Abstract
We report that metal salts composed of mixtures of anions of differing coordination strength can be used to increase the sensitivity and selectivity of adsorbate-induced anchoring transitions of liquid crystals (LCs) supported on surfaces decorated with the metal salts. Specifically, the dynamics of anchoring transitions triggered by the adsorbate dimethyl methylphosphonate (DMMP) on surfaces of aluminum (III) salts were analyzed within the framework of a model for mass transport to reveal that the sensitivity of a nitrile-containing nematic LC to DMMP increased from 250 parts-per- billion (ppb) to 25 ppb when the composition of the (counter) anion was changed from 100% perchlorate to 90% nitrate and 10% perchlorate (by mole percent). To provide insight into these observations, Polarization-Modulation Infrared Reflectance-Absorbance Spectroscopy (PM-IRRAS) was used to show that the intensity of the absorption band in the IR spectrum corresponding to the coordinated state of the nitrile group (but not the position of the peak) decreased with increase in mole fraction of the strongly coordinating anion (nitrate) in the anion mixture, thus suggesting that the addition of the strongly coordinating anion decreased the number of coordination interactions (per unit area of the interface) but not the strength of the individual coordination interactions between the metal cation and the LC. We also measured the incorporation of the nitrate anion into the metal salt to decrease the effect of humidity on the dynamic response of the LC to DMMP, a result that is consistent with weaker interactions between the nitrate anion and water as compared to the perchlorate anion and water. Finally, the bidentate anion acetylacetonate was measured to cause a similar increase in sensitivity to DMMP when mixed with perchlorate in a 1:1 ratio (the resulting sensitivity of the system to DMMP was 100 ppb). Overall, these results suggest that tailoring the identity of the anion represents a general and facile approach for tuning the orientational response of LCs supported on metal salts to targeted analytes.
Keywords: Liquid crystals, Gas sensor, Coordination interactions, Dimethyl methylphosphonate
Introduction
Nematic liquid crystals (LCs) are liquids that possess long-range orientational order. At a solid interface, the orientational ordering of the LC reflects intermolecular interactions between the mesogens and the solid.1–5 For example, strong coordination interactions between transition metal cations deposited on a surface (e.g., Cu2+ or Al3+) and nitrile-containing mesogens (i.e. 4-cyano-4’- pentylbiphenyl, 5CB) result in the homeotropic ordering (orientation parallel to surface normal) of the LC. Past studies have shown that the alignment of the LC is dependent on both the strength and number of coordination interactions between the LC and the metal salt (i.e., electron affinity of the cation in the salt).1, 6–14
Past studies have also shown that adsorbates introduced in the vapor phase above the LC film can competitively disrupt coordination interactions between the metal ions and the LC mesogens.1, 6, 15 This process occurs if the adsorbate coordinates more strongly with the metal ion as compared to the mesogen. Disruption of these coordination interactions by an adsorbate triggers a change in the ordering of the LC at the solid surface to a planar alignment (perpendicular to surface normal).1, 6, 8–9, 14–18 For example, the analyte dimethyl methylphosphonate (DMMP), when introduced as a vapor over the LC, triggers an ordering transition in a micrometer-thick film of nematic 5CB supported on a surface decorated with aluminum (III) perchlorate salts.12, 15 As detailed elsewhere, DMMP is widely used as a simulant of nerve agents including sarin. Detection of these types of analytes in the low parts-per-million (ppm) concentrations is important because the lethal concentration time for 50% of humans by inhalation of sarin is around 10–15 ppm min.19
The majority of the above described past studies have focused on high electron affinity cations, such as aluminum (III), nickel (II), and copper (II). The cations were deposited on surfaces as salts of weakly coordinating anions, such as perchlorate.6 We emphasize here that the choice of anion is important because the anion, in addition to the electron affinity of the metal cation, impacts the nature of the coordination interactions between the LC and metal cation.20 For example, metal salts composed of anions that coordinate more strongly than perchlorate (such as nitrate, chloride, or acetate) do not give rise to the homeotropic ordering seen on surfaces with metal perchlorates (planar anchoring is observed). Conversely, other weakly coordinating anions, including hexafluorophosphate (PF6−) and tetrafluoroborate (BF4−), have been observed to generate a homeotropic alignment of the LC when paired with a high electron affinity cation.20
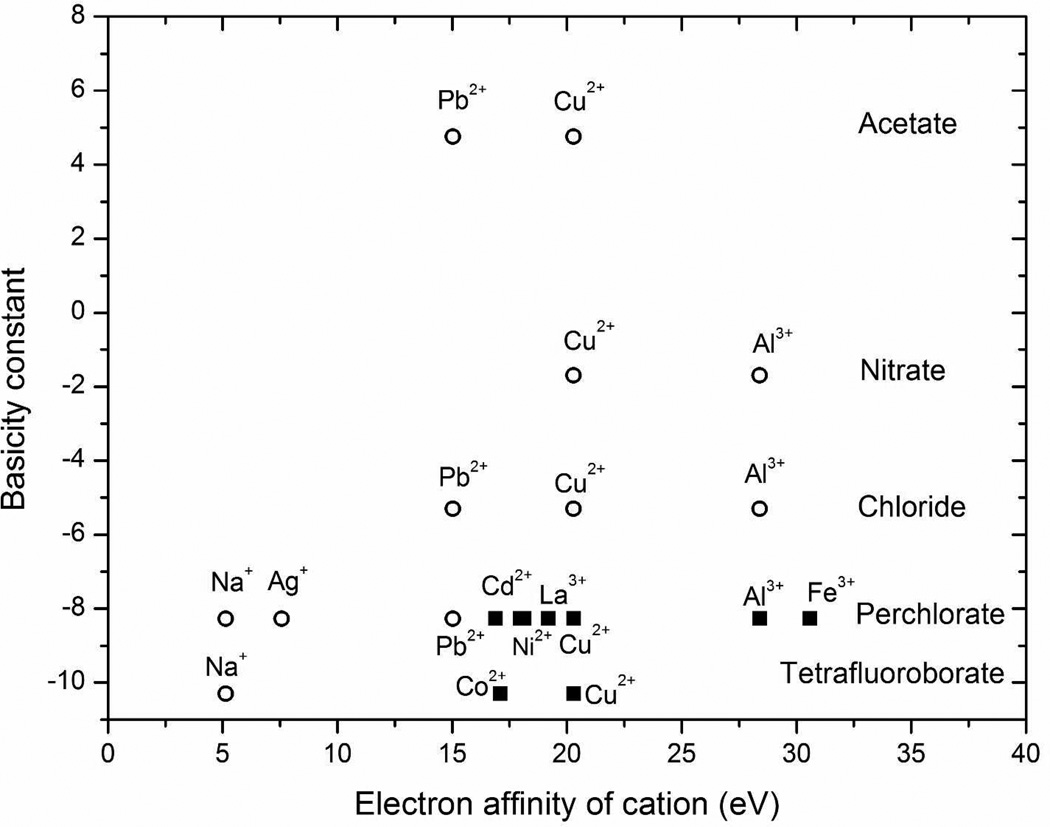
In general, the strength of a coordination complex formed between an anion and cation can be correlated with the basicity constant of the anion, a parameter that measures the chemical equilibrium of hydrogen between the anion and the hydrogen ion. A large basicity constant indicates an equilibrium that lies towards the complex with the hydrogen ion, which suggests strong coordination.21–28 Figure 1 shows that past observations of the effects of cation and anion type on the anchoring of nematic 5CB are well correlated by using metal ion electron affinity and anion basicity.6, 29 In this paper, we build from these prior observations to explore how mixtures of anions influence the anchoring of LCs and adsorbate-triggered anchoring transitions of LCs.
Figure 1.
Alignment of LC films supported on metal salt-decorated surfaces taken from various past studies.6, 29 The solid square data points (■) represent homeotropic alignment of the LC and the open circle data points (◦) represent a planar or tilted alignment of the LC.
The study reported herein is focused on aluminum (III) and copper (II) salts with mixed anions. These two cations were selected because they induce a homeotropic alignment of nematic 5CB when paired with weakly coordinating anions such as perchlorate. In addition, the coordination interactions formed between the perchlorate salts of these cations and 5CB are disrupted by the presence of DMMP.6 The strongly coordinating anions used in the present study are nitrate, chloride, and acetylacetonate, all of which possess basicity constant values that are larger than perchlorate (see Figure 1). The anion acetylacetonate was selected because it is a bidentate ligand.
This study reported below is divided into three main sections. The first section demonstrates that the alignment of a nitrile containing LC on a metal salt surface can be manipulated using a mixture of weakly and strongly coordinating anions. The second section shows that the anion composition of the metal salt supporting the LC film also impacts the sensitivity of the LC film to the vapor analyte DMMP. Insight into the origin of this effect is provided via the use of infrared spectroscopy. The third section reports an investigation of the impact of the anion composition on the effects of humidity (i.e., the extent to which the response of the system to DMMP is impacted by changes in humidity). Overall, the results of this study suggest that control of anion composition (using mixed anions) provides a facile method to tailor the interactions between a coordinating metal cation and a LC, and thus manipulate the anchoring of the LC and the orientational response of the LC to a vapor phase analyte.
Materials and Methods
Materials
11-Mercaptoundecanoic acid (MUA), aluminum (III) perchlorate nonahydrate, aluminum (III) nitrate nonahydrate, copper (II) perchlorate hexahydrate, and copper (II) chloride dehydrate salts were purchased from Sigma Aldrich (Milwaukee, WI). Aluminum (III) acetylacetonate was purchased from Alfa-Aesar (Ward Hill, MA). 5CB was purchased from EMD Chemicals (Gibbstown, NJ). Titanium (99.999%) and gold (99.999%) were purchased from Advanced Materials (Spring Valley, NY). Methanol and Fischer’s Finest glass slides were purchased from Fischer Scientific (Pittsburgh, PA). Absolute ethanol (anhydrous, 200 proof) was purchased from Pharmco-AAPER (Brookfield, CT). All chemicals and solvents were of analytical reagent grade and were used as received without any further purification. All deionized water used in the study possessed a resistivity of at least 18.2 MΩ cm.
Cleaning of Glass Substrates
Glass microscope slides were cleaned using acidic “piranha” solution 70:30 (% v/v) [H2SO4:H2O2]. Briefly, the glass slides were immersed in an acidic piranha bath at 60–80 °C for at least 1 h, and then rinsed in running deionized water for 2–3 min. The slides were then immersed in basic piranha 70:30 (% v/v) [KOH: H2O2] and heated to between 60 and 80 °C for at least 1 h. Finally, the slides were rinsed sequentially in deionized water, ethanol, and methanol, and then dried under a stream of nitrogen. The clean slides were stored in an oven at 110 °C. All other glassware was rinsed with distilled water and ethanol and dried under a gaseous stream of nitrogen.
Deposition of thin layers of gold
Semi-transparent films of gold with thicknesses of 200 Å were deposited onto piranha-cleaned glass slides (see above) mounted on a fixed holder within an electron beam evaporator (VEC-3000-C manufactured by Tekvac Industries, Brentwood, NY). The angle of incidence of the gold ranged from 0° to 15° on the slides (measured from surface normal). A layer of titanium (thickness 80 A) was used to promote adhesion between the glass microscope slides and the films of gold. The rates of deposition of gold and titanium were 0.2 Å/s. The pressure in the evaporator was maintained at less than 3 × 10−6 Torr before and during each deposition. The gold source was periodically cleaned by sequential immersion in aqua regia (70% HNO3, 30% HCl) and piranha solutions at 50 °C (30 min in each solution); see above for compositions. The cycle was repeated 3–4 times, rinsing the source between cycles in deionized water.
Preparation of functionalized gold surfaces
Carboxylic-acid-terminated self-assembled monolayers (SAMs) of 11-mercaptoundecanoic acid (MUA) were formed on gold-coated glass slides by immersing the slides overnight in an ethanolic solution containing 2 mM of MUA. The gold films were then rinsed with an excess of ethanol and dried under a stream of nitrogen. Mixtures of the metal salt were formed in an ethanolic solution in which the concentration of the metal cation remained constant at 5 mM. The metal salts were then deposited immediately onto the carboxylic-acid-terminated SAMs by spin coating the ethanolic salt solution at 3000 rpm for 60s (WS-400A-6NPP/Lite, Laurell Technologies, North Wales, PA).
Formation of Micrometer-Thick Films of LC
After coating the surfaces with metal salt, as described above, an 18 µm-thick gold-coated transmission electron microscopy (TEM) grid (Electron Microscopy Sciences, Hatfield, PA) was fastened to the salt-coated surface using a thin stainless steel plate (0.44 mm thickness). The stainless steel plate contained a 2-mm diameter hole that was aligned with the center of the TEM grid. The TEM grid contained square pores with lateral dimensions of 285 µm. The grid had an overall diameter of 3 mm. Both the TEM grid and stainless steel plate were dip-coated with a perfluorocarbon film (Nyebar Fluorocarbon Barrier Film, SmartGrease Company, Fairhaven, MA) to minimize wicking of the 5CB from the TEM grid. The grids were filled with LC using a microcapillary pipet at room temperature, taking care to fill only the middle squares of the TEM grid, so as to avoid wicking of the 5CB between the TEM grid and steel plate.
Exposure to DMMP
The sample, prepared as described above, was exposed to a stream of air containing DMMP within a flow cell that was constructed to direct the flow of air across the LC samples while permitting simultaneous observation of the samples through a polarized light microscope (CH40, Olympus, Melville, NY). The stream of gas containing DMMP was generated using a certified cylinder containing 10 ppm DMMP in nitrogen (Matheson Tri-Gas Inc, Eagan, MN). The gas of DMMP was diluted by air at a specified relative humidity (RH). The RH of the air was controlled using a portable dew point generator (LI-610, LI-COR Biosciences, Lincoln, NE). The temperature of the gas fed to the flow cell was maintained at room temperature (25 °C) and the RH was controlled to 30% unless indicated otherwise. The flow system was plumbed using 1/16” stainless steel Swagelok tubing (Badger Fluid System Technologies Milwaukee, WI). The flow rate of the gas through the exposure system was controlled using a series of rotameters (Aalborg Instruments & Controls, Inc., Orangeburg, NY). The volumetric flow rate of the 10 ppm DMMP stream from the cylinder was controlled and ranged between 0 to 150 mL/min, while the volumetric flow rate of the diluent air stream was controlled to range between 0 to 1000 mL/min. The concentration of DMMP used in the study reported in this paper ranged from 0.1 ppm to 1.25 ppm, while the overall volumetric flow rate of the gas stream passed over the samples remained constant at 1000 mL/min.
The flow cell was fabricated by machining a rectangular prism of aluminum metal. Glass windows allowed transmission of polarized light through the flow cell. The intensity of light that was transmitted through the LC sample in the flow cell was recorded using an Olympus camera (Olympus C2040Zoom, Melville, NY). The total volume of the flow cell was 10.5 cm3 (6 cm × 3.5 cm × 0.5 cm), giving rise to a gas residence time of ~0.6 sec when the flow rate was 1000 mL/min. The Reynolds number was calculated to be 51 for airflow within the flow cell at 1000 mL/min. This value corresponds to laminar flow.
Analysis of the polarized light images during exposure to DMMP
The images of the LC films viewed through cross-polars were analyzed by quantifying the increase in the average intensity of light transmitted through LC films hosted in four central squares of the TEM grid. The increase in intensity of light transmitted through the LC (quantified relative to the initial state of the LC) was normalized such that the initial intensity value corresponded to a value of 0% and the final state of the LC after exposure to DMMP corresponded to a value of 100%. The time of response (ton) of each LC sample was obtained by fitting the initial change in the normalized intensity of light transmitted through the sample to a straight line and calculating the time at which this line crossed 0%. Additional details can be found in a previous study.15
Measurement of the birefringence of films of 5CB
The birefringence of thin films of 5CB was measured for thin films confined between two metal salt decorated surfaces. These surfaces were decorated with salts with anions that varied in composition. The sandwich cells were separated by an approximately 18 µm thick Mylar spacer. The exact thickness of the sandwich cells was measured by using interferometry. The sandwich cells were then filled with 5CB and the retardance of the cells was measured using a compensator. From these values, the apparent birefringence of the samples was calculated and the value of the tilt angle of the 5CB was determined using the known birefringence of 5CB.30
Fourier Transform Polarization-Modulation IR Reflectance- Absorbance Spectroscopy (PM-IRRAS)
Substrates decorated with metal salts were prepared with the following modifications to the procedures described above. For PM-IRRAS, we deposited 1000 Å of gold onto silicon wafers (using 100 Å of titanium as an adhesion promoter) in order to obtain a reflective surface. In addition, we used a thin film of 4-n-octyl-4’-cyanobiphenyl (8CB) deposited onto the surface by spin coating a 1% (by weight) solution of 8CB in toluene at 3000 rpm for 1 minute. The IR spectra of 8CB on surfaces were obtained using a Nicolet Magna-IR 860 FT-IR spectrometer with a photoelastic modulator (PEM-90, Hinds Instruments, Hillsboro, OR), a synchronous sampling demodulator (SSD-100, GWC Technologies, Madison, WI), and a liquid- N2-cooled mercury cadmium telluride (MCT) detector. All spectra were recorded at an incident angle of 83° with the modulation centered at 2200 cm−1. All spectral data reported within this article were recorded within 500 cm−1 of the modulation center.
Results
1. Effect of anion composition of metal salts on the anchoring of nematic films of 5CB
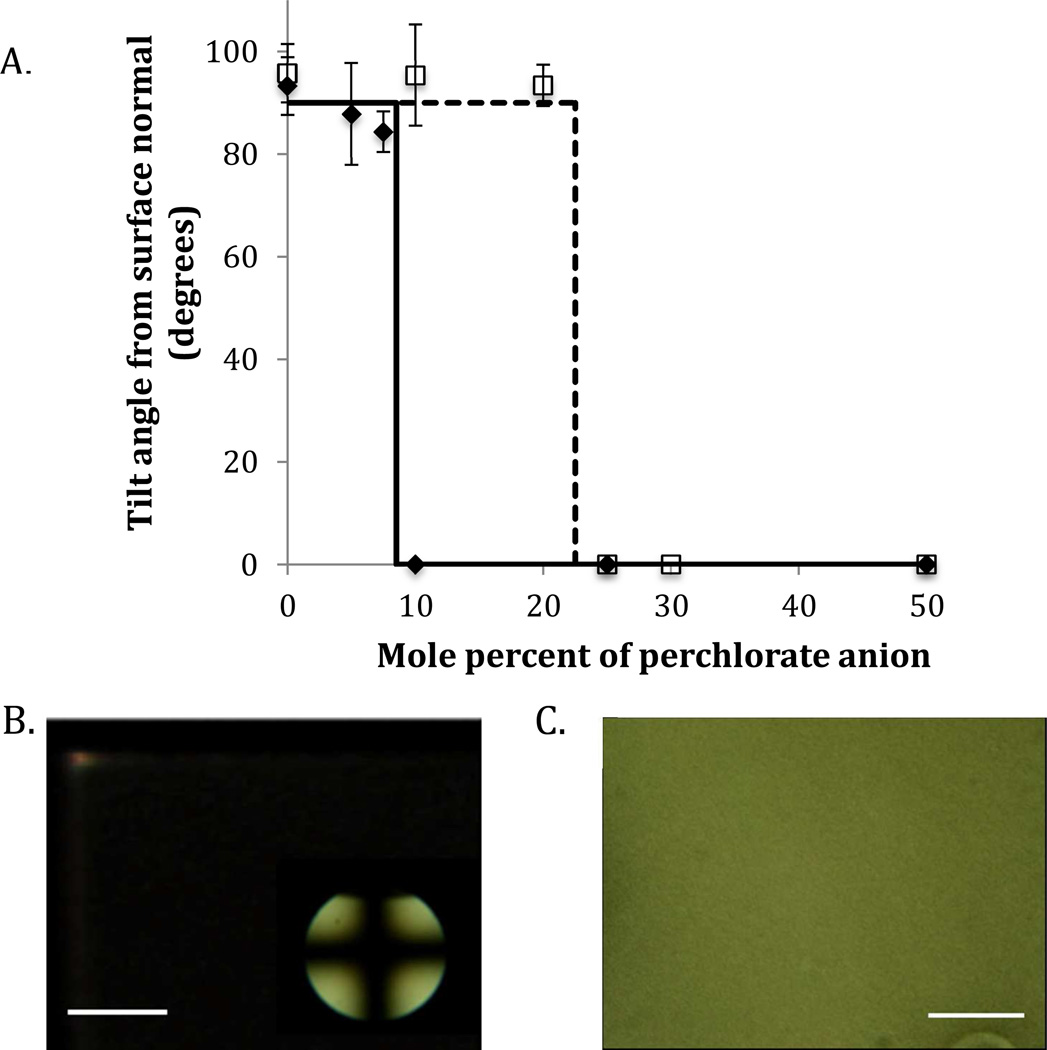
Our first set of measurements was designed to examine the influence of the anion of aluminum (III) salts on the alignment of supported films of 5CB. As noted in the Introduction, past studies have established that the electron affinity of the cation and basicity constant of the anion impact the alignment of supported films of 5CB. We also comment that the aluminum salts used in our experiments were purchased as hydrates (see Materials and Methods) and, as reported in previous studies,31–33 the first coordination shell of the cation will be partially or completely occupied by water. The coordination interactions of the nitrile group and the aluminum (III), which are evident in the orientational behavior of the nitrile-containing LCs on surfaces of aluminum (III), largely reflect interactions that occur in the second and third coordination spheres of the cation.1, 6, 8–9, 11–12, 15 In support of this interpretation, IR spectra of aluminum (III) perchlorate dissolved in deuterated acetonitrile in the presence of water show a small peak related to the first coordination sphere of aluminum (III) and a larger peak assigned to the second coordination shell.31 Our first set of results, as shown in Figure 2, expands upon these prior studies by investigating the anchoring of 5CB at the surface of aluminum (III) salts with mixed anions. For these studies, we confined a film of 5CB between two surfaces decorated with the metal salts. As described in the Methods section, we determined the distance between the two surfaces to be ~18 µm by using interferometry prior to filling the cavity defined by the two surfaces with 5CB. After filling, we measured the retardance of the film of 5CB and calculated the average birefringence as the ratio of the retardance of the 5CB film to the thickness of the film of LC. Finally, the birefringence was used to calculate the average tilt angle at the interface, as shown in Figure 2a.30
Figure 2.
(A) Tilt angle from surface normal for films of nematic 5CB anchored on surfaces decorated with aluminum (III) salts comprised of mixed anions (perchlorate/ nitrate, solid points; perchlorate/acetylacetonate, open squares). Polarized light micrographs of 5CB films anchored on surfaces that are coated with aluminum (III) salts with either (B) perchlorate or (C) nitrate anions. The conoscopic image in lower right of (B) confirms a uniform homeotropic alignment of the LC film. The scale bars represent 1 mm.
The anion combinations that we studied were mixtures of a weakly coordinating anion (perchlorate) and a more strongly coordinating anion (either nitrate or acetylacetonate). Figure 2a reveals that the tilt angle of a nematic film of 5CB at the aluminum (III) nitrate-coated surface is close to 90° from surface normal (within uncertainty in the measurement). We note that we attribute the measurements of an apparent tilt angle that is slightly greater than 90° to uncertainty in the measurement (and to correspond to planar anchoring of the LC). As the mole fraction of perchlorate in the salt increased to almost 10%, the tilt angle of the LC at the interface remained close to a value of 90°. However, at a critical composition of the anion (around 10% perchlorate, 90% nitrate by mole percent), we measured a discontinuous change in the alignment of the LC at the interface. For samples anchored on surfaces with salts containing more than 10% perchlorate, we measured the LC to adopt a homeotropic alignment (tilt angle of zero). This critical composition is shown in Figure 2a as the vertical solid black line. We also comment that when the compositions of the anions were close to the critical concentration we observed some variation of the anchoring of the LC across the sample, likely reflecting local variation in composition of the anions.
Polarized light micrographs of the LC films in contact with surfaces composed of 100% perchlorate and 100% nitrate are shown in Figure 2b and 2c, respectively. These optical micrographs are consistent with the tilt angles shown in Figure 2a. Figure 2b (100% perchlorate) shows a dark image when viewed through cross polarizers that is indicative of homeotropic alignment of the LC. This behavior is further confirmed by the cross-hair pattern obtained by using conoscopy (inset in Figure 2b). In contrast, as shown in Figure 2c, the 5CB films supported on metal salt films containing 100% of the strongly coordinating nitrate anion exhibited a bright appearance (consistent with planar anchoring of the LC). This general behavior was also observed for other metal salts such as mixtures of copper (II) perchlorate and copper (II) chloride (this system exhibited a critical composition that was similar to the aluminum (III) perchlorate/ nitrate system; data shown in supporting information).
Next, we characterized the mixed anion system comprised of perchlorate and acetylacetonate. The anion acetylacetonate was used because it is a bidentate anion (i.e., interacts with the cation at two independent coordination sites). Figure 2a shows that the acetylacetonate/perchlorate system exhibited a behavior similar to the nitrate/perchlorate system. However, a key difference is the observation of a critical composition that is almost double that of perchlorate (around 25% perchlorate, 75% acetylacetonate by mole percent) as compared to the nitrate-perchlorate system. This increase in the critical composition of the perchlorate anion is consistent with the idea that each individual acetylacetonate molecule interacts with the aluminum (III) cation at two different coordination sites. In brief, if we assume that the aluminum (III) cation is able to participate in six coordination interactions and the salt is electro-neutral, then each cation with three unidentate cations will have three coordination sites available for interaction with species such as the nitrile group of the LC. However, in the case of a bidentate anion, the anion occupies two coordination sites. This simple model leads to the conclusion, for example, that an aluminum (III) cation with one acetylacetonate and two perchlorate anions will have only two coordination sites available to interact with the other species. Our observation that a larger amount of perchlorate is needed in order to achieve homeotropic alignment of 5CB when using acetylacetonate is thus consistent with the bidentate nature of acetylacetonate ion occupying sites of potential coordination with the LC.
The results in Figure 2 demonstrate that the anchoring of the LC supported on the thin film of metal salt is dependent on the anion of the metal salt film. In general the anchoring of the LC at the metal salt interface is dependent on both the number of interactions and the strength of the individual interactions between the metal salt and LC. Two possible physical scenarios may, therefore, explain the observations shown in Figure 2. First, changes in the anion of the salt may influence the number of coordination interactions between the salt surface and the LC. Specifically, the more strongly coordinating anion may prevent the cation from participating in coordination interactions with the LC leading to fewer interactions (per unit area) between the metal salt and LC. Alternatively, the introduction of a strongly coordinating anion may decrease the strength of the individual interactions between the LC and the metal salt. This decrease in the strength of the individual interactions would not change the number of interactions at the interface. In this second scenario, there would exist a minimum strength of interactions between the metal salt and LC that is needed to cause the homeotropic alignment of the LC.
To distinguish between these two scenarios, we characterized the interactions between the nitrile group and the metal salt with mixed anions using PM-IRRAS. To obtain these results, it was necessary to make two changes to the experimental system. First, 5CB was replaced by the nitrile containing smectic 8CB to obtain stable thin films of the LC less than 1000 nm thick, as required for the PM-IRRAS measurements.8 Second, the aluminum (III) salts were exchanged for copper (II) salts of mixed anions (perchlorate and chloride) because the coordination interactions between copper perchlorate and 8CB have been well studied in the past,8 and because the nitrile absorption peak in the IR spectrum is very weak when using aluminum (III).31 As noted above, in the presence of water, past studies8, 31 have demonstrated the copper (II)-nitrile complex to give rise to a stronger absorption band than the aluminum (III)-nitrile complex because water largely occupies the first coordination sphere of aluminum (III) and thus the nitrile group coordinates through the second and third coordination spheres of aluminum (III). For this reason, we focused our IR studies on characterization of the effects of anion composition on the copper (II) system. Here we emphasize that we described experiments earlier in this paper in which the anchoring behavior of nematic 5CB as a function of the surface composition of anions was shown to be similar for copper (II) salts and aluminum (III) salts (the change in tilt occurs at approximately 10% perchlorate/ 90% chloride when using copper (II)).
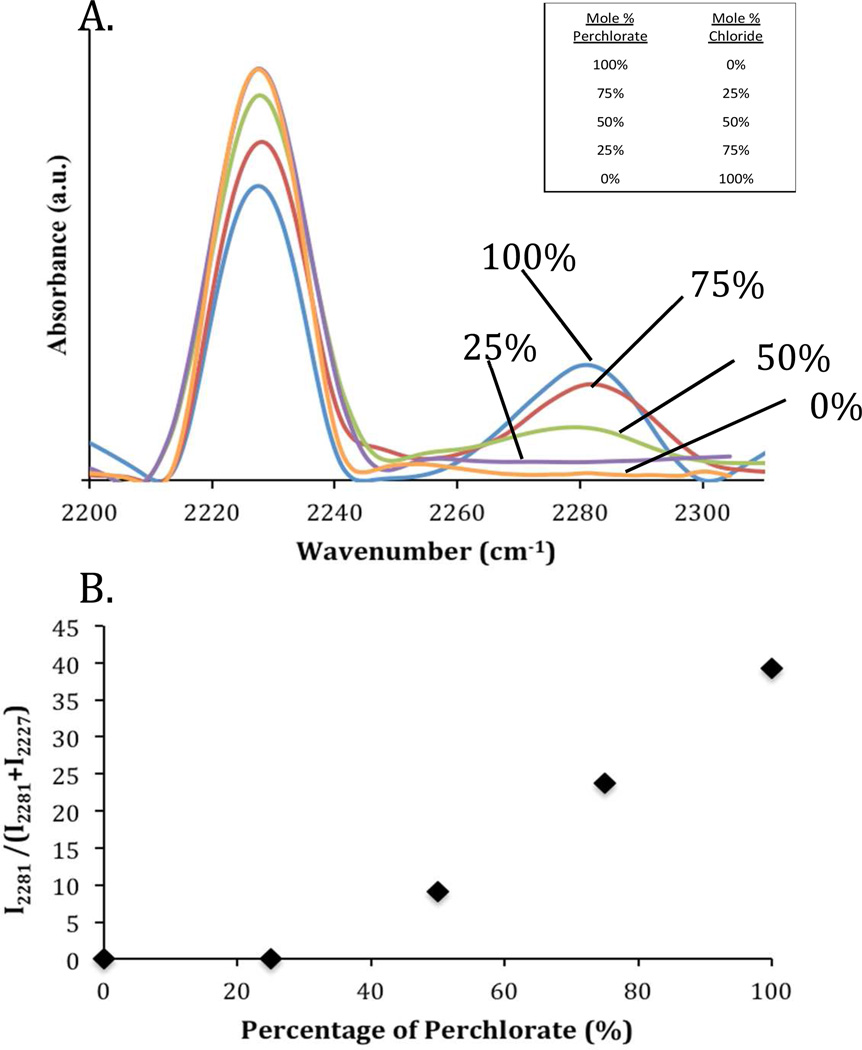
We measured IR spectra centered around the vibration of the nitrile group of 8CB in contact with copper (II) salts to characterize the intensity of the nitrile vibration frequencies in both the free and coordinated vibrational states. Figure 3 shows that, for 8CB in contact with 100% copper perchlorate, a free nitrile peak is observed at 2227 cm−1 and a coordinated nitrile peak is observed around 2281 cm−1, consistent with past studies.6, 8 The coordinated peak is attributed to 8CB interacting with the copper (II) perchlorate salts. When a strongly coordinating anion was introduced into the salt, the intensity of the peak due to the coordination interaction between the LC and copper cation decreased while the free nitrile peak increased. This result indicates that a smaller number of coordination interactions are formed between the metal salt surface and the 8CB as the fraction of non-coordinating anion in the salt was increased. We quantified this change by calculating the ratio of the intensity of the coordinated nitrile peak to the sum of the intensity of the peaks corresponding to coordinated and free nitrile groups (Figure 3b). At low percentages of the perchlorate anion (compositions that did not lead to a homeotropic alignment of the LC), the coordinated nitrile peak was no longer measurable. We also note that the position of the maximum in the intensity of the coordinated nitrile peak was measured to be located at 2279 cm−1 to 2281 cm−1. The location of the free nitrile peak remains constant at 2227 cm−1. A previous study has shown that the location of the peak corresponding to a coordinated nitrile shifts to longer wave numbers with increase in the electron affinity of the metal (i.e., stronger coordination6). Because there is very little shift in the position of the coordinated nitrile peak in the measurements in Figure 3 and a significant decrease in the intensity of the coordinated nitrile peak (as evidenced by the ratio of the coordinated nitrile peak to the total intensity shown in Figure 3b), the IR analysis supports the hypothesis that there is a decrease in the number of coordination interactions between the nitrile moiety and the metal salt as the composition of the anions changes from being perchlorate rich to nitrate rich.
Figure 3.
(A) PM-IRRAS spectra of 8CB films supported on copper (II) salts with mixed anions: blue (100% perchlorate), red (75% perchlorate and 25% chloride), green (50% perchlorate and 50% chloride), purple (25% perchlorate and 75% chloride), orange (100% chloride). (B) Ratio (expressed as a percentage) of the intensity of the coordinated nitrile peak at 2281 cm−1 to the sum of the intensity of the free nitrile peak at 2227 cm−1 and coordinated nitrile peak at 2281 cm−1, plotted as a function of the perchlorate as the anion. See (A) for details.
In summary, to obtain a homeotropic alignment of the LC at the interface, we conclude that a threshold number density of coordination interactions (number per unit area of interface) between the LC and metal cation on the surface is required. The density of interactions can be engineered by controlling the ratio of weakly and strongly coordinating anions.
2. Effect of metal salt anions on the response of nematic 5CB to DMMP
This section expands upon the observations presented in the previous section by reporting how the anion composition impacts the response of an aligned LC system to the vapor analyte DMMP. As noted in the Introduction, past studies have shown that DMMP, when transported from the vapor phase to the metal salt interface, can displace the LC from the coordination complex with the metal salt. These changes in the molecular interactions occurring at the interface cause the LC to undergo an anchoring transition. In experiments described below, we tested the proposition that a lower number density of coordination interactions between the metal salt and LC, achieved by using mixed anions, would result in an alignment of the LC that is more easily disturbed by DMMP.
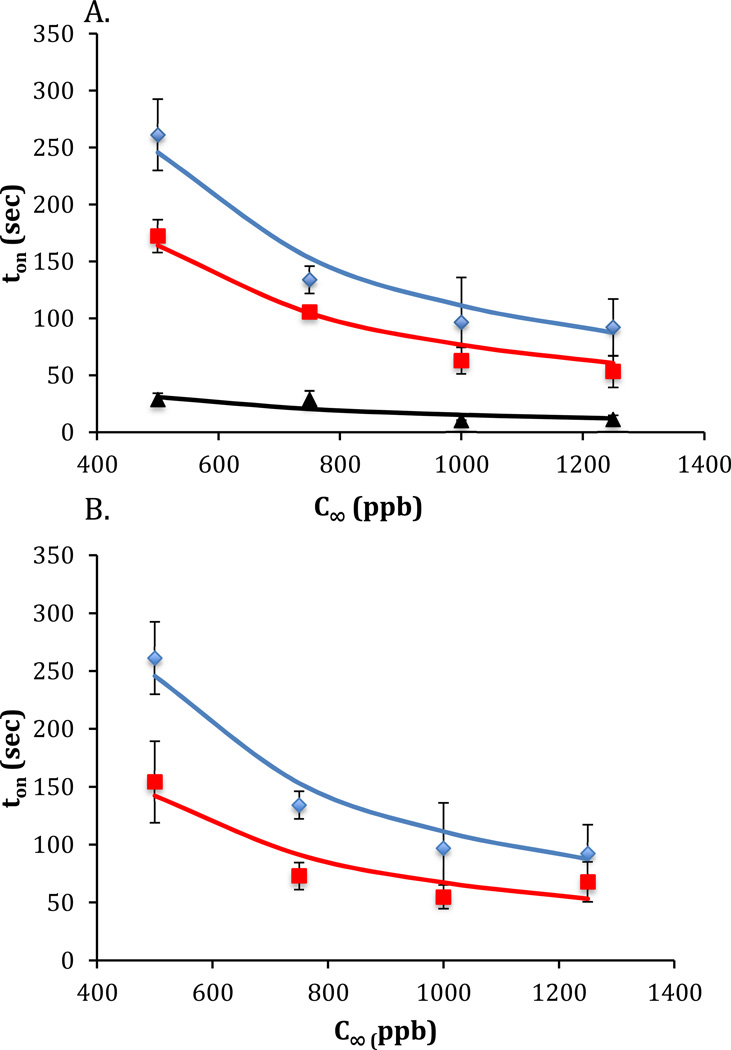
The optical response of a supported film of nematic 5CB to DMMP, plotted as a function of the composition of the anion of the aluminum salt, is shown in Figure 4. Figure 4a shows polarized light micrographs for situations in which the anion was 10% perchlorate and 90% nitrate, 50% perchlorate and 50% nitrate, or 100% perchlorate. Initially, the optical micrographs exhibit a dark appearance, consistent with homeotropic alignment of the 5CB at both the metal salt surface and air interface. In the presence of 1 ppm DMMP, the optical micrographs change to a bright appearance indicative of a change in the alignment of the LC at the metal salt surface from homeotropic to a planar or tilted alignment. As reported previously, this transition is a result of the disruption in the coordination complex formed between the metal salt and LC by DMMP.8 Inspection of Figure 4a reveals that the time required for a measurable change in the LC film to be observed decreases as the amount of perchlorate anion in the system is decreased. To quantify the effect of anion composition on the response, four central squares of each LC film were analyzed and the average intensity of light transmitted through cross polarizers was measured, as shown in Figure 4b (see Methods section). Figure 4b shows that the length of time required to measure an anchoring transition of the LC is influenced by the composition of the anion of the metal salt decorating the solid surface. Below we provide a quantitative analysis of the response shown in Figure 4b.
Figure 4.
(A) Polarized light images of the response of LC films exposed to a vapor of 1 ppm DMMP at 0 seconds (prior to exposure each sample was exposed to air at 30% R.H.). The LC films were supported on surfaces coated with aluminum (III) salts (with the indicated anion composition). The flow rate of the gas over the sample was 1000 mL/min. The number above each image indicates the time (in seconds) of exposure to the DMMP. (B) Normalized light intensities for the images shown in (A). The black data points indicate aluminum (III) salt composed of a 10% perchlorate and 90% nitrate anion mixture, the red data points indicate aluminum (III) salt composed of a 50% perchlorate and 50% nitrate anion composition, and the blue data points indicate aluminum (III) salt composed of a 100% perchlorate anion.
Previously, we established that the LC anchoring on a metal salt surface is not changed by DMMP until a critical concentration of DMMP, Cmin,LC, is accumulated within the LC film such that coordination of the LC and metal ion is disrupted.15 The value of Cmin,LC is linearly related to the minimum concentration of DMMP in the vapor phase, Cmin, based on Henry’s Law. An estimate of Cmin can be obtained by measuring the time of response, ton, of the LC sensor system as a function of the concentration of DMMP in the vapor phase (C∞) and analyzing the results using a simple mass transport model of the system. The relevant equation of the model that relates ton, C∞, and Cmin is
| (1) |
where the thickness of the 5CB film is δ5CB, the partitioning coefficient DMMP from air to LC is KH, and the overall mass transfer coefficient is, kov. Based on our prior studies, these parameters are estimated as: kov = 0.24 cm/s, KH = 5.3×10−5, and δ5CB = 18 µm.15
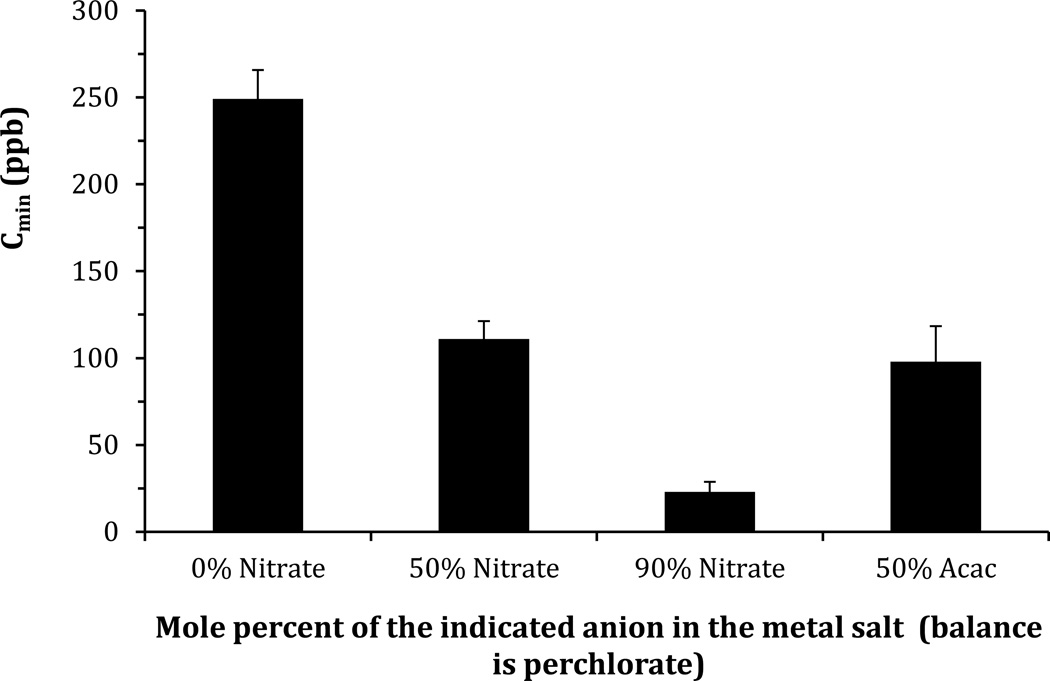
By varying C∞ and measuring the response time ton (Figure 5a) we determined Cmin for the LC films supported on metal salt films with three different mixed anion compositions (Figure 5a). The 100% perchlorate surface yielded a value of Cmin of 250 ppb, whereas a 50% perchlorate/50% nitrate surface lead to a Cmin of 110 ppb, and a 10% perchlorate/90% nitrate has the lowest Cmin at 25 ppb, as shown in Figure 6. Overall, this result indicates that the sensitivity of the LC system can be substantially improved by using mixtures of weakly and strongly coordinating anions.
Figure 5.
(A) The time of response (ton) of LC films to DMMP as a function of DMMP concentration. The LC films were supported on surfaces that were coated with aluminum (III) salts with different anion compositions; blue (100% perchlorate), green (50%/ 50% nitrate/ perchlorate), black (90%/ 10% nitrate/perchlorate) (B) Comparison of ton when using aluminum (III) salts with the anions being 100% perchlorate (blue) or 50%/ 50% perchlorate/acetylacetonate (red).
Figure 6.
The influence of anion composition on the sensitivity of LC films to DMMP. The sensitivity (Cmin) was determined using the model described in Equation 1 and the data shown in Figure 4. The error bars indicate the standard deviation of residuals from the fit in Figure 4.
To provide additional support for the above results, we measured the response of the LCs at the predicted sensitivities of the systems. The 100% perchlorate salt surfaces gave an optical response when exposed to a 300 ppb environment of DMMP, but no optical response was seen at lower concentrations such as at 200 ppb. In contrast, a 10% perchlorate/ 90% nitrate mixture generated an optical response of the LC when the sample was exposed to 100 ppb of DMMP. These results provide general support for our conclusions regarding sensitivities that are based on measurements of ton.
We also explored the extent to which the results above were seen in experiments performed with acetylacetonate. Figure 5b shows experimental results obtained with a mixture of 50% acetylacetonate and 50% perchlorate (by mole percent). From this data, Cmin was calculated to be 100 ppb. This sensitivity is improved relative to that of the 100% perchlorate sample and similar to that of a 50%/ 50% perchlorate/ nitrate mixture suggesting that the bidentate anion also improves the sensitivity of the LC based system to DMMP. Overall, the results described above indicate that the sensitivity of the LC-based system increases as the anions of the metal salt are enriched in the more strongly coordinating anion (Figure 6).
The general observations reported above are consistent with a simple equilibrium model of the ligand exchange reaction occurring at the interface. In brief, we considered the ligand exchange reaction occurring at the surface as:
| (2) |
where M-LC is the metal cation coordinated to the LC, DMMP is the concentration of free DMMP in the LC, M-DMMP is the metal cation coordinated to the DMMP molecules, and LC is the concentration of free mesogens within the system. We write the equilibrium constant for the ligand exchange as:
| (3) |
Because the ligand exchange reaction is occurring in the thin film of the salt, the number of binding sites remains constant and therefore the sum of [M-DMMP] and [M-LC] must be constant.
When the anchoring transition occurs in response to DMMP, we assume that binding of DMMP reduces the concentration of metal-LC interactions ([M-LC]) to a threshold level that we define as [M-LC]crit. This threshold concentration of metal ion-LC interactions is given by the result shown in Figure 2. For mixtures of perchlorate and nitrate, the change in orientation of the LC occurred when using 10% perchlorate and 90% nitrate counter anion. Thus, we can write [M-LC]crit as [M]xcrit, where [M] is the total metal binding sites in the salt film, and xcrit is the fraction of the total metal binding sites in the salt film that are accessible to the LC (i.e., xcrit=0.1 for perchlorate and nitrate). We calculate the number of [M-DMMP] interactions at the anchoring transition induced by DMMP as [M]xperc - [M]xcrit, where xperc is the mole fraction perchlorate in the mixed anion in the experiment of interest (in general, xperc≠xcrit). The concentration of DMMP at which the anchoring transition takes place is defined above as Cmin (calculated by equation 1). We assume that the concentration of the free LC, [LC], does not change significantly, and thus rewrite equation 3 as:
| (4) |
Equation 4 predicts that Cmin plotted versus (xperc – xcrit)/(xcrit) is a straight line passing through the origin. Such a fit was obtained using the data in Figure 6 (see Figure S2 of supporting information). This agreement between the model and data is consistent with a ligand exchange reaction in which one mesogen is replaced by one DMMP molecule in the coordination sphere of the aluminum (III). We end this discussion by noting that the above-described model is a very simple one that contains many assumptions, including that the equilibrium constant does not change as the mole fraction of perchlorate varies. Although simple, the model demonstrates the physical principles that underlie the dependence of the sensitivity of the LC films to DMMP on the total number of interactions between the surface and LC.
3. Influence of the metal salt anion on the effects of humidity
Whereas the studies described above were focused on the competitive interactions of DMMP and 5CB with aluminum (III) ions, the presence of other ligands that can potentially coordinate with aluminum (III) may influence the anchoring transitions induced by DMMP. For example, the coordination interactions of the nitrile moiety of acetonitrile with aluminum (III) perchlorate have been reported to be perturbed by high concentrations of water.31–37 On the other hand, a past study of a metal salt-LC-DMMP system showed that ordering of the LC was not disrupted by concentrations of water up to 75% RH.14 Below we expand upon these results by investigating the effects of humidity on the dynamics of DMMP-induced anchoring transitions (ton), measured as a function of the composition of the anions in the aluminum (III) salt used to support the LC film.
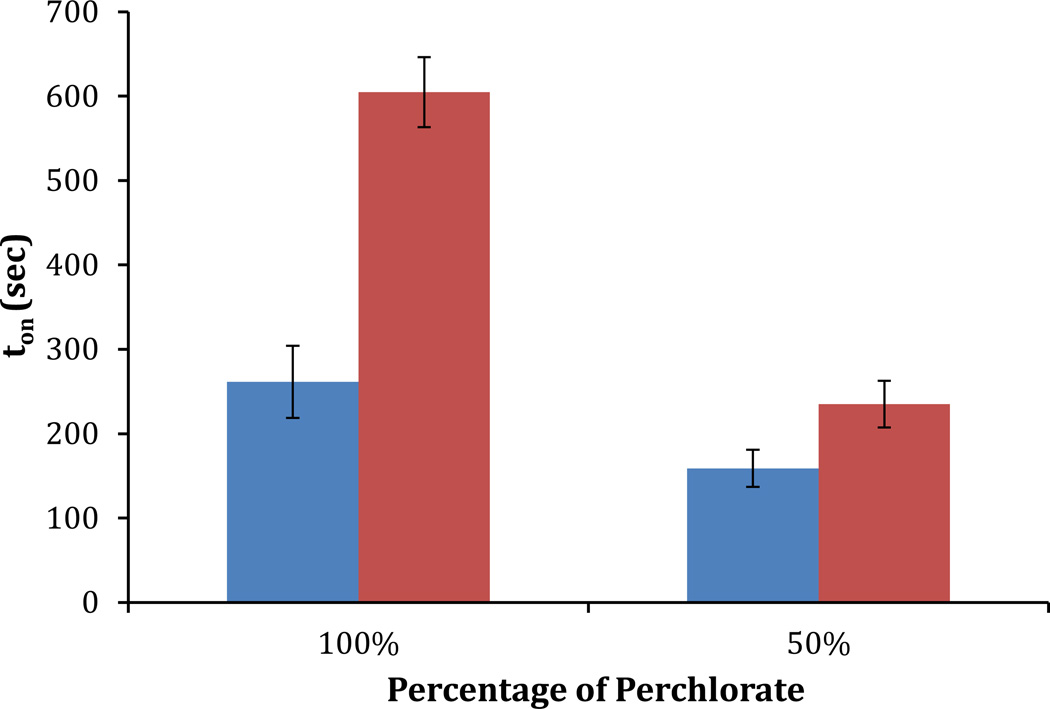
Figure 7 shows ton for the 100% perchlorate system and a 50% perchlorate/ 50% nitrate system when using 30% RH and 60% RH and a concentration of 500 ppb DMMP. Inspection of Figure 7 reveals the effect of a change in RH on the response of the LC to DMMP to be lower for the samples containing 50%/ 50% nitrate/ perchlorate as compared to 100% perchlorate. We propose that this increased tolerance to water is due to the weaker interactions of water with the nitrate anion as compared to the perchlorate anion. Water is known to have weaker interactions with the nitrate anion because of the lower polarizability of the nitrate anion in comparison to the perchlorate anion.38 We speculate that an accumulation of water around the aluminum (III) that is mediated by the perchlorate slows the ligand exchange process involving the DMMP, as is reflected in the dynamics of the anchoring transitions shown in Figure 7. Although the molecular-level details of the mechanism by which the substitution of nitrate for perchlorate changes the influence of water remain to be fully elucidated, the main conclusion of the experiment reported in this section is that changes in the composition of anions can be used to tailor the tolerance of LC-based sensors to potentially interfering compounds.
Figure 7.
Measured values of ton for a 100% perchlorate or a 50% perchlorate/ 50% nitrate salt surfaces (mole percent) under 30% humidity (blue) and 60% humidity (red) conditions at a concentration of 500 ppb DMMP.
Conclusions
The key result presented in this paper is that manipulation of the composition of mixed anions can be used to improve the sensitivity of adsorbate-induced anchoring transitions involving films of LCs supported on surfaces decorated with metal salts. Our results, which are supported by IR spectroscopic studies, suggest that the fraction of strongly coordinating anion within the salt can be used to regulate the number of coordination sites available to both the LC and DMMP. Furthermore, although the number of accessible coordinate sites on the metal cation on the surface does change with the choice of anion, our IR measurements suggest that the strength of the interactions is not substantially perturbed. Our results demonstrate that mixtures of anions can be used to improve the sensitivity of LCs to targeted adsorbates by at least an order of magnitude. Furthermore, preliminary results also support the proposition that the anion composition can also be used to minimize the effects of potentially interfering compounds in designs of LC-based sensors. Overall, our results provide guidance for the design of responsive materials based on adsorbate-induced changes in the orientations of LCs.
Supplementary Material
Acknowledgements
The authors thank Mr. John Cannon for help fabricating the metal plates and the flow cell. The study was funded primarily by the National Science Foundation (DMR-1121288, Materials Research Science and Engineering Center), with additional partial support from the Army Research Office (W911NF-11-1-0251 and W911NF-10-1-0181) and the National Institute of Health grant CA108467 and CA105730.
Footnotes
Supporting Information:
The tilt angle of the LC in contact with copper (II) perchlorate/ chloride salts and the fit of the equilibrium model to the experimental data are shown in the supporting information. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Shah RR, Abbott NL. Science. 2001;293:1296–1299. doi: 10.1126/science.1062293. [DOI] [PubMed] [Google Scholar]
- 2.Shah RR, Abbott NL. Langmuir. 2003;19:275–284. [Google Scholar]
- 3.Jerome B. Rep. Prog. Phys. 1991;54:391–451. [Google Scholar]
- 4.Yokoyama H. Mol. Cryst. Liq. Cryst. 1988;165:265–316. [Google Scholar]
- 5.Sanda PN, Dove DB, Ong HL, Jansen SA, Hoffmann R. Phys. Rev. A. 1989;39:2653. doi: 10.1103/physreva.39.2653. [DOI] [PubMed] [Google Scholar]
- 6.Yang KL, Cadwell K, Abbott NL. J. Phys. Chem. B. 2004;108:20180–20186. [Google Scholar]
- 7.Sridharamurthy SS, Cadwell KD, Abbott NL, Jiang H. Smart Mater. Struct. 2008;17:4. [Google Scholar]
- 8.Cadwell KD, Alf ME, Abbott NL. J. Phys. Chem. B. 2006;110:26081–26088. doi: 10.1021/jp063211k. [DOI] [PubMed] [Google Scholar]
- 9.Cadwell KD, Lockwood NA, Nellis BA, Alf ME, Willis CR, Abbott NL. Sens. Actuators, B. 2007;128:91–98. [Google Scholar]
- 10.Cheng DM, Sridharamurthy SS, Hunter JT, Park JS, Abbott NL, Jiang HR. J. Microelectromech. Syst. 2009;18:973–982. [Google Scholar]
- 11.Hunter JT, Abbott NL. Liquid Crystal-Based Chemical Sensors. In: Li Q, editor. Liquid Crystals Beyond Displays: Chemistry, Physics and Applications. Hoboken, New Jersey: Wiley; 2012. pp. 485–505. [Google Scholar]
- 12.Hunter JT, Pal SK, Abbott NL. ACS Appl. Mater. Interfaces. 2010;2:1857–1865. [Google Scholar]
- 13.Pal SK, Acevedo-Velez C, Hunter JT, Abbott NL. Chem. Mater. 2010;22:5474–5482. [Google Scholar]
- 14.Yang KL, Cadwell K, Abbott NL. Sens. Actuators, B. 2005;104:50–56. [Google Scholar]
- 15.Hunter JT, Abbott NL. Sens. Actuators, B. 2013;183:71–80. [Google Scholar]
- 16.Wang PH, Yu JH, Zhao YB, Li ZJ, Li GQ. Sens. Actuators, B. 2011 [Google Scholar]
- 17.VanTreeck HJ, Most DR, Grinwald BA, Kupcho KA, Sen A, Bonds MD, Acharya BR. Sens. Actuators, B. 2011;158:104–110. doi: 10.1016/j.snb.2012.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namkung J, Zou Y, Abu-Abed A, Lindquist RG. IEEE Sens. J. 2010;10:1479–1485. [Google Scholar]
- 19.Stewart CE. Weapons of mass casualties and terrorism response handbook. Sudbury, MA: Jones & Bartlett Learning; 2006. [Google Scholar]
- 20.Sen A, Acharya BR. Liq. Cryst. 2011;38:495–506. [Google Scholar]
- 21.Tatsuya Urabe TTMT. J. Mass Spectrom. 2009;44:193–202. doi: 10.1002/jms.1485. [DOI] [PubMed] [Google Scholar]
- 22.Edwards JO. J. Am. Chem. Soc. 1954;76:1540–1547. [Google Scholar]
- 23.Parr RG, Pearson RG. J. Am. Chem. Soc. 1983;105:7512–7516. [Google Scholar]
- 24.Pearson RG. J. Chem. Educ. 1968;45:581–587. [Google Scholar]
- 25.Pearson RG. J. Chem. Educ. 1968;45:643–648. [Google Scholar]
- 26.Pearson RG. Inorg. Chem. 1988;27:734–740. [Google Scholar]
- 27.Pearson RG. Chem. Br. 1991;27:444–447. [Google Scholar]
- 28.Yamada S, Tanaka M. J. Inorg. Nucl. Chem. 1975;37:587–589. [Google Scholar]
- 29.Acharya ASBR. Liq. Cryst. 2011;38:495–506. [Google Scholar]
- 30.Lockwood NA, Gupta JK, Abbott NL. Surf. Sci. Rep. 2008;63:255–293. [Google Scholar]
- 31.Jamroz D, Wojcik M, Lindgren J, Stangret J. J. Phys. Chem. B. 1997;101:6758–6762. [Google Scholar]
- 32.Jamroz D, Stangret J, Lindgren J. J. Am. Chem. Soc. 1993;115:6165–6168. [Google Scholar]
- 33.Ruben Y, Reuben J. J. Phys. Chem. 1976;80:2394–2400. [Google Scholar]
- 34.Supran LD, Sheppard N. Chem. Commun. (London) 1967:832–834. [Google Scholar]
- 35.Cho HG, Andrews L. J. Phys. Chem. A. 2010;114:5997–6006. doi: 10.1021/jp1012686. [DOI] [PubMed] [Google Scholar]
- 36.O'Brien JF, Alei M. J. Phys. Chem. 1970;74:743–746. [Google Scholar]
- 37.Richardson D, Alger TD. J. Phys. Chem. 1975;79:1733–1739. [Google Scholar]
- 38.Atkins P, De Paula J. Physical Chemistry. 7 ed. New York: Oxford University Press; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.