Abstract
On a global basis, at least 15 million individuals suffer some form of a stroke every year. Of these individuals, approximately 800,000 of these cerebrovascular events occur in the United States (US) alone. The incidence of stroke in the US has declined from the third leading cause of death to the fourth, a result that can be attributed to multiple factors that include improved vascular disease management, reduced tobacco use, and more rapid time to treatment in patients that are clinically appropriate to receive recombinant tissue plasminogen activator. However, treatment strategies for the majority of stroke patients are extremely limited and represent a critical void for care. A number of new therapeutic considerations for stroke are under consideration, but it is the mammalian target of rapamycin (mTOR) that is receiving intense focus as a potential new target for cerebrovascular disease. As part of the phosphoinositide 3-kinase (PI 3-K) and protein kinase B (Akt) cascade, mTOR is an essential component of mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) to govern cell death involving apoptosis, autophagy, and necroptosis, cellular metabolism, and gene transcription. Vital for the consideration of new therapeutic strategies for stroke is the ability to understand how the intricate and complex pathways of mTOR signaling sometimes lead to disparate clinical outcomes.
Keywords: Akt, apoptosis, autophagy, Deptor, mammalian target of rapamycin (mTOR), mLST8, mSIN1, mTORC1, mTORC2, necroptosis, oxidative stress, PI 3-K, PRAS40, Protor-1, p70S6K, Raptor, Rictor, SGK1, stroke
Incidence and Current Therapeutic Strategies for Cerebrovascular Disease
For 2011, an approximate 1% decrease in the age-adjusted death rate was reported for the United States population derived from information on mortality data for the years of 2000 through 2011 (1). Life expectancy is now believed to be approaching almost 80 years for all individuals. The five leading causes of death are cardiac disease, cancer, chronic lower respiratory disease, stroke, and traumatic accidents (2). Interestingly in this analysis, stroke is no longer ranked as the third leading cause of death. A number of factors may have contributed to this lower ranking for stroke that include improved long-term care with disorders tied to hypertension and low-density lipoprotein cholesterol management, reduction in tobacco consumption, improved public education and awareness for the need for rapid treatment of cerebrovascular disorders, and improved management of metabolic disorders such as diabetes (3, 4). Furthermore, treatment with recombinant tissue plasminogen activator in an applicable sub-group of patients that requires a narrow therapeutic window also has led to a reduction in mortality and morbidity in patients presenting with stroke (5, 6). However, overall therapeutic strategies for patients presenting with stroke remain limited for the majority of patients. A number of new therapeutic considerations for stroke, ischemic vascular disease, and central nervous system inflammation under investigation focus upon cytokines (7–19), growth factors (20), progenitor cells (21), normobaric hyperoxia (22), metallic ions (23), cellular metabolism (24), small molecular regulators of hypoxia inducible factor (25), tissue kallikrein (26), and retinoblastoma protein (27). Yet, gaining exceptional and more recent interest as a novel strategy for stroke and cerebrovascular disease is the role of the mammalian target of rapamycin (mTOR) (28–30).
Mechanistic Avenues of Consideration for mTOR
mTOR (also known as the mechanistic target of rapamycin and FK506-binding protein 12-rapamycin complex-associated protein 1) is a 289-kDa serine/threonine protein kinase. It was initially isolated in yeast in Saccharomyces cerevisiae with the identification of the genes TOR1 and TOR2 that encode two isoforms in yeast Tor1 and Tor2 (31). A single gene FRAP1 encodes mTOR in mammals, is ubiquitously expressed throughout the body, and modulates metabolism, cellular survival, gene transcription, and cytoskeletal components (29, 32–36).
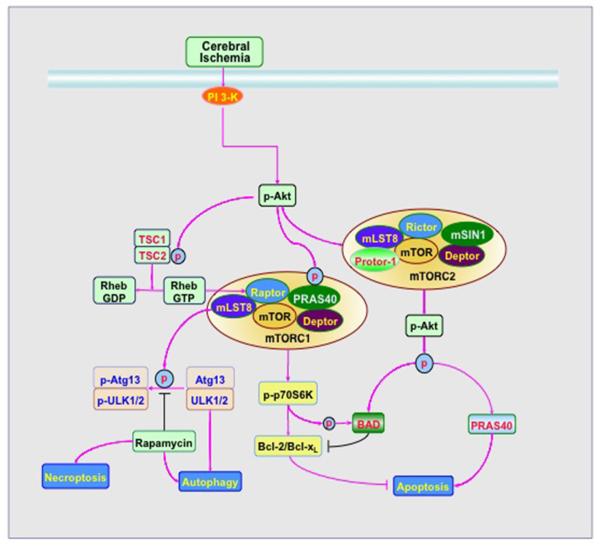
The protein complexes mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) each contain the protein mTOR and have been identified based on their components and their sensitivity to rapamycin (28, 35–39) (Figure 1). mTORC1 has the regulatory-associated protein of mTOR (Raptor) protein. Phosphorylation of Raptor that controls mTORC1 activity can proceed through a number of pathways that involve the protein Ras homologue enriched in brain (Rheb) (40). Rheb phosphorylates Raptor residue serine863 as well as other residues that include serine859, serine855, serine877, serine696, and threonine706. mTORC1 activity can be limited if serine863 remains unphosphorylated, (41). mTOR itself can phosphorylate Raptor following stimulation by insulin. In contrast, rapamycin, a macrolide antibiotic from Streptomyces hygroscopicus, inhibits mTOR activity (41). mTORC1 is more sensitive to the inhibitory effects of rapamycin than mTORC2 (42). Rapamycin inhibits mTORC1 by binding to immunophilin FK-506-binding protein 12 (FKBP12) that attaches to FKBP12 - rapamycin-binding domain (FRB) at the C-terminal of mTOR to prevent the phosphorylation of mTOR (43). Chronic exposure of rapamycin also can inhibit mTORC2 that may involve a mechanism that disrupts the assembly and the integrity of mTORC2 (42).
Figure 1. mTOR signaling in Stroke.
Activation of phosphoinositide 3 kinase (PI 3-K) following cerebral ischemia onset leads to phosphorylation and activation of Akt (protein kinase B). Subsequently, Akt activates mTORC1 through phosphorylating TSC2 and disrupting the interaction between TSC2 and TSC1. Akt also can directly phosphorylate proline rich Akt substrate 40 kDa (PRAS40) to reduce its binding to regulatory associated protein of mTOR (Raptor), lead to activation of mTORC1, and prevent apoptosis. mTORC1 phosphorylates the downstream target p70 ribosome S6 kinase (p70S6K) to phosphorylate pro-apoptotic protein BAD and increase the expression of Bcl-2/Bcl-xL which functions as an anti-apoptotic protein. mTORC1 activation also inhibits autophagic proteins autophagy related gene 13 (Atg13) and UNC-51 like kinase 1/2(ULK1/2) through phosphorylation to prevent autophagy. During the inhibition of mTOR with agents such as rapamycin, autophagy and necroptosis can be initiated.
The N-terminal portion of mTOR has at least a 20 HEAT (Huntingtin, Elongation factor 3, A subunit of Protein phosphatase-2A, and TOR1) repeat (34). This region promotes binding with two important, and mutually exclusive, regulatory proteins, Raptor (regulatory-associated protein of mTOR) and Rictor (rapamycin-insensitive companion of mTOR) (32, 44). It is the association with either Raptor or Rictor that determines whether mTOR is a component of mTORC1 or mTORC2.
mTORC1 consists of a number of other components in addition to Raptor that include the proline rich Akt substrate 40 kDa (PRAS40), Deptor (DEP domain-containing mTOR interacting protein), and mLST8/GβL (mammalian lethal with Sec13 protein 8, termed mLST8). PRAS40 competitively inhibits the binding of mTORC1 to Raptor (45). The maintenance of mTORC1 activity occurs through the inhibitory phosphorylation of PRAS40 by protein kinase B (Akt). PRAS40 is phosphorylated on several residues that include serine183, serine212, serine221, and threonine246 (46, 47). The serine sites are targets of mTOR and the residue of threonine246 is the phosphorylation target of Akt. The phosphorylation of PRAS40 leads to its dissociation with Raptor (48) and promotes the binding of PRAS40 to the cytoplasmic docking protein 14-3-3 (49–51). This removes PRAS40 from interacting with Raptor and facilitates the activation of mTORC1 (52). Deptor also is an inhibitory subunit of mTORC1. Deptor binds to the FAT domain of mTOR (for FKBP associated protein, Ataxia-telengiectasia, and Transactivation/transformation domain-associated protein) to inhibit the activity of mTORC1. In the absence of Deptor, the activity of Akt, mTORC1, and mTORC2 increase (53). mLST8 is a 36 kDa peripheral membrane protein that is a component of both mTORC1 and mTORC2. mLST8 promotes mTOR kinase activity with p70S6K and 4EBP1 (54), controls insulin signaling through the transcription factor FoxO3 (55), is necessary for the phosphorylation of Akt and protein kinase C-α (PKCα) (55), and is required for the association between Rictor and mTOR (55).
Two important targets of mTORC1 are p70S6K and the eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4EBP1) (33, 35). mTORC1 fosters mRNA biogenesis, translation of ribosomal proteins, and cell growth through p70S6K phosphorylation (56). Amino acids, such as glutamate and leucine, also have been shown to phosphorylate p70S6K. Through amino acid activation of the mTOR-p70S6K pathway, glutamate may control neuronal synaptic signaling (57) and leucine can decrease food intake (58). In contrast, phosphorylation of 4EBP1 results in its inactivation. When 4EBP1 is hypophosphorylated, it can block protein translation by binding to eukaryotic translation initiation factor 4 epsilon (eIF4E) through eIF4 gamma (eIF4G), a protein that helps transport mRNA to the ribosome. mTORC1 phosphorylation of 4EBP1 leads to the dissociation of 4EBP1 from eIF4E, allowing eIF4G to begin mRNA translation (59). Binding of 4EBP1 and p70S6K to Raptor can be prevented during activation of PRAS40.
Different from mTORC1, mTORC2 contains the rapamycin-insensitive companion of mTOR termed Rictor. Similar to mTORC1, mTORC2 has the components of mTOR, mLST8, and Deptor. mTORC2 contains additional components that are the mammalian stress-activated protein kinase interacting protein (mSIN1) and the protein observed with Rictor-1 (Protor-1). Rictor and mSIN1 can form the structural basis of mTORC2. mTORC2 utilizes Rictor to activate and phosphorylate Akt at Ser473, facilitating threonine308 phosphorylation by phosphoinositide-dependent kinase 1 (PDK1) (60). mSIN1 is necessary for mTORC2 to activate Akt. mTOR has also been shown to phosphorylate mSIN1, preventing the lysosomal degradation of mSIN1 (61). Protor-1 is a Rictor-binding subunit of mTORC2 that does not appear to alter other mTORC2 components in a way that would lead to the phosphorylation of Akt or PKCα. However, Protor-1 may function to activate serum and glucocorticoid induced protein kinase 1 (SGK1). Loss of Protor-1 in animal models reduces the hydrophobic motif phosphorylation of SGK1 and its substrate NRDG1 (N-Myc downregulated gene 1 in the kidney) (62).
Targets of mTORC2 are Akt, protein kinase C alpha (PKCα), P-Rex1, P-Rex2, Rho GTPases, and SGK1. mTORC2 promotes cell survival through the activation of Akt and uses PKCα for cytoskeleton remodeling. mTORC2 phosphorylates and activates SGK1, is a member of the protein kinase A/protein kinase G/protein kinase C (AGC) family of protein kinases, and is activated by growth factors to control ion transport and growth (63). mTORC2 modulates cell migration through activating Rac guanine nucleotide exchange factors P-Rex1 and P-Rex2 and uses Rho signaling during cell-to-cell contact (64). P-Rex1 and P-Rex2 are phosphorylated by Akt through mTORC2 acting as a catalytic complex and are linked to Rac activation and cell migration (64).
Akt activates mTORC1 in response to growth factors and several other Akt mediated pathways (33). Tuberous sclerosis complex (TSC) 1 (hamartin)/TSC2 (tuberin) complex is an inhibitor of mTORC1 and one of the targets of Akt for the regulation of mTORC1 activity. Although several regulatory phosphorylation sites are known to exist for TSC1, phosphorylation of TSC2 by Akt, extracellular signal-regulated kinases (ERKs), activating protein p90 ribosomal S6 kinase 1 (RSK1), AMP activated protein kinase (AMPK), or glycogen synthase kinase -3β (GSK-3β) appear to be more significant for controlling the TSC1/TSC2 complex. TSC2 functions as a GTPase-activating protein (GAP) converting a small G protein Ras homologue enriched in brain (Rheb-GTP) to the inactive GDP-bound form (Rheb-GDP). Once active, Rheb-GTP can directly interact with Raptor to activate mTORC1 and also regulate the binding of 4EBP1 to mTORC1 (65). Akt phosphorylates TSC2 on multiple sites that leads to the destabilization of TSC2 and disruption of its interaction with TSC1. The phosphorylation of TSC2 (serine939, serine981, and threonine1462) can increase its binding to protein 14-3-3 and lead to cellular sequestration, disruption of the TSC1/TSC2 complex, and subsequent activation of Rheb and mTORC1 (66). In contrast to mTORC1, TSC1/TSC2 fosters the activity of mTORC2 (67). Loss of a functional TSC1/TSC2 complex can lead to the loss of mTORC2 kinase activity in vitro (67). The TSC1/TSC2 complex can associate with mTORC2 to promote mTORC2 activity that involves the N-terminal region of TSC2 and the C-terminal region of Rictor.
On an additional note, it is important to recognize that mTOR signaling is part of cascade of pathways that include phosphoinositide 3 –kinase (PI 3-K), Akt, and AMPK (68). AMPK phosphorylates TSC2 to lead to increased GAP activity to turn Rheb-GTP into Rheb-GDP and thus inhibits the activity of mTORC1 (69). AMPK also can control TSC1/2 activity through RTP801 (REDD1/ product of the Ddit4 gene) (70). During hypoxia, AMPK activity can increase REDD1 expression to suppress mTORC1 activity by releasing TSC2 from its inhibitory binding to protein 14-3-3 (70). Increased AMPK activation has been shown to reduce myocardial infarct size in experimental models of diabetes (71). However, down-regulation of the AMPK pathway may be detrimental. For example, loss of AMPK activity can increase insulin resistance in skeletal muscle (72). In addition, the liver kinase B1 (LKB1) can regulate the activation of AMPK through phosphorylation (73). Loss of LKB1 impairs cardiac function during ischemic conditions (74), illustrating the importance of AMPK signaling in the mTOR pathway for the vascular system.
Cell Injury Through Apoptosis, Autophagy, and Necroptosis
Ischemic injury as aresult of oxidative stress in the brain (75, 76) can ultimately initiate programmed cell death pathways that oversee apoptosis, autophagy, and necroptosis (77–79) (Figure 1). In acute and chronic degenerative disorders, apoptosis, autophagy, and necroptosis have been associated with cell injury. Apoptotic DNA degradation and the presence of caspase 3 in neurons has been reported in the postmortem nigra of Parkinson's disease patients, suggesting that apoptosis results in neuronal cell death (80). Apoptotic DNA fragmentation (81) and caspase activation (82) also have been reported in the brains of patients with Alzheimer's disease as well as in cell models of Alzheimer's disease and cognitive loss (83–88). Apoptotic cell loss has also been associated with acute traumatic injury (84, 88).
mTOR has been shown through Akt to protect endothelial cells against apoptosis (89) and to block “pro-apoptotic” forkhead transcription factors, such as FoxO3a (89, 90). Inflammatory cells also can undergo apoptotic injury during oxidative stress if deprived of Akt and mTOR activation (86, 91). Apoptotic cell death in dopaminergic neurons can be blocked during application of agents that increase Akt and mTOR activity (92). Akt also functions to modulate apoptosis with mTOR through the inhibition of PRAS40. Phosphorylation of PRAS40 by Akt can block the activity of this substrate, lead to its dissociation from mTORC1 to promote mTOR activation and prevent apoptosis (49, 87, 93).
mTOR also can regulate apoptotic cell death through downstream signaling pathways such as p70S6K and BAD. Phosphorylation of BAD leads to the dissociation of this protein from the “anti-apoptotic” protein Bcl-2/Bcl-xL and increases BAD binding to protein 14-3-3. Activation of p70S6K promotes the phosphorylation of BAD in astrocytes to limit apoptotic cell injury (94). The activation of mTOR and p70S6K may also decrease apoptosis through pathways that can increase “anti-apoptotic” Bcl-2/Bcl-xL expression (94). In addition, insulin prevents apoptosis in rat retinal neuronal cells against serum deprivation through the activation of mTOR and p70S6K (95). Activation of p70S6K in the PI3-K/Akt pathway that may not involve BAD also can foster neuronal (96) and cardiac protection (97). Over-expression of wild type p70S6K or a rapamycin resistant form of the p70S6K kinase enhances the cytoprotective effect of insulin (95). Other growth factors similar to insulin, such as erythropoietin (EPO) (98), also have been reported to be dependent upon mTOR activation for cytoprotection against apoptosis (86, 99, 100).
Yet, activation of mTOR does not consistently block apoptosis. During Alzheimer's disease, post-mitotic neurons that attempt to enter the cell cycle do not replicate, but can result in apoptotic cell death (27, 101). In studies with amyloid oligomer exposure, neurons can be prevented from entering the cell cycle during the inhibition of mTOR and thus be protected from apoptosis (102).
In contrast to apoptosis that can lead to the ultimate destruction of a cell, autophagy allows cells to recycle cytoplasmic components and remove defective organelles for tissue remodeling. Of the three categories of autophagy that include microautophagy, macroautophagy, and chaperone-mediated autophagy, macroautophagy is the most prominent and involves the degradation of cytoplasmic material and the sequestration of the cytoplasmic protein and organelles into autophagosomes (78, 79, 103, 104). mTOR controls autophagy through the regulation of autophagic genes. mTOR phosphorylates the mammalian homologue of autophagy related gene 13 (Atg13) and the mammalian Atg1 homologue ULK1 and ULK2 to prevent the progression of autophagy (105). The focal adhesion kinase family interacting protein of 200 kDa (FIP200) has been identified as a ULK binding protein. FIP200 and Atg13 are vital for the stability and activation of ULK1. Mammalian Atg13 binds to ULK1/2 and FIP200 to activate ULKs and facilitates the phosphorylation of FIP200 by ULKs (105). It is believed that mTOR activation prevents autophagy in mammalian cells through the inhibition of the ULK-Atg13-FIP200 complex by phosphorylating Atg13 and ULKs.
In the nervous system, it remains unclear under what specific circumstances pathways such as autophagy may be beneficial. Autophagy can lead to cell death in cerebral astrocytes (106), in purkinje neurons (107), in sympathetic neurons (108), in cortical neurons (109), and in spinal cord motor neurons (110). Activation of the mTOR pathway that blocks autophagy may be necessary to protect against spinal cord injury (111) and maintain synaptic plasticity (112). Yet, in other scenerios, autophagy also may invoke a protective component (113). Trophic factor neuronal protection may be mediated through the induction of autophagy (114). Induction of autophagy also may be necessary with combined inhibition of mTOR signaling to improve cognitive function, limit amyloid (Aβ) cell injury (115), and clear mutant huntingtin in Huntington's disease (116). Activation of autophagy also may be required to protect against neuronal cell loss and α-synuclein toxicity in Parkinson's disease (117).
Given the varied outcomes that can occur with programmed cell death pathways, it may come as no surprise to learn that apoptosis and autophagy also share a complex relationship. For example, inhibition of mTOR activity in squamous carcinoma cell lines can lead to the combined activation of apoptosis and autophagy (118). Methamphatamine leads to cell death not only through apoptosis, but also through autophagy by inhibiting the disassociation of the Bcl-2/Beclin 1 complex (119). Bcl-2/Bcl-xL is an “anti-apoptotic” protein that blocks autophagy through its inhibitory interaction with Beclin 1 (120). Autophagy and apoptosis also may have opposing roles. Induction of apoptosis may conversely require the inhibition of autophagy (78, 121, 122).
Necroptosis is a regulated necrotic cell death pathway that is controlled by receptor-interacting protein (RIP-1 and RIP-3) kinases and cylindromatosis (turban tumor syndrome) (CYLD). Necroptosis can be closely tied to autophagy and has been shown that inhibition of mTOR in acute lymphoblastic leukemia leads to autophagy dependent cell loss with features that are consistent with necroptosis (77). In human carcinoma cell lines, agents that can slow cell cycle progression have been shown to be dependent upon necroptosis (123), suggesting a potential new pathway of treatment for neurodegenerative disorders such as Alzheimer's disease. Furthermore, the ability to proliferate by glioblastoma cells has been linked to a number of mechanisms that include inhibition of Akt, mTORC1 and mTORC2, cell-cycle block at G2-M, and the initiation of necroptosis and autophagy (124).
Targeting mTOR in Cerebrovascular Disease
Given the potential role of mTOR in a number of neurodegenerative disorders (29, 35, 36, 39, 125–130), new enthusiasm is now focusing upon the mTOR pathway for the treatment of cerebrovascular disease. In an experimental model of ischemic-reperfusion injury, the agent salvianolate has been shown to decrease stroke volume that was associated with the up-regulation of Golgi phosphoprotein-3 and mTOR phosphorylation (131). The neuroprotective agent ferulic acid reduces middle cerebral artery infarction in a rat experimental model with phosphorylation and activation of mTOR signaling to include mTOR and p70S6K (132). In animal models of ischemic preconditioning, activation of mTOR pathways are also believed to be necessary for neuroprotection. Phosphorylation and activation of mTOR has been observed during remote ischemic preconditioning of the hippocampus that was neuroprotective and improved memory function during global brain ischemia (133). In models of ischemic post-conditioning, long-term cerebral focal ischemic damage and neurological disability were reduced and mediated by enhanced Akt and mTOR activity (134).
Cerebrovascular protection in these models may be mediated in part through modulation of glutamate uptake and a reduction in excitotoxicity modulated through mTOR signaling in glia. During oxygen-glucose deprivation, the Akt-mTOR axis through mTORC1 and mTORC2 has been demonstrated to be necessary for glutamate transporter subtype 2 (GLT-1) expression that would promote glutamate uptake during brain ischemia and limit ischemic injury (135). Prior work supports such a premise with the illustration that mTOR signaling is vital to protect other non-neuronal cells such as those that involve microglia (86, 91, 100). An additional mechanism of protection through mTOR to consider involves small non-coding micro RNAs (miRNAs). In adult animal models of stroke, a small cohort of circulating miRNAs that were related to PI3-K, Akt, and mTOR were associated with a greater degree of neuroprotection in adult females, suggesting that miRNAs with the presence of a sex factor could offer clinical protection through mTOR signaling (136).
Despite the number of investigations that support a role for mTOR activation in neuroprotection during cerebral ischemia, other experimental studies offer a counter perspective. Some studies suggest that inhibition of mTOR through PRAS40 activation may reduce cerebral infarction through work that over-expresses PRAS40 as well as eliminates the presence of PRAS40 in murine models of stroke (137). However, other experimental models employing neuronal cell lines and microglia have shown that PRAS40 either in conjunction with the inhibition of mTOR signaling or independently can lead to detrimental cell injury and that cell protection requires the reduction of PRAS40 activity (49, 87). Studies also show that antagonism of the histamine H3 receptor leads to protection following cerebral ischemia and reperfusion through inhibition of mTOR phosphorylation and induction of autophagy (138). In hippocampal neurons, damage from excitotoxicity can be reduced with promotion of autophagy and mTOR inhibition (139). During oxygen-glucose deprivation in human umbilical vein endothelial cells, rapamycin with subsequent inhibition of mTOR protectes vascular cells from injury in conjunction with autophagy activation (140). Rapamycin also was found to be protective during oxygen-glucose deprivation in cortical neuronal cells. Application of rapamycin prevented the activation of mTORC1 and mTORC2 and led to increased neuronal survival (141). Using sub-lethal ischemic precondition to result in ischemic brain tolerance, rapamycin also was found to promote autophagy, reduce brain damage, and improve neurological scores that was suggested to be mediated through TSC1 (142).
The current work with examining the role of mTOR during cerebrovascular injury clearly suggests that a number of parameters may determine whether promotion of the mTOR signaling pathway or blockade of the mTOR axis is necessary for protection in the brain. Different experimental models as well multiple factors in clinical trials may impart variables that lead to a variety of outcomes. However, a case also can be made for the degree of mTOR activation that may be necessary to prevent or at least limit an injury process and subsequently lead to cellular and tissue protection. For example, activation of mTOR can prevent oxidative stress mediated autophagy in dopamine neurons (92). Yet, prolonged activation of mTOR can lead to dyskinesia in patients with Parkinson's disease (143). Furthermore, in chronic disorders such as Alzheimer's disease, it is the inhibition of mTOR with the activation of autophagy that may be necessary to impart clinical benefit (115).
Conclusions and Future Perspectives
Cerebrovascular disease is one of the five leading causes of disability and death in the US and affects over 800,000 people year at a cost of greater than 75 billion US dollars annually. Although the incidence of stroke has declined placing it in rank from the third leading cause of death to the fourth, multiple factors rather than any single entity are most likely contributors to this result. These factors would include improved management of vascular disease in patients, reduction of tobacco use, and more rapid time to treatment in patients during the initial onset of stroke. Yet, stroke continues to remain a significant cause of death and disability worldwide and the available treatments for stroke are markedly restricted. In addition, therapies such as recombinant tissue plasminogen activator are only applicable for a small subset of patients. New therapeutic strategies continue to be investigated for the treatment of stroke but none may be as groundbreaking as well as complex as those that focus upon mTOR signaling.
Targeting mTOR can offer a wide variety of outcomes that appear beneficial with either the activation or the inhibition of mTOR pathways, suggesting that the degree of mTOR activity may play a significant role in attempts to achieve neuroprotection during the treatment of stroke. However, current studies also indicate that mTOR most likely does not function in isolation and the axis that involves PI 3-K, Akt, and mTOR should be considered more broadly in relation to potential mechanisms that affect cellular survival. For example, regulation of the combined PI 3-K, Akt, and mTOR cascade has been shown to be important to promote increased radiosensitivity against tumor cell growth and the vascular supply of tumors (144). Furthermore, combined loss of 70S6K activity with the loss of the mTORC2 substrate Akt2 is necessary for defective insulin activity and β-cell function (145), suggesting that both of these pathways may require targeting when considering therapeutic strategies to maintain glycemic control. New studies also indicate that metformin may limit prostate cancer growth and disrupt membrane initiated androgen signaling through combined mTOR, 70S6K, and AMPK signaling (146). Additional studies also have highlighted the critical role of other linked pathways to the PI3-K, Akt, and mTOR axis for cellular survival and injury that involve wingless (Wnt) signaling (86, 100, 147–150), the CCN family (87, 151, 152), cytokines such as EPO (49, 86, 99, 100), sirtuins (153–155), forkhead transcription factors (55, 156–159), neurotransmitter modulation (160), and lipid metabolism (28).
One also must be cognizant of the potential for tumorigenesis with the activation of the mTOR pathway. Inhibition of tumor growth and development of metastases usually requires blockade of the proliferative mTOR pathway (39, 161–163). Experimental studies show that inhibition of mTOR can block lung cancer growth (39, 163), prostate cancer (164), breast cancer (165), and colorectal cancer (166). At the clinical level, rapamycin (sirolimus) and rapamycin derivative compounds (“rapalogs”) are currently approved by the Food and Drug Administration for the treatment of subependymal giant cell astrocytoma associated with tuberous sclerosis (everolimus) and neuroendocrine pancreatic tumors (everolimus) (35, 167, 168). As result, activation of mTOR signaling may foster protection against neurodegenerative disorders but such focus must also consider the potential for unintended and unchecked cellular growth. Ultimately, knowledge of how the intricate cellular signaling pathways of mTOR can lead to sometimes very different clinical outcomes will be essential for the development of clinical strategies for stroke that rely upon mTOR.
Acknowledgments
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
Conflict of Interest The authors declare no conflict of interest.
References
- 1.Minino AM. Death in the United States, 2011. NCHS data brief. 2013 Mar;(115):1–8. [PubMed] [Google Scholar]
- 2.Minino AM, Murphy SL. Death in the United States, 2010. NCHS data brief. 2012 Jul;(99):1–8. [PubMed] [Google Scholar]
- 3.Maiese K, Chong ZZ, Shang YC, Wang S. Novel directions for diabetes mellitus drug discovery. Expert opinion on drug discovery. 2012 Oct;24 doi: 10.1517/17460441.2013.736485. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pergola PE, White CL, Szychowski JM, Talbert R, Brutto OD, Castellanos M, et al. Achieved Blood Pressures in the Secondary Prevention of Small Subcortical Strokes (SPS3) Study: Challenges and Lessons Learned. Am J Hypertens. 2014 Mar;7 doi: 10.1093/ajh/hpu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Zhu G, Liu N, Zhang W. Low-dose tissue plasminogen activator is as effective as standard tissue plasminogen activator administration for the treatment of acute ischemic stroke. Curr Neurovasc Res. 2014 Feb;11(1):62–7. doi: 10.2174/1567202610666131126150043. [DOI] [PubMed] [Google Scholar]
- 6.Pineda D, Ampurdanes C, Medina MG, Serratosa J, Tusell JM, Saura J, et al. Tissue plasminogen activator induces microglial inflammation via a noncatalytic molecular mechanism involving activation of mitogen-activated protein kinases and Akt signaling pathways and AnnexinA2 and Galectin-1 receptors. Glia. 2012 Apr;60(4):526–40. doi: 10.1002/glia.22284. [DOI] [PubMed] [Google Scholar]
- 7.Caprara C, Grimm C. From oxygen to erythropoietin: relevance of hypoxia for retinal development, health and disease. Prog Retin Eye Res. 2012 Jan;31(1):89–119. doi: 10.1016/j.preteyeres.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Chang ZY, Yeh MK, Chiang CH, Chen YH, Lu DW. Erythropoietin protects adult retinal ganglion cells against NMDA-, trophic factor withdrawal-, and TNF-alpha-induced damage. PLoS One. 2013;8(1):e55291. doi: 10.1371/journal.pone.0055291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002 Dec 3;106(23):2973–9. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 10.Dimitrova KR, Leitman IM. Intramyocardial transplantation of endothelial progenitor cells and erythropoietin: a new scope for the treatment of cardiovascular disease. J Surg Res. 2013 Aug;183(2):550–2. doi: 10.1016/j.jss.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Fu W, Liao X, Ruan J, Li X, Chen L, Wang B, et al. Recombinant human erythropoietin preconditioning attenuates liver ischemia reperfusion injury through the phosphatidylinositol-3 kinase/AKT/endothelial nitric oxide synthase pathway. J Surg Res. 2013 Aug;183(2):876–84. doi: 10.1016/j.jss.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 12.Gut N, Piecha G, Aldebssi F, Schaefer S, Bekeredjian R, Schirmacher P, et al. Erythropoietin combined with ACE inhibitor prevents heart remodeling in 5/6 nephrectomized rats independently of blood pressure and kidney function. Am J Nephrol. 2013;38(2):124–35. doi: 10.1159/000353106. [DOI] [PubMed] [Google Scholar]
- 13.Guven Bagla A, Ercan E, Asgun HF, Ickin M, Ercan F, Yavuz O, et al. Experimental acute myocardial infarction in rats: HIF-1alpha, caspase-3, erythropoietin and erythropoietin receptor expression and the cardioprotective effects of two different erythropoietin doses. Acta histochemica. 2013 Sep;115(7):658–68. doi: 10.1016/j.acthis.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Han F, Yu H, Zheng T, Ma X, Zhao X, Li P, et al. Otoprotective effects of erythropoietin on Cdh23erl/erl mice. Neuroscience. 2013 May 1;237:1–6. doi: 10.1016/j.neuroscience.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikarashi N, Toba K, Kato K, Ozawa T, Oda M, Takayama T, et al. Erythropoietin, but Not Asialoerythropoietin or Carbamyl-Erythropoietin, Attenuates Monocrotaline-Induced Pulmonary Hypertension in Rats. Clin Exp Hypertens. 2012 May;4 doi: 10.3109/10641963.2012.681728. [DOI] [PubMed] [Google Scholar]
- 16.Kapoor S. Erythropoietin and its emerging neuroprotective effects. The International journal of neuroscience. 2013 Aug;123(8):591–2. doi: 10.3109/00207454.2013.772610. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Luo B, Han F, Li X, Xiong J, Jiang M, et al. Erythropoietin-derived nonerythropoietic Peptide ameliorates experimental autoimmune neuritis by inflammation suppression and tissue protection. PLoS One. 2014;9(3):e90942. doi: 10.1371/journal.pone.0090942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maiese K, Chong ZZ, Shang YC, Wang S. Erythropoietin: New Directions for the Nervous System. Int J Mol Sci. 2012;13:11102–29. doi: 10.3390/ijms130911102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. Jama. 2005 Jan 5;293(1):90–5. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonassen AK, Sack MN, Mjos OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res. 2001 Dec 7;89(12):1191–8. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- 21.Bennis Y, Sarlon-Bartoli G, Guillet B, Lucas L, Pellegrini L, Velly L, et al. Priming of late endothelial progenitor cells with erythropoietin before transplantation requires the CD131 receptor subunit and enhances their angiogenic potential. J Thromb Haemost. 2012 Sep;10(9):1914–28. doi: 10.1111/j.1538-7836.2012.04835.x. [DOI] [PubMed] [Google Scholar]
- 22.Nasrniya S, Bigdeli MR. Ischemic tolerance induced by normobaric hyperoxia and evaluation of group I and II metabotropic glutamate receptors. Curr Neurovasc Res. 2013 Feb;10(1):21–8. doi: 10.2174/156720213804805981. [DOI] [PubMed] [Google Scholar]
- 23.Pan R, Chen C, Liu WL, Liu KJ. Zinc promotes the death of hypoxic astrocytes by upregulating hypoxia-induced hypoxia-inducible factor-1alpha expression via poly(ADP-ribose) polymerase-1. CNS Neurosci Ther. 2013 Jul;19(7):511–20. doi: 10.1111/cns.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegel CS, McCullough LD. NAD+ and nicotinamide: sex differences in cerebral ischemia. Neuroscience. 2013 May 1;237:223–31. doi: 10.1016/j.neuroscience.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh N, Sharma G, Mishra V, Raghubir R. Hypoxia Inducible Factor-1: Its Potential Role In Cerebral Ischemia. Cell Mol Neurobiol. 2012 Feb;2 doi: 10.1007/s10571-012-9803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su J, Tang Y, Zhou H, Liu L, Dong Q. Tissue kallikrein protects neurons from hypoxia/reoxygenation-induced cell injury through Homer1b/c. Cell Signal. 2012 Nov;24(11):2205–15. doi: 10.1016/j.cellsig.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Yu Y, Ren QG, Zhang ZH, Zhou K, Yu ZY, Luo X, et al. Phospho-Rb mediating cell cycle reentry induces early apoptosis following oxygen-glucose deprivation in rat cortical neurons. Neurochem Res. 2012 Mar;37(3):503–11. doi: 10.1007/s11064-011-0636-6. [DOI] [PubMed] [Google Scholar]
- 28.Hao J, Zhu L, Li F, Liu Q, Zhao X, Liu S, et al. Phospho-mTOR: a novel target in regulation of renal lipid metabolism abnormality of diabetes. Exp Cell Res. 2013 Aug 15;319(14):2296–306. doi: 10.1016/j.yexcr.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013 Jan;19(1):51–60. doi: 10.1016/j.molmed.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan-Warren PJ, Berry M, Ahmed Z, Scott RA, Logan A. Exploiting mTOR signaling: a novel translatable treatment strategy for traumatic optic neuropathy? Invest Ophthalmol Vis Sci. 2013;54(10):6903–16. doi: 10.1167/iovs.13-12803. [DOI] [PubMed] [Google Scholar]
- 31.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991 Aug 23;253(5022):905–9. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 32.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10(11):868–80. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 33.Chong ZZ, Shang YC, Maiese K. Cardiovascular Disease and mTOR Signaling. Trends Cardiovasc Med. 2011 Jul;21(5):151–5. doi: 10.1016/j.tcm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chong ZZ, Shang YC, Wang S, Maiese K. A Critical Kinase Cascade in Neurological Disorders: PI 3-K, Akt, and mTOR. Future Neurol. 2012;7(6):733–48. doi: 10.2217/fnl.12.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maiese K, Chong ZZ, Wang S, Shang YC. Oxidant Stress and Signal Transduction in the Nervous System with the PI 3-K, Akt, and mTOR Cascade. International journal of molecular sciences. 2013;13(11):13830–66. doi: 10.3390/ijms131113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber JD, Gutmann DH. Deconvoluting mTOR biology. Cell Cycle. 2012 Jan 15;11(2):236–48. doi: 10.4161/cc.11.2.19022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curatolo P, Moavero R. mTOR inhibitors as a new therapeutic option for epilepsy. Expert review of neurotherapeutics. 2013 Jun;13(6):627–38. doi: 10.1586/ern.13.49. [DOI] [PubMed] [Google Scholar]
- 38.Uzdensky AB, Demyanenko SV, Bibov MY. Signal transduction in human cutaneous melanoma and target drugs. Curr Cancer Drug Targets. 2013 Oct;13(8):843–66. doi: 10.2174/1568009611313080004. [DOI] [PubMed] [Google Scholar]
- 39.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011 Jan;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, et al. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007 Nov 9;318(5852):977–80. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Lawrence JC, Jr, Sturgill TW, Harris TE. Mammalian target of rapamycin complex 1 (mTORC1) activity is associated with phosphorylation of raptor by mTOR. J Biol Chem. 2009 May 29;284(22):14693–7. doi: 10.1074/jbc.C109.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006 Apr 21;22(2):159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 43.Chen J, Zheng XF, Brown EJ, Schreiber SL. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4947–51. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: hitting the bull's-eye for neurological disorders. Oxid Med Cell Longev. 2010 Nov-Dec;3(6):374–91. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Zhang Q, Wen Q, Zheng Y, Philip L, Jiang H, et al. Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal. 2012 Jan;24(1):17–24. doi: 10.1016/j.cellsig.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 46.Oshiro N, Takahashi R, Yoshino K, Tanimura K, Nakashima A, Eguchi S, et al. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J Biol Chem. 2007 Jul 13;282(28):20329–39. doi: 10.1074/jbc.M702636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Harris TE, Lawrence JC., Jr Regulation of proline-rich Akt substrate of 40 kDa (PRAS40) function by mammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation. J Biol Chem. 2008 Jun 6;283(23):15619–27. doi: 10.1074/jbc.M800723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007 Mar 23;25(6):903–15. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Chong ZZ, Shang YC, Wang S, Maiese K. PRAS40 Is an Integral Regulatory Component of Erythropoietin mTOR Signaling and Cytoprotection. PLoS ONE. 2012;7(9):e45456. doi: 10.1371/journal.pone.0045456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, et al. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem. 2003 Mar 21;278(12):10189–94. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- 51.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007 Mar;9(3):316–23. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Harris TE, Roth RA, Lawrence JC., Jr PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007 Jul 6;282(27):20036–44. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- 53.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009 May 29;137(5):873–86. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003 Apr;11(4):895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 55.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006 Dec;11(6):859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 56.Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007 Aug;25(4):209–26. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- 57.Lenz G, Avruch J. Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. J Biol Chem. 2005 Nov 18;280(46):38121–4. doi: 10.1074/jbc.C500363200. [DOI] [PubMed] [Google Scholar]
- 58.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006 May 12;312(5775):927–30. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 59.Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998 Feb 15;12(4):502–13. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005 Feb 18;307(5712):1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 61.Chen CH, Sarbassov dos D. The mTOR (mammalian target of rapamycin) kinase maintains integrity of mTOR complex 2. J Biol Chem. 2011 Nov 18;286(46):40386–94. doi: 10.1074/jbc.M111.282590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pearce LR, Sommer EM, Sakamoto K, Wullschleger S, Alessi DR. Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem J. 2011 May 15;436(1):169–79. doi: 10.1042/BJ20102103. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008 Dec 15;416(3):375–85. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 64.Hernandez-Negrete I, Carretero-Ortega J, Rosenfeldt H, Hernandez-Garcia R, Calderon-Salinas JV, Reyes-Cruz G, et al. P-Rex1 links mammalian target of rapamycin signaling to Rac activation and cell migration. J Biol Chem. 2007 Aug 10;282(32):23708–15. doi: 10.1074/jbc.M703771200. [DOI] [PubMed] [Google Scholar]
- 65.Sato T, Nakashima A, Guo L, Tamanoi F. Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein. J Biol Chem. 2009 May 8;284(19):12783–91. doi: 10.1074/jbc.M809207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai SL, Tee AR, Short JD, Bergeron JM, Kim J, Shen J, et al. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006 Apr 24;173(2):279–89. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008 Jun;28(12):4104–15. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chong ZZ, Maiese K. Mammalian Target of Rapamycin Signaling in Diabetic Cardiovascular Disease. Cardiovasc Diabetol. 2012 Apr 30;11(1):45. doi: 10.1186/1475-2840-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003 Nov 26;115(5):577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 70.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008 Jan 15;22(2):239–51. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paiva MA, Rutter-Locher Z, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, et al. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011 Jun;300(6):H2123–34. doi: 10.1152/ajpheart.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, et al. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 2010 Oct;59(10):2426–34. doi: 10.2337/db09-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004 Mar 9;101(10):3329–35. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jessen N, Koh HJ, Folmes CD, Wagg C, Fujii N, Lofgren B, et al. Ablation of LKB1 in the heart leads to energy deprivation and impaired cardiac function. Biochim Biophys Acta. 2010 Jul-Aug;1802(7–8):593–600. doi: 10.1016/j.bbadis.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005 Feb;75(3):207–46. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 76.Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010 Mar;45(3):217–34. doi: 10.1016/j.exger.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonapace L, Bornhauser BC, Schmitz M, Cario G, Ziegler U, Niggli FK, et al. Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest. 2010 Apr;120(4):1310–23. doi: 10.1172/JCI39987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maiese K. The Many Facets of Cell Injury: Angiogenesis to Autophagy. Curr Neurovasc Res. 2012 Apr 19;9(2):1–2. doi: 10.2174/156720212800410911. [DOI] [PubMed] [Google Scholar]
- 79.Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert opinion on therapeutic targets. 2012 Dec;16(12):1203–14. doi: 10.1517/14728222.2012.719499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tatton NA. Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson's disease. Exp Neurol. 2000 Nov;166(1):29–43. doi: 10.1006/exnr.2000.7489. [DOI] [PubMed] [Google Scholar]
- 81.Broe M, Shepherd CE, Milward EA, Halliday GM. Relationship between DNA fragmentation, morphological changes and neuronal loss in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol. 2001 Jun;101(6):616–24. doi: 10.1007/s004010000337. [DOI] [PubMed] [Google Scholar]
- 82.Louneva N, Cohen JW, Han LY, Talbot K, Wilson RS, Bennett DA, et al. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer's disease. Am J Pathol. 2008 Nov;173(5):1488–95. doi: 10.2353/ajpath.2008.080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005 Dec;2(5):387–99. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim IK, Lee KJ, Rhee S, Seo SB, Pak JH. Protective effects of peroxiredoxin 6 overexpression on amyloid beta-induced apoptosis in PC12 cells. Free Radic Res. 2013 Oct;47(10):836–46. doi: 10.3109/10715762.2013.833330. [DOI] [PubMed] [Google Scholar]
- 85.Shang Y, Chong Z, Wang S, Maiese K. Tuberous sclerosis protein 2 (TSC2) modulates CCN4 cytoprotection during apoptotic amyloid toxicity in microglia. Curr Neurovasc Res. 2013;10(1):29–38. doi: 10.2174/156720213804806007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012 Mar 3;4(3):187–201. doi: 10.18632/aging.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shang YC, Chong ZZ, Wang S, Maiese K. WNT1 Inducible Signaling Pathway Protein 1 (WISP1) Targets PRAS40 to Govern beta-Amyloid Apoptotic Injury of Microglia. Curr Neurovasc Res. 2012 Aug 6;9(4):239–49. doi: 10.2174/156720212803530618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou TF, Yu JG. Recombinant human erythropoietin attenuates neuronal apoptosis and cognitive defects via JAK2/STAT3 signaling in experimental endotoxemia. J Surg Res. 2013 Jul;183(1):304–12. doi: 10.1016/j.jss.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 89.Dormond O, Madsen JC, Briscoe DM. The effects of mTOR-Akt interactions on anti-apoptotic signaling in vascular endothelial cells. J Biol Chem. 2007 Aug 10;282(32):23679–86. doi: 10.1074/jbc.M700563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO Relies upon Novel Signaling of Wnt1 that Requires Akt1, FoxO3a, GSK-3beta, and beta-Catenin to Foster Vascular Integrity During Experimental Diabetes. Curr Neurovasc Res. 2011 May 1;8(2):103–20. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007 Feb;19(2):263–72. [PMC free article] [PubMed] [Google Scholar]
- 92.Choi KC, Kim SH, Ha JY, Kim ST, Son JH. A novel mTOR activating protein protects dopamine neurons against oxidative stress by repressing autophagy related cell death. J Neurochem. 2010 Jan;112(2):366–76. doi: 10.1111/j.1471-4159.2009.06463.x. [DOI] [PubMed] [Google Scholar]
- 93.Thedieck K, Polak P, Kim ML, Molle KD, Cohen A, Jeno P, et al. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS One. 2007;2(11):e1217. doi: 10.1371/journal.pone.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pastor MD, Garcia-Yebenes I, Fradejas N, Perez-Ortiz JM, Mora-Lee S, Tranque P, et al. mTOR/S6 kinase pathway contributes to astrocyte survival during ischemia. J Biol Chem. 2009 Aug 14;284(33):22067–78. doi: 10.1074/jbc.M109.033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu X, Reiter CE, Antonetti DA, Kimball SR, Jefferson LS, Gardner TW. Insulin promotes rat retinal neuronal cell survival in a p70S6K-dependent manner. J Biol Chem. 2004 Mar 5;279:9167–75. doi: 10.1074/jbc.M312397200. by a. [DOI] [PubMed] [Google Scholar]
- 96.Faghiri Z, Bazan NG. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp Eye Res. 2010 Jun;90(6):718–25. doi: 10.1016/j.exer.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Medeiros C, Frederico MJ, da Luz G, Pauli JR, Silva AS, Pinho RA, et al. Exercise training reduces insulin resistance and upregulates the mTOR/p70S6k pathway in cardiac muscle of diet-induced obesity rats. J Cell Physiol. 2011 Mar;226(3):666–74. doi: 10.1002/jcp.22387. [DOI] [PubMed] [Google Scholar]
- 98.Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008 Apr;19(2):145–55. doi: 10.1016/j.cytogfr.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim J, Jung Y, Sun H, Joseph J, Mishra A, Shiozawa Y, et al. Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem. 2012 Jan;113(1):220–8. doi: 10.1002/jcb.23347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 Govern Pathways of mTOR, Apaf-1, and XIAP in Inflammatory Microglia. Curr Neurovasc Res. 2011 Oct 19;8(4):270–85. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chong ZZ, Li F, Maiese K. Attempted Cell Cycle Induction in Post-Mitotic Neurons Occurs in Early and Late Apoptotic Programs Through Rb, E2F1, and Caspase 3. Curr Neurovasc Res. 2006 Feb;3(1):25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bhaskar K, Miller M, Chludzinski A, Herrup K, Zagorski M, Lamb BT. The PI3K-Akt-mTOR pathway regulates Abeta oligomer induced neuronal cell cycle events. Molecular neurodegeneration. 2009;4:14. doi: 10.1186/1750-1326-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Balduini W, Carloni S, Buonocore G. Autophagy in hypoxia-ischemia induced brain injury. J Matern Fetal Neonatal Med. 2012 Apr;25(Suppl 1):30–4. doi: 10.3109/14767058.2012.663176. [DOI] [PubMed] [Google Scholar]
- 104.Deretic V, Jiang S, Dupont N. Autophagy intersections with conventional and unconventional secretion in tissue development, remodeling and inflammation. Trends Cell Biol. 2012 Jun 5; doi: 10.1016/j.tcb.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009 Apr;20(7):1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin AP, Liu CF, Qin YY, Hong LZ, Xu M, Yang L, et al. Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy. 2010 Aug 16;6(6):738–53. doi: 10.4161/auto.6.6.12573. [DOI] [PubMed] [Google Scholar]
- 107.Canu N, Tufi R, Serafino AL, Amadoro G, Ciotti MT, Calissano P. Role of the autophagic-lysosomal system on low potassium-induced apoptosis in cultured cerebellar granule cells. J Neurochem. 2005 Mar;92(5):1228–42. doi: 10.1111/j.1471-4159.2004.02956.x. [DOI] [PubMed] [Google Scholar]
- 108.Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci. 1999 Sep;14(3):180–98. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- 109.Wang JY, Xia Q, Chu KT, Pan J, Sun LN, Zeng B, et al. Severe global cerebral ischemia-induced programmed necrosis of hippocampal CA1 neurons in rat is prevented by 3-methyladenine: a widely used inhibitor of autophagy. J Neuropathol Exp Neurol. 2011 Apr;70(4):314–22. doi: 10.1097/NEN.0b013e31821352bd. [DOI] [PubMed] [Google Scholar]
- 110.Baba H, Sakurai M, Abe K, Tominaga R. Autophagy-mediated stress response in motor neuron after transient ischemia in rabbits. J Vasc Surg. 2009 Aug;50(2):381–7. doi: 10.1016/j.jvs.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 111.Hu LY, Sun ZG, Wen YM, Cheng GZ, Wang SL, Zhao HB, et al. ATP-mediated protein kinase B Akt/mammalian target of rapamycin mTOR/p70 ribosomal S6 protein p70S6 kinase signaling pathway activation promotes improvement of locomotor function after spinal cord injury in rats. Neuroscience. 2010 Sep 1;169(3):1046–62. doi: 10.1016/j.neuroscience.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 112.Ma T, Hoeffer CA, Capetillo-Zarate E, Yu F, Wong H, Lin MT, et al. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer's disease. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu B, Wen X, Cheng Y. Survival or death: disequilibrating the oncogenic and tumor suppressive autophagy in cancer. Cell death & disease. 2013;4:e892. doi: 10.1038/cddis.2013.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen A, Xiong LJ, Tong Y, Mao M. Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Molecular medicine reports. 2013 Oct;8(4):1011–6. doi: 10.3892/mmr.2013.1628. [DOI] [PubMed] [Google Scholar]
- 115.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Floto RA, Sarkar S, Perlstein EO, Kampmann B, Schreiber SL, Rubinsztein DC. Small molecule enhancers of rapamycin-induced TOR inhibition promote autophagy, reduce toxicity in Huntington's disease models and enhance killing of mycobacteria by macrophages. Autophagy. 2007 Nov-Dec;3(6):620–2. doi: 10.4161/auto.4898. [DOI] [PubMed] [Google Scholar]
- 117.Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J Neurosci. 2009 Oct 28;29(43):13578–88. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kapoor V, Zaharieva MM, Das SN, Berger MR. Erufosine simultaneously induces apoptosis and autophagy by modulating the Akt-mTOR signaling pathway in oral squamous cell carcinoma. Cancer Lett. 2012 Jun 1;319(1):39–48. doi: 10.1016/j.canlet.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 119.Nopparat C, Porter JE, Ebadi M, Govitrapong P. The mechanism for the neuroprotective effect of melatonin against methamphetamine-induced autophagy. J Pineal Res. 2010 Aug 24; doi: 10.1111/j.1600-079X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 120.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005 Sep 23;122(6):927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 121.Carayol N, Vakana E, Sassano A, Kaur S, Goussetis DJ, Glaser H, et al. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci U S A. 2010 Jul 13;107(28):12469–74. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luo S, Rubinsztein DC. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 2010 Feb;17(2):268–77. doi: 10.1038/cdd.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Werneck MB, Hottz E, Bozza PT, Viola JP. Cyclosporin A inhibits colon cancer cell growth independently of the calcineurin pathway. Cell Cycle. 2012 Nov 1;11(21):3997–4008. doi: 10.4161/cc.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu X, Chhipa RR, Nakano I, Dasgupta B. The AMPK Inhibitor Compound C Is a Potent AMPK-Independent Antiglioma Agent. Mol Cancer Ther. 2014 Mar;13(3):596–605. doi: 10.1158/1535-7163.MCT-13-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dello Russo C, Lisi L, Feinstein DL, Navarra P. mTOR kinase, a key player in the regulation of glial functions: Relevance for the therapy of multiple sclerosis. Glia. 2013 Mar;61(3):301–11. doi: 10.1002/glia.22433. [DOI] [PubMed] [Google Scholar]
- 126.Hyrskyluoto A, Reijonen S, Kivinen J, Lindholm D, Korhonen L. GADD34 mediates cytoprotective autophagy in mutant huntingtin expressing cells via the mTOR pathway. Exp Cell Res. 2012 Jan 1;318(1):33–42. doi: 10.1016/j.yexcr.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 127.James MF, Stivison E, Beauchamp R, Han S, Li H, Wallace MR, et al. Regulation of mTOR complex 2 signaling in neurofibromatosis 2-deficient target cell types. Mol Cancer Res. 2012 May;10(5):649–59. doi: 10.1158/1541-7786.MCR-11-0425-T. [DOI] [PubMed] [Google Scholar]
- 128.Sheng B, Liu J, Li GH. Metformin preconditioning protects Daphnia pulex from lethal hypoxic insult involving AMPK, HIF and mTOR signaling. Comp Biochem Physiol B Biochem Mol Biol. 2012 May 4; doi: 10.1016/j.cbpb.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 129.Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, et al. The Interaction between Early Life Epilepsy and Autistic-Like Behavioral Consequences: A Role for the Mammalian Target of Rapamycin (mTOR) Pathway. PLoS ONE. 2012;7(5):e35885. doi: 10.1371/journal.pone.0035885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Walker CL, Walker MJ, Liu NK, Risberg EC, Gao X, Chen J, et al. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS One. 2012;7(1):e30012. doi: 10.1371/journal.pone.0030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.You H, Li T, Zhang J, Lei Q, Tao X, Xie P, et al. Reduction in Ischemic Cerebral Infarction is Mediated through Golgi Phosphoprotein 3 and Akt/mTOR Signaling following Salvianolate Administration. Curr Neurovasc Res. 2014 Mar 7; doi: 10.2174/1567202611666140307124857. [DOI] [PubMed] [Google Scholar]
- 132.Koh PO. Ferulic acid attenuates focal cerebral ischemia-induced decreases in p70S6 kinase and S6 phosphorylation. Neurosci Lett. 2013 Oct 25;555:7–11. doi: 10.1016/j.neulet.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 133.Zare Mehrjerdi F, Aboutaleb N, Habibey R, Ajami M, Soleimani M, Arabian M, et al. Increased phosphorylation of mTOR is involved in remote ischemic preconditioning of hippocampus in mice. Brain Res. 2013 Aug 14;1526:94–101. doi: 10.1016/j.brainres.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 134.Xie R, Wang P, Ji X, Zhao H. Ischemic post-conditioning facilitates brain recovery after stroke by promoting Akt/mTOR activity in nude rats. J Neurochem. 2013 Dec;127(5):723–32. doi: 10.1111/jnc.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ji YF, Zhou L, Xie YJ, Xu SM, Zhu J, Teng P, et al. Upregulation of glutamate transporter GLT-1 by mTOR-Akt-NF-small ka, CyrillicB cascade in astrocytic oxygen-glucose deprivation. Glia. 2013 Dec;61(12):1959–75. doi: 10.1002/glia.22566. [DOI] [PubMed] [Google Scholar]
- 136.Selvamani A, Williams MH, Miranda RC, Sohrabji F. Circulating miRNA profiles provide a biomarker for severity of stroke outcomes associated with age and sex in a rat model. Clin Sci (Lond) 2014 Jan 15; doi: 10.1042/CS20130565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xiong X, Xie R, Zhang H, Gu L, Xie W, Cheng M, et al. PRAS40 plays a pivotal role in protecting against stroke by linking the Akt and mTOR pathways. Neurobiol Dis. 2014 Feb 27; doi: 10.1016/j.nbd.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yan H, Zhang X, Hu W, Ma J, Hou W, Zhang X, et al. Histamine H3 receptors aggravate cerebral ischaemic injury by histamine-independent mechanisms. Nature communications. 2014;5:3334. doi: 10.1038/ncomms4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kulbe JR, Mulcahy Levy JM, Coultrap SJ, Thorburn A, Bayern KU. Excitotoxic glutamate insults block autophagic flux in hippocampal neurons. Brain Res. 2014 Jan 13;1542:12–9. doi: 10.1016/j.brainres.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Urbanek T, Kuczmik W, Basta-Kaim A, Gabryel B. Rapamycin induces of protective autophagy in vascular endothelial cells exposed to oxygen-glucose deprivation. Brain Res. 2014 Mar 17;1553:1–11. doi: 10.1016/j.brainres.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 141.Fletcher L, Evans TM, Watts LT, Jimenez DF, Digicaylioglu M. Rapamycin treatment improves neuron viability in an in vitro model of stroke. PLoS One. 2013;8(7):e68281. doi: 10.1371/journal.pone.0068281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xia DY, Li W, Qian HR, Yao S, Liu JG, Qi XK. Ischemia preconditioning is neuroprotective in a rat cerebral ischemic injury model through autophagy activation and apoptosis inhibition. Braz J Med Biol Res. 2013 Jul;46(7):580–8. doi: 10.1590/1414-431X20133161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Santini E, Heiman M, Greengard P, Valjent E, Fisone G. Inhibition of mTOR signaling in Parkinson's disease prevents L-DOPA-induced dyskinesia. Science signaling. 2009;2(80):ra36. doi: 10.1126/scisignal.2000308. [DOI] [PubMed] [Google Scholar]
- 144.Fokas E, Yoshimura M, Prevo R, Higgins G, Hackl W, Maira SM, et al. NVPBEZ235 and NVP-BGT226, dual phosphatidylinositol 3-kinase/Mammalian target of rapamycin inhibitors, enhance tumor and endothelial cell radiosensitivity. Radiat Oncol. 2012 Mar 27;7(1):48–60. doi: 10.1186/1748-717X-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Treins C, Alliouachene S, Hassouna R, Xie Y, Birnbaum MJ, Pende M. The combined deletion of S6K1 and Akt2 deteriorates glycaemic control in high fat diet. Mol Cell Biol. 2012 Jul 30; doi: 10.1128/MCB.00514-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Malaguarnera R, Sacco A, Morcavallo A, Squatrito S, Migliaccio A, Morrione A, et al. Metformin inhibits androgen-induced IGF-IR upregulation in prostate cancer cells by disrupting membrane initiated androgren signaling. Endocrinology. 2014 Jan 17;:en20131925. doi: 10.1210/en.2013-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009 Sep 4;5(3):279–89. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gazitt Y, Kolaparthi V, Moncada K, Thomas C, Freeman J. Targeted therapy of human osteosarcoma with 17AAG or rapamycin: characterization of induced apoptosis and inhibition of mTOR and Akt/MAPK/Wnt pathways. Int J Oncol. 2009 Feb;34(2):551–61. [PubMed] [Google Scholar]
- 149.Sun J, Jin T. Both Wnt and mTOR signaling pathways are involved in insulin-stimulated proto-oncogene expression in intestinal cells. Cell Signal. 2008 Jan;20(1):219–29. doi: 10.1016/j.cellsig.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 150.Vigneron F, Dos Santos P, Lemoine S, Bonnet M, Tariosse L, Couffinhal T, et al. GSK-3beta at the crossroads in the signalling of heart preconditioning: implication of mTOR and Wnt pathways. Cardiovasc Res. 2011 Apr 1;90(1):49–56. doi: 10.1093/cvr/cvr002. [DOI] [PubMed] [Google Scholar]
- 151.Wang S, Chong ZZ, Shang YC, Maiese K. Wnt1 inducible signaling pathway protein 1 (WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL. Curr Neurovasc Res. 2012 Feb;9(1):20–31. doi: 10.2174/156720212799297137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 (CCN4) autoregulates its expression and nuclear trafficking of beta-catenin during oxidant stress with limited effects upon neuronal autophagy. Curr Neurovasc Res. 2012 Apr 4;9(2):89–99. doi: 10.2174/156720212800410858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert opinion on therapeutic targets. 2012 Feb;16(2):167–78. doi: 10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guo W, Qian L, Zhang J, Zhang W, Morrison A, Hayes P, et al. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res. 2011 Nov;89(11):1723–36. doi: 10.1002/jnr.22725. [DOI] [PubMed] [Google Scholar]
- 155.Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011 Nov;121(11):4477–90. doi: 10.1172/JCI46243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Leger B, Cartoni R, Praz M, Lamon S, Deriaz O, Crettenand A, et al. Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol. 2006 Nov 1;576(Pt 3):923–33. doi: 10.1113/jphysiol.2006.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008 May;14(5):219–27. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maiese K, Chong ZZ, Shang YC, Hou J. A “FOXO” in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009 May;29(3):395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Melnik BC, Zouboulis CC. Potential role of FoxO1 and mTORC1 in the pathogenesis of Western diet-induced acne. Experimental dermatology. 2013 May;22(5):311–5. doi: 10.1111/exd.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lakhani R, Vogel KR, Till A, Liu J, Burnett SF, Gibson KM, et al. Defects in GABA metabolism affect selective autophagy pathways and are alleviated by mTOR inhibition. EMBO molecular medicine. 2014 Feb 27; doi: 10.1002/emmm.201303356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maiese K. Therapeutic targets for cancer: current concepts with PI 3-K, Akt, & mTOR. The Indian journal of medical research. 2013 Feb;137(2):243–6. [PMC free article] [PubMed] [Google Scholar]
- 162.Roulin D, Cerantola Y, Dormond-Meuwly A, Demartines N, Dormond O. Targeting mTORC2 inhibits colon cancer cell proliferation in vitro and tumor formation in vivo. Mol Cancer. 2010;9:57. doi: 10.1186/1476-4598-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zou ZQ, Zhang LN, Wang F, Bellenger J, Shen YZ, Zhang XH. The novel dual PI3K/mTOR inhibitor GDC-0941 synergizes with the MEK inhibitor U0126 in non-small cell lung cancer cells. Mol Med Report. 2012 Feb;5(2):503–8. doi: 10.3892/mmr.2011.682. [DOI] [PubMed] [Google Scholar]
- 164.Melnik BC, John SM, Carrera-Bastos P, Cordain L. The impact of cow's milk-mediated mTORC1-signaling in the initiation and progression of prostate cancer. Nutr Metab (Lond) 2012;9(1):74. doi: 10.1186/1743-7075-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Karlsson E, Waltersson MA, Bostner J, Perez-Tenorio G, Olsson B, Hallbeck AL, et al. High-resolution genomic analysis of the 11q13 amplicon in breast cancers identifies synergy with 8p12 amplification, involving the mTOR targets S6K2 and 4EBP1. Genes Chromosomes Cancer. 2011 Oct;50(10):775–87. doi: 10.1002/gcc.20900. [DOI] [PubMed] [Google Scholar]
- 166.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011 May 1;71(9):3246–56. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Maiese K. Targeting Conserved Pathways: Mitochondrial Dysfunction and Beyond. Curr Neurovasc Res. 2012 Dec 13; doi: 10.2174/156720213804805954. [DOI] [PubMed] [Google Scholar]
- 168.Wang S, Wu J, Nie SD, Bereczki E, Pei JJ. Dysregulated mTOR-dependent signaling in neurodegeneration or carcinogenesis: implication for Alzheimer's disease and brain tumors. J Alzheimers Dis. 2013;37(3):495–505. doi: 10.3233/JAD-130641. [DOI] [PubMed] [Google Scholar]