Abstract
Background
Measurement of aerodigestive tract length is an important determinant for accurate placement of esophageal probes and gavage tubes at the desired location. The relationship of esophageal body, upper esophageal sphincter (UES) and lower esophageal sphincter (LES) lengths with somatic growth in neonates is not well understood.
Objectives
Our objectives were to (1) evaluate a relationship between segmental esophageal lengths and somatic growth parameters and (2) ascertain the relationship between segmental esophageal lengths and gestational age (GA) and postmenstrual age (PMA) in preterm and full-term born human neonates.
Design/Methods
One hundred esophageal manometry studies were performed in 75 infants (30–60 weeks PMA) and the high-pressure zones of LES and UES identified. The distance from nares to LES and from nares to UES, esophageal body length, length of UES and LES derived from the manometry studies were correlated with somatic growth parameters. Growth rate of different esophageal segments was also determined in 26 subjects that underwent longitudinal studies. Analysis of variance and linear regression analysis were performed.
Results
Seventy-five neonates of 23.0–40.6 weeks gestational age (0.6–4.4 kg) were studied at 29.1–58.6 weeks PMA (1.0–6.4 kg). Significant correlation (P < 0.001) of PMA and physical growth parameters with the growth of nares-LES (R2 = 0.8), esophageal body length (R2 = 0.6) and nares-UES (R2 = 0.4) were noted. Nares-to-LES length increased at a rate of 0.25 cm/wk PMA during 33.0–36.0 weeks of age.
Conclusions
In vivo esophageal segmental lengths correlated strongly with somatic growth parameters and PMA in neonates. We speculate that this approach has many practical applications with the use of esophageal probes and catheters.
Keywords: Esophagus, Neonate, Growth, Upper esophageal, sphincter, Lower esophageal sphincter
BACKGROUND
Measurement of accurate esophageal length in neonates is an important determinant for various clinical procedures such as placement of pharyngeal or esophageal probes at the desired location (pH or impedance probes, gavage tubes). Current methods to locate the probe include radiologically determined gastroesophageal junction, acid-base interphase and manometry (1—3). However, each method has limitations and needs additional expense and expertise. Fluoroscopic and en doscopic localization techniques have not proven sufficiently accurate and have been abandoned (3,4). Miscalculation of distance can cause incorrect placement of probes following unreliable methods and leads to unreliable interpretation and possible incorrect management strategies (3). Pehl et al. (5) suggested that esophageal manometry is necessary to ensure a valid pH probe placement.
Several investigators correlated the length from nares to lower esophageal sphincter (LES) with physical growth parameters in different age groups using different methods. The following 3 studies are applicable to the neonates. Strobel et al. (6) performed manometry studies in 119 North American children ranging from 3 weeks to 235 months; Omari et al. (7) performed manometry studies in 156 Australian premature infants between 26 and 40 weeks postmenstrual age (PMA); Emmerson et al. (2) studied 26 infants (24—35 weeks gestational age, GA) in United Kingdom using manometry, acid-alkali interphase and x-rays. There were disagreements between these studies and may be due to differences in methodologies, different age groups or their developmental stages, or anthropometric variations. Furthermore, none of these studies examined the relationship between the somatic growth parameters and the proximal aerodigestive tract, that is, nares to upper esophageal sphincter (UES) length.
This study was undertaken to determine the relationship between (1) somatic growth parameters and segmental esophageal lengths and (2) the GA and PMA and segmental esophageal lengths.
MATERIALS AND METHODS
Subjects
A total of 100 esophageal manometry studies in 75 neonates (23.0–40.6 weeks gestation, birth weight 0.6–4.4 kg, 35 males and 40 females) were performed between 29.1 and 58.6 weeks PMA (1.0–6.4 kg) for ongoing esophageal motility protocols. GA among infants was determined by maternal history and obstetric data. PMA was calculated by adding chronological age to GA. All infants were examined by the principal investigator (S.R.J.) and the attending neonatologist and were deemed healthy at study. All subjects were of appropriate growth for GA at birth and were not receiving prokinetics or acid-suppressive therapies at the time of evaluation. Exclusion criteria were as follows: congenital anatomical malformations, chromosomal anomalies, hypoxic ischemic injury, tracheostomy, gastrostomy, hydrocephalus and seizure disorders. No sedation was used in these studies. These studies were approved by the institutional review boards at the 2 study sites: (1) Columbus Children's Hospital at Columbus, OH, and (2) Children's Hospital of Wisconsin, Milwaukee, WI. Informed consents were obtained from parents.
Manometric Methods
The esophageal manometric methods adapted for neonates have been described and validated by us before (8,9). Briefly, the esophageal manometry catheter assembly with dual sleeves and 4 side-ports and a terminal gastric recording port was passed nasally with the infant in supine position. The catheter assembly was connected to the pneumohydraulic micromano-metric water perfusion system via the resistors (Dentsleeve International, Mui Scientific, Ontario, Canada) and pressure transducers (Ohmeda TNF-R disposable pressure transducers) and amplifiers (UPS 2020, MMS medical instruments, New Hampshire). The response rate using technique was 300 mm Hg per seconds. All studies were done in the same manner, with the transducers at the level of the subject's esophagus (mid-axillary line). Respiratory patterns and vital signs were recorded concurrent with manometry.
Manometric Technique and Determination of Esophageal Length and Sphincter Parameters
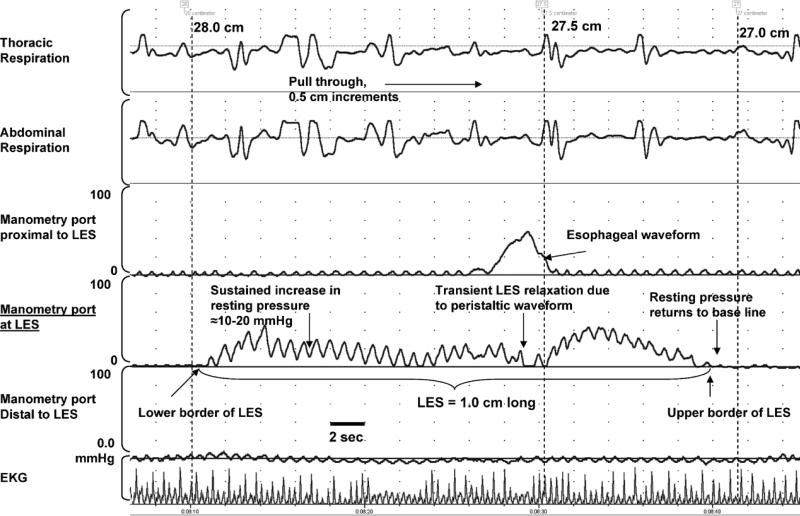
All studies were done in the same manner. Manometric catheter was passed nasally into the stomach. During station pull through, the catheter was withdrawn at 0.5-cm intervals with a pause of at least 15 seconds at each station. The high-pressure zones of the LES were identified by noticing a consistent increase in pressure of more than 5.0 mm Hg above the baseline for at least 15 seconds along with the respiratory artifact (Fig. 1). In addition, the respiratory inversion point in the same pressure channel was also identified. The length of LES was determined as the distance between the inferior and superior limits of the high-pressure zones (Fig. 1). Similarly, while continuing the pull through proximally, reemergence of the high-pressure zone determined the UES length. The resting pressure of the sphincters was measured in their respective sleeves after the pull through when subjects were quiet and resting comfortably. To determine reliable and accurate basal resting sphincteric pressures, we measured sphincteric pressures (using upper and lower esophageal manometric sleeves) only after approx imately 15 to 20 minutes after pull through when infants were comfortable. The basal pressures were determined during a resting phase of esophageal quiescence (Fig. 2). All measurements were taken at end-expiration points with use of concurrent respiratory measurements (Fig. 2). All manometric data were measured at end-expiration which was determined with use of concurrent thoracic and abdominal respiratory motion (Fig. 1). Finally, based on the distance traversed by the catheter, the lengths from the nares to LES and nares to UES and the esophageal body length were calculated. These segmental lengths were then correlated with somatic growth parameters.
FIG. 1.
Manometric determination of LES high-pressure zones during the pull through. Three manometric ports are shown with concurrent electrocardiogram and thoracic and abdominal respiration. The length of LES (1.0 cm) was determined as the distance in centimeters (0.5-cm increments) between the inferior and superior limits of the high-pressure zones. The determination of UES high-pressure zones was also done in a similar fashion.
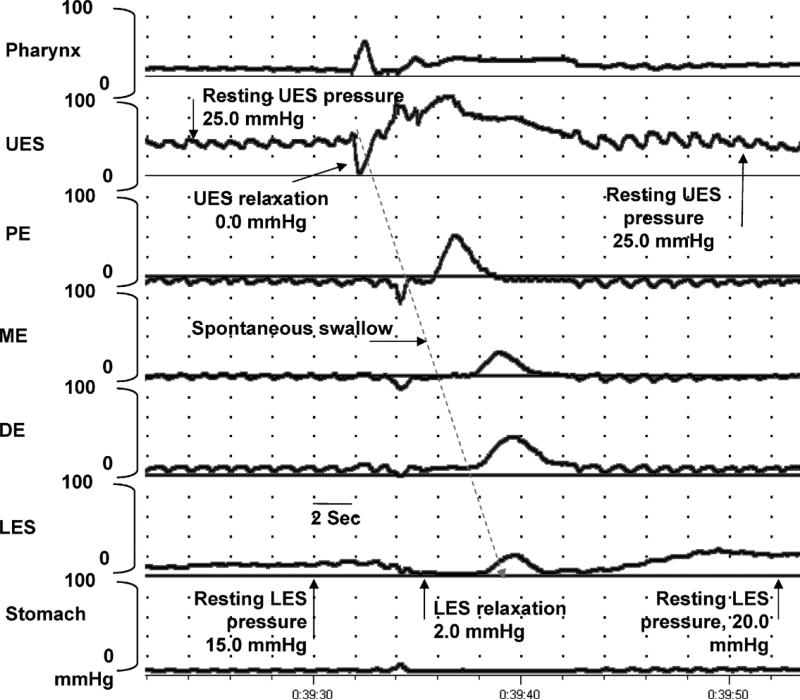
FIG. 2.
Manometric tracing showing characteristics of the LES and UES high-pressure zones function during a spontaneous swallow. Note the resting pressure before and after swallow. The lower and upper sphincteric pressure relaxes transiently to accommodate swallow and regain the resting tone as the spontaneous swallow transits through the respective esophageal segments.
Somatic Growth Parameters
Physical measurements were taken at study using standard nursery practices. Weight, length and head circumference were measured using electronic scales, stadiometer and measuring tapes, respectively. Body surface area (BSA) was calculated based on height and weight using a standard formula (✓ {height (cm) × weight (kg)} / 3600)(10,11). Measurements at birth were obtained from obstetric and birthing records.
Statistical Analysis
Association between esophageal length parameters (nares to LES, nares to UES, esophageal body length) and somatic growth variables were assessed using SPSS 13.0 (Statistical Program for Social Sciences, SPSS Inc, Chicago, IL). Regression analysis was performed to determine the best-fit correlation of resting LES pressure, resting UES pressure, and LES and UES lengths with PMA, GA, weight, length, head circumference and BSA as independent variables. Pairwise comparison was also made in the longitudinal data from 26 premature neonates that were studied twice. All data are presented as mean ± SD, unless stated otherwise. P value of less than 0.05 was considered significant.
RESULTS
Demographic Characteristics
Among the 75 neonates, the ethnic distribution was 12% African Americans and 88% whites; among whom were 8% Hispanics and 92% non-Hispanics. At birth, all the neonates had appropriate weight, length and head circumference for GA. The birth weight (1.62 T 0.89 kg), birth length (39.7 ± 6.3 cm), head circumference (27.78 ± 3.7 cm) and BSA (0.13 ± 0.04 m2) individually correlated linearly (R2 > 0.850, P > 0.001) with GA (30.8 ± 4.8 weeks, mean ± SD). The 1- and 5-min APGAR scores were 6 and 8 (median), respectively.
At the time of study, subjects were of appropriate growth for age, and their weight (2.5 ± 1.0 kg), length (45.7 ± 5.0 cm), head circumference (32.4. ± 3.8 cm) and BSA (0.19 ± 0.04 m2) individually correlated linearly (R2 > 0.706, P < 0.001) with the PMA (38.2 ± 5.6 weeks).
Relationship Between Nares-to-LES Length and Somatic Growth Parameters
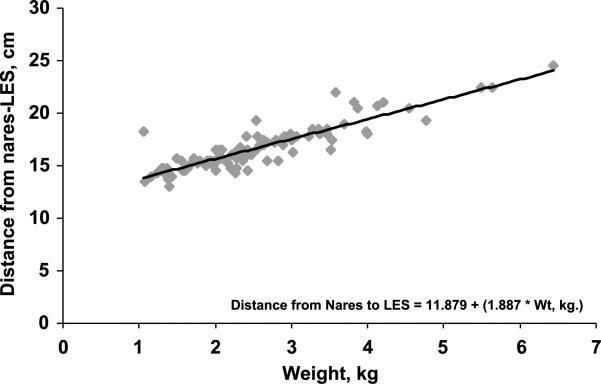
The relationship (P < 0.001) between somatic growth parameters and the distance between the nares and superior border of LES is shown in Table 1. Furthermore, the best linear correlation was evident with body weight and BSA (R2 = 0.793, P < 0.001; Fig. 3).
TABLE 1.
Prediction of distance from nares to superior border of LES based on growth parameters
| Somatic growth variables | Nares-to-LES distance | R 2 | P |
|---|---|---|---|
| PMA, wk | 5.531 + (0.291 * PMA) | 0.578 | <0.001 |
| Weight, kg | 11.879 + (1.887 * Wt) | 0.793 | <0.001 |
| Length, cm | –0.0936 + (0.367 * Lt) | 0.683 | <0.001 |
| BSA, m2 | 8.766 + (44.420 * BSA) | 0.793 | <0.001 |
| FOC, cm | 1.517 + (0.468 * FOC) | 0.608 | <0.001 |
LES, lower esophageal sphincter; PMA, postmenstrual age; BSA, body surface area; FOC, fronto-occipital circumference.
FIG. 3.
Relationship between nares and LES distance as a function of body weight was found to be linear (R2 = 0.793, P < 0.001). Similar relationship with BSA was also noted.
Relationship Between Esophageal Body Lengths and Somatic Growth Parameters
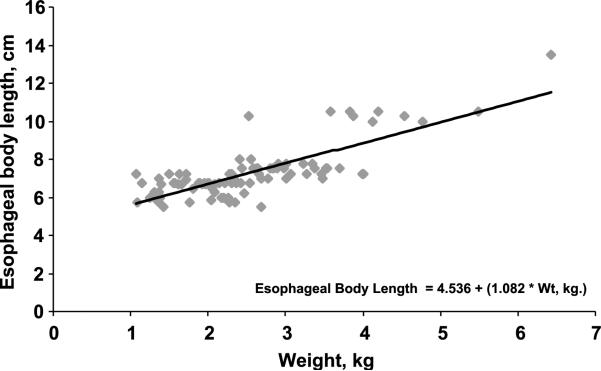
The relationship between somatic growth parameters and the esophageal body length (between the lower border of UES and upper border of LES) are shown in Table 2. The best correlation was seen with body weight (R2 = 0.619, P < 0.001; Fig. 4).
TABLE 2.
Prediction of the esophageal length based on growth parameters
| Demographic characteristics | True esophageal body length | R 2 | P |
|---|---|---|---|
| PMA, wk | 1.249 + (0.157 * PMA) | 0.412 | <0.001 |
| Weight, kg | 4.536 + (1.082 * Wt) | 0.619 | <0.001 |
| Length, cm | –2.445 + (0.213 * Lt) | 0.593 | <0.001 |
| BSA, m2 | 2.829 + (25.109 * BSA) | 0.618 | <0.001 |
| FOC, cm | – 0.368 + (0.237 * FOC) | 0.393 | <0.001 |
PMA, postmenstrual age; BSA, body surface area; FOC, fronto-occipital circumference.
FIG. 4.
Relationship between esophageal body length as a function of body weight was found to be linear (R2 = 0.619, P < 0.001).
Relationship Between Nares-to-UES Length and Somatic Growth Parameters
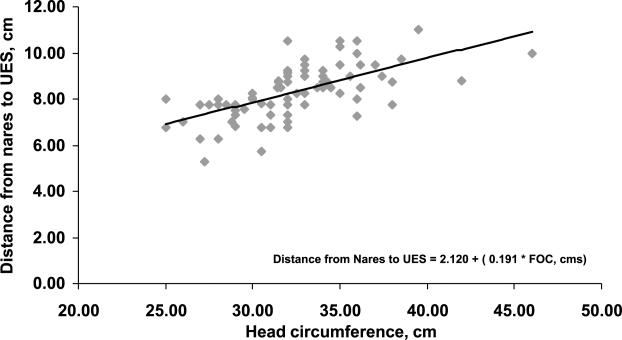
The relationship between somatic growth parameters and the distance between the nares and superior border of UES are shown in Table 3. FUrthermore, the best linear correlation was evident with body weight and BSA (R2 = 0.401, P < 0.001; Fig. 5).
TABLE 3.
Prediction of distance from nares to superior border of UES based on growth parameters
| Demographic characteristics | Nares-to-LES distance | R 2 | P |
|---|---|---|---|
| PMA, wk | 4.164 + (0.110 * PMA) | 0.313 | <0.001 |
| Weight, kg | 6.596 + (0.696 * Wt) | 0.399 | <0.001 |
| Length, cm | 2.607 + (0.125 * Lt) | 0.325 | <0.001 |
| BSA, m2 | 5.481 + (16.121 * BSA) | 0.400 | <0.001 |
| FOC, cm | 2.120 + (0.191 * FOC) | 0.401 | <0.001 |
LES, lower esophageal sphincter; PMA, postmenstrual age; BSA, body surface area; FOC, fronto-occipital circumference.
FIG. 5.
Relationship between nares-UES distance length and head circumference was found to be linear (R2 = 0.401, P < 0.001).
Relationship Between the Segmental Esophageal Lengths and GA and PMA
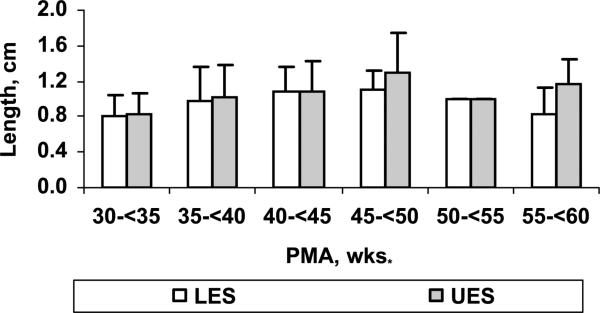
Relationship was noted between PMA at study (Tables 1, 2 and 3; linear regression, P < 0.001) but not with GA at birth for nares-to-LES length, esophageal body length, nares-to-UES length and sphincteric lengths. We then stratified the measured UES and LES lengths into 5-week intervals across the age spectrum based on the PMA at study. The intergroup comparisons between the PMA were significant for both UES and LES lengths (analysis of variance, P < 0.05; Fig. 6).
FIG. 6.
The intergroup comparisons between the PMA were significant for both UES and LES lengths (analysis of variance, P < 0.05).
Longitudinal Comparison of Esophageal Body, UES and LES Growth
Data from 26 preterm neonates that were studied twice were analyzed for the changes in segmental esophageal body growth with somatic growth and increment in PMA (Table 4). Between this period, nares to LES and nares to UES distance increased by 0.25 and 0.18 cm/wk, respectively. LES sphincteric length increased 0.07 cm/wk, and UES sphincteric length increased 0.06 cm/wk PMA.
TABLE 4.
Longitudinal data on patients (n = 26)
| Time 1 | Time 2 | P * | |
|---|---|---|---|
| Physical characteristics | |||
| PMA, wk | 33.32 ± 1.65 | 36.32 ± 1.92 | 0.001 |
| Body weight at study, kg | 1.64 ± 0.36 | 2.2 ± 0.42 | 0.001 |
| Body length at study, cm | 41.42 ± 2.99 | 44.04 ± 2.45 | 0.01 |
| BSA at study, m2 | 0.13 ± 0.02 | 0.16 ± 0.02 | 0.001 |
| Head circumference at study, cm | 28.71 ± 2.18 | 32.41 ± 3.29 | 0.001 |
| Esophageal characteristics | |||
| LES length, cm | 0.8 ± 0.29 | 1.02 ± 0.32 | 0.015 |
| UES length, cm | 0.88 ± 0.34 | 1.06 ± 0.34 | 0.047 |
| Nares-LES-UB, cm | 15.08 ± 1.14 | 15.84 ± 1.11 | 0.023 |
| Nares-UES-UB, cm | 7.56 ± 1.05 | 8.11 ± 0.69 | 0.031 |
| True esophageal body length, cm | 6.57 ± 0.51 | 6.66 ± 0.54 | 0.515 |
Paired t test.
PMA, postmenstrual age; BSA, body surface area; LES, lower esophageal sphincter; UES, upper esophageal sphincter; UB, upper border.
DISCUSSION
In this study, we systematically determined the anatomic growth of the aerodigestive tract based on esophageal manometric characteristics of UES and LES in premature and full-term born human neonates during maturation. The novel cardinal findings of this study are as follows: (1) a linear relationship between all the somatic growth parameters and the aerodigestive tract, that is, distance from nares to LES, esophageal body and nares to UES; (2) a linear relationship between the somatic growth parameters and the UES and LES lengths; (3) a significant relationship between the above-mentioned segmental esophageal parameters and PMA but not GA; (4) the data from a large cohort of premature infants from the Midwest United States; (5) the use of micromanometric techniques in the evaluation of aerodigestive tract; and (6) a significant increase in esophageal and sphincteric growth was observed over a period of maturation from 33 to 36 weeks PMA. Furthermore, a lack of relationship with GA may be due to the fact that the studies were performed at a later time during which period postnatal physiology and growth may have influenced the measurements. This is the first systematic study that has evaluated the segmental growth of human aerodigestive tract in vivo and provided both cross-sectional and longitudinal data across the neonatal age spectrum using reliable methods applicable for the neonatal demographics within the United States.
The findings from this study may have far-reaching applications in neonatal practice. LES is an important first line of defense against gastroesophageal reflux (GER)(12). In addition, increase in length of LES will increase the length of distal high-pressure zone, as shown in this study. Thus, one of the antireflux barriers improves with maturation. Recently, Wenzel et al. and Sifrim et al. have shown a significant association between the height of refluxate and associated GER symptoms (18,19). As described before, (20–23) GER signs and symptoms improve during infancy, suggesting resolution of GER. It has been speculated by these authors that improvement in GER happen with growth, but the mechanisms responsible for this change are not clear. The increase in anatomical dimensions of the aerodigestive tract during maturation may in part contribute to these observations.
The manometric methods used in this study to evaluate esophageal motor function are the methods of choice (3,9,24) and have been used by others and us (2,7–9,13). However, use of an open-ended catheter, (13) or a single manometric port (2), can be inaccurate for recording sphincter dimensions, and both such methods are not suitable for the purpose of our study. Our manometric methods (8,9) are similar to the one used by Omari et al. (7) and our results on the nares-to-LES length also show a linear correlation. Other investigators (6,14,15) used different methods to assess the nares-to-LES length, the accuracy of which is uncertain owing to variability in subject-ages and methods; however, they also found a correlation in ages ranging from infancy to adolescence. Our results are accurate and distinct from other studies, in that they are based on a single method performed in neonates during postnatal maturation (less than 60 weeks PMA). Furthermore, all our studies were performed at the bed side without the need for additional radiological procedures. This unique approach minimizes distress, resource utilization and expense due to neonatal transport to radiology suites. Although this procedure is not readily available, esophageal manometry has advantages in further evaluation of esophageal motor function and length. We speculate that this approach has many practical applications with the use of esophageal probes and catheters.
The differences between our study and that of other neonatal studies (2,7) may be due to anthropometric differences which may also contribute to the differences in organ growth and maturation or simply be due to the nature of surviving premature neonates in the 21st century. Ours is the first study to evaluate neonates wherein the ethnic distribution closely matches with that of US population. Specifically, the distribution of the study population was comparable to the population of United States (75% whites, 12.3% African American and 12.7% other categories; among whom there were 12.5% Hispanics and 87.5% non-Hispanics)(16).
The longitudinal data from this study suggest an increase in esophageal dimensions with postnatal growth. During evaluation by esophageal manometry, the change in length of the sphincters or the esophageal body can also be included in follow-up studies, thus determining changes in the basal antireflux mechanisms due to treatment strategies. It is logical to speculate in premature infants that the organ growth occurs with physical growth characteristics in children (25). However, pertinent to the aerodigestive tract, the anatomical definitions of different segments (nares-to-UES length, length of UES, esophageal body length, nares-to-LES length and length of LES) are not known in the preterm infant population. Our study shows new data about different segments of aerodigestive tract. Assessment of the change in proximal aerodigestive tract length (nares-UES length) over time is also a valuable addition, particularly in dysmorphic infants requiring evaluation for dysphagia.
Application of these measurements will allow the following: (1) modification in esophageal probe designs; (2) accurate placement of pH probes at 87% of the nares-LES length, thus ensuring recording in the distal esophagus (17); and (3) proper placement of gavage tubes into stomach, minimizing consequences of tube malposition.
In conclusion, a significant linear correlation of lengthening of the neonatal aerodigestive tract with postmenstrual age and growth parameters were noted. We speculate that application of this approach will be cost-effective and will minimize the need for confirmation of esophageal probe position by x-rays or fluoroscopy.
Acknowledgments
Grant Support: Supported in part by NIH grants RO3 DK 061502 and RO1 DK 068158 (awarded to S.R.J.).
Location of Study: Columbus Children's Hospital, Columbus, OH and Children's Hospital of Wisconsin, Milwaukee, WI.
REFERENCES
- 1.DiLorenzo C, Hillemeier C, Hyman P, et al. Manometry studies in children: minimum standards for procedures. Neurogastroenterol Motil. 2002;14:411–20. doi: 10.1046/j.1365-2982.2002.00347.x. (Review) [DOI] [PubMed] [Google Scholar]
- 2.Emmerson AJ, Chant T, May J, Vales P. Assessment of three methods of pH probe positioning in preterm infants. J Pediatr Gastroenterol Nutr. 2002;35:69–72. doi: 10.1097/00005176-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 3.American Gastroenterological Association Medical Position Statement: guidelines on the use of esophageal PH recording. Gastroenterology. 1996;110:1981. doi: 10.1053/gast.1996.1101981. [DOI] [PubMed] [Google Scholar]
- 4.Klauser AG, Schindlbeck NE, Müller-Lissner SA. Esophageal 24-hour pH monitoring: is prior manometry necessary for correct positioning of the electrode? Am J Gastroenterol. 1990;85:1463–7. [PubMed] [Google Scholar]
- 5.Pehl C, Boccali I, Hennig M, Schepp W. pH probe positioning for 24-hour pH-metry by manometry or pH step-up. Eur J Gastroenterol Hepatol. 2004;16:375–82. doi: 10.1097/00042737-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Strobel CT, Byrne WJ, Ament ME, Euler AR. Correlation of esophageal lengths in children with height: application to the Tuttle test without prior esophageal manometry. J Pediatr. 1979;94:81–4. doi: 10.1016/s0022-3476(79)80361-3. [DOI] [PubMed] [Google Scholar]
- 7.Omari TI, Benninga MA, Haslam RR, Barnett CP, Davidson GP, Dent J. Lower esophageal sphincter position in premature infants cannot be correctly estimated with current formulas. J Pediatr. 1999;135:522–5. doi: 10.1016/s0022-3476(99)70179-4. [DOI] [PubMed] [Google Scholar]
- 8.Jadcherla SR, Duong HQ, Hoffmann RG, Shaker R. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr. 2003;143:31–8. doi: 10.1016/S0022-3476(03)00242-7. [DOI] [PubMed] [Google Scholar]
- 9.Jadcherla SR, Duong HQ, Hofmann C, Hoffmann R, Shaker R. Characteristics of upper oesophageal sphincter and oesophageal body during maturation in healthy human neonates compared with adults. Neurogastroenterol Motil. 2005;17:1–8. doi: 10.1111/j.1365-2982.2005.00706.x. [DOI] [PubMed] [Google Scholar]
- 10.Mosteller RD. Simplified calculation of body surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. (Letter) [DOI] [PubMed] [Google Scholar]
- 11.Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 1970;54:225–35. [PubMed] [Google Scholar]
- 12.Mittal RK, McCallum RW. Characteristics and frequency of transient relaxations of the lower esophageal sphincter in patients with reflux esophagitis. Gastroenterology. 1988;95:593–9. doi: 10.1016/s0016-5085(88)80003-9. [DOI] [PubMed] [Google Scholar]
- 13.Newell SJ, Sarkar PK, Durbin GM, Booth IW, McNeish AS. Maturation of the lower oesophageal sphincter in the preterm baby. Gut. 1988;29:167–72. doi: 10.1136/gut.29.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Putnam PE, Orenstein SR. Determining esophageal length from crown-rump length. J Pediatr Gastroenterol Nutr. 1991;13:354–9. doi: 10.1097/00005176-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Jolley SG, Tunell WP, Carson JA, et al. The accuracy of abbreviated esophageal pH monitoring in children. J Pediatr Surg. 1984;19:848–53. doi: 10.1016/s0022-3468(84)80383-8. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Census Bureau [August 12, 2005];Census Data on Population Change, Race and Age. 2000 Available at: http://www.census.gov/main/www/cen2000.html.
- 17.Euler AR, Ament ME. Detection of gastroesophageal reflux in the pediatric-age patient by esophageal intraluminal pH probe measurement (Tuttle test). Pediatrics. 1977;60:65–8. [PubMed] [Google Scholar]
- 18.Sifrim D. Relevance of volume and proximal extent of reflux in gastro-oesophageal reflux disease. Gut. 2005;54:175–8. doi: 10.1136/gut.2004.043984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenzl TG, Schneider S, Scheele F, Silny J, Heimann G, Skopnik H. Effects of thickened feeding on gastroesophageal reflux in infants: a placebo-controlled crossover study using intraluminal impedance. Pediatrics. 2003;111:e355–e9. doi: 10.1542/peds.111.4.e355. [DOI] [PubMed] [Google Scholar]
- 20.Sutphen JL. Pediatric gastroesophageal reflux disease. Gastroenterol Clin North Am. 1990;19:617–29. (Review) [PubMed] [Google Scholar]
- 21.Orenstein SR. Infantile reflux: different from adult reflux. Am J Med. 1997;103:114S–9S. doi: 10.1016/s0002-9343(97)00335-5. [DOI] [PubMed] [Google Scholar]
- 22.Nelson SP, Chen EH, Syniar GM, Christoffel KK. One-year follow-up of symptoms of gastroesophageal reflux during infancy. Pediatric Practice Research Group. Pediatrics. 1998;102:E67. doi: 10.1542/peds.102.6.e67. [DOI] [PubMed] [Google Scholar]
- 23.Nelson SP, Chen EH, Syniar GM, Christoffel KK. Prevalence of symptoms of gastroesophageal reflux during infancy. A pediatric practice-based survey. Pediatric Practice Research Group. Arch Pediatr Adolesc Med. 1997;151:569–72. doi: 10.1001/archpedi.1997.02170430035007. [DOI] [PubMed] [Google Scholar]
- 24.Kahrilas PJ, Quigley EMM. American Gastroenterological Association Medical Position Statement: guidelines on the use of esophageal pH recording. Gastroenterology. 1996;110:1981–96. doi: 10.1053/gast.1996.1101981. [DOI] [PubMed] [Google Scholar]
- 25.Ganong WF. Review of Medical Physiology. 22 ed. McGraw-Hill Medical; New York: 2005. [Google Scholar]