Abstract
Background
Several human studies have demonstrated the feasibility of intraarterial delivery of mitoxantrone in systemic malignancies. Computational models predict that intraarterial bolus injection of mitoxatrone during transient cerebral hypoperfusion will enhance brain tissue drug deposition compared to injections during normal blood flow.
Objective
To assess whether transient reduction in cerebral blood flow would enhance the delivery of mitoxantrone. This is accomplished by obtaining real-time measurements of mitoxantrone concentrations in brain tissues using a novel optical pharmacokinetics technique, based on reflectance spectroscopy.
Methods
The blood-brain-barrier of anesthetized rabbits was disrupted by intracarotid injection of mannitol (8 ml, 25% over 40 seconds). Thereafter, animals received 3 mg of mitoxantrone injection during normal perfusion (n=5) or cerebral hypoperfusion (CHP) that was induced by contralateral arterial occlusion and systemic hypotension (n=8).
Results
CHP significantly decreased the cerebral blood flow, allowing a longer exposure time of the drug. It was determined that therapeutic concentrations of mitoxantrone were achieved in both groups, however, hypoperfusion did not increase the tissue concentrations of mitoxantrone after 20 minutes.
Conclusion
These results demonstrate the effective delivery of mitoxantrone by the intraarterial route, after blood-brain-barrier disruption, but the predicted benefits of flow reduction for improving intraarterial deposition of mitoxantrone was not evident.
Keywords: intracarotid, mitoxantrone, optical, pharmacokinetics
Introduction
Computer models of intraarterial drug delivery suggest that tissue drug deposition is improved when intraarterial drugs are injected during transient cerebral hypoperfusion (CHP).1 The availability of small balloon tipped catheters makes it feasible for modulating blood flow in distal regions of the brain.2 Transient cessation of blood flow, either locally or systemically, is often used during neurosurgical and endovascular interventions while treating potentially life threatening vascular malformations and brain aneurysms.3 We therefore hypothesized that intraarterial bolus injections of mitoxantrone will significantly improve tissue drug deposition. Mitoxantrone, a water-soluble, natural-product-derived, non-cell cycle specific chemotherapeutic drug, normally does not cross the intact blood-brain-barrier (BBB). The drug is clinically effective against a range of tumors such as leukemia, colon and breast cancers, as well as melanomas. Early reports suggest that local mitoxantrone prolongs survival in a rodent glioma tumor model.4 Intratumoral mitoxatrone has been used to improve survival of patients with recurrent gliomas. 5
However, the use of mitoxantrone in treating brain tumors is limited by the poor traversement of the BBB. Several studies have demonstrated the feasibility of intraarterial mitoxantrone delivery for treating human lymphangiosarcoma, melanoma7, and breast,7, 8 colorectal9 and pancreatic10 cancers. Thus, methods to improve mitoxantrone delivery to brain tumors, such as by the intraarterial route, are of direct clinical interest. The method of “optical pharmacokinetics” (OP) has been recently developed for minimally-invasive monitoring of local drug concentrations in tissue.11, 12 The OP method consists of placing a fiberoptic probe at the surface of the tissue to be interrogated, and the measurements are obtained in less than 50 ms. The OP technique has been used to measure tissue concentrations of chemotherapeutic drugs in peripheral tissue and implanted tumors.13 Similar ability to track brain tissue drug concentrations by optical methods provides a valuable tool to understand intraarterial drug kinetics.14 In this project we utilized the OP technique to determine whether tissue deposition of mitoxantrone could be augmented by intraarterial injection during CHP.
Methods
Animal preparation
After approval of the investigation protocol by the Institutional Animal Care and Use Committees of both Boston University and Columbia University, studies were conducted on New Zealand White rabbits, 1.5–2 kg in weight. Rabbits, like primates, have a clear separation of the internal and external carotid irrigations. The large size of their skull is convenient for placing laser Doppler (LD) or optical pharmacokinetic (OP) probes for cerebral blood flow and drug measurements, respectively. Therefore, rabbits are well-suited for intracarotid drug delivery experiments. After placement of an intravenous line, the animals were anesthetized with 0.2–0.5 ml boluses of intravenous 1% propofol (Diprivan®, Astra Zeneca, Willmington, DL). Subsequently, through a tracheotomy, the animals were ventilated by Harvard small animal ventilator, to produce an end-tidal CO2 of 35±5 mm Hg. Anesthesia was maintained with continuous propofol infusion at a rate of 20–30 mg/hr. The surgical preparation of the animals consisted of cannulation of the femoral and common carotid arteries. The internal carotid artery of the animal was carefully isolated using the retinal discoloration test.16 The contra-lateral carotid artery was carefully dissected and secured within a silastic loop, such that the vessel could be occluded without disturbing the animal in the stereotactic frame.
For the placement of LD and OP probes, the animals were placed prone in a stereotactic frame. Through a midline incision the skull was exposed. Electroencephalographic leads were secured to the skull with 1.5 mm stainless steel screws. The skull was gently milled down, such that cerebral arteries could be seen through the inner table. The LD probes were secured in plastic retainers that were glued to the skull. The OP probe was manipulated with a Kite micro-manipulator, so that the probe tip was in gentle contact with the thinned skull over the brain. An esophageal temperature probe monitored the core temperature of the animal. The EEG activity, mean femoral arterial pressure, heart rate, CBF from the left and right hemispheres and the ventilatory parameters were recorded by Mac-Lab data (AD Instruments) collection system.
Optical Pharmacokinetics
The method of Optical Pharmacokinetics (OP) is a mode of diffuse reflectance spectroscopy with a specific geometry, which enables the noninvasive real-time measurement of drug concentrations in-situ. An abbreviated description of the method is provided here. The OP method determines drug concentrations by measuring the change of the wavelength-dependent total absorption coefficient of the tissue, and can be used for determining the concentrations of drugs that have an optical absorption band within the wavelength range from visible to near-infrared. Our particular instrument was sensitive to wavelengths of a broader range (450–850 nm). An optical fiber probe, comprising separate illumination and collection fibers, is placed in gentle contact with the tissue surface. The diffusely backscattered light samples the underlying tissue, Fig. 1. Optical absorption spectrum of mitoxantrone is distinct from that of hemoglobin, and oxy-hemoglobin, Fig. 2; therefore, it is well suited for tissue concentration measurements by the OP method. The underlying optical-physics concepts for the OP method have been described in detail in earlier publications by Mourant et al, 11 who have reported the preclinical measurements of chemotherapeutic drug concentrations in animal models in the peripheral tissues. It was shown that for an appropriate range of fiber separations, D, between light delivery and collection fibers, the pathlength of the collected photons, L, is insensitive to variations in scattering properties for the range of scattering parameters typically found in tissue. As employed in these studies, the fiber separation is 2 mm, and the depth of sensitivity is 2–3 mm. The OP device was calibrated with a tissue phantom model using light scattering properties of intralipid, Fig. 3. To establish the correlation between measured and dissolved concentration of mitoxantrone, known amounts of mitoxantrone 0.312 μg/ml to 5 μg/ml were dissolved in 1% intralipid fat emulsion. A linear correlation was observed between the measured and dissolved concentrations of mitoxantrone.
Fig. 1.
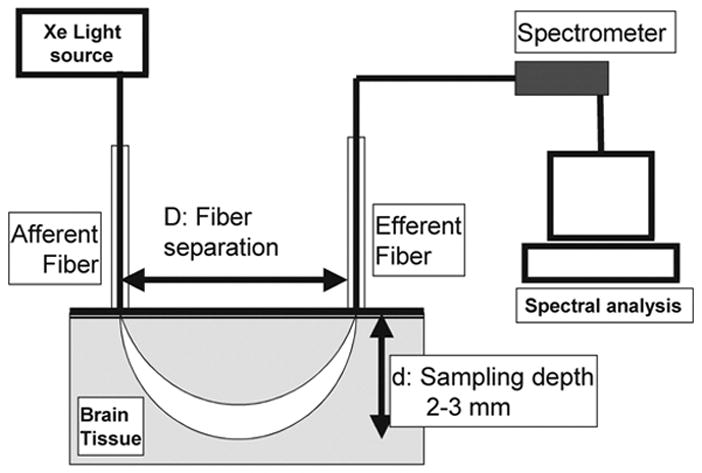
Set-up for the diffuse reflectance spectroscopy system
Fig. 2.

Absorption Spectrum for mitoxantrone, deoxyhemoglobin and oxyhemoglobin normalized to the peak of each spectra.
Fig. 3.
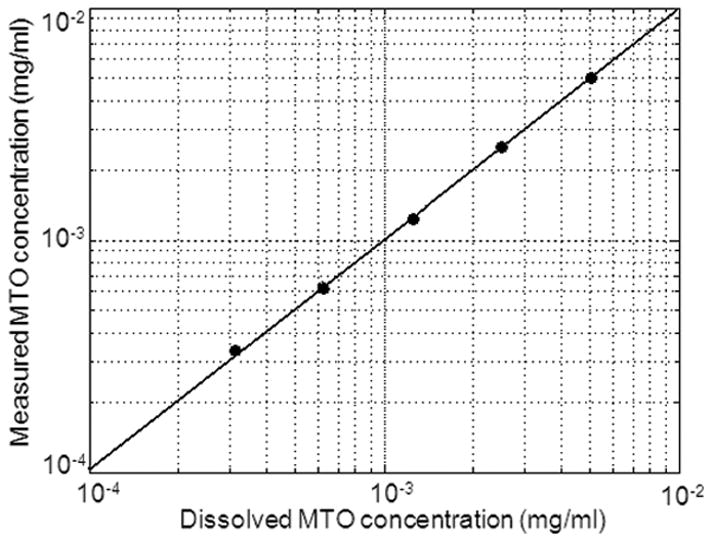
Correlation between measured vs. known concentrations of MTO in a tissue phantom consisting of 1% intralipid fat emulsion. The highest concentration tested was 5 μg/ml and the lowest 0.312 μg/ml. A linear correlation was observed between known and measured concentrations.
Cerebral hypoperfusion (CHP)
Bilateral occlusion of carotid arteries is well tolerated in rabbits. Therefore, to significantly decrease the CBF we used bilateral ICA occlusion with systemic hypotension by intravenous adenosine (20–30 mg) and esmolol (20–30 mg) to transiently decrease CBF to 10–30% of the baseline value. Typically with these doses of the drugs, during CHP the mean arterial pressure decreased from 95–100 mm Hg to 20–30 mm Hg. The heart rate decreased from 225–220 to 75–125 beats/min; and CBF decreased to 10–30% of the baseline value. The end-tidal CO2 decreased from 40–50 mm Hg to 20–30 mm Hg. 17, 18 Arterial occlusion is released after 2 minutes, such that the heart rate and end-tidal CO2, mean arterial pressure and CBF recover rapidly in minutes. Effects of adenosine and esmolol resolve in 5 minutes without any inotropic drugs.17, 18
Drug delivery protocol
In preliminary studies published earlier, we first determined the need to disrupt the BBB to increase tissue deposition of mitoxatrone (Fig. 4).15 In this publication we are determining whether transient cerebral hypoperfusion would augment tissue deposition of mitoxantrone. We randomized New Zealand white rabbits 3–4 lb in weight, into two groups that received intraarterial injections of mitoxantrone (3 mg as 0.1% solution in normal saline injected over 1 min) with or without CHP. The BBB was disrupted in all animals with intraarterial injection of 25% mannitol 8 ml, injected over 40 seconds.19,20
Fig. 4.
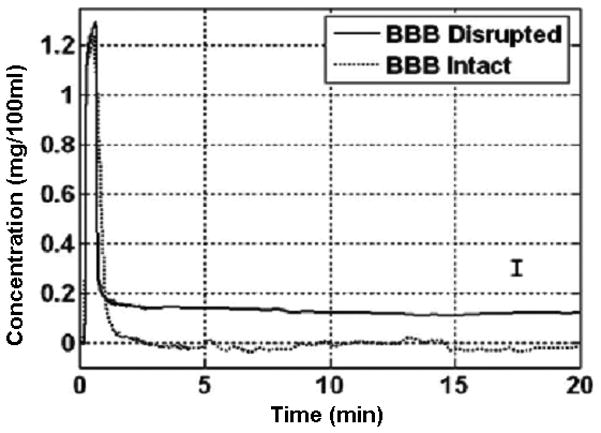
The effect of BBB disruption on the uptake of brain tissue mitoxantrone as reported in an earlier publication by Reif et al.14
Outcome data
Mitoxantrone concentration-time curve parameters that were analyzed included: (i) baseline concentration, (ii) peak concentration, (iii) area under the concentration time curve, and (iv) final brain tissue concentration at 20 minutes after drug. The hemodynamic data for analysis included the heart rate, mean arterial pressure, ipsilateral cerebral blood flow, core temperature, respiratory rate, and end-tidal carbon-dioxide tension. The data were collected in real time but were analyzed at six specific time points: (i) baseline, (ii) after intracarotid mannitol injection, (iii) CHP before injection of mitoxantrone, (iv) after injection of mitoxantrone, (v) five minutes after the start of drug bolus injection, and (vi) 20 minutes after drug bolus.
Data Analysis
The data were analyzed by factorial and repeated measures ANOVA. For single factor comparisons, CHP vs. normal perfusions (NP), a p-value of 0.05 was considered significant to compare the two groups. The Bonoferroni-Dunn test was used to correct for multiple comparisons between six different stages of CHP-assisted mitoxantrone delivery; a p-value of 0.0033 was considered significant for multiple comparisons.
Results
The study was completed in a total of 13 animals, which were randomly assigned to the CHP (n=8) and normal perfusion (n=5) groups. Significant changes in cerebral and systemic hemodynamics occurred during cerebral hypoperfusion. There were statistically significant decreases in heart rate, mean arterial pressure, laser Doppler flow, and end-tidal carbon-dioxide concentrations during CHP compared to baseline and recovery as well as the normal perfusion group. The CBF rapidly recovered within 2 minutes after drug injection, corresponding to the release of contralateral ICA occlusion. The hemodynamic values returned to 10% of the baseline within 5 minutes of drug injection without any pharmacological intervention. Subsequently, the hemodynamic parameters remained stable for the remainder of the experiment, (Fig. 5, Table 1). The peak concentrations of mitoxantrone were not affected by cerebral hypoperfusion 11.16 ± 01.61 vs 11.20 ± 3.18 μg/gm of tissue, P=0.98. The initial peak duration was slightly increased in the CHP group vs. NP but was not statistically significant, 85±33 vs 56±36s, respectively, P=0.17. There was a trend towards an increase in the area under the concentration time curve during CHP vs. NP, 928 ± 353 vs. 594 ± 306 μg/g *min, P=0.11. However, 20 minutes after drug infusion the tissue concentrations were comparable in the CHP vs. NP groups, 1.64 ± 0.841 vs. 1.80 ± 1.09 μg/gm, P=0.77 (Fig 6).
Fig. 5.
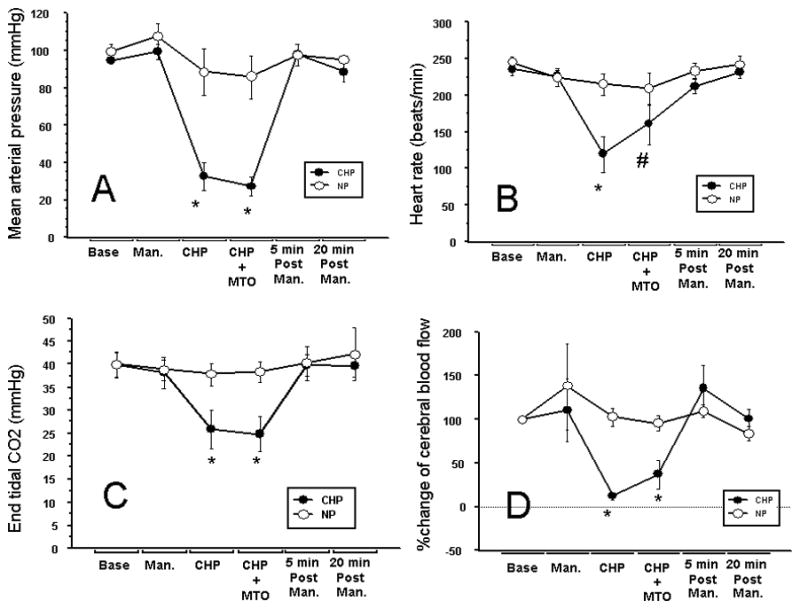
Cerebral Hypoperfusion (CHP)- assisted delivery of mitoxantrone (0.1%*3ml) Bolus Hemodynamic data: Temp and RR no change across and between groups of rabbits normal perfusion (NP=5, open circles) and cerebral hypoperfusion (CHP=8, solid circles). Measurements at six time points: baseline (BASE), after injection of IC-mannitol (MAN.), during CHP before drug (CHP), with Mitoxantrone (CHP+MTO), 5 min post drug (5min. Post Man.), 20 min post drug (20min Post Man.). A: Mean arterial pressure (mm hg), B: heart rate (bpm), C: End-tidal carbon-dioxide tension (mm Hg) and D: %-Δ cerebral blood flow from baseline. Transient but significant changes in hemodynamic parameters were seen during CHP at the time intracarotid mitoxantrone was injected.
Table 1.
Changes in physiological parameters during transient cerebral hypoperfusion
| Base | IC-Mannitol | CHP | CHP+ IC-MTO | 5 min Post MTO | 20 min post MTO | ||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | CHP | 36.1±1.1 | 36.2 ±1.1 | 36.1±1.1 | 36.1±1.1 | 36.1±1.1 | 36.1±1.1 |
| NP | 36.1±1.0 | 35.1 ± 1.3 | 36.2±0.8 | 36.2±0.8 | 36.2±0.8 | 36.3±1.1 | |
| Resp. rate (Breaths/min) | CHP | 62±15 | 62±15 | 62±15 | 62±15 | 62±15 | 62±15 |
| NP | 64±6 | 64±6 | 64±6 | 64±6 | 64±6 | 64±6 | |
| Heart rate (beats/min) | CHP | 235±24 | 226±23 | 120±71@* | 160±75** | 212±28 | 231±20 |
| NP | 246±17 | 226±27 | 215±32 | 212±28 | 234±22 | 242±26 | |
| Mean arterial pressure (mm Hg) | CHP | 95±6 | 100±12 | 32±21@* | 27±14@** | 98±17 | 88±16 |
| NP | 99±10 | 107±16 | 89±26 | 86±26 | 97±4 | 95±5 | |
| End-tidal CO2 (mm Hg) | CHP | 40±7 | 38±10 | 26±11@* | 25±11@** | 40±7 | 40±7 |
| NP | 40±6 | 39±5 | 38±5 | 38±5 | 40±8 | 42±13 | |
| %Δ cerebral blood flow from baseline | CHP | 100±0 | 134±85 | 13±8@* | 44±46@** | 136±74 | 101±74 |
| NP | 100±0 | 138±110 | 104±23 | 96±19 | 110±16 | 84±20 |
Table legend: Abbreviations: CHP: transient cerebral hypoperfusion (n=8); NP: normal perfusion (n=5); IC: intracarotid; MTO: mitoxantrone; IC: intracarotid.
Symbols:
significant difference between CHP and NP (P<0.05);
Significant difference between CHP and other stages of the experiment (P<0.0033);
significant differences between MTO and other stages of the experiment.
Fig. 6.
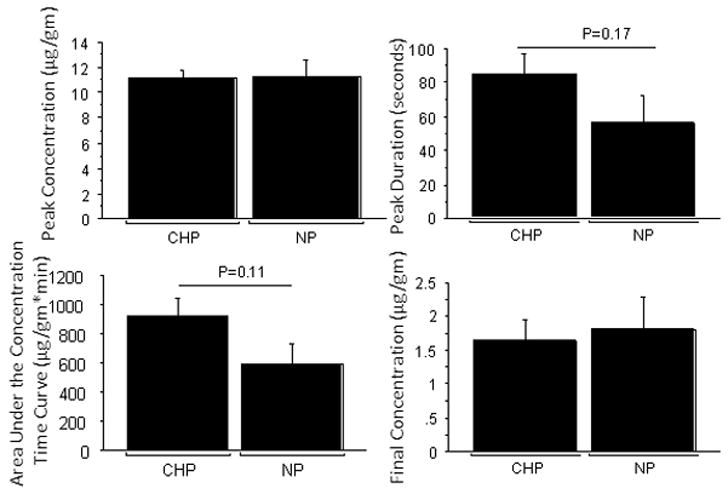
Drug Concentration parameters: The peak concentration (μg/g, A) and peak duration (seconds, B) was comparable between the Cerebral Hypoperfusion (CHP, n=8) and the normal perfusion (NP, n=5) groups but the area under the concentration time curve (μg/g*min) was showed a trend towards an increase with CHP (C). The final concentrations (μg/g, D) at the end of twenty minutes were similar in the NP and CHP groups. In animals with breeched BBB, there seems to be no sustained benefit of injecting mitoxantrone during CHP.
Discussion
There were two main outcomes of this study. First, the study demonstrated that it is possible to track local tissue concentrations of drugs in real time by optical means without invading the cranial vault. Second, the concentration of mitoxantrone in the brain tissue achieved by BBB disruption and bolus injection was in the therapeutic range (>50 ng/gm of tissue);19 although concurrent manipulation of blood flow in this animal model did not further augment tissue concentrations of mitoxantrone 20 minutes after injection.
Transient CHP is routinely used during neurological and endovascular surgery when blood flow to the brain is momentarily interrupted to permit surgical interventions such as during clipping of aneurysms20, deployment of stents21 or treating high flow cerebral arteriovenous malformations.3, 22 The injection of drugs during CHP increases tissue drug deposition in two ways. First, it increases the transit time through the cerebral circulation by as much as 50–100X.23 Second, the rapid injection of drug overwhelms the blood flow, displacing blood and minimizing protein binding, resulting in a significant increase in free drug concentrations.24 Whether CHP can improve drug delivery and outcome in tumor models and patients remains to be seen, however, risks benefits of the procedure will have to be assessed against the course of relentless and fatal disease.
The failure to observe an increased tissue deposition of mitoxantrone during CHP is in contrast to our observations with other drugs that are lipid soluble. For example, in earlier experiments electrophysiological effects of intracarotid anesthetics were augmented 5–10 fold when injections were made during cerebral hypoperfusion. 17,18 Similarly, tissue concentration of carmustine, 5 minutes after intracarotid drug injection, increased 4 to 7 fold when the drug was injected during CHP compared to a conventional 20 min intraarterial infusion with normal blood flow. 27 However, both carmustine and anesthetic drugs are highly lipid soluble compared to mitoxantrone. It is possible that during CHP, highly lipophilic drugs like propofol, pentothal and carmustine are able to rapidly diffuse through the brain parenchyma, but hydrophilic mitoxantrone does not, thereby limiting its uptake by the tissue, Fig. 7. Furthermore, there is evidence to suggest that the capillary area available for the diffusion of lipid soluble drugs is 100x greater than that available for the uptake of water soluble drugs, therefore the uptake of mitoxantrone might be more restricted in no or low flow state since only a small amount of drug in the capillary will be permitted to diffuse out.28 Fig. 7 depicts a conceptual pharmacokinetics model at the tissue level.
Fig. 7.
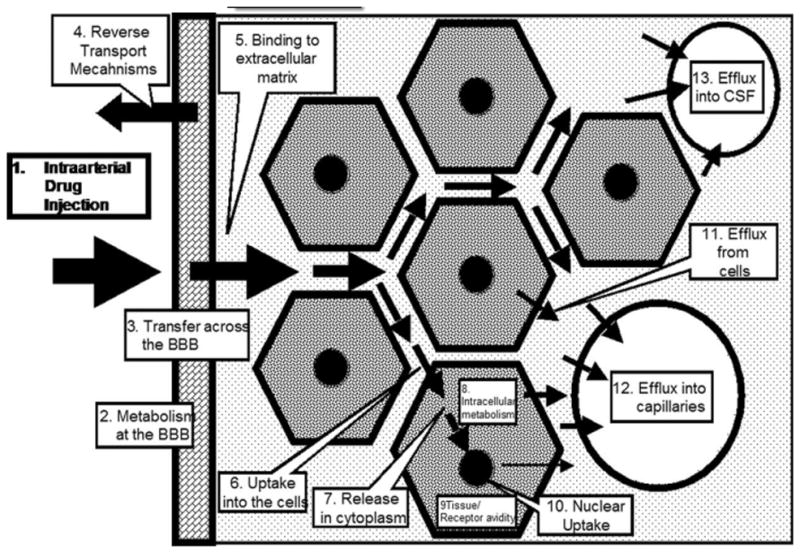
Conceptual micro-pharmacokinetic events affecting intraarterial drug delivery. Barriers to drug delivery are not restricted the blood brain barrier alone. While diffusion of small lipid soluble molecules might be unrestricted most chemotherapeutic drugs face additional hurdles to reach the target site.
The complex micro-pharmacokinetics of intraarterial bolus injections is difficult to investigate Fig. 7. The optical pharmacokinetics technique that calculates the average concentration of drug within a volume of tissue cannot be used to validate this model. However, the OP method provides the temporal resolution to understand intraarterial drug kinetics. With bolus intraarterial injections, the tissue concentrations of drugs change rapidly, and measuring such changes is beyond the time-resolution of conventional tissue sampling techniques, either by undertaking multiple tissue biopsies or with microdialysis, and would also be difficult by imaging techniques. With systemic drug delivery, when concentration parameters in various compartments are stable, arterial blood drug concentrations can be equated to brain drug concentrations by correcting for the blood:brain partition ratio. However, to understand bolus kinetics, real-time measurements of drug concentration are required. Although such measurements are feasible by using radioactive drugs, and in some instances by magnetic resonance imaging, neither of these technologies can be widely employed nor used in conventional laboratory settings. Optical measurements, therefore, offer valuable insights into drug delivery to the brain.
The principal limitation with optical pharmacokinetics is that drugs must have an optical absorption band in the 600–950 nm range, preferably one that does not directly coincide with that of hemoglobin and oxyhemoglobin absorption bands. Some anthracycline planar anti-cancer drugs like mitoxantrone, daunorubicin, and doxorubicin have spectral signatures that easily permit OP measurements of drug concentrations. For other chemotherapeutic agents one possible way to overcome this problem is to tag the molecule with a chromophore, as long as the chromophore does not significantly alter the pharmacology. In some cases, like paclitaxel, tagging is relatively easy without altering its pharmacology, and tagged versions are available commercially. Others, like methotrexate, can be tagged with fluorescein. Another way to overcome this limitation is to develop models of drug delivery that use dyes with known pharmacokinetic properties. While optical measurements do not compromise the structural integrity of the brain tissue, the technique, even in rabbits, does require shaving of the skull. The geometry of the current probe limits its use to a sampling depth of 2–3 mm. For clinical use in human research, either the probe geometry will have to be revised or implantable fiberoptic probes would have to be developed. The current technology could be used to understand pharmacokinetics of certain chemotherapeutic drugs when the brain tissue is exposed during surgery.
One of the limitations in the present study is that we used a standard dose of mannitol to disrupt the BBB. Although this experimental step was common to both groups, a variable degree of BBB disruption could affect the resulting tissue concentration of drugs. Human data suggest that such variability is less likely to affect carotid injection compared to vertebral artery injection.29 For the future, the ability of the OP method to track multiple chromophores could enable us to measure concentrations of tracers of BBB disruption such as Evan’s blue or indocyanine green and thereby normalize tissue mitoxantrone concentrations to that of a tracer that can quantify BBB disruption. Such an approach will be invaluable in the treatment of brain tumors, in experimental or clinical settings, due to the variable degree of blood brain barrier disruption around tumor tissue. We are currently undertaking such investigations.
It is feasible to track tissue concentrations of mitoxantrone after intracarotid injection of the drug by the method of optical pharmacokinetics. Significant deposition of mitoxantrone in the brain tissue was evident after bolus injection of the drug following hyperosmotic disruption of the BBB. Contrary to what computer modeling suggests and our own previous observations, cerebral hypoperfusion does not appear to augment tissue deposition of mitoxantrone after intraarterial injections. Improvements of intraarterial drug delivery will require adjuvant strategies, such as the increased lipid solubility of the mitoxatrone, through liposomal formulations to improve intraarterial drug delivery.
Summary.
Using a novel optical method of measuring concentrations of mitoxantrone in brain tissues, it was determined that although therapeutic concentrations of mitoxantrone could be achieved with intraarterial drug delivery after blood-brain-barrier disruption, the reduction of cerebral blood flow during mitoxantrone injection did not further increase the brain tissue deposition of the drug. These results suggest that the predicted benefits of flow reduction for improving intraarterial deposition of the drugs might not be universal and adjuvant and/or alternate strategies are needed to improve mitoxantrone delivery.
Acknowledgments
Financial support: This work was supported in part by the NCI R01 (R01-CA- 12500, SJ) and NCI grants (R01-CA82104; U54-CA104677, IB)
Footnotes
No reprints will be available upon request
References
- 1.Dedrick RL. Arterial drug infusion: pharmacokinetic problems and pitfalls. J Natl Cancer Inst. 1988 Mar 16;80(2):84–89. doi: 10.1093/jnci/80.2.84. [DOI] [PubMed] [Google Scholar]
- 2.Pile-Spellman J, Young WL. Materials and methods for interventional neuroradiology (INR) (Chapter 18.2) In: Taveras JM, editor. Neuroradiology. 3. Baltimore: Williams & Wilkins; 1996. pp. 1047–1073. [Google Scholar]
- 3.Pile-Spellman J, Young WL, Joshi S, et al. Adenosine-induced cardiac pause for endovascular embolization of cerebral arteriovenous malformations: technical case report. Neurosurgery. 1999 Apr;44(4):881–886. doi: 10.1097/00006123-199904000-00117. discussion 886–887. [DOI] [PubMed] [Google Scholar]
- 4.Yemisci M, Bozdag S, Cetin M, et al. Treatment of malignant gliomas with mitoxantrone-loaded poly (lactide-co-glycolide) microspheres. Neurosurgery. 2006 Dec;59(6):1296–1302. doi: 10.1227/01.NEU.0000245607.99946.8F. discussion 1302–1293. [DOI] [PubMed] [Google Scholar]
- 5.Boiardi A, Eoli M, Salmaggi A, et al. Efficacy of intratumoral delivery of mitoxantrone in recurrent malignant glial tumours. J Neurooncol. 2001 Aug;54(1):39–47. doi: 10.1023/a:1012510513780. [DOI] [PubMed] [Google Scholar]
- 6.Durr FE. Biologic and biochemical effects of mitoxantrone. Semin Oncol. 1984 Sep;11(3 Suppl 1):3–10. [PubMed] [Google Scholar]
- 7.Nitz U, Havenith B, Rost B, Mosny D, Ellerbrok G. Locoregional intra-arterial chemotherapy of primary incurable local recurrence of breast cancer. Geburtshilfe Frauenheilkd. 1993 Nov;53(11):760–767. doi: 10.1055/s-2007-1023722. [DOI] [PubMed] [Google Scholar]
- 8.Gorich J, Tomczak R, Gabelmann A, Wisianowski C, Kramer S. Intraarterial chemotherapy in cases of breast cancer. Radiologe. 1999 Sep;39(9):790–794. doi: 10.1007/s001170050577. [DOI] [PubMed] [Google Scholar]
- 9.Vaglini M, Cascinelli F, Chiti A, et al. Isolated pelvic perfusion for the treatment of unresectable primary or recurrent rectal cancer. Tumori. 1996 Sep-Oct;82(5):459–462. doi: 10.1177/030089169608200510. [DOI] [PubMed] [Google Scholar]
- 10.Papachristou E, Link KH, Schoenberg MH. Regional celiac artery infusion in the adjuvant treatment of pancreatic cancer. Anticancer Res. 2003 Mar-Apr;23(2A):831–834. [PubMed] [Google Scholar]
- 11.Mourant JR, Johnson TM, Los G, Bigio IJ. Non-invasive measurement of chemotherapy drug concentrations in tissue: preliminary demonstrations of in vivo measurements. Physics in Medicine & Biology. 1999;44(5):1397–1417. doi: 10.1088/0031-9155/44/5/322. [DOI] [PubMed] [Google Scholar]
- 12.Bigio IJ, Bown SG. Spectroscopic sensing of cancer and cancer therapy: current status of translational research. Cancer Biol Ther. 2004 Mar;3(3):259–267. doi: 10.4161/cbt.3.3.694. [DOI] [PubMed] [Google Scholar]
- 13.Kanick SC, Eiseman JL, Joseph E, Guo J, Parker RS. Noninvasive and nondestructive optical spectroscopic measurement of motexafin gadolinium in mouse tissues: comparison to high-performance liquid chromatography. J Photochem Photobiol B. 2007 Sep 25;88(2–3):90–104. doi: 10.1016/j.jphotobiol.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Reif R, Wang M, Joshi S, A’Amar O, Bigio IJ. Optical method for real-time monitoring of drug concentrations facilitates the development of novel methods for drug delivery to brain tissue. J Biomed Opt. 2007 May-Jun;12(3):034036. doi: 10.1117/1.2744025. [DOI] [PubMed] [Google Scholar]
- 15.Breidenbach M, Rein D, Schmidt T, et al. Intra-arterial mitoxantrone and paclitaxel in a patient with Stewart-Treves syndrome: selection of chemotherapy by an ex vivo ATP-based chemosensitivity assay. Anticancer Drugs. 2000 Apr;11(4):269–273. doi: 10.1097/00001813-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Joshi S, Wang M, Hartl R. Retinal discoloration test. J Cereb Blood Flow Metab. 2004 Mar;24(3):305–308. doi: 10.1097/01.WCB.0000107731.66603.18. [DOI] [PubMed] [Google Scholar]
- 17.Joshi S, Wang M, Etu JJ, Nishanian EV, Pile-Spellman J. Cerebral blood flow affects dose requirements of intracarotid propofol for electrocerebral silence. Anesthesiology. 2006 Feb;104(2):290–298. doi: 10.1097/00000542-200602000-00014. discussion 295A. [DOI] [PubMed] [Google Scholar]
- 18.Joshi S, Wang M, Etu JJ, Pile-Spellman J. Reducing cerebral blood flow increases the duration of electroencephalographic silence by intracarotid thiopental. Anesth Analg. 2005 Sep;101(3):851–858. doi: 10.1213/01.ANE.0000160583.42078.B2. table of contents. [DOI] [PubMed] [Google Scholar]
- 19.Green RM, Stewart DJ, Hugenholtz H, Richard MT, Thibault M, Montpetit V. Human central nervous system and plasma pharmacology of mitoxantrone. J Neurooncol. 1988;6(1):75–83. doi: 10.1007/BF00163544. [DOI] [PubMed] [Google Scholar]
- 20.Schick U, Dohnert J, Meyer JJ, Vitzthum HE. Effects of temporary clips on somatosensory evoked potentials in aneurysm surgery. Neurocrit Care. 2005;2(2):141–149. doi: 10.1385/NCC:2:2:141. [DOI] [PubMed] [Google Scholar]
- 21.Plaschke K, Bockler D, Schumacher H, Martin E, Bardenheuer HJ. Adenosine-induced cardiac arrest and EEG changes in patients with thoracic aorta endovascular repair. Br J Anaesth. 2006 Mar;96(3):310–316. doi: 10.1093/bja/ael002. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto T, Young WL, Aagaard BD, Joshi S, Ostapkovich ND, Pile-Spellman J. Adenosine-induced ventricular asystole to induce transient profound systemic hypotension in patients undergoing endovascular therapy. Dose-response characteristics. Anesthesiology. 2000 Oct;93(4):998–1001. doi: 10.1097/00000542-200010000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Joshi S. Electrocerebral silence after intracarotid propofol injection is a function of transit time. Anesth Analg. 2007 Jun;104(6):1498–1503. doi: 10.1213/01.ane.0000264089.72804.54. table of contents. [DOI] [PubMed] [Google Scholar]
- 24.Jones DR, Hall SD, Jackson EK, Branch RA, Wilkinson GR. Brain uptake of benzodiazepines: effects of lipophilicity and plasma protein binding. J Pharmacol Exp Ther. 1988;245(3):816–822. [PubMed] [Google Scholar]


