldato P.; Sowers A. L.; Yeh G. C.; Moody T. W.; Wink D. A.; Ramchandran R.; Roberts D. D. Modulation of angiogenesis by dithiolethione-modified NSAIDs and valproic acid. Br. J. Pharmacol. 2007, 151, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Rossoni G.; Sparatore A.; Lee L. C.; Del Soldato P.; Moore P. K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radical Biol. Med. 2007, 42, 706–719. [DOI] [PubMed] [Google Scholar]
- Sparatore A.; Perrino E.; Tazzari V.; Giustarini D.; Rossi R.; Rossoni G.; Erdmann K.; Schroder H.; Del Soldato P. Pharmacological profile of a novel H2S-releasing aspirin. Free Radical Biol. Med. 2009, 46, 586–592. [DOI] [PubMed] [Google Scholar]
- Shukla N.; Rossoni G.; Hotston M.; Sparatore A.; Del Soldato P.; Tazzari V.; Persad R.; Angelini G. D.; Jeremy J. Y. Effect of hydrogen sulphide-donating sildenafil (ACS6) on erectile function and oxidative stress in rabbit isolated corpus cavernosum and in hypertensive rats. BJU Int. 2009, 103, 1522–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L.; Cirino G.; Santagada V.; Caliendo G.. Hydrogen Sulfide Derivatives of Non-Steroidal Anti-Inflammatory Drugs. WO2008/009127 A1, 2008.
- Wallace J. L.; Caliendo G.; Santagada V.; Cirino G. Markedly reduced toxicity of a hydrogen sulphide-releasing derivative of naproxen (ATB-346). Br. J. Pharmacol. 2010, 159, 1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombkowski R. A.; Russel M. J.; Olson K. R. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 2004, 286, R678–685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.