Abstract
The synthesis and antiplasmodial and antimycobacterial evaluation of two new series of nitroimidazole and nitroimidazooxazine derivatives is described. The majority of these compounds, especially hybrids 9d, 9f, and 14b, exhibited potent activity against the chloroquine-resistant K1 strain of Plasmodium falciparum. Furthermore, a notable number from the tetrazole series were significantly more active against M. tuberculosis than kanamycin, a standard TB drug.
Keywords: Nitroimidazoles, nitroimidazooxazines, antiplasmodial, antimycobacterial activity
According to the World Health Organization (WHO), nearly one-third of the world’s population harbors Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB).1 TB is the second leading cause of death due to an infectious organism:2 current WHO estimates stand at 9.2 million new cases and 1.8 million deaths annually.1,3 Malaria, on the other hand, infects about 255 million people worldwide, resulting in 781 000 deaths, mostly to children under 5 years and pregnant women.4 Currently, there are no effective vaccines against these pathogens and treatment success in some areas remains low due to poor management and patient noncompliance.5 This situation is further exacerbated by the increasing prevalence of multi- (MDR) and extensive-drug resistant (XDR) strains of Mtb.6 Therefore, there is an urgent need for new, fast acting, and more efficacious antimalarial and anti-TB therapies to replace the existing drug regimens.
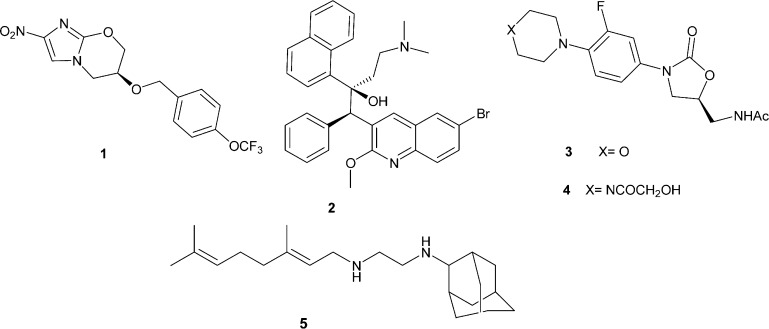
Although there has been no introduction of a new anti-TB drug over the last 40 years, a number of different classes of compounds are undergoing clinical development,2 namely nitroimidazooxazines (PA-824, 1),7 diarylquinolines (TMC207, 2),8 oxazolidinones (eperesol, 3, and linezolid, 4),9 and ethylenediamines (SQ109, 5)10 (Figure 1). The use of the antibiotic metronidazole in the management of anaerobic bacterial and protozoan infections has reinvigorated interest in the nitroimidazole scaffold over the past decade.11,12 Nitroimidazoles (e.g., 1) are pro-drugs that are highly effective against both the replicating and nonreplicating persistent forms of Mtb, and their activity is believed to arise as a result of metabolic activation of the nitro group, which leads to the generation of nitric oxide as an active species.13 The main drawbacks of 1 are its poor aqueous solubility and its propensity to bind to proteins in human plasma.3,14 Various measures have been undertaken in addressing these shortcomings, such as the synthesis of biphenyl analogues of 1,15 the use of urea, carbamate, and amide linkers in the place of the benzyl ether,16 and the hybridization of 1 with oxazolidinone,17 among others. The most promising results from these studies were achieved when the benzyl group of 1 was replaced with various (hetero)biaryl side-chains and amide groups. It was noted that compounds that contain these scaffolds exhibited better in vitro and in vivo potencies and improved absorption, distribution, metabolism, and excretion (ADME) properties compared to those of 1.18–21
Figure 1.
Chemical structures of lead TB compounds in clinical development.
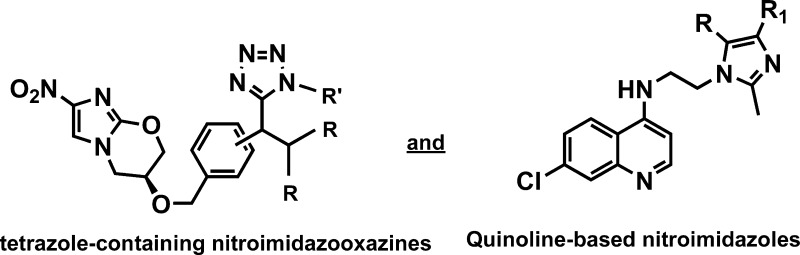
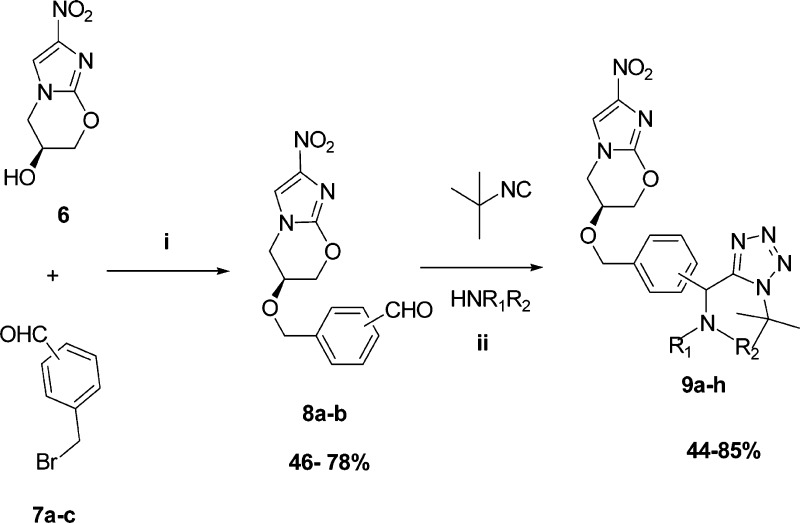
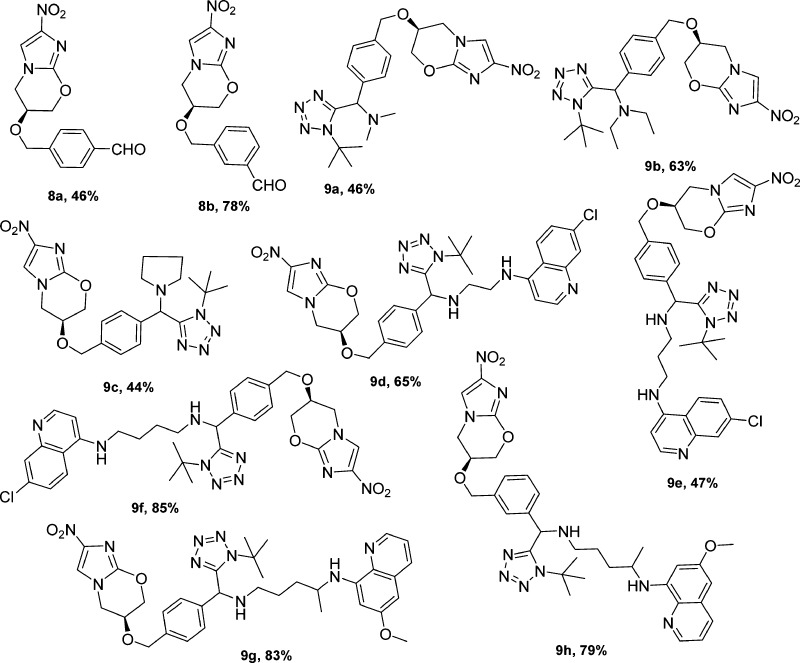
In this context, we desired to investigate the antiplasmodial and antimycobacterial properties of new analogs that contain the key nitroimidazole pharmacophore. The first series was designed to contain a tetrazole moiety in the place of the lipophilic trifluoromethoxy group of 1, as it was hypothesized that the inclusion of this moiety will significantly aid in improving the physicochemical properties. These tetrazole-containing compounds were synthesized in three-steps; the first involved the synthesis of aralkyl halides 7a–c from known literature methods.22 These aralkyl halides were then reacted with the commercially available nitroimidazooxazine-alcohol (6) in the presence of sodium hydride7,23 to afford aldehydes 8a–b (meta and para) (Scheme 1). The reaction failed in the case of the ortho-aldehyde, presumably due to steric hindrance. Aldehydes 8a–b were then subjected to the modified TMSN3–Ugi multicomponent reaction (MCR)24 involving amines and the convertible tert-butyl isocyanide. Amine inputs included primaquine and 4-aminoquinoline diamines. Target compounds 9a–h were obtained in moderate to excellent yields (Figure 2) and diastereoselectivity (based on the 1H NMR); compounds 9a–f were obtained exclusively as single diastereomers while 9g and 9h were obtained as 1:1 diastereomeric mixtures, owing to the fact that the commercial primaquine salt used in this study is racemic.
Scheme 1.
Figure 2.
Structures and yields of MCR intermediates and target compounds.
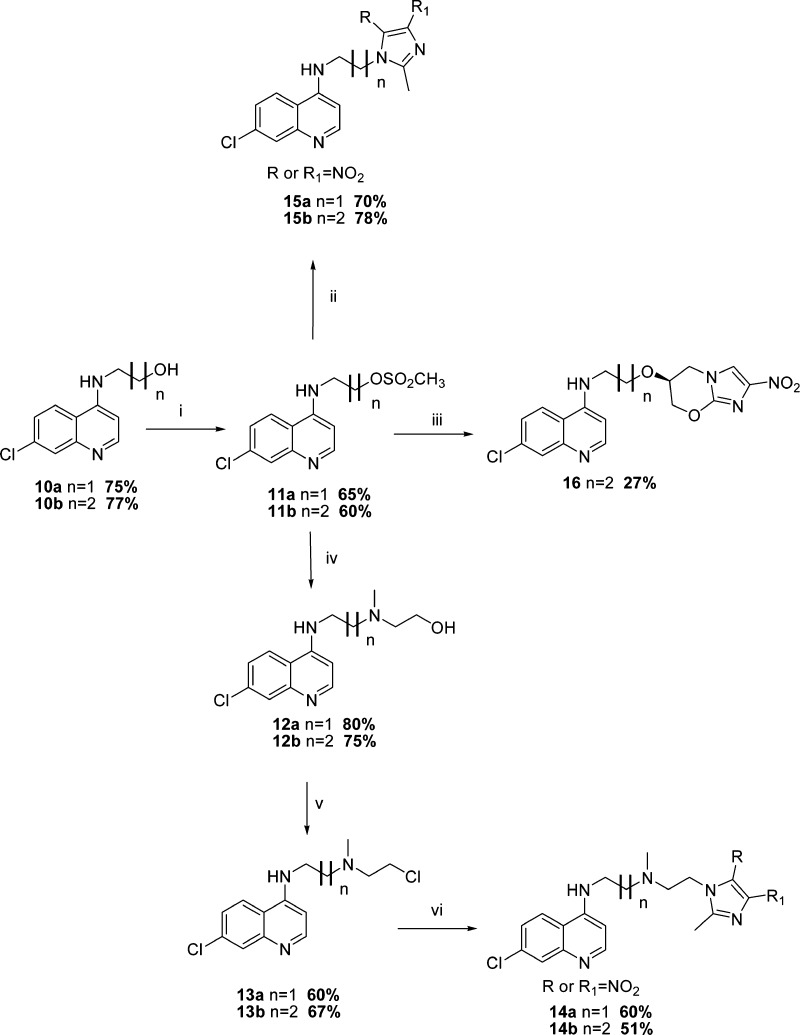
The synthesis of the second series of compounds, 14a–b, 15a–b, and 16, began with the synthesis of intermediates 10a and 10b as described by Chauhan and co-workers25 (Scheme 2). These intermediates were then mesylated to the corresponding methanesulfonic acid esters 11a and 11b, which in turn underwent nucleophilic substitution reaction with 2-methyl-4-nitro-1H-imidazole to yield hybrids 15a–b in reasonable yields. Hybrid 16 was obtained via the sodium hydride mediated reaction of 11b with alcohol 6. In addition, the methanesulfonic acid esters 11a–b were also reacted with N-(methyl)ethanolamine to furnish intermediates 12a–b, which on chlorination using thionyl chloride yielded 13a–b. These chloride derivatives were subsequently reacted with 2-methyl-4-nitro-1H-imidazole in the presence of anhydrous potassium carbonate to afford the isomeric mixture of 14a and 14b (Scheme 2).
Scheme 2.
All synthesized compounds were evaluated in vitro for their antiplasmodial (against the multidrug-resistant K1 strain) and antimycobacterial activity (against the drug-sensitive H37Rv Mtb strain). Chloroquine, primaquine, kanamycin, and streptomycin were used as positive controls, and the results are tabulated in Table 1.
Table 1. In Vitro Antimycobacterial and Antiplasmodial Activity of the Synthesized Compounds.
| P. falciparum IC50 (μM) | M. tuberculosis MIC99 (μM) | cytotoxicity IC50 (μM) | ||
|---|---|---|---|---|
| entry | K1 | H37Rv | L6 | SIa |
| 6 | >270 | 40 | 330.6 | |
| 8a | ndb | 0.25 | nd | |
| 8b | 62.62 | 0.625 | 34.3 | 0.54 |
| 9a | 15.79 | 0.625 | 176.9 | 11.2 |
| 9b | 7.95 | 0.313 | 112.7 | 14.2 |
| 9c | 8.70 | 0.313 | 75.23 | 8.65 |
| 9d | 0.100 | 0.313 | 24.6 | 246 |
| 9e | 0.485 | nd | 41.52 | 85 |
| 9f | 0.164 | 0.625 | 29.3 | 175 |
| 9g | 2.042 | 0.625 | 75.9 | 37 |
| 9h | 0.985 | 5 | 142.8 | 145 |
| 10a | 1.468 | >160 | 233.17 | 159 |
| 10b | 1.086 | >160 | 178.3 | 164 |
| 12a | 0.078 | >160 | 77.57 | 995 |
| 12b | 0.391 | >160 | 24.75 | 63 |
| 14a | 0.812 | >160 | 33.69 | 41.5 |
| 14b | 0.0943 | >160 | 17.6 | 186.6 |
| 15a | 11.063 | >160 | 140.8 | 13 |
| 15b | 4.25 | >160 | 136.8 | 32.2 |
| 16 | 10.52 | 1.25 | 134.5 | 12.8 |
| 17ac | 0.298 | >160 | 21.22 | 71.2 |
| 8a/17ad | 0.303 | 0.25 | 20.41 | 67.4 |
| 8a/17bd | 1.874 | 0.25 | 21.89 | 11.7 |
| 8a/17cd | 2.514 | 0.25 | 11.81 | 4.69 |
| 8a/primaquinec | 0.900 | 0.5 | 29.00 | 32.2 |
| 8b/primaquinec | 0.721 | 0.625 | 22.94 | 31.8 |
| chloroquine | 0.213 | >160 | ||
| primaquine | 0.643 | 80 | ||
| podophyllotoxin | 0.0193 | |||
| kanamycin | 5.40 | |||
| streptomycin | 0.27 |
Selective indices [(IC50 L6 cell-line)/IC50 (K1)].
nd: not determined.
Structures of quinoline diamines 17a–c can be found in the Supporting Information (section 1.10).
Equimolar combination of individual components.
Hybrid compounds 9d–h and 14a–16 showed antiplasmodial IC50 values in the low micromolar range. More specifically, the PA-824–chloroquinoline hybrids 9d (IC50 = 0.100 μM) and 9f (IC50 = 0.164 μM) and the methylnitroimidazole–chloroquinoline hybrid 14b (IC50 = 0.094 μM) were the most active, endowing superior activity than both chloroquine (IC50 = 0.213 μM) and primaquine (IC50 = 0.643 μM). Moreover, hybrids 9d, 9f, and 14b demonstrated an improved activity over their intermediates, exemplified by 8b and quinoline diamine 17a, and the equimolar combination of the individual components, an indication of a synergistic effect. Additionally, the PA-824–chloroquinoline hybrids were found to be more efficacious than the PA-824–primaquine hybrids [9g (IC50 = 2.042 μM) and 9h (IC50 = 0.985 μM)], and the latter hybrids showed an antagonistic effect. The covalent attachment of methylnitroimidazole to intermediates 11a and 11b induced a significant reduction in antiplasmodial activity, as seen in 15a and 15b. Interestingly, the methylnitroimidazole derivative 14b displayed a 4-fold increase in potency compared to intermediate 12b whereas the opposite was observed for hybrid 14a, which had a 10-fold reduction in potency compared to 12a. The 2- and 4-carbon spacer appeared to be more favored in PA-824-chloroquinoline hybrids whereas the 3-carbon spacer was favorable in the methylnitroimidazole–chloroquinoline hybrids. The selective indices of the most active compounds were closer to or greater than 100, suggesting that these compounds are more selective toward the chloroquine-resistant Plasmodium falciparum parasite.
Against replicating H37Rv M. tuberculosis, the MCR series intermediates and target tetrazoles (8 and 9) exhibited MIC99 values ranging between 0.25 and 1.25 μM, except for 9e and 9h, while from the second series only hybrid 16 showed potent antimycobacterial activity. Also, compounds 9a–d, 9f–g, and 16 were more active than the standard TB drug, kanamycin (MIC99 = 5.40 μM), and the two antimalarial drugs, chloroquine (MIC99 > 160 μM) and primaquine (MIC99 = 80 μM). Interestingly, hybridization was found to be less beneficial for antimycobacterial activity, as evidenced by the antagonistic effect in both the PA-824-aminoquinoline and PA-824-primaquine hybrids. Notably, an increase in the length of the alkyl side-chain of the PA-824-chloroquinoline hybrids resulted in reduced activity.
In summary, we have designed and synthesized new nitroimidazole and nitroimidazooxazine derivatives, and these were screened for antiplasmodial and antimycobacterial activity. The majority of these compounds, especially hybrids 9d, 9f, and 14b, exhibited potent activity against the K1 strain of P. falciparum, with IC50 values in the low micromolar range. Furthermore, compounds from the MCR series possessed superior antimycobacterial activity, with MIC99 values in the region of 0.25–125 μM while only one compound, 16, from the second series showed an appreciable activity. Furthermore, the majority of the active compounds were more efficacious than kanamycin, a standard TB drug, in these assays
Acknowledgments
We are grateful to Dr. Marcel Kaiser (Swiss Tropical and Public Health Institute) for antiplasmodial and cytotoxicity assays.
Supporting Information Available
Synthetic experimental procedures, characterization of final compounds, and details regarding biological assays. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
All authors have given approval to the final version of the manuscript.
We thank the University of Cape Town Department of Chemistry Equity Development Program scholarship and the South African National Research foundation (NRF) for financial support. The University of Cape Town, South African Medical Research Council, and South African Research Chairs Initiative of the Department of Science and Technology administered through the NRF is also gratefully acknowledged (K.C.).
The authors declare no competing financial interest.
Supplementary Material
References
- WHO report, Global Tuberculosis Control (2011); http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf (Last accessed 23.08.2012).
- Koul A.; Arnoult E.; Lounis N.; Guillemont J.; Andries K. The challenge of new drug discovery for tuberculosis. Nature Res. Rev. 2011, 469, 483–490. [DOI] [PubMed] [Google Scholar]
- Marriner G. A.; Nayyar A.; Uh E.; Wong S. Y.; Mukherjee T.; Via L. E.; Carroll M.; Edwards R. L.; Grubber T. D.; Choi I.; Lee J.; Arora K.; England K. D.; Boshoff H. I. M.; Barry C. E. III. The medicinal chemistry of tuberculosis chemotherapy. Top. Med. Chem. 2011, 7, 47–124. [Google Scholar]
- Burrows J. N.; Chibale K.; Wells T. N. C. The state of the art in antimalarial drug discovery and development. Curr. Top. Med. Chem. 2011, 11, 1226–1254. [DOI] [PubMed] [Google Scholar]
- Johnson R.; Streicher E. M.; Louw G. E.; Warren R. M.; van Helden P. D.; Victor T. C. Drug resistance in Mycobacterium tuberculosis. Curr. Issue Mol. Biol. 2006, 8, 97–111. [PubMed] [Google Scholar]
- Gandhi N. R.; Nunn P.; Dheda K.; Schaaf H. S.; Zignol M.; van Sooligen D.; Jensen P.; Bayona J. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 2010, 375, 1830–1843. [DOI] [PubMed] [Google Scholar]
- Denny W. A.; Palmer B. D. The nitroimidazooxazine (PA-824 and analogues): Structure-activity relationship and mechanistic studies. Fut. Med. Chem. 2010, 2, 1295–1304. [DOI] [PubMed] [Google Scholar]
- Protopopova M.; Hanrahan C.; Nikonenko B.; Samala R.; Chen P.; Gearhart J.; Einck L.; Nancy C. A. Identification of new antitubercular drug candidate, SQ109, from a combinatorial library of 1.2-ethylenediamens. J. Antimicrob. Chemother. 2005, 56, 968–974. [DOI] [PubMed] [Google Scholar]
- Chan E. D.; Iseman M. D. Multidrug-resistant and extensively drug-resistant tuberculosis: A review. Curr. Opin. Infect. Dis. 2008, 21, 587–595. [DOI] [PubMed] [Google Scholar]
- Brennan P. J. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis 2003, 83, 91–97. [DOI] [PubMed] [Google Scholar]
- Tonelli M.; Simone M.; Tosso B.; Novelli F.; Biodo V. Antiviral activity of benzimidazole derivatives. II. Antimalarial activity of 2-phenylbezimidazole derivatives. Bioorg. Med. Chem. 2010, 18, 2937–2953. [DOI] [PubMed] [Google Scholar]
- Lin P. L.; Dartois V.; Johnston P. J.; Janssen C.; Via L.; Goodwin M. B.; Klein E.; Barry C. E. III; Flynn J. L. Metronidazole prevents reactivation of latent Mycobacterium tuberculosis infection in macaques. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 14188–14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland H. S.; Blaser A.; Kmentova I.; Franzblau S. G.; Wan B.; Wang Y.; Ma Z.; Palmer B. D.; Denny W. A.; Thompson A. M. Synthesis and structure-activity relationships of antitubercular 2-nitroimidazooxazines bearing heterocyclic side chains. J. Med. Chem. 2010, 53, 855–866. [DOI] [PubMed] [Google Scholar]
- Burman W. J. Rip Van Winkle Up: Development of tuberculosis treatment in the 21st century. Clin. Infect. Dis. 2010, 50, S165–S172. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Coates A. R. M.; Mitchison D. A. Comaprison of the sterilizing activation of the nitroimidazopyran PA-824 and moxifloxacin against persistent Mycobacterium tuberculosis. Int. J. Tuberculosis Lung Dis. 2008, 12, 69–73. [PubMed] [Google Scholar]
- Silvestri R.; Artico M.; De Martino G.; Ragno K.; Massa S.; Loddo K.; Murgioni C.; Loi A. G.; LaColla P.; Pani A. Synthesis, biological evaluation, and binding mode of novel 1-[2-(Diarylmethoxy)ethyl]-2-methyl-5-nitoimidazoles targeted at HIV-1 reverse transcriptase. J. Med. Chem. 2002, 45, 1567–1576. [DOI] [PubMed] [Google Scholar]
- Kim P.; Manjunatha U. H.; Singh R.; Patel S.; Jiricek J.; Keller T. H.; Boshoff H. I. M.; Barry C. E. III; Dowd C. S. Structure-activity relationships of antitubercular nitroimidazoles. 1. Structural features associated with Aerobic and Anaerobic activity of 4- and 5-nitromidazoles. J. Med. Chem. 2009, 52, 1317–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z. C.; Genliang L.; Combrink K.; Chen D. D.; Song M.; Wang J.; Ma Z.; Palmer B. D.; Blaser A.; Thompson A. M.; Kmentova I.; Sunderland H. S.; Denny W. A.. Bicyclic nitroimidazoles covalently linked to substituted phenyl oxazolidinones. PCT Int. Appl. WO2009120789, 2009.
- Palmer B. D.; Thompson A. M.; Sunderland H. S.; Blaser A.; Kmentova I.; Franzblau S. G.; Wan B.; Wang Y.; Ma Z.; Denny W. A. Synthesis and structure-activity studies of biphenyl analogues of the tuberculosis drug (6S)-2-Nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824). J. Med. Chem. 2010, 53, 282–294. [DOI] [PubMed] [Google Scholar]
- Thompson A. T.; Sunderland H. S.; Palmer B. D.; Kmentova I.; Blaser A.; Franzblau S. G.; Wan B.; Wang Y.; Ma Z.; Denny W. A. Synthesis and structure-activity relationships of varied ether linked analogues of the tuberculosis drug (6S)-2-Nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824). J. Med. Chem. 2011, 54, 6563–6568. [DOI] [PubMed] [Google Scholar]
- Blaser A.; Palmer B. D.; Sunderland H. S.; Kmentova I.; Franzblau S. G.; Wan B.; Wang Y.; Ma Z.; Denny W. A. Synthesis and structure-activity relationships for amide-, carbonate-, and urea-linked analogues of the tuberculosis drug (6S)-2-Nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824). J. Med. Chem. 2012, 55, 312–326. [DOI] [PubMed] [Google Scholar]
- Illiashevsky O.; Amir L.; Glaser R.; Marks R. S.; Lemcoff N. G. Synthesis, characterization and protein binding properties of supported dendrons. J. Mater. Chem. 2009, 16, 6616–6622. [Google Scholar]
- Jiricek J.; Patel S.; Keller T. H.; Barry C. E. III; Dowd C. S.. Nitroimidazole compounds. PCT Int. Appl. WO20080275035, 2008.
- Tukulula M.; Little S.; Gut J.; Rosenthal P. J.; Wan B.; Franzblau S. G.; Chibale K. The design, synthesis, in silico ADME profiling, antiplasmodial and antimycobacterial evaluation of new arylamino quinoline derivatives. Eur. J. Med. Chem. 2012, 57, 259–267. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Srivastava K.; Kumar S. R.; Puri S. K.; Chauhan P. M. S. Synthesis of new 4-aminoquinolines and quinoline-acridine hybrids as antimalarial agents. Bioorg. Med. Chem. Lett. 2010, 20, 7059–7063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.