Abstract
GPR40 (FFAR1 or FFA1) is a target of high interest being pursued to treat type II diabetes due to its unique mechanism leading to little risk of hypoglycemia. We recently reported the discovery of AM-1638 (2), a potent full agonist of GPR40. In this report, we present the discovery of GPR40 full agonists containing conformationally constrained tricyclic spirocycles and their structure–activity relationships leading to more potent agonists such as AM-5262 (26) with improved rat PK profile and general selectivity profile. AM-5262 enhanced glucose stimulated insulin secretion (mouse and human islets) and improved glucose homeostasis in vivo (OGTT in HF/STZ mice) when compared to AM-1638.
Keywords: GPR40, full agonist, spirocycles, tricyclic, AM-5262, AM-1638, AMG 837, FFAR1, FFA1
Type II diabetic patients lose their ability to maintain glucose homeostasis due to defects in both insulin secretion and action.1 GPR40 (FFAR1 or FFA1) is a G-protein-coupled receptor, primarily expressed in pancreatic islet β-cells and intestinal enteroendocrine cells.2 When activated by medium to long chain fatty acids, GPR40 elicits increased insulin secretion from islet β-cells only in the presence of elevated glucose levels.3 This unique mechanism to treat type II diabetes potentially mitigates the risk of hypoglycemia seen with standard insulin secretagogues and has triggered significant efforts at identifying therapeutic agents utilizing this target.4−21
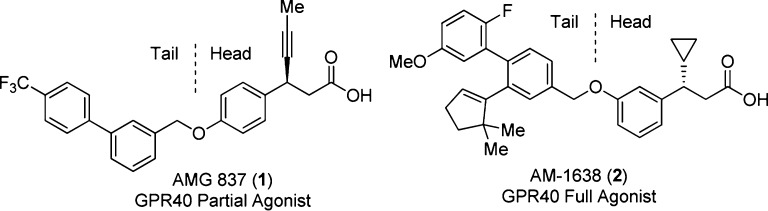
We previously described the discovery of a GPR40 partial agonist AMG 837 (1, Figure 1)16 and a GPR40 full agonist AM-1638 (2).21 AM-1638 showed greater antidiabetic efficacy in several rodent models21,22 and provided compelling evidence that GPR40 full agonists can afford access to a powerful mechanism for maintaining glycemic control and great potential for the treatment of type II diabetic patients.
Figure 1.
GPR40 partial agonist AMG 837 and full agonist AM-1638.
In this letter, we describe further optimization beyond AM-1638. We took on an approach to add conformational constraints, especially at the flexible phenylpropanoic acid region (subsequently referred to as the “head group”, Figure 1).
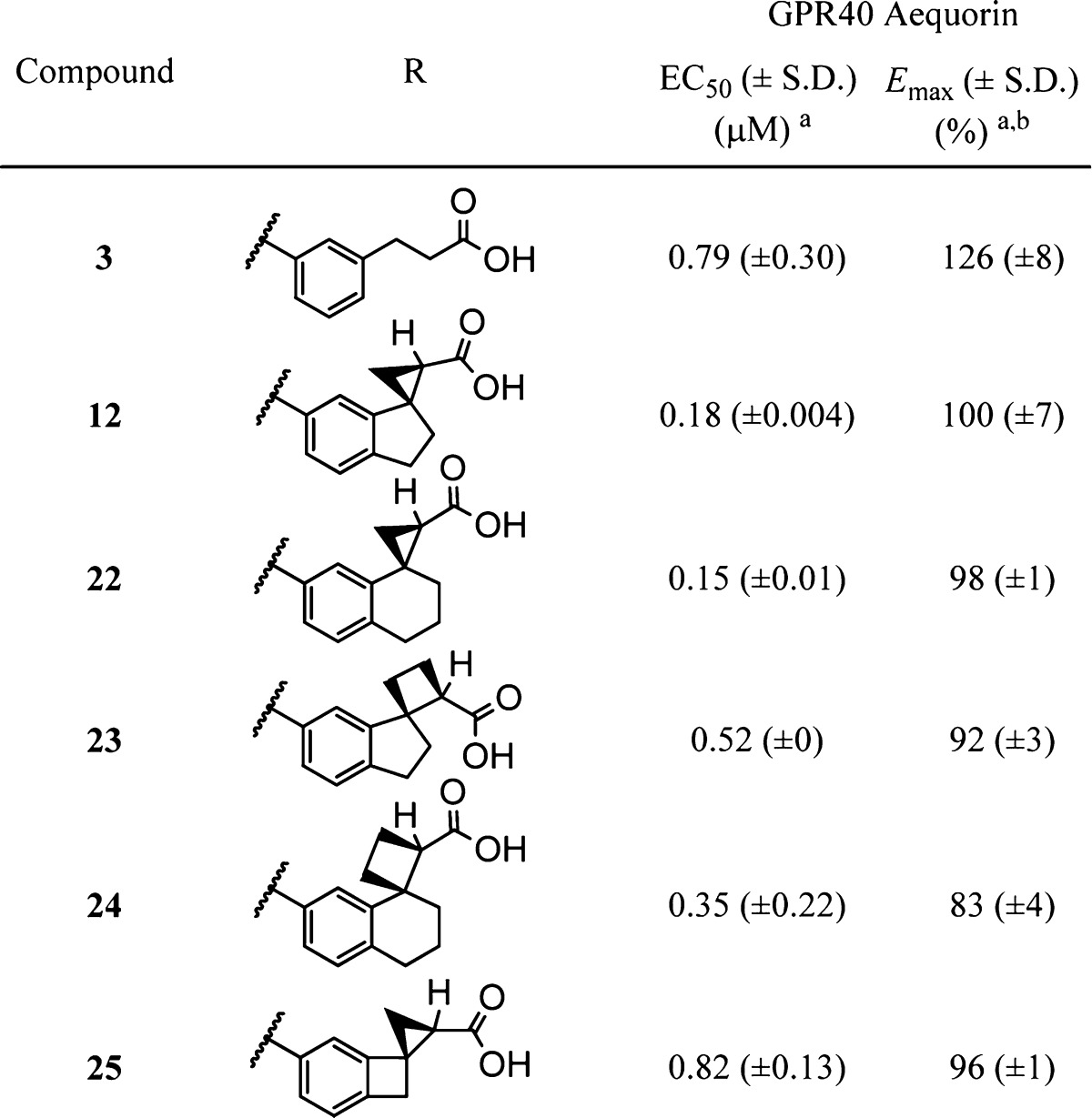
Our efforts to define conformational constraints that maintain full agonist activity are shown in Table 1. Compared to phenylpropanoic acid (3), constraining the phenyl with a fused 5-membered ring (4) showed a modest improvement in potency. To further constrain the carboxylic acid, we explored fusing a cyclopropane to the cyclopentane ring of the 2,3-dihydro-1H-indene (4). Incorporation of cyclopropane through the fused connection (examples 5–8) proved to be detrimental to potency, although 8 still retained full agonism. However, switching to a spiro-cyclopropane attached to the dihydroindene (examples 9–12) resulted in increased potency (12 vs 4), while maintaining full agonism on GPR40. Note that only one of the four possible stereoisomers maintains both high potency and full efficacy on the target. To probe whether the tricyclic constraint was necessary, we eliminated the cyclopentane ring and made 2-phenylcyclopropanecarboxylic acids 13 and 14. This change resulted in a 10-fold loss in potency and reduced efficacy.
Table 1. Identification of Spirocyclic Head Groups.
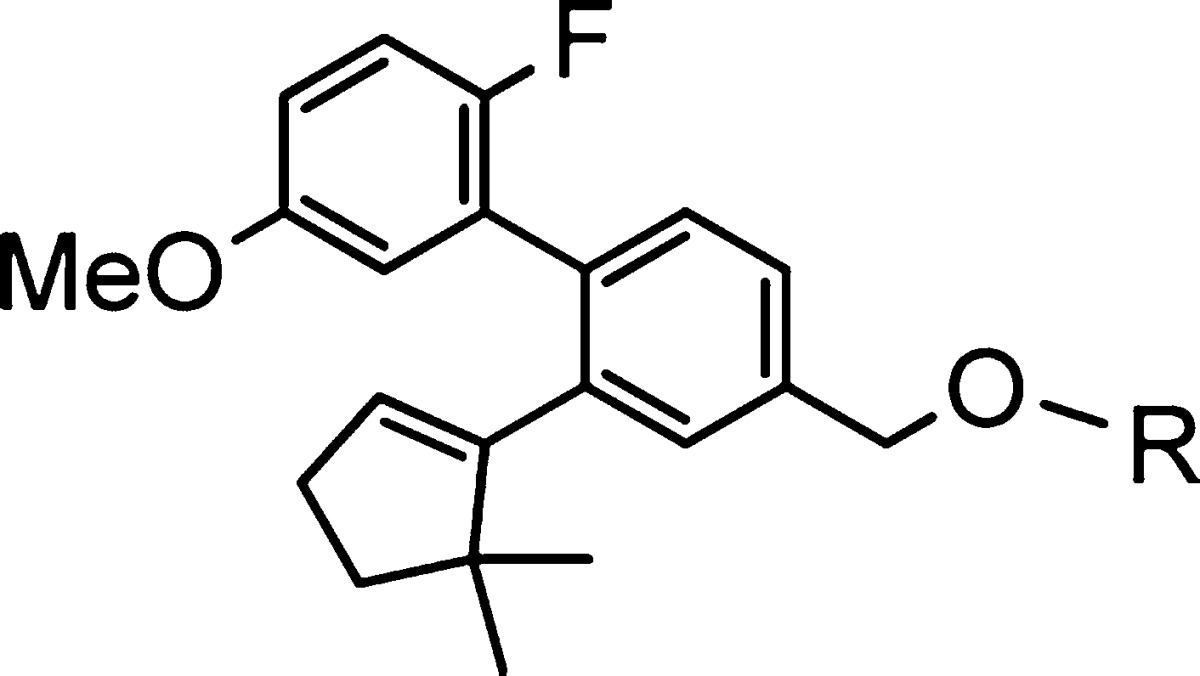

Mean of at least two runs.
% maximal efficacy compared to 2.
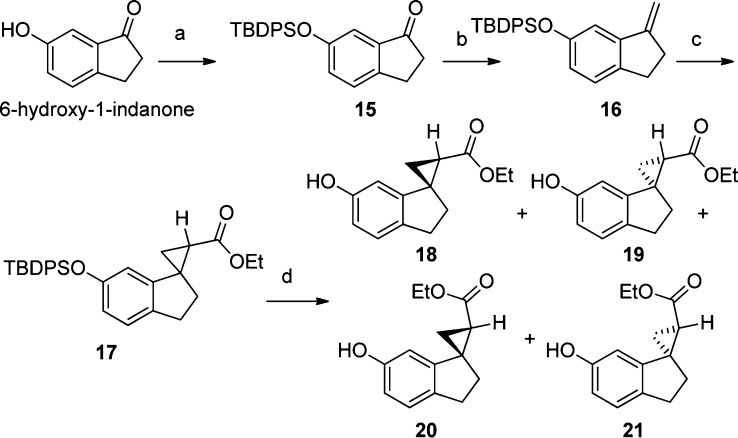
A large scale synthetic route to prepare head groups shown in examples 9–12 was developed (Scheme 1). Commercially available 6-hydroxy-1-indanone (250 g) was protected with tert-butyldiphenylsilyl group, followed by a Wittig reaction to deliver the terminal olefin 16 in quantitative yields for both steps. Rhodium acetate catalyzed cyclopropanation with ethyl diazoacetate resulted in 17 in 80% yield. Deprotection followed by chiral separation delivered the head groups 18–21.
Scheme 1.
The relative configurations of the head groups shown in compounds 5–12 were assigned based on NMR studies. Absolute configurations were defined based on comparison of experimental and calculated vibrational circular dichroism (VCD)23 spectra and optical rotations.24 The configuration of head group 19 (in compound 11, Table 1) was further verified by X-ray analysis of its brominated analogue.25
We then turned our attention to optimize the sizes of the spirocyclic rings (Table 2). Compared to the spiro[2,4]heptane in 12, the spiro[2,5]octane in 22 showed equal potency and efficacy. Other variations on ring sizes (23–25) showed decreased potencies and thus indicated less optimal conformational restrictions than 12 and 22; however, they were still equal to or better than the flexible phenylpropanoic acid 3.
Table 2. Optimization of Spirocyclic Ring Sizes.
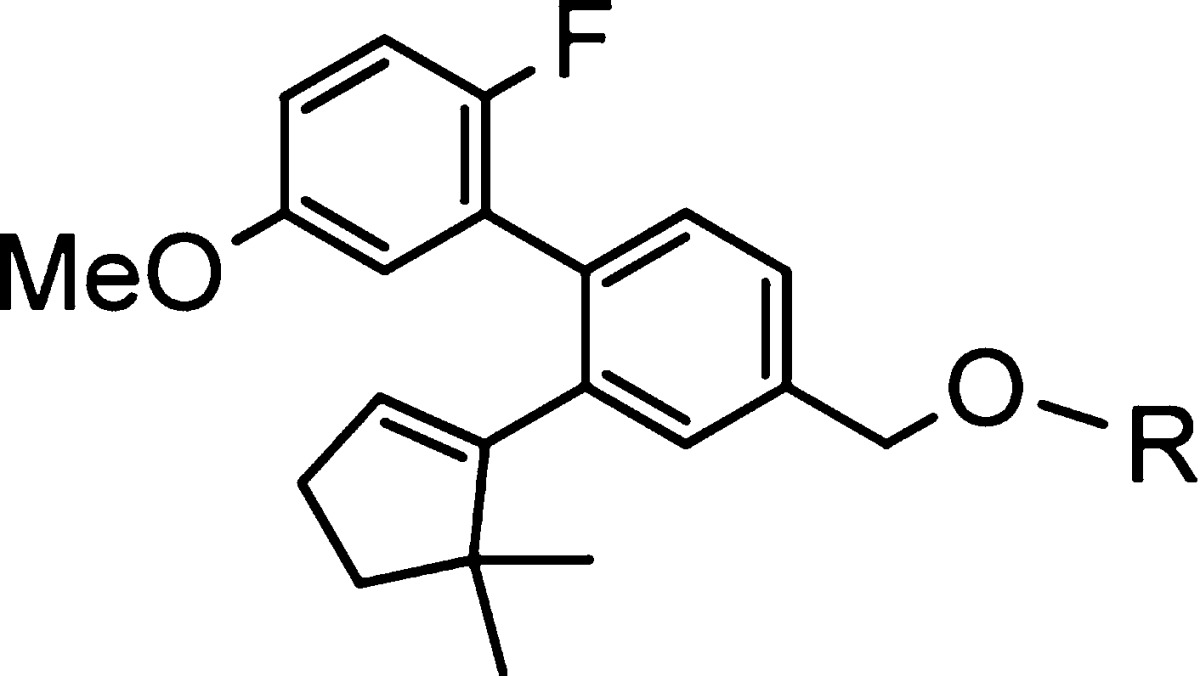
Mean of at least two runs.
% maximal efficacy compared to 2.
Reduction of the cyclopentene in the tail region of 12 resulted in potency improvement for 26, but not its diastereoisomer 27, although both have full efficacy (Table 3). The spiro[2,5]octanes (28–30) again showed similar potency as spiro[2,4]heptane (26).
Table 3. Combination of Best Head and Tail Groups.

Mean of at least two runs.
% maximal efficacy compared to 2.
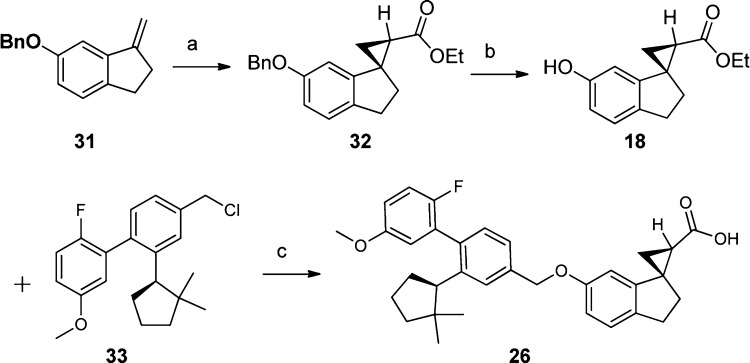
The preparation of these spirocyclic GPR40 full agonists is exemplified by the synthesis of 26 (Scheme 2). Asymmetric approaches to 18 were explored, and the chiral ruthenium bis(oxazolinyl) pyridine catalyst reported by Nishiyama26 was found to be most effective to deliver the 18 precursor 32 in 94% yield with 90% ee. Coupling the head group 18 with tail group 33 was accomplished at room temperature with Cs2CO3 in DMSO, followed by hydrolysis with aqueous LiOH and MeOH to deliver 26 in one pot.
Scheme 2.
Highly conformationally constrained molecules, like 26 and 30, were expected to improve off-target selectivity.27 Both 26 and 30 showed better selectivity than 2 against a broad panel of 101 GPCRs, ion channels, transporters, and enzymes at 10 μM (Table 4, additional detail in Supporting Information). Compound 2 showed greater than 70% inhibition compared to controls for the adenosine A1 receptor, thyrotropin releasing hormone TRH1 receptor, the norepinephrine transporter, the cholecystokinin CCK2 receptor, the histamine H4 receptor, the dopamine D1 receptor, and the kappa opioid receptor; while 26 and 30 displayed less than 70% inhibition against these receptors.
Table 4. Binding Activity Screen against 101 Receptors.
Percent inhibition of control values or control specific binding.
Compound 2 has medium clearance in both rats (Cl = 0.91 L/h/kg) and cynomolgus monkeys (cyno, Cl = 0.81 L/h/kg). Table 5 showed that 26 improved clearance in rat (0.25 L/h/kg) and that 30 improved clearance by 5–7-fold in both rat and cyno.
Table 5. Improvement in Pharmacokinetic Properties.
| compd | species | Cla (L/h/kg) | Vdssa (L/kg) | iv t1/2a (h) | % Fb |
|---|---|---|---|---|---|
| 2 | rat | 0.91 | 1.1 | 1.8 | 15 |
| cynoc | 0.81 | 2.0 | 2.1 | 71 | |
| 26 | rat | 0.25 | 0.7 | 4.2 | 28 |
| cynoc | 0.84 | 1.4 | 2.2 | 69 | |
| 30 | rat | 0.17 | 0.8 | 4.1 | 61 |
| cynoc | 0.11 | 0.4 | 3.7 | 44 |
iv dose at 0.5 mg/kg.
Oral dose at 2 mg/kg.
Cynomolgus monkey.
Binding data obtained from reciprocal competition experiments demonstrated that similarly to AM-1638 (2), AM-5262 (26) exhibits positive cooperativity and occupies a different binding site from AMG 837 (Figure 2).28 Conversely, experiments with radiolabeled AM-1638 clearly showed that AM-5262 binds at the same site as AM-1638, further providing evidence of allosteric behavior with AMG 837.
Figure 2.
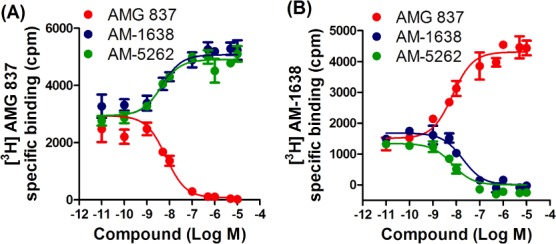
Binding of AM-5262 was examined in cross-interaction experiments with [3H] AMG 837 (A) and [3H] AM-1638 (B). Data are expressed as cpm specific binding and shown as means ± SEM of 2–3 independent experiments. The curves are fitted to an allosteric ternary complex model.
GPR40 full agonists stimulate incretin and insulin secretion in vitro and in vivo.22 The activity of AM-5262 was compared head-to-head with AM-1638 in isolated primary cells from rodents and humans. AM-5262 stimulated GLP-1 and GIP secretion from rat intestinal cells, and the potency of AM-5262 was 2–5-fold greater than AM-1638 (Figure 3A,B). AM-5262 potentiated glucose stimulated insulin secretion from both mouse (Figure 3C) and human islets (Figure 3D), and the activity was greater than that of exendin-4 in human islets.
Figure 3.
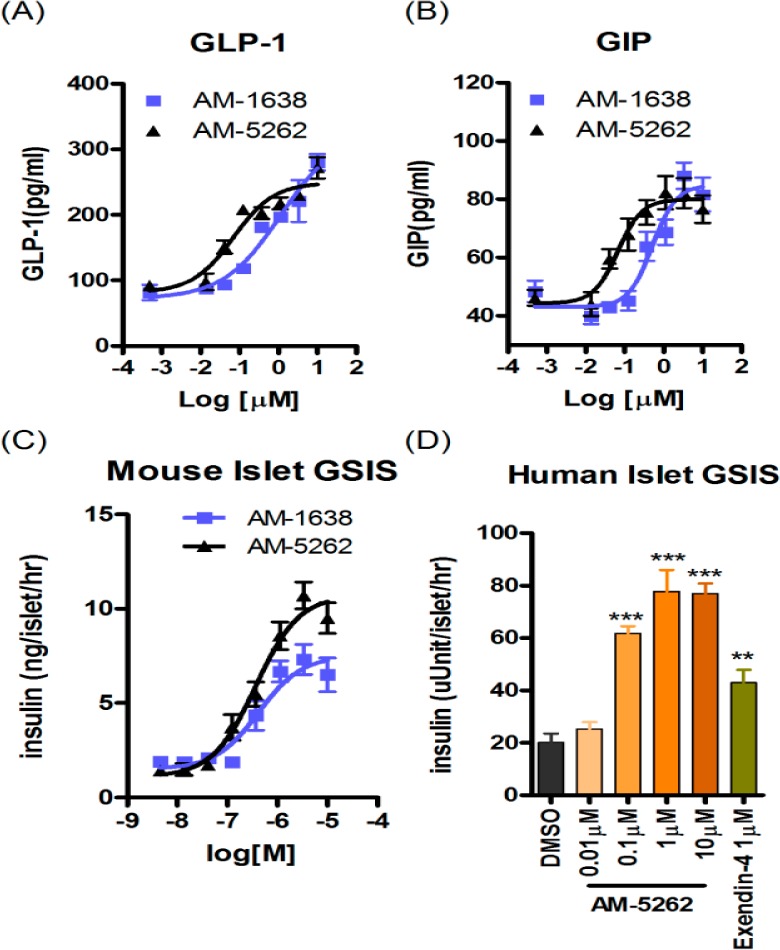
Characterization of AM-5262 activity in isolated primary cells. Stimulation of (A) GLP-1 and (B) GIP secretion by AM-1638 and AM-5262 from rat fetal intestinal cells. Potentiation of glucose-dependent insulin secretion from (C) mouse and (D) human islets in response to indicated treatments.
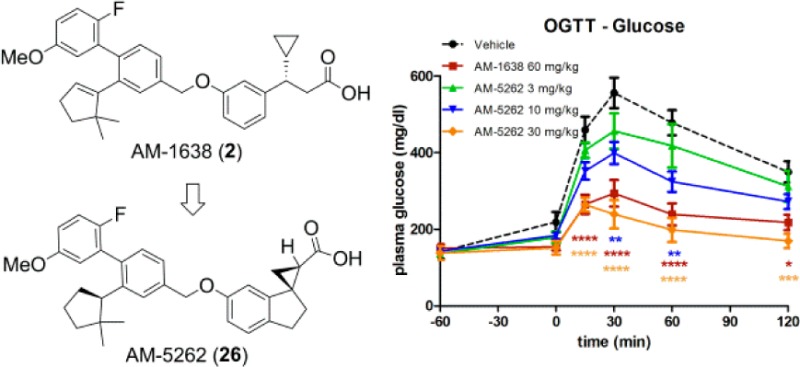
When compared for their ability to improve glycemic control in HF/STZ type II diabetic mice,22 AM-5262 showed similar efficacy at 30 mg/kg dose compared to AM-1638 at 60 mg/kg during an oral glucose tolerance test (OGTT, Figure 4A). AM-1638 displays maximal efficacy at 60 mg/kg in this model. Glucose AUC improved ∼48% in response to 30 mg/kg AM-5262, and this was associated with an increase in insulin secretion (Figure 4B). Total plasma levels of drugs were measured one hour post-dose (Figure 4C). Total drug plasma levels were 6–7-fold lower for AM-5262 at 30 mg/kg compared to AM-1638 at 60 mg/kg, suggesting that AM-5262 displays greater potency in vivo.
Figure 4.
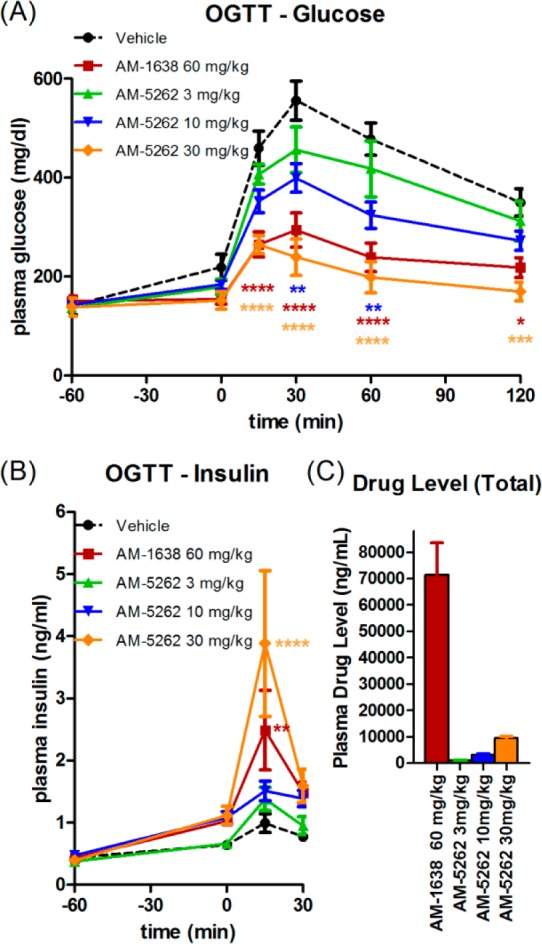
AM-5262 improves glucose metabolism in high-fat fed/STZ treated mice. (A) Glucose and (B) insulin levels during an OGTT in response to treatments indicated in the figure legends. Compounds were dosed orally at −60 min, and oral glucose was administered at 0 min. (C) Total (bound + free) drug levels in the plasma 1 h post-drug dose during the OGTT.
In conclusion, we have described the discovery and SAR of potent GPR40 full agonists with highly conformationally constrained tricyclic head groups. These compounds demonstrated improved rat PK and off-target selectivity profiles compared to their unconstrained parent compounds. AM-5262 (26) binds to the same ligand site on GPR40 as AM-1638 and has enhanced glucose stimulated insulin secretion (mouse and human islets) and improved glucose homeostasis in vivo (OGTT in HF/STZ mice).
Supporting Information Available
Experimental procedures, analytical data, and receptor screening data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- Defronzo R. A. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes. Diabetes 2009, 58, 773–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y.; Kawamata Y.; Harada M.; Kobayashi M.; Fujii R.; Fukusumi S.; Ogi K.; Hosoya M.; Tanaka Y.; Uejima H.; Tanaka H.; Maruyama M.; Satoh R.; Okubo S.; Kizawa H.; Komatsu H.; Matsumura F.; Noguchi Y.; Shinohara T.; Hinuma S.; Fujisawa Y.; Fujino M. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 2003, 422, 173–176. [DOI] [PubMed] [Google Scholar]
- Stoddart L. A.; Smith N. J.; Milligan G. International union of pharmacology. LXXI. Free fatty acid receptors FFA1, -2, and -3: pharmacology and pathophysiological functions. Pharmacol. Rev. 2008, 60, 405–417. [DOI] [PubMed] [Google Scholar]
- For a review, see:Medina J. C.; Houze J. B. GPR40 (FFAR1) modulators. Annu. Rep. Med. Chem. 2008, 43, 75–85. [Google Scholar]
- For a review on patents, see:Bharate S. B.; Nemmani K. V.; Vishwakarma R. A. Progress in the discovery and development of small-molecule modulators of G-protein-coupled receptor 40 (GPR40/FFA1/FFAR1): An emerging target for type 2 diabetes. Expert Opin. Ther. Pat. 2009, 19, 237–264. [DOI] [PubMed] [Google Scholar]
- Garrido D. M.; Corbett D. F.; Dwornik K. A.; Goetz A. S.; Littleton T. R.; McKeown S. C.; Mills W. Y.; Smalley T. L.; Briscoe C. P.; Peat A. J. Synthesis and activity of small molecule GPR40 agonists. Bioorg. Med. Chem. Lett. 2006, 16, 1840–1845. [DOI] [PubMed] [Google Scholar]
- McKeown S. C.; Corbett D. F.; Goetz A. S.; Littleton T. R.; Bigham E.; Briscoe C. P.; Peat A. J.; Watson S. P.; Hickey D. M. B. Solid phase synthesis and SAR of small molecule agonists for the GPR40 receptor. Bioorg. Med. Chem. Lett. 2007, 17, 1584–1589. [DOI] [PubMed] [Google Scholar]
- Song F.; Lu S.; Gunnet J.; Xu J. Z.; Wines P.; Proost J.; Liang Y.; Baumann C.; Lenhard J.; Murray W. V.; Demarest K. T.; Kuo G.-H. Synthesis and biological evaluation of 3-aryl-3-(4-phenoxy)-propionic acid as a novel series of G protein-coupled receptor 40 agonists. J. Med. Chem. 2007, 50, 2807–2817. [DOI] [PubMed] [Google Scholar]
- Christiansen E.; Urban C.; Merten N.; Liebscher K.; Karlsen K. K.; Hamacher A.; Spinrath A.; Bond A. D.; Drewke C.; Ullrich S.; Kassack M. U.; Kostenis E.; Ulven T. Discovery of potent and selective agonists for the free fatty acid receptor 1 (FFA1/GPR40), a potential target for the treatment of type II diabetes. J. Med. Chem. 2008, 51, 7061–7064. [DOI] [PubMed] [Google Scholar]
- Zhou C. Y.; Tang C.; Chang E.; Ge M.; Lin S. N.; Cline E.; Tan C. P.; Feng Y.; Zhou Y. P.; Eiermann G. J.; Petrov A.; Salituro G.; Meinke P.; Mosley R.; Akiyama T. E.; Einstein M.; Kumar S.; Berger J.; Howard A. D.; Thornberry N.; Mills S. G.; Yang L. H. Discovery of 5-aryloxy-2,4-thiazolidinediones as potent GPR40 agonists. Bioorg. Med. Chem. Lett. 2010, 20, 1298–1301. [DOI] [PubMed] [Google Scholar]
- Negoro N.; Sasaki S.; Mikami S.; Ito M.; Suzuki M.; Tsujihata Y.; Ito R.; Harada A.; Takeuchi K.; Suzuki N.; Miyazaki J.; Santou T.; Odani T.; Kanzaki N.; Funami M.; Tanaka T.; Kogame A.; Matsunaga S.; Yasuma T.; Momose Y. Discovery of TAK-875: A potent, selective, and orally bioavailable GPR40 agonist. ACS Med. Chem. Lett. 2010, 1, 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen E.; Due-Hansen M. E.; Urban C.; Merten N.; Pfleiderer M.; Karlsen K. K.; Rasmussen S. S.; Steensgaard M.; Hamacher A.; Schmidt J.; Drewke C.; Petersen R. K.; Kristiansen K.; Ullrich S.; Kostenis E.; Kassack M. U.; Ulven T. Structure–activity study of dihydrocinnamic acids and discovery of the potent FFA1 (GPR40) agonist TUG-469. ACS Med. Chem. Lett. 2010, 1, 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S.; Kitamura S.; Negoro N.; Suzuki M.; Tsujihata Y.; Suzuki N.; Santou T.; Kanzaki N.; Harada M.; Tanaka Y.; Kobayashi M.; Tada N.; Funami M.; Tanaka T.; Yamamoto Y.; Fukatsu K.; Yasuma T.; Momose Y. Design, synthesis, and biological activity of potent and orally available G protein-coupled receptor 40 agonists. J. Med. Chem. 2011, 54, 1365–1378. [DOI] [PubMed] [Google Scholar]
- Walsh S. P.; Severino A.; Zhou C. Y.; He J. F.; Liang G. B.; Tan C. P.; Cao J.; Eiermann G. J.; Xu L.; Salituro G.; Howard A. D.; Mills S. G.; Yang L. H. 3-Substituted 3-(4-aryloxyaryl)-propanoic acids as GPR40 agonists. Bioorg. Med. Chem. Lett. 2011, 21, 3390–3394. [DOI] [PubMed] [Google Scholar]
- Christiansen E.; Urban C.; Grundmann M.; Due-Hansen M. E.; Hagesaether E.; Schmidt J.; Pardo L.; Ullrich S.; Kostenis E.; Kassack M. U.; Ulven T. Identification of a potent and selective free fatty acid receptor 1 (FFA1/GPR40) agonist with favorable physicochemical and in vitro ADME properties. J. Med. Chem. 2011, 54, 6691–6703. [DOI] [PubMed] [Google Scholar]
- Houze J. B.; Zhu L.; Sun Y.; Akerman M.; Qiu W.; Zhang A. J.; Sharma R.; Schmitt M.; Wang Y.; Liu J.; Liu J.; Medina J. C.; Reagan J. D.; Luo J.; Tonn G.; Zhang J.; Lu J. Y.-L.; Chen M.; Lopez E.; Nguyen K.; Yang L.; Tang L.; Tian H.; Shuttleworth S. J.; Lin D. C. H. AMG 837: A potent, orally bioavailable GPR40 agonist. Bioorg. Med. Chem. Lett. 2012, 22, 1267–1270. [DOI] [PubMed] [Google Scholar]
- Negoro N.; Sasaki S.; Ito M.; Kitamura S.; Tsujihata Y.; Ito R.; Suzuki M.; Takeuchi K.; Suzuki N.; Miyazaki J.; Santou T.; Odani T.; Kanzaki N.; Funami M.; Tanaka T.; Yasuma T.; Momose Y. Identification of fused-ring alkanoic acids with improved pharmacokinetic profiles that act as G protein-coupled receptor 40/free fatty acid recptor 1 agonists. J. Med. Chem. 2012, 55, 1538–1552. [DOI] [PubMed] [Google Scholar]
- Mikami S.; Kitamura S.; Negoro N.; Sasaki S.; Suzuki M.; Tsujihata Y.; Miyazaki T.; Ito R.; Suzuki N.; Miyazaki J.; Santou T.; Kanzaki N.; Funami M.; Tanaka T.; Yasuma T.; Momose Y. Discovery of phenylpropanoic acid derivatives containing polar functionalities as potent and orally bioavailable G protein coupled receptor 40 agonists for the treatment of type 2 diabetes. J. Med. Chem. 2012, 55, 3756–3776. [DOI] [PubMed] [Google Scholar]
- Negoro N.; Sasaki S.; Mikami S.; Ito M.; Tsujihata Y.; Ito R.; Suzuki M.; Takeuchi K.; Suzuki N.; Miyazaki J.; Santou T.; Odani T.; Kanzaki N.; Funami M.; Morohashi A.; Nonaka M.; Matsunaga S.; Yasuma T.; Momose Y. Optimization of (2,3-dihydro-1-benzofuran-3-yl)acetic acids: discovery of a non-free fatty acid-like, highly bioavailable G protein-coupled receptor 40/free fatty acid receptor 1 agonist as a glucose-dependent insulinotropic agent. J. Med. Chem. 2012, 55, 3960–3974. [DOI] [PubMed] [Google Scholar]
- Christiansen E.; Due-Hansen M. E.; Urban C.; Grundmann M.; Schröder R.; Hudson B. D.; Milligan G.; Cawthorne M. A.; Kostenis E.; Kassack M. U.; Ulven T. Free fatty acid receptor 1 (FFA1/GPR40) agonists: mesylpropoxy appendage lowers lipophilicity and improves ADME properties. J. Med. Chem. 2012, 55, 6624–6628. [DOI] [PubMed] [Google Scholar]
- Brown S. P.; Dransfield P. J.; Vimolratana M.; Jiao X.; Zhu L.; Pattaropong V.; Sun Y.; Liu J.; Luo J.; Zhang J.; Wong S.; Zhuang R.; Guo Q.; Li F.; Medina J. C.; Swaminath G.; Lin D. C.-H.; Houze J. B. Discovery of AM-1638: A potent and orally bioavailable GPR40/FFA1 full agonist. ACS Med. Chem. Lett. 2012, 3, 726–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J.; Swaminath G.; Brown S. P.; Zhang J.; Guo Q.; Chen M.; Nguyen K.; Tran T.; Miao L.; Dransfield P. J.; Vimolratana M.; Houze J. B.; Wong S.; Toteva M.; Shan B.; Li F.; Zhuang R.; Lin D. C.-H. A potent class of GPR40 full agonists engages the enteroinsular axis to promote glucose control in rodents. PLoS One 2012, 7, e46300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P. J.; Devlin F. J.; Pan J.-J. The determination of the absolute configurations of chiral molecules using vibrational circular dichroism (VCD) spectroscopy. Chirality 2008, 20, 643–663. [DOI] [PubMed] [Google Scholar]
- Stephens P. J.; Devlin F. J.; Cheeseman J. R.; Frisch M. J.; Rosini C. Determination of absolute configuration using optical rotation calculated using density functional theory. Org. Lett. 2002, 4, 4595–4598. [DOI] [PubMed] [Google Scholar]
- X-ray data was collected by Frederick J. Hollander and Antonio DiPasquale at UC Berkeley and submitted to the Cambridge Structural Database (www.ccdc.cam.ac.uk): CCDC 903315.
- Nishiyama H.; Itoh Y.; Matsumoto H.; Park S. B.; Itoh K. New chiral ruthenium bis(oxazolinyl)pyridine catalyst. Efficient asymmetric cyclopropanation of olefins with diazoacetates. J. Am. Chem. Soc. 1994, 116, 2223–2224. [Google Scholar]
- Mann A.Conformational Restriction and/or Steric Hindrance in Medicinal Chemistry. In The Practice of Medicinal Chemistry, 2nd ed.; Wermuth C. G., Ed.; Academic Press: London, U.K., 1996; pp 233–250. [Google Scholar]
- Lin D. C.-H.; Guo Q.; Luo J.; Zhang J.; Nguyen K.; Tran T.; Dransfield P. J.; Brown S. P.; Houze J. B.; Vimolratana M.; Jiao X. Y.; Wang Y.; Birdsall N. J.; Swaminath G. Identification and pharmacological characterization of multiple allosteric binding sites on the FFA1 Receptor. Mol. Pharmacol. 2012, 82, 843–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.