Abstract
Enhancing the engraftment of hematopoietic stem cells (HSC) is especially important when times to engraftment are prolonged due either to limiting numbers of HSC in the donor graft or to intrinsic slower engrafting time of the tissue sources of HSC. Both inhibition of Dipeptidylpeptidase (DPP) 4/CD26 and treatment of cells with 16,16 dimethyl prostaglandin E2 (dmPGE2) have been shown to enhance hematopoietic stem cell engraftment in murine transplantation models and have been evaluated in clinical settings for their influence on engraftment of cord blood cells, a tissue source of HSC known to manifest an extended time to engraftment of donor cells compared to that of bone marrow (BM) and mobilized peripheral blood for hematopoietic cell transplantation (HCT). Herein, we present new experimental data, using a CD45+ head-to head congenic model of donor mouse BM cells for engraftment of lethally-irradiated mice, demonstrating that similar levels of enhanced engraftment are detected by pulsing donor BM cells with Diprotin A, a DPP4 inhibitor, or with dmPGE2 prior to infusion, or by pretreating recipient mice with sitagliptin, also a DPP4 inhibitor, by oral gavage. Moreover, the combined effects of pretreating the donor BM cells with dmPGE2 in context of pretreating the recipient mice with sitagliptin after administration of a lethal dose of radiation resulted in significantly enhanced competitively repopulating HCT compared to either treatment alone. This information is highly relevant to the goal of enhancing engraftment in human clinical HCT.
Keywords: Hematopoietic Stem Cells, Engraftment, Bone Marrow, Cord Blood, Prostaglandin E2, Dipeptidylpeptidase 4
INTRODUCTION
Hematopoietic cell transplantation (HCT), first pioneered using bone marrow (BM) in the late 1950s and 1960s and later with mobilized peripheral blood stem cells (PBSC) in the 1980s, has been a lifesaving curative procedure to treat patients with malignant and non-malignant hematologic disorders and inborn errors of metabolism [1,2]. Successful HCT requires rigorous human leukocyte antigen (HLA)-matching of donors and recipients, but not all patients in need of an HCT have adequately HLA-matched allogeneic donors available at the precise time the cells are needed for transplantation. Since the late 1980’s, human cord blood (CB) has served as an alternative source of hematopoietic stem and progenitor cells (HSPC) for HCT in over 30,000 transplants [3] since the initial laboratory [4] and clinical [5] studies identified CB as a source of transplantable HSC.
As a source of HSPC, CB has a number of significant advantages over BM or PBSC for HCT [6]. Cryopreserved and previously HLA-typed cells are readily and quickly availability in CB banks. CB has been stored for over 20 years with efficient recovery of HSPC [7], providing donor cells for patients who require an HCT but for whom a suitably HLA-matched donor cannot be obtained through BM registries quickly enough or at all. An additional advantage of CB is lowered graft vs. host disease (GVHD) compared to BM donor grafts, which has allowed the clinical use of more HLA-disparate grafts [6]. One disadvantage of CB HSPC compared to BM or PBSC however, is an inherent functional difference that results in a slower time to neutrophil, platelet and immune cell recovery, a phenomenon noted for HCT in both pediatric and adult recipients, regardless of whether the recipients receive a single or double CB HCT [3,6], which translates into longer hospital stays post-transplant. In addition, single CB HCT in adults has been associated with an increased rate of graft rejection compared to that of BM.
Various preclinical and clinical efforts have been evaluated with the goal to enhance the time to engraftment of CB cells by either increasing the homing capabilities of the donor HSC, or by increasing numbers of HSPC through ex vivo expansion [3,6]. Recently, new preclinical and clinical studies have shown that enhanced hematopoietic stem cell (HSC) engraftment can be obtained by inhibition of Dipeptidylpeptidase (DPP) 4 or pulse exposure of cells to prostaglandin E2 (PGE2) treatment. These novel strategies to enhance HSPC engraftment are the focus of this present report.
DPP4 is found on the cell surface as CD26, and within cells [8,9]. Short-term pretreatment of relatively unseparated donor mouse BM or human CB CD34+ cells with Diprotin A, a DPP4 inhibitor, results in enhanced engraftment of these cells respectively in lethally-irradiated mouse BM recipients in both competitive and non-competitive HSC assays [10], and in sublethally-irradiated immune-deficient mice [11,12]. Enhanced engraftment of untreated mouse BM cells has also been shown when recipient mice are treated sitagliptin, an orally active DPP4 inhibitor originally approved for and used in the treatment of type II diabetes [13]. Sitagliptin has recently been used in a clinical setting to pre-treat adult recipients with end-stage leukemia and lymphoma prior to single unit CB HCT [14,15]. PGE2 is the primary eicosanoid produced by oxidation of arachidonic cascade and is the most abundant eicosanoid [16,17]. We have previously shown that PGE2 has pleiotropic effects on hematopoiesis, affecting HSC and HPC function. PGE2 inhibits myelopoiesis in vitro and in vivo [18–21] but promotes erythroid and multipotential colony formation in vitro [22,23]. Short-term ex vivo exposure of mouse or human bone marrow cells to PGE2 stimulated proliferation, cycling and differentiation of quiescent cells leading to an increase in cycling HPC, suggesting that PGE enhances HSC function [24,25]. Recently, the stimulatory effects of dmPGE2 on long-term repopulating HSC were demonstrated in zebrafish and mice [26,27], leading to assessment of its effects in the clinical setting of double CB HCT [28].
While the effects of DPP4 inhibition or exposure to dmPGE2 have been evaluated independently, the comparative or combination effects of these compounds have not previously been reported. Herein, we report the effects of treating donor cells ex-vivo with Diprotin A or dmPGE2, prior to engraftment in lethally-irradiated mice or after exposing recipient mice to sitagliptin, using a congenic mouse model of BM HCT.
METHODS AND MATERIALS
Animals
C57Bl/6 (CD45.2+) mice, 6–12 weeks old, were purchased from Jackson Laboratories (Bar Harbor, Maines). B6.SJL-PtrcAPep3B/BoyJ (BOYJ) (CD45.1) mice, 6–12 weeks old, were either purchased from Jackson Laboratories or bred in our NIDDK Center of Excellence in Molecular Hematology-and NCI-Designated Indiana University Simon Cancer Center-sponsored animal core facility. C57Bl/6 X BOYJ-F1 (CD45.1/CD45.2) mice were bred in-house. The Animal Care and Use Committee of IUSM approved all protocols.
Compounds
Diprotin A was purchased from Peptides International (Louisville, KY). Sitagliptin was purchased from Merck (Rahway, NJ). 16,16 dimethyl Prostaglandin E2 (dmPGE2) was purchased from Cayman Chemicals (Ann Arbor, MI). dmPGE2 was reduced to dryness under N2 on ice and reconstituted in 100% ethanol and stored at −20 °C prior to use.
Treatment of cell grafts ex vivo
Treatment of donor cells or recipient mice and engrafting studies were generally done as previously reported [10,13,27] and as specifically noted in the Figure Legends. Briefly, for Figure 3, C57Bl/6 (CD45.2+) BM cells were treated with Diprotin A (5mM/106 cells) or with control diluent for 1 hour at room temperature prior to washing the cells 2X and admixing with untreated B6.BoyJ competitor (CD45.1+) donor cells at a 1:1 ratio. Similarly, C57Bl/6 BM cells were incubated with dmPGE2 as previously described [27], (1μM/106 cells for 1 hour at 4 °C with vortexing of cells every 30 minutes) and washed 2X prior to admixing with CD45.1+ competitor donor cells at a 1:1 ratio. For combination treatment, BM cells were treated first with dmPGE2 and then sequentially with Diprotin A. In all cases, two hundred fifty thousand total treated or untreated BM cells were injected i.v., into lethally-irradiated (950cGy gamma radiation) dual CD45.2+/CD45.1+ (F1) recipient mice. In some cases, cells were infused 24 hours later into F1 mice that were orally gavaged with control diluent or with 200 μg sitagliptin/mouse 4 hours after radiation. Vehicle-treated cells were pulsed with the equivalent concentration of ethanol.
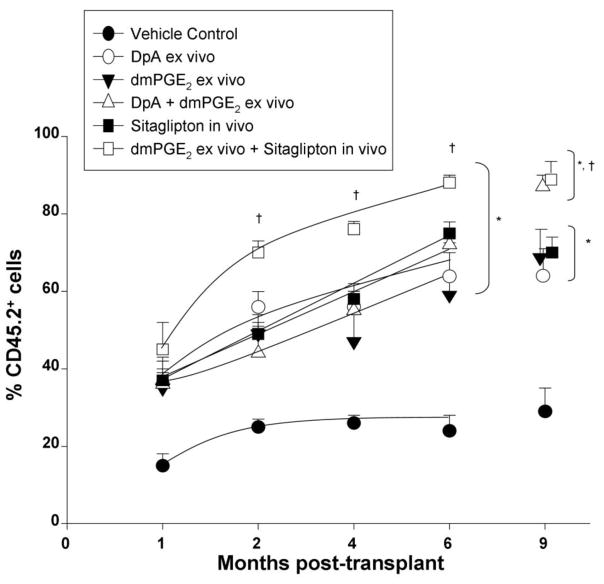
Figure 3.
Comparative donor mouse BM cell engraftment into lethally-irradiated mice in the setting of competition with congenic competitor cells. Unseparated donor C57Bl/6 (CD45.2+) cells were treated with control diluent or with either Diprotin A (5mM/106 cells) for 1 hour at room temperature), dmPGE2 (1μM/106 cells for 1 hour at 4°C), or with Diprotin A and then with dmPGE2, prior to washing cells 2X and infusing cells at a 1:1 ratio with B6.BoyJ (CD45.1+) competitor cells (2.5×105 cells each) 24 hours later into (dual CD45.2+/CD45.1+ F1) recipient mice that had been lethally irradiated with 950cGy gamma radiation) and 4 hours after radiation orally gavaged one time with either control diluent or 200 μg sitagliptin. Results are shown as mean ± 1SEM for 5 mice in group each assayed individually. * = all points p<0.05 versus vehicle control; † = p<0.05 versus all other treatment groups; as determined by ANOVA with Tukey post-hoc test.
Competitive Transplantation
Competitive transplantation was performed on a head-to head basis as previously described [10,13,27]. Five mice per treatment group were used in all experiments to assess the mean percent chimerism at various times after HCT. The proportion of CD45.1, CD45.2, and CD45.1/CD45.2 cells in peripheral blood was determined monthly by flow cytometry. CD45.1-PE and CD45.2-FITC were purchased from BD Biosciences, San Jose, Ca.
Statistics
Students t test was used to determine statistical differences between two treatment groups within an experiment for Figures 1 and 2. For Figure 3, statistical comparisons on monthly chimerism in competitive assays were done by ANOVA with Tukey post-hoc test in GraphPad InStat, GraphPad Software Inc., San Diego, CA.
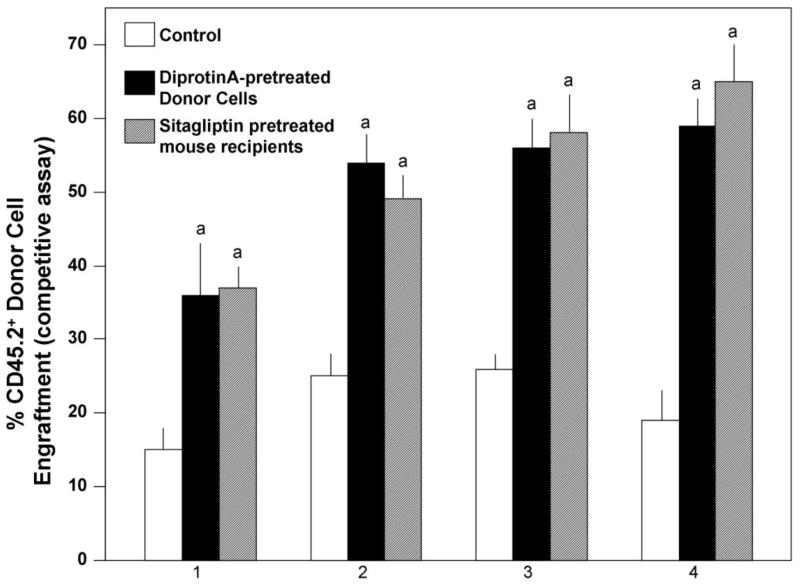
Figure 1.
Comparison of DPP4 inhibitor-treated donor mouse BM cells and DPP4 inhibitor treated recipient mice on engraftment in the setting of competitive repopulation using congenic mice. Mouse C57Bl/6 (CD45.2+) BM cells were treated with Diprotin A (5mM/106 cells) or with control diluent for 1 hour at room temperature prior to washing the cells 2X and mixing the donor cells at a 1:1 ratio with untreated B6.BoyJ competitor (CD45.2+) BM cells prior to infusing the donor and competitor cells i.v. at 2×105 cells each into lethally-irradiated (950cGy gamma radiation) dual CD45.2+/CD45.1+ (F1) recipient mice. Cells were infused 24 hours later into F1 mice that were orally gavaged with control diluent or with 200 μg sitagliptin/mouse 4 hours after radiation. Results are shown as mean ±1SEM for 5 mice per group each assayed individually. a, significantly different from controls (control treated donor cells into non-sitagliptin treated recipients), p<0.001.
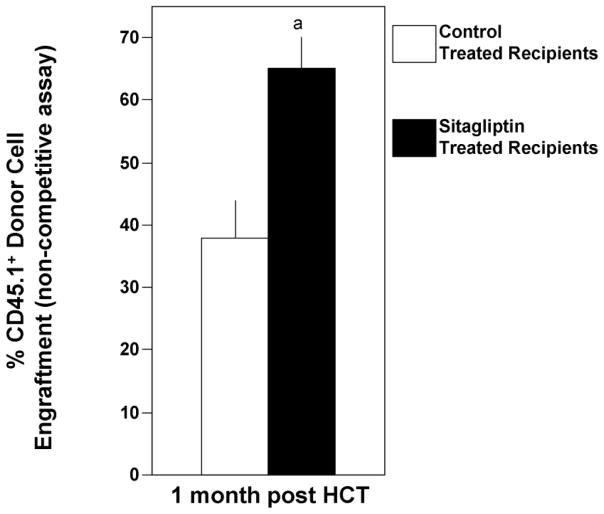
Figure 2.
Influence of oral treatment of lethally-irradiated mouse recipients on engraftment of mouse BM cells in a short-term non-competitive assay. One hundred thousand B6.BoyJ (CD45.1+) donor cells were injected i.v. into C57Bl/6 (CD45.2+) recipient mice 24 hours after 950cGy radiation. The recipient mice were treated 4 hours after radiation with either oral administered control diluent or 200 μg sitagliptin. Results are shown as mean percent engraftment ±1SEM for 5 mice/group at one month post donor BM cell infusion with all mice surviving. a, significantly different from control treated recipients, p<0.001.
RESULTS
Comparative Effects of Diprotin A-Pretreated Donor Cells or Sitagliptin-Treated Recipients on Engraftment of Mouse BM Cells in a Competitive Assay
We have previously reported that Diprotin A-treated mouse donor cell infusion into non-sitagliptin-treated irradiated recipients, and non-treated donor mouse BM infusion into sitagliptin-treated irradiated recipient mice both result in enhanced HSPC engraftment in a competitive stem cell engrafting model [10,13]. However, comparison of the two treatment modalities “head to head” has not previously been reported. We therefore transplanted 2×105 C57Bl/6 (CD45.2+) BM cells treated with Diprotin A (5mM/106 cells) for 1 hour at room temperature admixed with untreated B6.BoyJ competitor (CD45.2+) BM cells donor cells at a 1:1 ratio prior to i.v. injection into untreated lethally-irradiated (950cGy gamma radiation) dual CD45.2+/CD45.1+ (F1) recipient mice, or 2×105 C57Bl/6 (CD45.2+) BM cells treated with control diluent admixed with untreated B6.BoyJ competitor (CD45.2+) BM cells donor cells at a 1:1 ratio into F1 mice that were orally gavaged with control diluent or 200μg sitagliptin/mouse 4 hours after radiation (Figure 1). Pre-treatment of donor cells with Diprotin A or pre-treatment of recipients with sitagliptin significantly enhanced engraftment, but there was no statistically significant difference (p>0.05) between engraftment of Diprotin A pretreated donor cells into control recipients and control treated donor cells into sitagliptin-treated recipients.
Influence of Sitagliptin Treated Recipient Mice on Short-Term Engraftment of BM in a Non-Competitive Assay
Sitagliptin treatment of recipient mice has been shown to enhance engraftment of donor mouse HSC in a competitive assay [13], however, competitive transplant assays do not represent the scenario of limiting HSPC number. To mimic the condition of low HSC number, we chose a low donor cell number (105 cells) that bordered on being just sufficient to repopulate hematopoiesis and allow survival of the irradiated mice. As shown in Figure 2 for short-term engraftment (1 month), survival and donor BM cell engraftment occurred in both test groups, but significantly higher engraftment was observed when the cells were injected into lethally-irradiated mice that had been treated with sitagliptin 4 hours after the dose of radiation, and 16 hours prior to the donor cell infusion.
Effects of Treating Donor Cells with Diprotin A or dmPGE2, Alone and in Combination, on Engraftment, and Effects of Infusing dmPGE2 treated Donor Cells into Sitagliptin Treated Recipients
In order to determine how pretreatment of donor cells with Diprotin A compared to cells treated with dmPGE2, donor BM cells were either pulse exposed to Diprotin A, dmPGE2 or both, prior to being washed and infused i.v. into control recipient mice not pre-exposed to sitagliptin in a head –to –head competitive transplant assay in F1 hybrid mice (Figure 3). Treatment of donor cells with either Diprotin A or dmPGE2 significantly enhanced engraftment at all time points compared to control treated donor cells. Combined treatment of donor cells with both Diprotin A and dmPGE2 significantly enhanced engraftment at all time points compared to control treated donor cells but did not result in enhanced engraftment compared to cells pretreated with either Diprotin A or dmPGE2 alone. The level of enhanced engraftment by Diprotin A or dmPGE2 alone or in combination was equivalent to that seen when control treated cells were infused into sitagliptin pretreated recipients (Figure 3). Pulse treatment of donor cells with dmPGE2 and infusion of these cells into sitagliptin-pretreated recipients resulted in the highest levels of engraftment at months 2, 4, 6 and 9 with statistically significant higher chimerism noted between combined ex vivo dmPGE2 and in vivo sitagliptin treatment compared to dmPGE2 or sitagliptin alone.
DISCUSSION
Our results confirm the enhanced engraftment of mouse BM cells by pretreatment with either Diprotin A [10] or dmPGE2 [26,27] ex vivo, and for recipient mice treated with sitagliptin in vivo [13]. In addition, we demonstrate under conditions of head-to-head comparison in our experimental transplant system, that each treatment modality results in approximately equal enhanced engrafting capacity, and that pretreating the donor cells with a combination of Diprotin A and dmPGE2 ex vivo does not further enhance the engrafting capability of the donor mouse BM cells beyond that of pretreating with either agent alone. This lack of enhanced engraftment capacity with combined pre-treatment of donor cells with Diprotin A and dmPGE2 may reflect the fact that both treatments are known to enhance the homing capabilities of donor HSC to the BM of the recipient mice through effects on the CXCR4/SDF-1 axis [10,27]. It is of course possible that further modification to the methodology we used may increase the effectiveness of each procedure alone, or the combined effects of these procedures. However, most importantly, we found that pretreating donor cells with dmPGE2 ex vivo and injecting these cells into sitagliptin treated recipients results in the highest levels of HSPC engraftment, suggesting that in this treatment modality, in vivo inhibition of DPP4 may provide additional benefit outside the CXCR4/SDF-1 axis, perhaps by preventing N-terminal degradation of hematopoietic cytokines by DPP4 and preserving their biological activity, as we recently described [13].
In our studies where donor mouse BM cells were pulsed with Diprotin A and infused into mice in a setting of competitive HSC repopulation, it is clear that the Diprotin A treated cells had an engrafting advantage. However, in such a competitive HSC repopulating situation it is perhaps less clear why the C57Bl/6 donor cell population, but not the B6.BoyJ competitors, would show enhancement when the recipients, but not the donor cells were treated to decrease DPP4 enzymatic activity. The most likely explanation for this outcome is that C57Bl/6 BM cells are better competitors than B6.BoyJ BM cells, a finding previously reported by others [29] and that the C57Bl/6 BM cells having a competitive advantage anyway are more responsive to the effects of in vivo DPP4 inhibition by sitagliptin. In fact, giving donor CB cells an advantage for engraftment in context of clinical HCT by first pretreating these cells with dmPGE2 might allow them to be more potent engrafting cells when placed into an in vivo environment in which DPP4 enzymatic activity was decreased by pretreating the recipient with sitagliptin, perhaps mimicking the situation of the most competitive cells benefiting from a reduced DPP4 enzymatic environment in vivo.
Finding means to enhance the engrafting capability of limiting numbers of donor cells for HCT is of high practical importance. This is especially so for human CB HCT, and for human HCT when numbers of BM cells are limiting, for example in cases when BM is collected from a young child for use in an older higher weight sibling. Hence, our finding in mice of the combination treatment modality of short-term ex vivo exposure to dmPGE2 ex vivo and their administration to sitagliptin-treated recipient mice is of interest, since clinical trials using dmPGE2 treated cells in context of a double CB HCT [28], and sitagliptin-treated recipients in context of a single CB HCT [14] have been separately reported. The studies of sitagliptin treated recipients with leukemia and lymphoma receiving a single CB unit HCT were found to be safe and showed some modest improvement in time to neutrophil engraftment compared to reported comparable data taking into consideration the disease status of recipients, the HLA-disparity between donor CB cells and recipients, and the numbers of donor CB cells transplanted [14]. However, it was not clear that the improvement in time to engraftment was statistically significant. It was learned in this study that the administration of sitagliptin to the recipients (1X/day for 4 days) was suboptimal with regards to decreasing the enzymatic activity of DPP4 compared to that obtained in reports of sitagliptin treatment of normal healthy volunteers [14,15]. This discrepancy in amount of reduction in DPP4 enzymatic activity noted in our study in recipients with leukemia and the intensive conditioning regimen used compared to that of normal healthy volunteers may reflect the possibility that the disease status or conditioning of the patients prior to CB HCT elicited release of high levels of DPP4 that were not able to be effectively down modulated long enough by giving the patients sitagliptin 1X/day. Current studies are addressing this issue by giving the conditioned patients sitagliptin twice a day for 4 days. The clinical report for dmPGE2-treated donor CB cell engraftment in context of a double CB HCT demonstrated a significant decrease in time to neutrophil engraftment for the dmPGE2-treated CB unit [28]. This encouraging decrease in time to engraftment, however, will need to be substantially enhanced, and eventually will have to be shown in context of a single CB HCT to have a major impact in reducing the costs of CB HCT. Ultimately, to reduce the overall costs of CB HCT, simple procedures to accelerate time to neutrophil, platelet, and immune cell recovery will have to be adapted, and the use of dmPGE2-treated donor cells engrafting sitagliptin-treated recipients may be one way to accomplish this relevant end-point.
CONCLUSIONS
In summary, our studies demonstrated that pre-treating donor mouse BM cells with dmPGE2 and infusing them into sitagliptin treated irradiated recipient mice allowed for enhanced engraftment of donor cells compared to that shown for each treatment alone. Thus, it seems reasonable to propose that one might be able to more effectively accelerate time to donor cell engraftment, especially in the context of single CB HCT, if the CB cells were first exposed to pre-treatment with dmPGE2 and these cells then infused into conditioned patients treated with sitagliptin.
Acknowledgments
These studies were supported by NIH grants R01 HL112669, R01 HL67384, R01 HL056416, P01 DK090948 to HEB, and R01 HL096305 to LMP. Flow cytometry was performed in the Flow Cytometry Resource Facility of the Indiana University Simon Cancer Center (NCI P30 CA082709). Additional core support was provided by a Center of Excellence in Hematology grant P01 DK090948 to HEB. We thank Giao Hangoc, DVM for his help in setting up the experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas ED. A history of allogeneic hematopoietic cell transplantation. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. 4. Blackwell Publishing Co. Ltd; United Kingdom: 2009. pp. 3–7. [Google Scholar]
- 2.Armitage JO. The history of autologous hematopoietic cell transplantation. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. 4. Blackwell Publishing Co. Ltd; United Kingdom: 2009. pp. 8–14. [Google Scholar]
- 3.Broxmeyer HE, Farag SS, Rocha V. Cord Blood Hematopoietic Cell Transplantation. In: Appelbaum FR, Forman Sj, Negrin RS, Antin JH, editors. Thomas’ Hematopoietic Cell Transplantation. 5. Wiley-Blackwell; Oxford, England: 2013. In Press. [Google Scholar]
- 4.Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 6.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122:491–498. doi: 10.1182/blood-2013-02-453175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broxmeyer HE, Lee MR, Hangoc G, et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood. 2011;117:4773–4777. doi: 10.1182/blood-2011-01-330514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ou X, O’Leary HA, Broxmeyer HE. Implications of DPP4 modification of proteins that regulate stem/progenitor and more mature cell types. Blood. 2013;122:161–169. doi: 10.1182/blood-2013-02-487470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Leary H, Ou X, Broxmeyer HE. The role of dipeptidyl peptidase 4 in hematopoiesis and transplantation. Curr Opin Hematol. 2013;20:314–319. doi: 10.1097/MOH.0b013e32836125ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christopherson KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 11.Campbell TB, Hangoc G, Liu Y, et al. Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice. Stem Cells Dev. 2007;16:347–354. doi: 10.1089/scd.2007.9995. [DOI] [PubMed] [Google Scholar]
- 12.Christopherson KW, Paganessi LA, Napier S, Porecha NK. CD26 inhibition on CD34+ or lineage− human umbilical cord blood donor hematopoietic stem cells/hematopoietic progenitor cells improves long-term engraftment into NOD/SCID/Beta2null immunodeficient mice. Stem Cells Dev. 2007;16:355–360. doi: 10.1089/scd.2007.9996. [DOI] [PubMed] [Google Scholar]
- 13.Broxmeyer HE, Hoggatt J, O’Leary HA, et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med. 2012;18:1786–1796. doi: 10.1038/nm.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farag SS, Srivastava S, Messina-Graham S, et al. In vivo DPP-4 inhibition to enhance engraftment of single-unit cord blood transplants in adults with hematological malignancies. Stem Cells Dev. 2013;22:1007–1015. doi: 10.1089/scd.2012.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velez de MN, Strother RM, Farag SS, et al. Modelling the Sitagliptin Effect on Dipeptidyl Peptidase-4 activity in adults with haematological malignancies after umbilical cord blood haematopoietic cell transplantation. Clin Pharmacokinet. 2013 Oct 19; doi: 10.1007/s40262-013-0109-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami M, Kudo I. Prostaglandin E synthase: a novel drug target for inflammation and cancer. Curr Pharm Des. 2006;12:943–954. doi: 10.2174/138161206776055912. [DOI] [PubMed] [Google Scholar]
- 17.Serhan CN, Levy B. Novel pathways and endogenous mediators in anti-inflammation and resolution. Chem Immunol Allergy. 2003;83:115–145. doi: 10.1159/000071558. [DOI] [PubMed] [Google Scholar]
- 18.Kurland JI, Broxmeyer HE, Pelus LM, et al. Role for monocyte-macrophage-derived colony-stimulating factor and prostaglandin E in the positive and negative feedback control of myeloid stem cell proliferation. Blood. 1978;52:388–407. [PubMed] [Google Scholar]
- 19.Pelus LM, Broxmeyer HE, Kurland JI, Moore MA. Regulation of macrophage and granulocyte proliferation. Specificities of prostaglandin E and lactoferrin. J Exp Med. 1979;150:277–292. doi: 10.1084/jem.150.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelus LM, Broxmeyer HE, Moore MA. Regulation of human myelopoiesis by prostaglandin E and lactoferrin. Cell Tissue Kinet. 1981;14:515–526. doi: 10.1111/j.1365-2184.1981.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 21.Gentile P, Byer D, Pelus LM. In vivo modulation of murine myelopoiesis following intravenous administration of prostaglandin E2. Blood. 1983;62:1100–1107. [PubMed] [Google Scholar]
- 22.Lu L, Pelus LM, Broxmeyer HE. Modulation of the expression of HLA-DR (Ia) antigens and the proliferation of human erythroid (BFU-E) and multipotential (CFU-GEMM) progenitor cells by prostaglandin E. Exp Hematol. 1984;12:741–748. [PubMed] [Google Scholar]
- 23.Lu L, Pelus LM, Piacibello W, et al. Prostaglandin E acts at two levels to enhance colony formation in vitro by erythroid (BFU-E) progenitor cells. Exp Hematol. 1987;15:765–771. [PubMed] [Google Scholar]
- 24.Pelus LM. Association between colony forming units-granulocyte macrophage expression of Ia-like (HLA-DR) antigen and control of granulocyte and macrophage production. A new role for prostaglandin E. J Clin Invest. 1982;70:568–578. doi: 10.1172/JCI110649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelus LM. CFU-GM expression of Ia-like, HLA-DR, antigen: An association with the humoral control of human granulocyte and macrophage production. Exp Hematol. 1982;10:219–231. [Google Scholar]
- 26.North TE, Goessling W, Walkley CR, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cutler C, Multani P, Robbins D, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122:3074–3081. doi: 10.1182/blood-2013-05-503177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waterstrat A, Liang Y, Swiderski CF, et al. Congenic interval of CD45/Ly-5 congenic mice contains multiple genes that may influence hematopoietic stem cell engraftment. Blood. 2010;115:408–417. doi: 10.1182/blood-2008-03-143370. [DOI] [PMC free article] [PubMed] [Google Scholar]