Abstract
Bone marrow-mesenchymal stromal cells (BM-MSCs) have immunosuppressive properties and have been used in cell therapies as immune regulators for the treatment of graft-versus-host disease. We have previously characterized several biological properties of MSCs from placenta (PL) and umbilical cord blood (UCB), and compared them to those of BM—the gold standard. In the present study, we have compared MSCs from BM, UCB, and PL in terms of their immunosuppressive properties against lymphoid cell populations enriched for CD3+ T cells. Our results confirm the immunosuppressive potential of BM-MSCs, and demonstrate that MSCs from UCB and, to a lesser extent PL, also have immunosuppressive potential. In contrast to PL-MSCs, BM-MSCs and UCB-MSCs significantly inhibited the proliferation of both CD4+ and CD8+ activated T cells in a cell–cell contact-dependent manner. Such a reduced proliferation in cell cocultures correlated with upregulation of programmed death ligand 1 on MSCs and cytotoxic T lymphocyte-associated Ag-4 (CTLA-4) on T cells, and increased production of interferon-γ, interleukin-10, and prostaglandin E2. Importantly, and in contrast to PL-MSCs, both BM-MSCs and UCB-MSCs favored the generation of T-cell subsets displaying a regulatory phenotype CD4+CD25+CTLA-4+. Our results indicate that, besides BM-MSCs, UCB-MSCs might be a potent and reliable candidate for future therapeutic applications.
Introduction
Mesenchymal stromal cells (MSCs) comprise a heterogeneous population of multipotent progenitors that possess four biological properties that make them special candidates for cell therapy: a broad differentiation potential, the capacity to produce and secrete factors that promote tissue remodeling, low immunogenicity, and immunosuppressive properties [1,2]. Regarding this last property, MSCs can interact with both innate and adaptive immune cells and thus exert profound effects on immune responses [3–5]; in particular, MSCs affect T-cell proliferation and differentiation primarily through the production of immunosuppressive molecules and the generation of regulatory T cells (Tregs) [6–9].
Several studies using peripheral blood mononuclear cells (PBMC) have demonstrated MSCs involvement in T-cell immunosuppression [4,5,8,10–12]. However, few studies have been performed with enriched populations of CD3+ T cells [10,13,14]. This is important because CD4+ and CD8+ T cells are the major effector cells in immunological diseases such as graft-versus-host disease (GVHD) [15], and thus it is important to determine the immunosuppression properties of MSCs on these populations and determine their potential for cell therapies.
Bone marrow (BM) is the main source of MSCs [15]; BM-MSCs have been used in cell therapy protocols to reduce GVHD [15,16]. However, BM presents some disadvantages, such as the difficulty in finding donors, the cost and invasiveness of the collection procedure, and age-related decreases in MSCs numbers [17]. Due to all of these factors, it is important to obtain MSCs from sources other than BM. Our research group has obtained MSCs from umbilical cord blood (UCB) and placenta (PL); both of these sources are easily accessible and pose no risk to the donor. In a previous study, we showed that UCB-MSCs and PL-MSCs have morphological and immunophenotypic properties in addition to adipogenic, osteogenic, and chondrogenic differentiation capacities similar to those of BM-MSCs [18]. However, we do not know whether these alternatively sourced cells have the same immunosuppressive potential as BM-MSCs, and thus it is important to determine which of them may be the best MSCs source for use in immunosuppressive cell therapy protocols.
MSCs have been suggested to affect T-cell proliferation through both cell contact-dependent and independent mechanisms. Programmed death ligand 1 (PD-L1) and human leukocyte antigen-G1 (HLA-G1) expression have been linked to the cell contact-dependent mechanisms [8,19–21], while transforming growth factor beta (TGF-β), hepatocyte growth factor, interleukin-10 (IL-10), indoleamine 2,3-dioxygenase (IDO), nitric oxide, prostaglandin E2 (PGE2), and human leukocyte antigen-G5 (HLA-G5) have been identified as secreted factors [1,5,8]. Currently, there is controversy regarding the need for direct contact between MSCs and T cells to inhibit T-cell proliferation [4,8,11,19–23]. Additionally, studies of activation marker expression are also controversial. Some studies have shown that BM-MSCs prevent the expression of the early activation markers CD25 and CD69 on phytohemagglutinin (PHA)-stimulated CD4+ T cells [10,24]. Others have observed that MSCs do not affect activation marker expression on T cells [4,12]. Further, the effects of UCB-MSCs and PL-MSCs on activation marker expression have not been reported.
It is commonly accepted that MSCs-mediated immunosuppression can be accomplished by lymphocyte populations known as Tregs. However, there are also conflicting reports on this subject. Some authors have suggested that MSCs promote the generation of CD4+CD25+ forkhead box P3 (Foxp3) or CD4+CD25highFoxp3+ T-cell populations [7,8,25–27], while others indicate that BM-MSCs-induced Tregs populations are not Foxp3+ [4,28]. Apparently, these contradictory findings might be due to the T-cell populations used and the absence or presence of cell contact with BM-MSCs [9]. Further, a role for Tregs in MSCs-mediated immunosuppression has been suggested by the increase of CD4+CD25+CTLA-4+ Tregs found in cocultures of allogeneic MSCs and mixed lymphocyte reactions (MLR) [6]. Thus, in addition to the expression of surface membrane molecules and soluble mediators, MSC-mediated immunosuppression could be amplified by Tregs activity.
In an attempt to contribute to our understanding of the immunosuppressive properties of MSCs from BM, UCB, and PL against CD3+ T cells on the basis of these notions, we performed a comparative study of MSCs from three sources with regard to their potential for the inhibition of proliferation, effects on activation markers, and expression of immunosuppressive membrane and secreted molecules. We also assessed the capacity of MSCs to generate T-cell subsets displaying a regulatory phenotype in cocultures. To our knowledge, this is the first study comparing the immunosuppressive properties of MSCs from three sources to determine which one would be the most appropriate for in vivo immunoregulatory applications.
Methodology
Isolation and culture of MSCs derived from BM, UCB, and PL
BM samples were obtained from 5 volunteer donors according to the ethical guidelines of the Villacoapa Hospital, Mexican Institute for Social Security (IMSS). UCB (n=5) and PL (n=5) samples were collected according to the institutional guidelines of Troncoso Hospital, IMSS. The isolation of mononuclear cells (MNC) from three sources was performed as previously described [18]. MNC from BM, UCB, or PL were resuspended in low glucose Dulbecco's modified Eagle's medium (Gibco BRL, Rockville, MD) that was supplemented with 10% fetal bovine serum (FBS; Gibco BRL), 4 mM l-glutamine, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 100 μg/mL of gentamicin (all reagents were obtained from Gibco BRL); the cells were seeded at a density of 0.2×106 cells/cm2 in T-25 culture flasks (Corning, Inc./Costar, New York, NY). After a 4-day culture, the nonadherent cells were removed and fresh medium was added to the cultures. Once the cultures reached 80% confluence, the cells were harvested with trypsin (0.05% trypsin, 0.53 mM EDTA; Gibco BRL), and subcultured at a density of 0.002×106 cells/cm2 in T-75 flasks (Corning, Inc./Costar). At the second passage, the cells were harvested, analyzed, and cryopreserved for future use.
Characterization of MSCs
Immunophenotypic characterization and differentiation capacities of MSCs were performed according to previously described protocols [18]. FITC, PE, or APC-conjugated monoclonal antibodies against CD73, CD90, and CD45 (BD Biosciences, San Diego, CA) CD105, CD13, CD14 (Caltag, Buckingham, United Kingdom), HLA-ABC, HLA-DR, CD31, and CD34 (Invitrogen, Carlsbad, CA) were used for immunophenotypic characterizations as described in the Flow Cytometric Analysis section.
Adipogenic and osteogenic differentiation was induced with Stem Cells Kits™ (Stemcell Technologies, Inc., Vancouver, BC, Canada). Adipogenic differentiation was determined by visualizing the presence of Oil Red O-stained (Sigma-Aldrich, St. Louis, MO) lipid vacuoles. Osteogenic differentiation was assessed by the detection of von Kossa-stained calcium deposits and alkaline phosphatase staining. Chondrogenic differentiation was induced with a commercial induction medium (Cambrex Bio Science, Walkersville, Inc., Walkersville, MD) that was supplemented with 10 ng/mL of TGF-β (Cambrex Bio Science). The resulting micromasses were fixed, embedded, and sliced. Cross sections were stained with Alcian blue dye (Sigma-Aldrich).
CD3+ T-cell collection
PBMC were obtained from the peripheral blood samples of three volunteer donors by density gradient with Ficoll-Paque Plus (specific gravity <1.077g/mL; GE Healthcare Bio-Sciences AB, Uppsala, Sweden). CD3+ T cells were obtained by separation with human CD3 MicroBeads and MACS MS columns (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the supplier's instructions. The purity of the obtained CD3+ T-cell suspensions was determined by flow cytometry as described in the Flow Cytometric Analysis section, and only suspensions with purity greater than 97% were used in the experiments. CD3+ T cells were maintained in RPMI medium (RPMI 1640, 10% fetal calf serum, 2 mM l-glutamine, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 100 μg/mL of gentamicin; all reagents were obtained from Gibco BRL) for 24 h.
Stimulation of T cells
Cultured CD3+ T cells were activated in RPMI medium with Dynabeads CD3/CD28 T-cell expander (Invitrogen) at a 1:1 ratio (25 μL of dynabeads/1×106 T cells). Fifteen hours before harvesting the cultures (3–4 days of activation), the cells were labeled with 10 μM of 5-bromo-2-deoxyuridine (BrdU; BD Biosciences) to determine cell proliferation as described in the Flow Cytometric Analysis section.
Cocultures of MSCs/CD3+ T cells
Cocultures of MSCs/CD3+ T cells were performed in the presence or absence of cell–cell contact. Cocultures with cell–cell contact were performed in 24-well plates. CD3+ T cells (1.5×105) were seeded and activated as described in Stimulation of T Cells section in the absence or presence of 1.5×105 MSCs from BM, UCB, or PL (MSC:T-cell ratio, 1:1). Antibody-coated beads were present during T-cell cocultures with MSCs. Cultures without cell–cell contact were performed in transwell culture chambers (Corning, Inc./Costar). MSCs from BM, UCB, or PL (1.5×105 cells) were placed into the lower chambers, and CD3+ T cells (1.5×105 cells) were placed into the upper chambers; the chambers were separated by a semipermeable membrane with a 0.4 mm pore size. CD3+ T cells that were activated in the absence of MSCs were used as positive controls for proliferation, cytokine secretion, and surface molecule expression. BM-MSCs, UCB-MSCs, or PL-MSCs that were cultured in the absence of T cells were used as controls for the basal MSCs expression of surface molecules and secreted factors. The cultures were maintained for 1–4 days to permit concurrent evaluations of proliferation and molecule expression by flow cytometry and cytokine secretion (see Flow Cytometric Analysis, Quantitative Cytokine Analysis, and Quantitative PGE2 Measurement sections).
Proliferation assays
After 3 or 4 days of T-cell activation, the cells were labeled with 10 μM BrdU (BD Pharmingen, San Diego, CA) at 15 h before harvesting. CD3+, CD4+, and CD8+ T-cell proliferation rates were assessed with a BrdU Flow Kit (BD Pharmingen) according to the supplier's instructions. CD3+ T cells that were activated in the absence of MSCs were used as positive controls and were set at 100% proliferation. The levels of proliferation observed in the cocultures were normalized to this control.
Flow cytometric analysis
In addition to monoclonal antibodies against surface markers characteristic of MSCs, we also used FITC, PE, or APC-conjugated monoclonal antibodies against CD3, CD4, CD8, CD25, CD69, cytotoxic T lymphocyte-associated Ag-4 (CTLA-4), BrdU (BD Biosciences), Foxp3 (eBioscience, San Diego, CA), and their respective isotype controls to characterize T cells and also PE anti-PD-L1 (BD Biosciences) was used for MSCs. A total of 1–2×105 cells, previously blocked with Fc receptor blocker (Blocking Reagent Human; Miltenyi Biotec), were resuspended in 100 μL of phosphate-buffered saline (PBS) with 3% FBS and 1 mM EDTA and were incubated with the appropriate antibodies for 20–30 min; the cells were subsequently washed with 1 mL of PBS (with 3% FBS and 1 mM EDTA) and fixed with FACS Lysing Solution (BD Biosciences). Cytofix/Cytoperm buffer (BD Pharmingen) was used according to the supplier's instructions to permit the detection of intracellular CTLA-4. The Foxp3 Fixation/Permeabilization Concentrate and Diluent Kit (eBioscience) was used to detect intracellular Foxp3. The labeled cells were analyzed on a Coulter Epics Altra Flow Cytometer (Beckman Coulter, Brea, CA), and at least 10,000 events were collected per sample. The data were analyzed with FlowJo 2.6 software. The percentages of positive cells and mean fluorescence intensities (MFI) were obtained.
Quantitative cytokine analysis
To quantify the secreted cytokine concentrations, supernatants were obtained from several culture conditions, including nonactivated CD3+ T cells or MSCs in the absence of T cells (negative controls) and activated CD3+ T cells in the absence of MSCs (positive control). The supernatants were stored at −70°C until use. Cytokine analyses were performed with a cytometric bead array (BD Biosciences), according to the supplier's instructions.
Quantitative PGE2 measurement
PGE2 concentrations in the supernatants were assessed by enzyme-linked immunosorbent assay (ELISA) with a Prostaglandin E2 Human ELISA Kit (Invitrogen), according to the supplier's instructions. The supernatant from MSCs that were cultured in the absence of activated CD3+ T cells was used as control.
Statistical analysis
Data are expressed as the mean and standard error of mean calculated from 3 to 10 independent experiments. Statistical analyses were performed using SPSS statistics 20.0. Comparisons between groups were performed by Kruskal–Wallis test followed by Mann–Whitney U test. A P value<0.05 was considered to be significant.
Results
Immunophenotype and differentiation capacity of BM-MSCs, UCB-MSCs, and PL-MSCs
Individual experiments from BM-MSCs (n=5), UCB-MSCs (n=5), and PL-MSCs (n=5) displayed immunophenotypes and differentiation capacities similar to those reported previously [18]. MSCs from the three sources expressed high levels of the characteristic MSCs surface markers CD105, CD90, and CD73 as established by the International Society for Cellular Therapy (ISCT) [29]. Further, the MSCs expressed low levels of HLA-ABC, were HLA-DR-negative, and did not express hematopoietic markers such as CD34, CD45, and CD14 or endothelial markers such as CD31. Meanwhile, MSCs from the three sources were capable of osteogenic and chondrogenic differentiation; however, only BM- and PL-MSCs showed adipogenic potential, unlike UCB-MSCs, which had no such potential (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd).
CD4+ and CD8+ T-cell proliferation is inhibited by cocultivation and cellular contact with BM-MSCs and UCB-MSCs
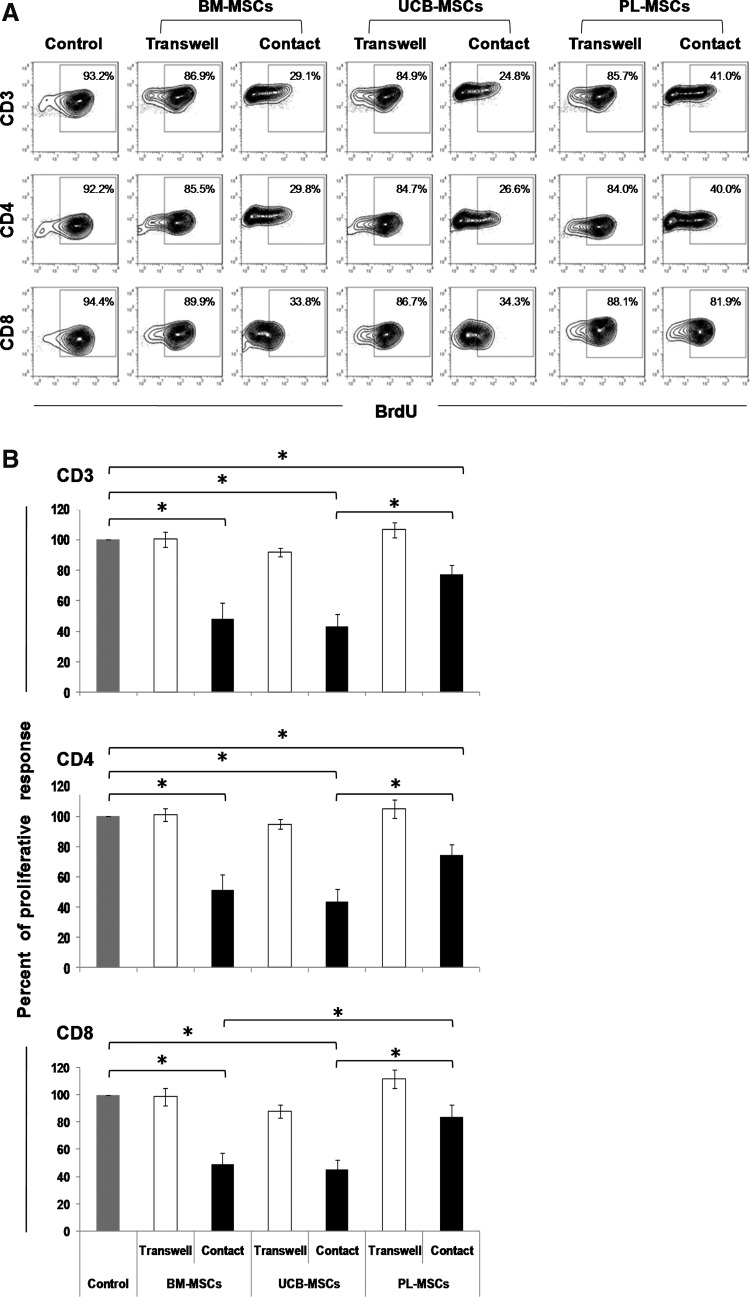
BM-MSCs, UCB-MSCs, and PL-MSCs have been shown to decrease the proliferation of mitogen or alloantigen-activated PBMC [4,30,31]. Some studies have demonstrated the importance of the type of population evaluated (PBMC or CD3+ T cells) and the presence of cell contact in cocultures with MSCs in BM-MSCs-mediated immunosuppression [9,14]. Thus, we decided to analyze the direct effect of MSCs on a CD3+ T-cell-enriched population to reduce the interference from other cellular components such as B cells, monocytes, and dendritic cells. We isolated CD3+ T cells (purity ≥97%) that were activated with anti-CD3/CD28 in the presence or absence of BM-MSCs, UCB-MSCs, or PL-MSCs, and either allowed (Contact) or prevented cell–cell contact (Transwell). Proliferation of activated T cells cultured in absence of MSCs was considered 100% of proliferation response (positive control). No incorporation of BrdU was observed in negative control (nonactivated T cells cultured in absence of MSCs; data not shown). Figure 1A and B show that BM- and UCB-MSCs significantly reduced proliferation of CD3+ (48.2%±10.1% and 42.9%±7.8%, respectively; P<0.05), CD4+ (51.2%±10.4% and 43.6%±8.2%, respectively; P<0.05), and CD8+ (49.0%±8.3% and 44.8%±7.4%, respectively; P<0.05) T cells, while PL-MSCs only reduced the proliferation of CD3+ (77.0%±6.2%; P<0.05) and CD4+ (74.5%±6.8%; P<0.05) T cells, but not CD8+ (83.7%±8.8%) T cells. It is worth mentioning that these reductions were only observed in cocultures with cell–cell contact (Supplementary Fig. S2). Additionally, in UCB-MSCs cocultures, we observed a greater reduction in the proliferation of CD3+, CD4+, and CD8+ T cells, compared with PL-MSC cocultures (P<0.05); however, in BM-MSCs cocultures, we only observed a decrease in proliferation of CD8+ T cells when compared with the PL-MSCs cocultures (P<0.05). These results suggest that BM-MSCs and UCB-MSCs have a greater ability to inhibit T-cell proliferation than do PL-MSCs and that contact between the T cells and MSCs is necessary for such inhibition.
FIG. 1.
BM-MSCs and UCB-MSCs decrease both CD4+ and CD8+ T-cell proliferation in a cell contact-dependent manner. Anti-CD3/CD28-activated CD3+ T cells were cocultured in the presence of BM-MSCs, UCB-MSCs, or PL-MSCs at a 1:1 ratio of MSCs:T cells. Cocultures were prepared in the presence (black bars) or absence (white bars) of cell–cell contact; the latter were performed with transwell chambers. Proliferation of activated CD3+ T cells cultured in absence of MSCs was the positive control (100% proliferation, n=10, gray bars). The percentage of BrdU incorporation was determined in the CD3+, CD4+, and CD8+ populations after 3–4 days of culture. (A) Representative dot plots from an experiment. (B) Data are shown as the mean±SEM for the percentages of proliferation (BM-MSCs: n=10 with cell–cell contact, n=5 transwell; UCB-MSCs: n=10 with cell–cell contact, n=5 transwell; and PL-MSCs: n=10 with cell–cell contact, n=5 transwell. Individual experiments). *Indicates a statistically significant difference with P<0.05. BM-MSCs, bone marrow mesenchymal stromal cells; PL, placenta; UCB, umbilical cord blood; BrdU, 5-bromo-2-deoxyuridine; SEM, standard error of mean.
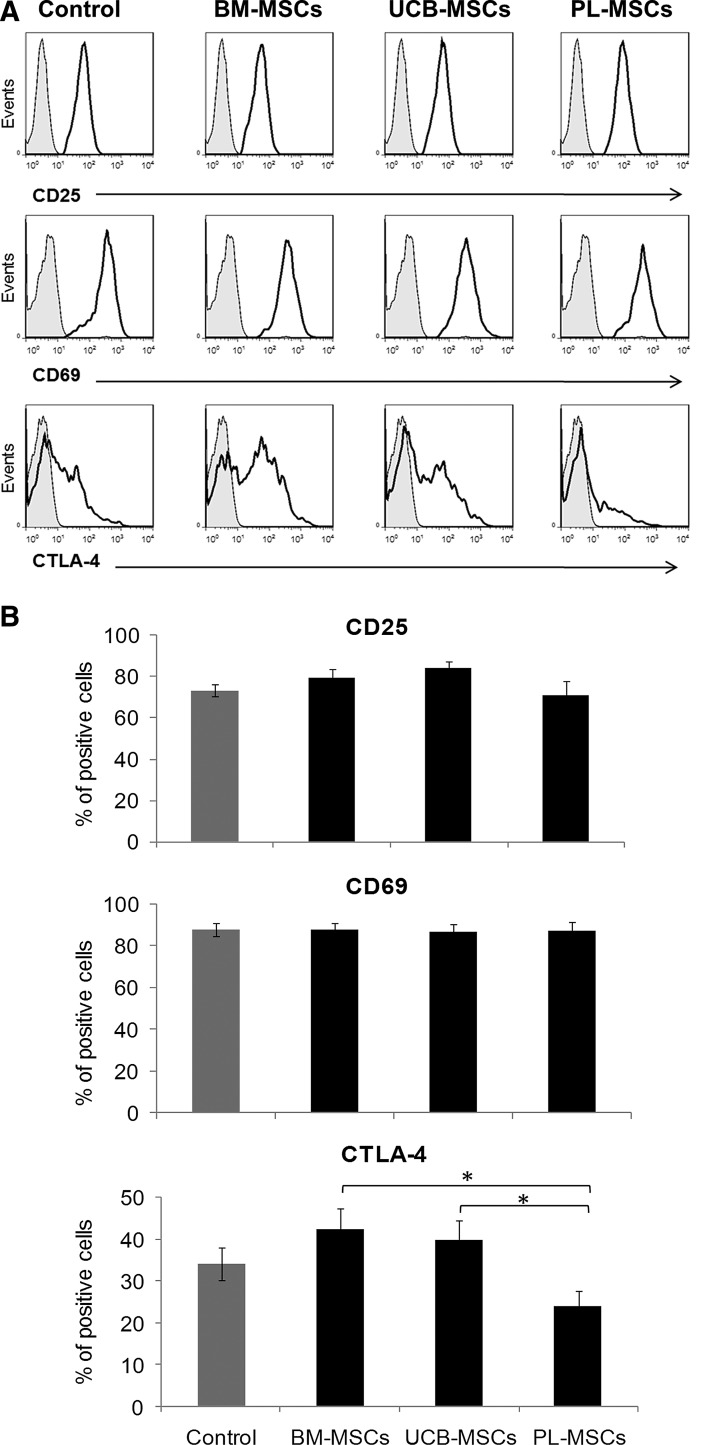
Effect of BM-, UCB-, and PL-MSCs on T-cell activation
Antigenic or mitogenic T-cell stimulation rapidly induces the expression of the activation markers CD25, CD69, and CTLA-4 [32]. Because we observed inhibited T-cell proliferation in cocultures with cell contact between MSCs and T cells, we decided to examine whether the presence of MSCs in these cocultures could affect the expression of CD25, CD69, and CTLA-4. Expression of such molecules on activated T cells cultured in absence of MSCs was considered as the positive control (Control). Basal expression of molecules in nonactivated T cells was considered as negative control (data not shown). We found that CD25 and CD69 expression was unaffected by any of the MSCs from three sources (Fig. 2A, B). On the other hand, we noted that CTLA-4 expression was not modified in the BM-MSCs and UCB-MSCs cocultures (Fig. 2B). Interestingly, we observed significant decrease in CTLA-4 expression on T cells in PL-MSCs cocultures (23.8%±3.7%) when compared with BM-MSCs and UCB-MSCs cocultures (42.3%±5.0% and 39.5%±4.8%, respectively; P<0.05) (Fig. 2B). No significant differences were observed in MFI values (data not shown).
FIG. 2.
MSCs effects on CD25, CD69, and CTLA-4 expression. Anti-CD3/CD28-activated CD3+ T cells were cocultured in the absence or presence of BM-MSCs, UCB-MSCs, or PL-MSCs at a 1:1 ratio of MSCs:T cells (black bars). Cocultures were performed with cell–cell contact. Molecule expression on activated T cells cultured in absence of MSCs was the positive control (Control: CD25 n=7, CD69 n=5, and CTLA-4 n=10, gray bars). The percentages of marker expression were determined in the CD3+ population after 48 h (CD25) or 24 h (CD69 and CTLA-4). (A) Representative histograms for CD25, CD69, and CTLA-4. (B) Data are shown as the mean±SEM of the percentages of expression (CD25: BM-, UCB-, or PL-MSCs n=7 individual experiments for each cell source; CD69: BM-, UCB-, or PL-MSCs n=7 individual experiments for each cell source and CTLA-4 BM-, UCB-, or PL-MSCs n=10 individual experiments for each cell source). *Indicates a statistically significant difference with P<0.05.
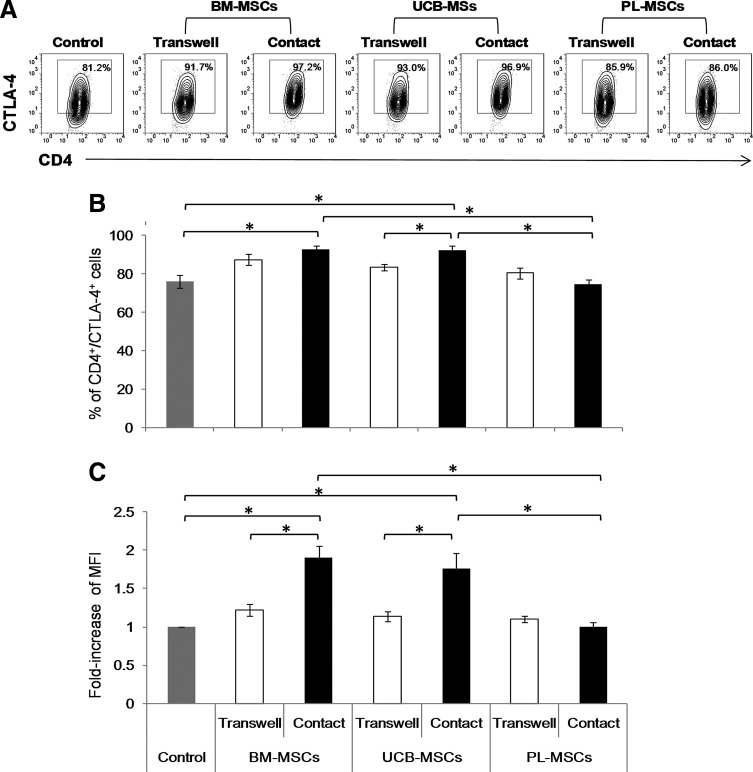
Effects of MSCs on the expression of the immunosuppressive molecules CTLA-4 and PD-L1
Because CTLA-4 is an important negative regulator of immune responses and achieves maximum expression levels between 24 and 48 h postactivation [33,34], we decided to analyze whether the previously observed changes in CTLA-4 expression remained at 3 days of culture and whether the presence or absence of cell–cell contact affected this expression. We performed cocultures with and without cell–cell contact and measured the percentages and MFI of CTLA-4-positive cells in the CD4+ population (Fig. 3A). Expression of CTLA-4 in activated T cells cultured in absence of MSCs was considered as the positive control (Control). Basal expression of molecules in nonactivated T cells was considered as negative control (data not shown). We observed that in cocultures with cell–cell contact, BM-MSCs and UCB-MSCs significantly enhanced both the percentage of CD4+CTLA-4+ cells (92.6%±1.8% and 92.1%±2.5%, respectively) when compared with control (76.0%±3.4%; P<0.05) (Fig. 3B) and the CTLA-4 MFI (1.9- and 1.8-fold, respectively; P<0.05) of the CD4+CTLA-4+ population (Fig. 3C). Meanwhile, in cocultures without cell–cell contact, no increases were observed in the percentage of CD4+CTLA-4+ or the CTLA-4 MFI and the MFI values were significantly lower than in cell–cell contact cocultures of BM-MSCs and UCB-MSCs (P<0.05) (Fig. 3B, C). We also observed that PL-MSCs did not increase CTLA-4 expression levels, and the CTLA-4 percentage and MFI increases in the PL-MSCs cocultures with contact were significantly lower (74.7%±2.3% and MFI:1.1-fold) than those in the BM-MSCs (92.6%±1.8% and 1.9-fold; P<0.05) and UCB-MSCs cocultures (92.1%±2.5% and 1.8-fold; P<0.05) (Fig. 3B, C). These results suggest the need for MSCs–T-cell contact to promote increased CTLA-4 expression and that CTLA-4 may contribute to the reduced proliferation rates observed in these cocultures.
FIG. 3.
BM-MSCs and UCB-MSCs increase the frequency and intensity of CTLA-4 expression on CD4+CTLA-4+ T cells. Anti-CD3/CD28-activated CD3+ T cells were cocultured in the absence or presence of BM-MSCs, UCB-MSCs, or PL-MSCs at a 1:1 ratio of MSCs:T cells. Cocultures were performed with and without (Transwell, white bars) cell–cell contact (black bars). CTLA-4 expression on activated T cells cultured in absence of MSCs was the expression control (Control, n=7, gray bars). The percentages of CTLA-4+ cells and the fold changes the CTLA-4 MFI were determined in the CD4+ population after 3 days of culture. (A) Representative dot plots from an experiment. (B) Data are shown as the mean±SEM of the percentage of CD4+CTLA-4+ and (C) the fold increases in the CTLA-4 MFI in the CD4+CTLA-4+ population. BM-MSCs: n=7 with cell–cell contact, n=7 transwell; UCB-MSCs: n=7 with cell–cell contact, n=7 transwell; and PL-MSCs: n=7 with cell–cell contact, n=7 transwell (Individual experiments). *Indicates a statistically significant difference with P<0.05. MFI, mean fluorescence intensity.
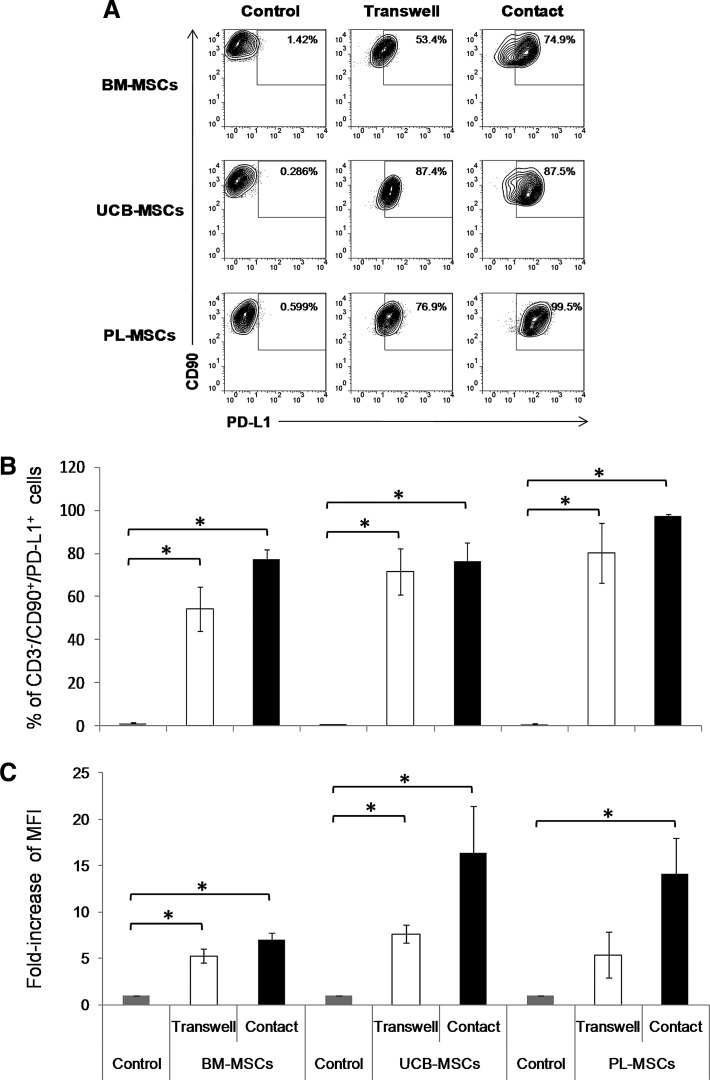
On the other hand, because cell contact was found to be very important for the inhibition of T-cell proliferation, we decided to evaluate the expression of PD-L1, a molecule that affects T-cell inhibition in a cell contact-dependent manner [20]. Thus, we analyzed PD-L1 expression on the MSCs present in the cocultures with and without cell contact (Fig. 4A). The percentage and MFI of PD-L1+ MSCs cultured in absence of activated T cells, was considered as the basal expression of this molecule (Control). The results showed that the presence of activated T cells increased the percentage of PD-L1+ MSCs (CD3-CD90+PD-L1+; P<0.05) in the cocultures, regardless of cell contact: BM-MSCs (control: 1.5%±0.3%, transwell: 54.3%±10.2%, and contact: 77%±4.6%), UCB-MSCs (control: 0.11%±0.03%, transwell: 71%±10.7%, and contact: 76.3%±8.7%), and PL-MSCs (control: 0.29%±0.11%, transwell: 80.0%±13.8%, and contact: 97.4%±1.0%) (Fig. 4B). Similar results were obtained from an analysis of the PD-L1 MFI, for which increases (P<0.05) were observed in BM-MSCs (transwell: 5-fold and contact: 7-fold), UCB-MSCs (transwell: 7-fold and contact: 16-fold), and PL-MSCs (transwell: 5-fold and contact: 14-fold) (Fig. 4C). These results suggest that PD-L1 might be involved in MSC-mediated immunosuppressive activity through cell–cell contact. Further, PD-L1 expression was increased in the cultures regardless of cell contact; this suggests that this increase might be due to the presence of a soluble mediator that stimulates PD-L1 expression on MSCs.
FIG. 4.
PD-L1 expression is increased in MSCs in the presence of activated CD3+ T cells. BM-MSCs, UCB-MSCs, or PL-MSCs were cocultured in the absence or presence of anti-CD3/CD28-activated CD3+ T cells at a 1:1 ratio of MSCs:T cells. Cocultures were performed with (Contact, black bars) and without (Transwell, white bars) cell–cell contact. PD-L1 expression on MSCs cultured in absence of activated T cells was considered as basal expression of such molecule (Control: BM-MSCs n=6, UCB-MSCs n=5, PL-MSCs n=4, gray bars). The percentages of PD-L1+ cells and the fold increase in the PD-L1 MFI were determined in the CD3-CD90+ population after 3 days of culture. (A) Representative dot plots from an experiment. (B) Data are shown as the mean±SEM of the percentages of CD3-CD90+PD-L1+ cells and (C) the fold increases in PD-L1 MFI in the CD3-CD90+PD-L1+ population. BM-MSCs: n=6 with cell–cell contact, n=6 Transwell; UCB-MSCs: n=5 with cell–cell contact, n=5 transwell; and PL-MSCs: n=4 with cell–cell contact, n=4 transwell (Individual experiments). *Indicates a statistically significant difference with P<0.05. PD-L1, programmed death ligand 1.
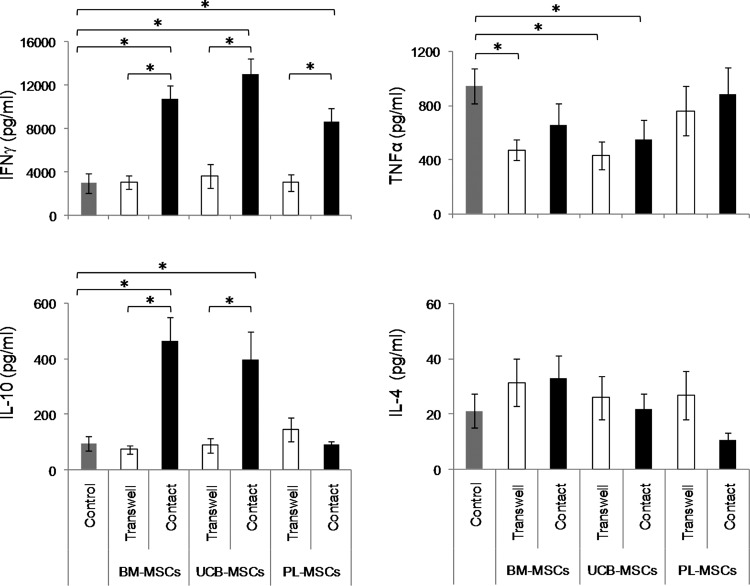
Decreased tumor necrosis factor alfa and increased interferon-γ and IL-10 levels in cocultures with BM-MSCs and UCB-MSCs
Previous studies have demonstrated the importance of interferon gamma (IFNγ) for PD-L1 expression on BM-MSCs [20] and PL-MSCs [35], and for the induction of the immunosuppressive properties of MSCs, either alone or in the presence of other pro-inflammatory cytokines such as tumor necrosis factor alfa (TNFα). Further, the decreased proliferation rates observed in cocultures could be related to the presence of immunosuppressive Th2-like cytokines such as IL-4 and IL-10 [2,4]. Based on the above findings, we decided to evaluate the presence of cytokines that might be involved in the immunosuppressive responses of the MSCs from the three sources. Thus, we determined the concentrations of INFγ, TNFα, IL-10, and IL-4 in the cocultures with and without cell contact. Concentration of cytokines detected in conditioned medium of activated T cells cultured in absence of MSCs was considered as positive control (Control). We detected higher concentrations of IFNγ and IL-10 in cocultures with cell contact in the presence of BM-MSCs (IFNγ: 10,761.4±1,192 pg/mL, IL-10: 463±87 pg/mL; P<0.05) and UCB-MSCs (IFNγ: 13,031.9±1,409.8 pg/mL, IL-10: 393±103 pg/mL; P<0.05), compared with control cultures (IFNγ: 2,976±936 pg/mL, IL-10: 95±25 pg/mL). However, in PL-MSCs cocultures we detected only an increase in INFγ (8,690±1,147 pg/mL; P<0.05), but not in IL-10 (88.3±12.2 pg/mL) (Fig. 5), compared with control cultures. In cocultures with transwell chambers, the IFNγ (BM-MSCs: 3,047±593 pg/mL, UCB-MSCs: 3,596±1,101 pg/mL, and PL-MSCs: 3,023±749 pg/mL) and IL-10 concentrations (BM-MSCs: 72±14 pg/mL, UCB-MSCs: 87±27 pg/mL, and PL-MSCs: 143±42 pg/mL) were similar to those observed in activated T cells in the absence of MSCs. We observed a tendency to decline TNFα secretion in cocultures with cell contact and with transwell chambers in presence of BM-MSCs (contact: 652±160 pg/mL; transwell: 479±76 pg/mL; P<0.05) and UCB-MSCs (contact: 544±151 pg/mL and transwell: 430±105 pg/mL; P<0.05) compared with control cultures (944±130 pg/mL). Moreover, no significant changes were observed in the concentrations of IL-4 under any of the culture conditions (Fig. 5). These results suggest that BM-MSCs and UCB-MSCs, when in contact with activated T cells, induced secretion of both IFNγ and IL-10; PL-MSCs, on the other hand, only induced INFγ secretion. Further, in contrast to PL-MSCs, both BM-MSCs and UCB-MSCs decline TNFα secretion.
FIG. 5.
IFNγ and IL-10 expression are strongly induced in cell-contact cocultures with BM-MSCs and UCB-MSCs. Anti-CD3/CD28-activated CD3+ T cells were cocultured in the absence or presence of BM-MSCs, UCB-MSCs or PL-MSCs at a 1:1 ratio of MSCs:T cells. Cocultures were performed with (black bars) and without (Transwell, white bars) cell–cell contact. IFNγ, IL-10, TNFα and IL-4 concentrations in cell-free supernatants were determined with a cytometric bead array after 3 days of culture. Cytokines concentration detected in conditioned medium of activated T cells cultured in absence of MSCs, were considered as basal expression of such cytokines (Control, n=7, gray bars). Data are shown as the mean±SEM of the cytokine concentrations (BM-MSCs: n=7 with cell–cell contact, n=7 transwell; UCB-MSCs: n=7 with cell–cell contact, n=7 transwell and PL-MSCs: n=7 with cell–cell contact, n=7 transwell. Individual experiments). *Indicates a statistically significant difference with P<0.05. IFNγ, interferon-γ; IL, interleukin; TNFα, tumour necrosis factor alfa.
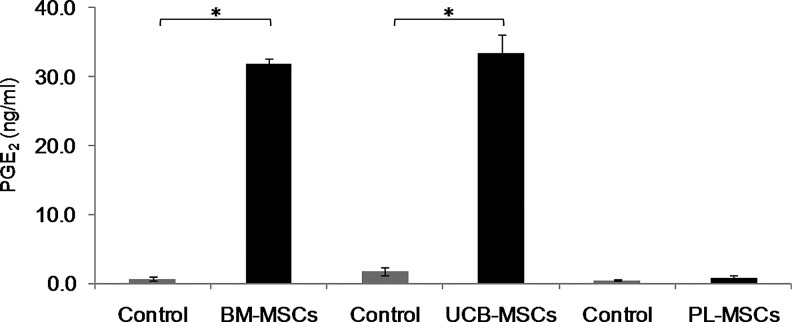
BM-MSCs and UCB-MSCs induce PGE2 secretion
PGE2 is a mediator that affects T-cell activation, proliferation, and differentiation; as such, it has been shown to decrease proliferation, stimulate IL-4 and IL-10 secretion, and promote adaptive Tregs (CD4+CD25+Foxp3+) differentiation [36]. Because we detected increased IFNγ concentrations in the BM-MSCs and UCB-MSCs cocultures and because BM-MSCs secrete PGE2 in response to IFNγ stimulation [2], we decided to evaluate the PGE2 concentrations in MSCs and activated T-cell cocultures with cell contact. Concentration of PGE2 detected in conditioned medium of MSCs cultured in absence of activated T cells, was considered as the basal expression of this molecule (Control). We observed that PGE2 secretion was significantly increased only in the BM-MSCs and UCB-MSCs cocultures (32.0±0.6 ng/mL and 33.5±2.6 ng/mL, respectively; P<0.05), while the PGE2 levels in the PL-MSCs cocultures remained similar to those observed in the control cultures (0.8±0.3 ng/mL and 0.5±0.14 ng/mL, respectively) (Fig. 6). These results indicate different PGE2 induction potentials between BM-MSCs/UCB-MSCs and PL-MSCs and suggest that PGE2 might be involved in the inhibition of T-cell proliferation in the BM-MSCs and UCB-MSCs cocultures with cell contact.
FIG. 6.
PGE2 expression is induced in BM-MSCs and UCB-MSCs cocultures. BM-MSCs, UCB-MSCs, or PL-MSCs were cocultured in the absence or presence of anti-CD3/CD28-activated CD3+ T cells at a 1:1 ratio of MSCs:T cells. Cocultures were performed with cell–cell contact (black bars). PGE2 concentrations in cell-free supernatants were determined by ELISA after 3 days of culture. PGE2 concentrations detected in conditioned medium of MSCs cultured in absence of activated T cells were considered as basal expression of such molecule (Control, gray bars). Data are shown as the mean±SEM (Control n=3, BM-MSCs n=3, UCB-MSCs n=3, and PL-MSCs n=3). Individual experiments (evaluations were performed in duplicate). *Indicates a statistically significant difference with P<0.05. PGE2, prostaglandin E2; ELISA, enzyme-linked immunosorbent assay.
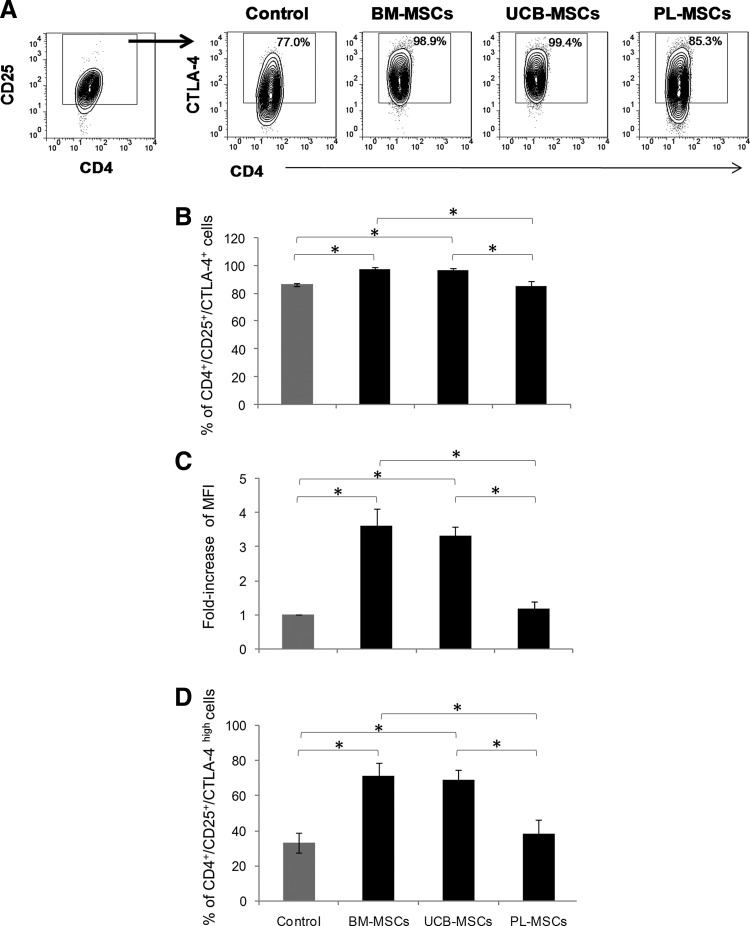
BM-MSCs and UCB-MSCs induce generation of T-cell subsets displaying a regulatory phenotype
Several studies have indicated that IFNγ, IL-10, and PGE2 are involved in Tregs induction [8,9,37]. Because we detected increased levels of these mediators in the BM-MSCs and UCB-MSCs cocultures with cell contact, which were concomitant with the inhibited CD4+ and CD8+ T-cell proliferation and increased CD4+CTLA-4+ T-cell frequencies, we determined whether these findings were related to the generation of CD4+CD25+Foxp3+ Tregs. Because CTLA-4 is a molecule that is constitutively expressed on Tregs and is essential to their function [38], we also analyzed the generation of CD4+CD25+CTLA-4+ and CD8+CD25+CTLA-4+ T-cell subsets displaying a regulatory phenotype [6]. Thus, the T-cell populations from the MSCs cell contact cocultures were obtained. The percentages of Foxp3+ cells were determined within the CD4+CD25+ T-cell fraction, and the percentages of CTLA-4+ and CTLA-4high cells were determined within the CD4+CD25+ and CD8+CD25+ fractions. The percentage of Foxp3+, CTLA-4+, and CTLA-4high in addition to MFI of CTLA-4+ of CD4+CD25+ T-cell fraction from activated T cells cultured in absence of MSCs was considered as the basal population of T-cell displaying a regulatory phenotype (Control).
We found no significant increases in CD4+CD25+Foxp3+ Tregs generation; however, there was a tendency toward reduced percentages of this population in the PL-MSCs cocultures, although this was not statistically significant (data not shown). In contrast, we noted that the presence of either BM-MSCs or UCB-MSCs significantly increased the percentage of CD4+CD25+CTLA-4+ cells over the control (98%±0.6%, 97%±0.9% and 86%±1.2%, respectively; P<0.05) (Fig. 7A, B), and the CTLA-4 MFI in CD4+CD25+ T cells (2.6-fold and 2.3-fold over the control, respectively; P<0.05) (Fig. 7A, C). We also noted that both types of MSCs induced an increase in the percentage of CD4+CD25+ CTLAhigh cells (control: 32.8%±5.7%, BM-MSCs: 71.1%±7.5%, and UCB-MSCs: 69%±5.5%; P<0.05) (Fig. 7D). In contrast, there were no significant increases in the CD4+CD25+CTLA-4+ population with regard to percentage (Control: 86.5%±1.2%, PL-MSCs: 85.6%±3.3%) or MFI (control: 1-fold and PL-MSCs: 1.2-fold) in the PL-MSCs cocultures. Similarly, we observed no increase in the percentage of CD4+CD25+CTLAhigh cells in the PL-MSCs cocultures (Control: 32.8%±5.7% and PL-MSCs: 37.9%±8.3%). Interestingly, the values obtained for the percentages and expression levels of CTLA-4 in the PL-MSC cocultures were significantly lower (CD4+CD25+CTLA-4+: 85.6%±3.3%, MFI: 1.2-fold, and CD4+CD25+CTLAhigh: 37.9%±8.3%; P<0.05) than those from the BM-MSCs (CD4+CD25+CTLA-4+: 98%±0.6%, MFI: 3.6-fold, and CD4+CD25+CTLAhigh: 71.1%±7.5%) and UCB-MSC cocultures (CD4+CD25+CTLA-4+: 96.9%±0.9%, MFI: 3.3-fold, CD4+CD25+CTLAhigh: 69%±5.5%) (Fig. 7). However, we did not observe changes in CTLA-4 on the CD8+ T cells from any of the tested conditions (data not shown).
FIG. 7.
BM-MSCs and UCB-MSCs induce the generation of T-cell subsets displaying a regulatory phenotype. Anti-CD3/CD28-activated CD3+ T cells were cocultured in the absence or presence of BM-MSCs, UCB-MSCs, or PL-MSCs at a 1:1 ratio of MSCs:T cells. Cocultures were performed with cell–cell contact (black bars). The percentages of the CTLA-4+ and CTLA-4high populations and the fold changes in the CTLA-4 MFI were determined in the CD4+CD25+ population after 3 days of culture. CD4+CD25+CTLA-4+ T cells detected in activated T cells cultured in absence of MSCs were considered as control (Control, gray bars). (A) Representative dot plots from an experiment. (B) Data are shown as the mean±SEM of the percentages of CD4+CD25+CTLA-4+, (C) fold changes in the CTLA-4 MFI in CD4+CD25+CTLA-4+ cells and (D) CD4+CD25+CTLA-4high cells. Control n=4, BM-MSCs n=4, UCB-MSCs n=4, and PL-MSCs n=4 (Individual experiments). *Indicates a statistically significant difference with P<0.05.
Discussion
MSCs have been reported to be immunosuppressive. Thus, BM-MSCs have been used to treat GVHD, a condition in which donor T cells are the principal effector cells in an immune response against the host tissues [39]. Although BM is the main source of MSCs, it has some disadvantages such as a lack of donors, a difficult harvesting procedure, and reduced numbers of MSCs with age [17]. We have shown that MSCs from alternative sources such as UCB and PL have morphologic and immunophenotypic characteristics and differentiation capacities similar to those of BM-MSCs [18]. Several authors have reported the immunosuppressive effects of BM-MSCs [1,2,4,8,10,11,22]; however, it is little known whether UCB-MSCs and PL-MSCs shared the immunosuppressive properties of BM-MSCs and could thus be considered as alternative sources of MSCs for clinical protocols. Some studies of MSCs from adult [BM and adipose tissue (AT)] and neonatal sources (UCB and PL) have compared different characteristics regarding morphology, expansion potential, multiple differentiation capacity, immunophenotype, and hematopoietic support capacity. No significant differences concerning the morphology, immunophenotype, osteogenic, and chondrogenic differentiation capacity were observed [18,40], however, UCB-MSCs showed no adipogenic differentiation capacity but in contrast showed higher proliferation capacity than BM-MSCs and AT-MSCs, whereas BM-MSCs possessed the shortest culture period and the lowest proliferation capacity [40]. Further, both UCB-MSCs and BM-MSCs are superior to MSCs from AT for maintenance of primitive hematopoietic progenitor cells [41]. This study is the first to compare the immunosuppressive properties of BM-MSCs, UCB-MSCs, and PL-MSCs on an enriched population of CD3+ T cells while under identical culture conditions.
MSCs from the three sources met the criteria established by the ISCT [29] with regard to immunophenotype, osteogenic, and chondrogenic differentiation capacities; however, as we have previously demonstrated [18], UCB-MSCs showed no adipogenic capacity, unlike BM-MSCs and PL-MSCs, a fact that has been corroborated by other research groups [40]; further, we observed that PL-MSCs has a lower adipogenic capacity than BM-MSCs. Interestingly, MSCs from both neonatal sources do not show the same adipogenic capacity than those from adult source, this aspect could be related to the fact that in adult stage adipocytes formation is increased in BM and therefore BM-MSCs from adults would have higher tendency toward adipogenic lineage compared with MSCs from neonatal sources, which could be predisposed to regenerate supporting tissues (bone and cartilague) important for such developmental stage; this hypothesis is supported by previous studies that suggest differences in adipogenic capacity between MSCs from children and adults [42].
Most previous studies analyzed the immunosuppressive capacity of BM-MSCs and other alternative sources such as amnios, PL, Wharton's jelly, and umbilical cord on alloantigen or PHA-activated PBMC [4,8,12,13,43]. These studies are important because MSCs act on many of the leukocyte subsets involved directly or indirectly in regulating the immune response in vivo [2,3,24,44]. However, few studies have analyzed BM- and PL-MSCs-mediated immunosuppression on CD3+ T-cell-enriched populations [10,14,25,45], which is important in the context of GVHD, because they are the major effector cells in this disease [39]. Additionally, the immunosuppressive effects of UCB-MSCs on this T-cell population remain unknown. To assess this, we analyzed the immunosuppressive effects of MSCs from the three sources on the proliferation of a CD3+ T-cell-enriched population. We noted that BM-MSCs, UCB-MSCs, and to a lesser extent PL-MSCs, suppressed the proliferation of anti-CD3/CD28-activated CD3+ T cells only in the presence of direct contact between the two cell populations; similar results were previously obtained with mouse BM-MSCs [23]. Moreover, several studies have reported that a lack of cellular contact affects MSCs-mediated immunosuppression because the reduction of activated T-cell proliferation was less apparent [8,20–22,30,46,47].
Despite evidence of the importance of cell contact in MSCs immunosuppression, little is known about mechanisms involved in this process. In this regard it has been shown that expression of adhesion molecules ICAM-I and VCAM-I on MSCs decline proliferation of splenocytes activated with anti-CD3 antibodies [48] and further, both adhesion molecules have the capacity to induce CTLA-4 expression on T cells [49], which is involved in inhibition of T-cell proliferation [33,34]. In addition, cell contact between MSCs and T cells increase expression of IL-10, HLA-G1, and HLA-G5 immunosuppression molecules [7,8,19,21], which are involved in the inhibition of T-cell proliferation. It appears that HLA-G1, whose expression is increased on MSCs cocultured with activated T cells [21], is the molecule responsible to stimulate initial secretion of IL-10, which stimulate HLA-G5 secretion in MSCs and through a positive feedback mechanism stimulate secretion of IL-10. In fact, it has been shown that increase in HLA-G5 secretion is more evident in cell contact [8].
When we examined whether CD4+ and CD8+ T-cell populations are equally affected by MSCs from the three sources, we found that BM-MSCs and UCB-MSCs significantly reduced the proliferation of both CD4+ and CD8+ T cells, whereas PL-MSCs only reduced the proliferation of CD4+ T cells. These observations are consistent with previous research, in which BM-MSCs and UCB-MSCs were found to have similar immunosuppressive capacities on PBMC in MLR and PHA activation assays [30,50]. Similarly, Chang et al. [35] used an alloantigen-activated PBMC model to demonstrate that PL-MSCs more efficiently suppressed the proliferation of CD4+ T cells relative to CD8+ T cells. Our results demonstrate for the first time that under identical culture conditions, BM-MSCs and UCB-MSCs have a greater potential for CD3+ T-cell immunosuppression than PL-MSCs. Similar results were obtained from MSCs from Wharton's jelly, which decline proliferation of PHA- activated PBMC similar to those of BM-MSCs [51].
The observed inhibited T-cell proliferation might be related to the downregulation of activation markers such as CD25, CD69, and CTLA-4. Several studies have described the effect of BM-MSCs on the expression of these molecules [4,10,12,24]; however, it is not known whether UCB-MSCs and PL-MSCs have a similar effect. We found that MSCs from the three sources did not affect CD25 and CD69 expression on activated CD3+ T cells, which was consistent with previous reports from BM-MSCs [4,12].
Meanwhile, we noted that BM-MSCs and UCB-MSCs did not affect CTLA-4 expression at 24 h, unlike PL-MSCs, which significantly decreased CTLA-4 expression. It was previously demonstrated that BM-MSCs do not affect CTLA-4 in activated CD4+ and CD8+ T-cell-enriched populations [4], which is consistent with our results. The CTLA-4 expression trends, which were first observed at 24 h of culture with MSCs from the three sources, were most evident after 3 days of culture; this effect was only observed in cell-contact cocultures, wherein we found significant increases in CTLA-4 expression in activated CD4+ T cells in the presence of BM-MSCs and UCB-MSCs, but not PL-MSCs. It is noteworthy that the increased CTLA-4 expression is consistent with the greater ability of both the BM-MSCs and UCB-MSCs to reduce T-cell proliferation; this suggests the involvement of CTLA-4+ Tregs populations in this inhibitory process.
Because we observed reduced T-cell proliferation only in the cell-contact cocultures, we assumed that PD-L1, a molecule involved in MSC-mediated immunosuppression through a cell contact-dependent mechanism [20], might be upregulated on MSCs. Our results demonstrated that cocultured MSCs from the 3 sources had increased PD-L1 expression levels regardless of cell contact. Similar results have been observed in BM-MSCs [20], PL-MSCs [35], and Wharton's jelly [47]; however, this is the first study to show this increase in UCB-MSCs.
IFNγ can act as an immunosuppressive cytokine and as such, is capable of directly inhibiting proliferation and inducing T-cell apoptosis [37,52], stimulating the synthesis of immunosuppressive molecules such as IDO, PGE2, and PD-L1 in MSCs [1,2,20], and facilitating the immunosuppressive properties of MSCs [4,5,53,54]. Thus, we decided to analyze IFNγ expression in our cocultures with and without cell contact. We detected significant increases in the IFNγ concentrations in the supernatants from cell contact cocultures in the presence of MSCs derived from the three sources, which is correlated with the increase observed in PD-L1 expression in this culture condition. Interestingly, we also observed increased PD-L1 expression levels in the cocultures regardless of cell contact, which is likely because, even at low concentrations, IFNγ can stimulate PD-L1 expression on MSCs, as previously demonstrated in BM-MSCs and umbilical cord-MSCs [47,55]. It is worth mentioning that in PL-MSCs cocultures, IFNγ might stimulate the secretion of IDO [35], an enzyme that depletes tryptophan and subsequently promotes the inhibition of CD4+ and CD8+ T-cell proliferation; however, it is has been suggested that CD8+ T cells are more resistant to IDO-mediated immunosuppression than CD4+ T cells [56,57], which might explain why we observed reduced CD4+ but not CD8+ T-cell proliferation in the PL-MSCs cocultures. Unlike PL-MSCs, in BM-MSCs and UCB-MSCs cocultures, besides participation of PD-L1, other molecules and immunosuppressor populations seem to be involved. These results suggest that INFγ and PD-L1 are not the only molecules that are involved in the immunosuppressor effect of MSCs from the three sources. Our results seem to contradict previous reports in which the presence of MSCs from BM, UCB, PL, AT, and Wharton's jelly, either did not affect or decrease IFNγ secretion by activated T cells [20,45,58,59]; however, it was recently reported that MSCs-mediated effects on IFNγ secretion depend on the T-cell source and the type of activation and the presence of MSCs even favor the secretion of IFNγ by CD3+ T cells activated with anti-CD3/CD28 [14].
All of these results support our observations in which MSCs increase IFNγ secretion through contact with activated T cells; apparently, this correlates with reduced T-cell proliferation, in which PD-L1 could actively participate by cell contact, as previously demonstrated in BM-MSCs and PL-MSCs [20,52]. The IFNγ-mediated regulation of PD-L1 and its involvement in immunosuppression mediated by UCB-MSCs are ongoing studies in our laboratory.
Similarly, the decreased proliferation observed in the cocultures could be related to the presence of immunosuppressive cytokines. It has been shown that the activation of T cells in the presence of BM-MSCs reduces the secretion of Th1 proinflammatory cytokines (TNFα and IFNγ) and induces the secretion of Th2 anti-inflammatory cytokines (IL-4 and IL-10) [2]. Further, IFNγ, along with TNFα, increases MSCs-mediated immunosuppression by favoring the secretion of immunosuppressive molecules such as PGE2 [2,4]. Therefore, along with the IFNγ secretion patterns in our cocultures, we also analyzed TNFα, IL-10, and IL-4 secretion. The results revealed no significant increases in levels of IL-4; it suggests that a Th2 type response is not generated, however, we observed a decrease in secretion of TNFα in cocultures in presence of BM-MSCs and UCB-MSCs, which suggest that Th1 type response could be decreased as has been previously shown [2]. Moreover, we observed a significant increase in IL-10 secretion in the cell-contact cocultures with BM-MSCs and UCB-MSCs and interestingly we did not observe changes in TNFα and IL-10 secretion patterns in cocultures with PL-MSCs, which are related to their low immunosuppressive potential.
Through antibody studies, it has been shown that IL-10 is important in BM-MSC-mediated immunosuppression; this cytokine is also involved in Tregs generation [5,8]. Previous reports have shown that the inhibition of cell contact between BM-MSCs and T cells affects IL-10 secretion [7,8,19]. Thus in our system, BM-MSCs and UCB-MSCs-mediated immunosuppression through cell contact could be facilitated by similar mechanisms because both types of MSCs promoted CTLA-4 expression on CD4+ T cells and increased IL-10 and IFNγ concentrations in the coculture supernatants; in contrast, such results were not observed in the PL-MSCs cocultures. Moreover, IFNγ stimulates PGE2 secretion from MSCs. PGE2 is an important lipid factor in the MSCs-mediated immunosuppression of T cells because it decreases T-cell proliferation, stimulates IL-4 and IL-10 secretion, and promotes adaptive CD4+CD25+Foxp3+ Tregs generation [36]. We detected increased PGE2 concentrations in cell-contact cocultures with BM-MSCs and UCB-MSCs. Apparently, the observed increases in PGE2 might be due to IFNγ, as previously suggested by studies of BM-MSCs [2,5,11]; however, no increase in PGE2 was observed in PL-MSCs cocultures, which indicates that INFγ is not the only molecule that induce PGE2 secretion; thus, probably TGF-β and epidermal growth factor could be secreted and participate in such an induction, as previously demonstrated in amnion cells [60]. Currently, the role of PGE2 in PL-MSCs-mediated immunosuppression remains unpublished, and our results suggest that PGE2 might be involved in immunosuppression mediated by BM-MSCs and UCB-MSCs but not by PL-MSCs. Similarly, previous reports have shown the role of PGE2 in Wharton's jelly-MSCs-mediated immunosuppression [61].
Because we observed high concentrations of IFNγ, IL-10, and PGE2 in the cell-contact cocultures with BM-MSCs and UCB-MSCs, and because these mediators favor Tregs generation [8,9,37], we decided to determine the presence of Tregs in our cocultures. We did not detect any increases in the generation of CD4+CD25+Foxp3+ and CD8+CD25+CTLA-4+ with any of the MSCs types; however, we did observe significant increases in the CD4+CD25+CTLA-4+ T-cell subsets displaying a regulatory phenotype in BM-MSCs and UCB-MSCs cocultures; this latter finding is consistent with previous studies [6,50]. To our knowledge, this is the first study to analyze the ability of PL-MSCs to induce Tregs generation, and we observed that under our conditions, these cells are unable to generate CD4+CD25+CTLA-4+ T-cell subsets displaying a regulatory phenotype; this finding is consistent with the reduced ability of these cells to suppress T-cell proliferation in our study. CTLA-4 has been suggested to induce IDO expression in CD4+ T cells; this might have occurred in our system because this effect is IFNγ-dependent [57], and we detected increased amounts of IFNγ in the cocultures that also generated T-cell subsets displaying a regulatory phenotype.
MSCs-mediated immunosuppression through the involvement of Tregs has been recently demonstrated. In an in vivo model of arteriosclerosis transplant, it was demonstrated that local administration of BM-MSCs prevents the disease condition by increasing the local concentrations of IFNγ and IL-10, cytokines produced by Tregs like TR1 [62]. Further in vitro studies showed that the presence of BM-MSCs favors the generation of TR1, which have an IL-10+IFNγ+CD4+ phenotype [28]. Our laboratory is currently determining whether these Tregs are generated in our cultures and their participation in MSCs-mediated immunosuppression.
Although we found differences between PL-MSCs and BM-MSCs, it seems that the immunosuppression potential is not related with age, because such potential was similar between UCB-MSCs and BM-MSCs. Nevertheless, it has been shown that during aging principally three aspects in MSCs are affected: (1) proliferation, (2) differentiation capacity, and (3) genome stability [63], which influence MSCs quality, although it has suggested that decline in tissue regeneration capacity during aging may be in part due to MSCs present in such tissues [17,64]. Our results suggest that immunosuppression capacity is not affected by age.
In summary, our study shows that PL-MSCs possess less immunosuppressive potential than do BM-MSCs and UCB-MSCs, however for the three sources, T-cell contact is essential for immunosuppression process. Unlike PL-MSCs, BM-MSCs and UCB-MSCs reduce both CD4+ and CD8+ T-cell proliferation, increase IL-10 and PGE2 expression and induce generation of CD4+CD25+CTLA-4+ T-cell subsets displaying a regulatory phenotype. Interestingly, BM-MSCs and UCB-MSCs have similar immunosuppressive properties. To our knowledge, this is the first study in which the immunosuppressive properties of MSCs from BM, UCB, and PL are compared under identical culture conditions, using a CD3+ T-cell-enriched population. Our results suggest that UCB-MSCs, rather than PL-MSCs, would be a good alternative to BM-MSCs in cell therapy protocols for the treatment of immunological diseases such as GVHD, graft rejection, or autoimmune diseases.
Supplementary Material
Acknowledgments
We thankfully acknowledge the excellent technical assistance of Martina Flores Jiménez, Guadalupe Alarcón Santos, Erika Hernández Estévez, Karina Estrada González, Ileana Mondragón García, Juan de Dios Moreno Alvarez, Luis Chávez Sánchez, Rosana Pelayo Camacho, and Lourdes Andrea Arriaga Pizano. Grant Sponsor: we are indebted to CONACYT for support to J.J.M.M. (grant no. 87183) and IMSS support to J.J.M.M. (grant no. 1159) and A.M.G (grant no. 1014) are gratefully acknowledged. This article constitutes a partial fulfillment of the Graduate Program in Biological Sciences of the National Autonomous University of México (UNAM). Marta E. Castro-Manrreza acknowledges the scholarship and financial support provided by the National Council of Science and Technology (CONACyT) and the Graduate Program in Biological Sciences of UNAM for the training received during the studies.
Author Disclosure Statement
The authors declare that they have no competing financial interests.
References
- 1.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W. and Dilloo D. (2004). Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenasemediated tryptophan degradation. Blood 103:4619–4621 [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal S. and Pittenger MF. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822 [DOI] [PubMed] [Google Scholar]
- 3.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD. and Mao N. (2005). Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105:4120–4126 [DOI] [PubMed] [Google Scholar]
- 4.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, et al. (2006). Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24:386–398 [DOI] [PubMed] [Google Scholar]
- 5.Ryan JM, Barry F, Murphy JM. and Mahon BP. (2007). Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol 149:353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, et al. (2005). Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 90:516–525 [PubMed] [Google Scholar]
- 7.Prevosto C, Zancolli M, Canevali P, Zocchi MR. and Poggi A. (2007). Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica 92:881–888 [DOI] [PubMed] [Google Scholar]
- 8.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, et al. (2008). Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+ CD25highFOXP3+ regulatory T cells. Stem Cells 26:212–222 [DOI] [PubMed] [Google Scholar]
- 9.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP. and Mahon BP. (2009). Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+ CD25(High) forkhead box P3+regulatory T cells. Clin Exp Immunol 156:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Blanc K, Rasmusson I, Gotherstrom C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C. and Ringden O. (2004). Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol 60:307–315 [DOI] [PubMed] [Google Scholar]
- 11.Rasmusson I, Ringden O, Sundberg B. and Le Blanc K. (2005). Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res 305:33–41 [DOI] [PubMed] [Google Scholar]
- 12.Ramasamy R, Tong CK, Seow HF, Vidyadaran S. and Dazzi F. (2008). The immunosuppressive effects of human bone marrow-derived mesenchymal stem cells target T cell proliferation but not its effector function. Cell Immunol 251:131–136 [DOI] [PubMed] [Google Scholar]
- 13.Najar M, Raicevic G, Boufker HI, Fayyad KH, De BC, Meuleman N, Bron D, Toungouz M. and Lagneaux L. (2010). Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: combined comparison of adipose tissue, Wharton's jelly and bone marrow sources. Cell Immunol 264:171–179 [DOI] [PubMed] [Google Scholar]
- 14.Kronsteiner B, Wolbank S, Peterbauer A, Hackl C, Redl H, van Griensven M. and Gabriel C. (2011). Human mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev 20:2115–2026 [DOI] [PubMed] [Google Scholar]
- 15.Kuzmina LA, Petinati NA, Parovichnikova EN, Lubimova LS, Gribanova EO, Gaponova TV, Shipounova IN, Zhironkina OA, Bigildeev AE, et al. (2012). Multipotent mesenchymal stromal cells for the prophylaxis of acute graft-versus-host disease—a phase II study. Stem Cells Int 2012:968213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philippe B, Luc S, Valérie PB, Jérôme R, Alessandra BR. and Louis C. (2010). Culture and use of mesenchymal stromal cells in phase I and II clinical trials. Stem Cells Int 2010:503593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caplan AI. (2009). Why are MSCs therapeutic?. New data: new insight. J Pathol 217:318–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montesinos JJ, Flores-Figueroa E, Castillo-Medina S, Flores-Guzmán P, Hernández-Estévez E, Fajardo-Orduña G, Orozco S. and Mayani H. (2009). Human mesenchymal stromal cells from adult and neonatal sources: comparative analysis of their morphology, immunophenotype, differentiation patterns and neural protein expression. Cytotherapy 11:163–176 [DOI] [PubMed] [Google Scholar]
- 19.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R. and Pennesi G. (2005). Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol 35:1482–1490 [DOI] [PubMed] [Google Scholar]
- 20.Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, Shen B, Yin S, Liu W, Cui L. and Li N. (2008). A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res 18:846–857 [DOI] [PubMed] [Google Scholar]
- 21.Giuliani M, Fleury M, Vernochet A, Ketroussi F, Clay D, Azzarone B, Lataillade JJ. and Durrbach A. (2011). Long-lasting inhibitory effects of fetal liver mesenchymal stem cells on T-lymphocyte proliferation. PLoS One 6:e19988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S. and Gianni AM. (2002). Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838–3843 [DOI] [PubMed] [Google Scholar]
- 23.Xu G, Zhang L, Ren G, Yuan Z, Zhang Y, Zhao RC. and Shi Y. (2007). Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res 17:240–248 [DOI] [PubMed] [Google Scholar]
- 24.Groh ME, Maitra B, Szekely E. and Koc ON. (2005). Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol 33:928–934 [DOI] [PubMed] [Google Scholar]
- 25.Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, Sportoletti P, Falzetti F. and Tabilio A. (2008). Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol 36:309–318 [DOI] [PubMed] [Google Scholar]
- 26.Ghannam S, Pene J, Torcy-Moquet G, Jorgensen C. and Yssel H. (2010). Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol 185:302–312 [DOI] [PubMed] [Google Scholar]
- 27.Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R. and Le Blanc K. (2011). The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood 117:4826–4835 [DOI] [PubMed] [Google Scholar]
- 28.Hsu WT, Lin CH, Chiang BL, Jui HY, Wu KK. and Lee CM. (2013). Prostaglandin E2 potentiates mesenchymal stem cell-induced IL-10+IFN-γ+CD4+ regulatory T cells to control transplant arteriosclerosis. J Immunol 190:2372–2380 [DOI] [PubMed] [Google Scholar]
- 29.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj. and Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S. and Xu J. (2009). The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology 126:220–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li CD, Zhang WY, Li HL, Jiang XX, Zhang Y, Tang PH. and Mao N. (2005). Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell Res 15:539–547 [DOI] [PubMed] [Google Scholar]
- 32.Wang M, Windgassen D. and Papoutsakis ET. (2008). Comparative analysis of transcriptional profiling of CD3+, CD4+ and CD8+ T cells identifies novel immune response players in T-cell activation. BMC Genomics 9:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wing K, Yamaguchi T. and Sakaguchi S. (2011). Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol 32:428–433 [DOI] [PubMed] [Google Scholar]
- 34.Jago CB, Yates J, Câmara NO, Lechler RI. and Lombardi G. (2004). Differential expression of CTLA-4 among T cell subsets. Clin Exp Immunol 136:463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang CJ, Yen ML, Chen YC, Chien C, Huang HI, Bai CH. and Yen BL. (2006). Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells 24:2466–2477 [DOI] [PubMed] [Google Scholar]
- 36.Kalinski P. (2012). Regulation of immune responses by prostaglandin E2. J Immunol 188:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng G, Gao W, Strom TB, Oukka M, Francis RS, Wood KJ. and Bushell A. (2008). Exogenous IFN-gamma ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3+ regulatory T cells. Eur J Immunol 38:2512–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wing K. and Sakaguchi S. (2010). Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol 11:7–13 [DOI] [PubMed] [Google Scholar]
- 39.Duffner UA, Maeda Y, Cooke KR, Reddy P, Ordemann R, Liu C, Ferrara JL. and Teshima T. (2004). Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol 172:7393–7398 [DOI] [PubMed] [Google Scholar]
- 40.Kern S, Eichler H, Stoeve J, Klüter H. and Bieback K. (2006). Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301 [DOI] [PubMed] [Google Scholar]
- 41.Wagner W, Roderburg C, Wein F, Diehlmann A, Frankhauser M, Schubert R, Eckstein V. and Ho AD. (2007). Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells 25:2638–2647 [DOI] [PubMed] [Google Scholar]
- 42.Muraglia A, Cancedda R. and Quarto R. (2000). Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci 113:1161–1166 [DOI] [PubMed] [Google Scholar]
- 43.Manochantr S, U-pratya Y, Kheolamai P, Rojphisan S, Chayosumrit M, Tantrawatpan C, Supokawej A. and Issaragrisil S. (2013). Immunosuppressive properties of mesenchymal stromal cells derived from amnion, placenta, Wharton's jelly and umbilical cord. Intern Med J 43:430–439 [DOI] [PubMed] [Google Scholar]
- 44.Cutler AJ, Limbani V, Girdlestone J. and Navarrete CV. (2010). Umbilical cord-derived mesenchymal stromal cells modulate monocyte function to suppress T cell proliferation. J Immunol 185:6617–6623 [DOI] [PubMed] [Google Scholar]
- 45.Luan X, Li G, Wang G, Wang F. and Lin Y. (2013). Human placenta-derived mesenchymal stem cells suppress T cell proliferation and support the culture expansion of cord blood CD34+ cells: a comparison with human bone marrow-derived mesenchymal stem cells. Tissue Cell 45:32–38 [DOI] [PubMed] [Google Scholar]
- 46.Li CD, Zhang WY, Li HL, Jiang XX, Zhang Y, Tang PH. and Mao N. (2005). Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell Res 15:539–547 [DOI] [PubMed] [Google Scholar]
- 47.Tipnis S, Viswanathan C. and Majumdar AS. (2010). Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunol Cell Biol 88:795–806 [DOI] [PubMed] [Google Scholar]
- 48.Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C. and Shi Y. (2010). Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol 184:2321–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Damle NK, Klussman K, Leytze G, Myrdal S, Aruffo A, Ledbetter JA. and Linsley PS. (1994). Costimulation of T lymphocytes with integrin ligands intercellular adhesion molecule-1 or vascular cell adhesion molecule-1 induces functional expression of CTLA-4, a second receptor for B7. J Immunol 152:2686–2697 [PubMed] [Google Scholar]
- 50.Avanzini MA, Bernardo ME, Cometa AM, Perotti C, Zaffaroni N, Novara F, Visai L, Moretta A, Del Fante C, et al. (2009). Generation of mesenchymal stromal cells in the presence of platelet lysate: a phenotypic and functional comparison of umbilical cord blood- and bone marrow-derived progenitors. Haematologica 94:1649–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasanna SJ, Gopalakrishnan D, Shankarand SR. and Vasandan AB. (2010). Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One 5:e9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu Y. and Waller EK. (2009). Dichotomous role of interferon-gamma in allogeneic bone marrow transplant. Biol Blood Marrow Transplant 15:1347–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B. and Bartholomew A. (2008). IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol 38:1745–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deuse T, Stubbendorff M, Tang-Quan K, Phillips N, Kay MA, Eiermann T, Phan TT, Volk HD, Reichenspurner H, Robbins RC. and Schrepfer S. (2011). Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant 20:655–667 [DOI] [PubMed] [Google Scholar]
- 55.Mourez R, François M, Boivin MN, Stagg J. and Galipeau J. (2007). Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J Immunol 179:1549–1558 [DOI] [PubMed] [Google Scholar]
- 56.Jones BJ, Brooke G, Atkinson K. and McTaggart SJ. (2007). Immunosuppression by placental indoleamine 2,3-dioxygenase: a role for mesenchymal stem cells. Placenta 28:1174–1181 [DOI] [PubMed] [Google Scholar]
- 57.Boasso A, Herbeuval JP, Hardy AW, Winkler C. and Shearer GM. (2005). Regulation of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells. Blood 105:1574–1581 [DOI] [PubMed] [Google Scholar]
- 58.Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, Lee JE, Kim YJ, Yang SK, et al. (2009). Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol 259:150–156 [DOI] [PubMed] [Google Scholar]
- 59.Chiesa S, Morbelli S, Morando S, Massollo M, Marini C, Bertoni A, Frassoni F, Bartolomé ST, Sambuceti G, Traggiai E. and Uccelli A. (2011). Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc Natl Acad Sci U S A 108:17384–17389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kniss DA, Zimmerman PD, Fertel RH. and Iams JD. (1993). Transforming growth factor-beta potentiates epidermal growth factor-induced prostaglandin E2 production in amnion cells. Prostaglandins 45:27–33 [DOI] [PubMed] [Google Scholar]
- 61.Chen K, Wang D, Du WT, Han ZB, Ren H, Chi Y, Yang SG, Zhu D, Bayardand F. and Han ZC. (2010). Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol 135:448–458 [DOI] [PubMed] [Google Scholar]
- 62.Jui HY, Lin CH, Hsu WT, Liu YR, Hsu RB, Chiang BL, Tseng WY, Chen MF, Wu KK. and Lee CM. (2012). Autologous mesenchymal stem cells prevent transplant arteriosclerosis by enhancing local expression of interleukin-10, interferon-γ, and indoleamine 2,3-dioxygenase. Cell Transplant 21:971–984 [DOI] [PubMed] [Google Scholar]
- 63.Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, Benes V, Blake J, Huber FX, et al. (2009). Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One 4:e5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raggi C. and Berardi AC. (2012). Mesenchymal stem cells, aging and regenerative medicine. Muscles Ligaments Tendons J 2:239–242 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.