Abstract
The performance of completely biological, decellularized engineered allografts in a sheep model was evaluated to establish clinical potential of these unique arterial allografts. The 4-mm-diameter, 2–3-cm-long grafts were fabricated from fibrin gel remodeled into an aligned tissue tube in vitro by ovine dermal fibroblasts. Decellularization and subsequent storage had little effect on graft properties, with burst pressure exceeding 4000 mmHg and the same compliance as the ovine femoral artery. Grafts were implanted interpositionally in the femoral artery of six sheep (n=9), with contralateral sham controls (n=3). At 8 weeks (n=5) and 24 weeks (n=4), all grafts were patent and showed no evidence of dilatation or mineralization. Mid-graft lumen diameter was unchanged. Extensive recellularization occurred, with most cells expressing αSMA. Endothelialization was complete by 24 weeks with elastin deposition evident. These completely biological grafts possessed circumferential alignment/mechanical anisotropy characteristic of native arteries and were cultured only 5 weeks prior to decellularization and storage as “off-the-shelf” grafts.
Introduction
Atissue-engineered small-diameter artery (tissue-engineered vascular graft [TEVG]) remains the holy grail of vascular tissue engineering.1 Synthetic materials are well known to perform poorly in small-diameter (<6 mm) coronary and peripheral artery bypass applications typically because of acute thrombogenicity of the graft and anastomotic intimal hyperplasia, compounded by minimal spontaneous endothelialization.2 Autologous vessels, which are the standard of care at present, are of limited quantity, especially in patients with diffuse atherosclerosis who have had previous bypass procedures.1 Various approaches have been used to fabricate TEVG,3–9 recently reviewed.3 Most notably in terms of clinical promise, TEVGs derived from rolling a fibroblast-produced “cell sheet” into a tube5,7,8,10 and from smooth muscle cells (SMCs) seeded onto a degradable synthetic polymer tube4,6,9 have resulted in successful implantation.
In contrast to these approaches, our approach utilizes the biopolymer fibrin (in hydrogel form) as the starting scaffold,11 which eliminates risk of any adverse host reaction to residual synthetic material. Another benefit of fibrin is the ability to control alignment of cell-produced extracellular matrix, thereby creating circumferential alignment—a prominent feature of the arterial media, which dictates mechanical properties of the artery.12 Previously, we reported developing a fibrin-based TEVG from human dermal fibroblasts with circumferential alignment and physiological burst strength.13
In this study, we evaluated decellularized TEVG fabricated from allogeneic fibroblasts in the ovine model. This animal model presents a high propensity for calcification.14 In addition to the assessment of graft thrombogenicity and patency, sheep have frequently been used as a model for anastomotic intimal hyperplasia.15 Anticoagulation therapy was used for the entire implant duration in order to evaluate host remodeling without the possibility of clotting. Herein, we report implantation of TEVG fabricated in vitro in 5 weeks from only biological components, following their decellularization and storage, into the arterial circulation of a large-animal model up to 6 months. These “off-the-shelf” grafts were monitored with ultrasonography and characterized with cellular, biochemical, and biomechanical analyses at implantation and explantation. Preliminary results from this study were previously presented.16
Materials and Methods
Cell culture
Ovine dermal fibroblasts (oDFs; Coriell Institute) were maintained in a 50/50 mixture of Dulbecco's modification of Eagle's medium and Ham's F12 cell culture medium (DMEM/F12; Cellgro) supplemented with 15% fetal bovine serum (FBS; Thermo-Fisher Scientific), 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated at 37°C in 100% humidity and 5% CO2, passaged at ∼100% confluency, and harvested for use at passage 7.
TEVG fabrication
oDF-seeded fibrin gel was formed by adding thrombin (Sigma) and calcium chloride in 20 mM HEPES-buffered saline to a suspension of oDF (Coriell) in bovine fibrinogen (Sigma). The final component concentrations of the cell suspension were as follows: 4 mg/mL fibrinogen, 1.1 U/mL thrombin, 5.0 mM Ca++, and 1 million cells/mL. Cell suspensions were mixed and injected into a tubular mold with 9-cm length, 12.5-mm outer diameter, and 4-mm inner diameter containing a glass mandrel pretreated with a 5% Pluronic F-127 solution. Subsequently, grafts were cultured in DMEM supplemented with 10% FBS (HyClone), 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco), 2 μg/mL insulin (Sigma), and 50 μg/mL ascorbic acid (Sigma). After an initial 2-week maturation period, the grafts were transferred to a custom pulsed-flow-stretch bioreactor for an additional 3-week maturation period as previously described.13 The final dimensions of each graft were 3–4 cm in length, 4-mm inner diameter, and ∼500 μm in thickness.
TEVG decellularization
After 5 weeks of culture as described earlier, grafts were rinsed in PBS and incubated on an orbital shaker for 6 h with 1% sodium dodecyl sulfate (SDS; Sigma) in distilled water. The SDS solution was changed three times, at 30 min, 1 h, and 4 h. The grafts were rinsed in PBS and incubated with 1% Triton X-100 (Sigma) in distilled water for 30 min, extensively washed with PBS for 72 h, and then incubated in deoxyribonuclease enzyme (Worthington Biochemical) in DMEM supplemented with 10% FBS for 4 h. Decellularized grafts were stored at 4°C in PBS until implantation. Following decellularization, a subset of grafts was analyzed for mechanical and biochemical properties.
TEVG mechanical property measurement
TEVGs were mounted in a system designed for pressurizing individual grafts to failure. Each graft was cannulated and tested for compliance and burst strength as described previously.13 Additionally, TEVG samples were tested for suture retention and tensile properties.13
Immunoblot analysis
Immunoblot analyses were performed as previously described.17 To evaluate the efficacy of decellularization, cellular and decellularized tissue samples were probed with antibodies for β-actin (A5441; Sigma) and β-2-microglobulin (15976; Abcam) and developed using electrochemiluminescence.
Tissue compositional property measurement
The collagen content was quantified using a hydroxyproline assay previously described, assuming 7.46 mg collagen per mg of hydroxyproline.18 The insoluble elastin content was quantified using a ninhydrin-based assay as previously described.19 The sample volume was calculated using the measured length, width, and thickness of the strips determined with a force probe during tensile testing.13 The cell content was quantified with a modified Hoechst assay for DNA assuming 7.7 pg of DNA per cell.20
Implant procedures
All protocols and procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC). Surgeries were performed by the University of Minnesota Experimental Surgical Services. Grafts were evaluated in a total of six animals utilizing both femoral arteries. Animals were sedated with 0.04 mg/kg intramuscular atropine and 10 mg/kg intramuscular ketamine. Following sedation, a jugular vein IV was placed and animals were anesthetized with 10–12 mg/kg intravenous propofol, and maintained on isoflurane during surgery. Interpositional femoral artery implants were performed in all cases following resection of a short segment of the artery (1–2 cm). Grafts were secured using running 7-0 prolene sutures. Grafts were implanted for either 8 weeks (n=5 grafts, n=3 sham control) into four sheep or 24 weeks (n=4 grafts) into two sheep in order to compare the progression of host-cell remodeling. For the duration of these studies, animals were maintained on daily subcutaneous injections of enoxaparin (1 mg/kg) beginning immediately postoperatively, as well as aspirin (80 mg/day) and clopidogrel (75 mg/day) beginning 3 days prior to surgery. Ultrasound measurement and quantification were performed at 1, 8, and 20 weeks. Prior to sacrifice, angiography was performed at 8 and 24 weeks.
Explant analysis
Grafts were cut into ring sections and imaged. The two end-rings and mid-graft ring were fixed for histological analyses and the remainder of the rings were distributed for scanning electron microscopy (SEM), mechanical, and biochemical analyses.
Histology
Grafts preimplant and postexplant were fixed in 4% paraformaldehyde, embedded in OCT (Tissue-Tek), and frozen in liquid N2. Sections of 9-μm thickness were stained with Lillie's trichrome, Von Kossa, picrosirius red (PSR), and Verhoeff-Van Gieson (VVG). For PSR-stained sections, images were taken with the sections placed between crossed plane polarizers. Samples for SEM imaging were fixed as described previously21 and imaged using a Hitachi S-4700 Scanning Electron Microscope at 3.0 kV.
Immunostaining
Histological sections of grafts were stained for αSMA (A5228; Sigma), calponin (0095R; Bioss), vWF (ab6994; Abcam), Ki67 (ab15580; Abcam), CD3 (C7930; Sigma), CD11b (C2262-36L; US Biological), CD45 (C2399-07B; US Biological), and elastin (ab21599; Abcam).
All samples were blocked with 5% normal donkey serum, incubated in primary antibody at 5 μg/mL, and stained with a Cy2-, 3-, or 5-conjugated, species-matched secondary antibody (Jackson Immunoresearch).13 Nuclei were counterstained with Hoechst 33342 (H3570; Invitrogen).
Statistics
For all experiments, statistical significance of differences between groups was determined using Student's t-test for two treatments and one-way analysis of variance for more than two treatments with the Tukey post hoc test in GraphPad Prism® software for Windows. Any reference to a difference in the “Results” and “Discussion” sections implies statistical significance at the level p<0.05. In all cases, where the difference was significant, symbols are used to indicate the difference and explained in the figure captions.
Results
TEVG properties and decellularization
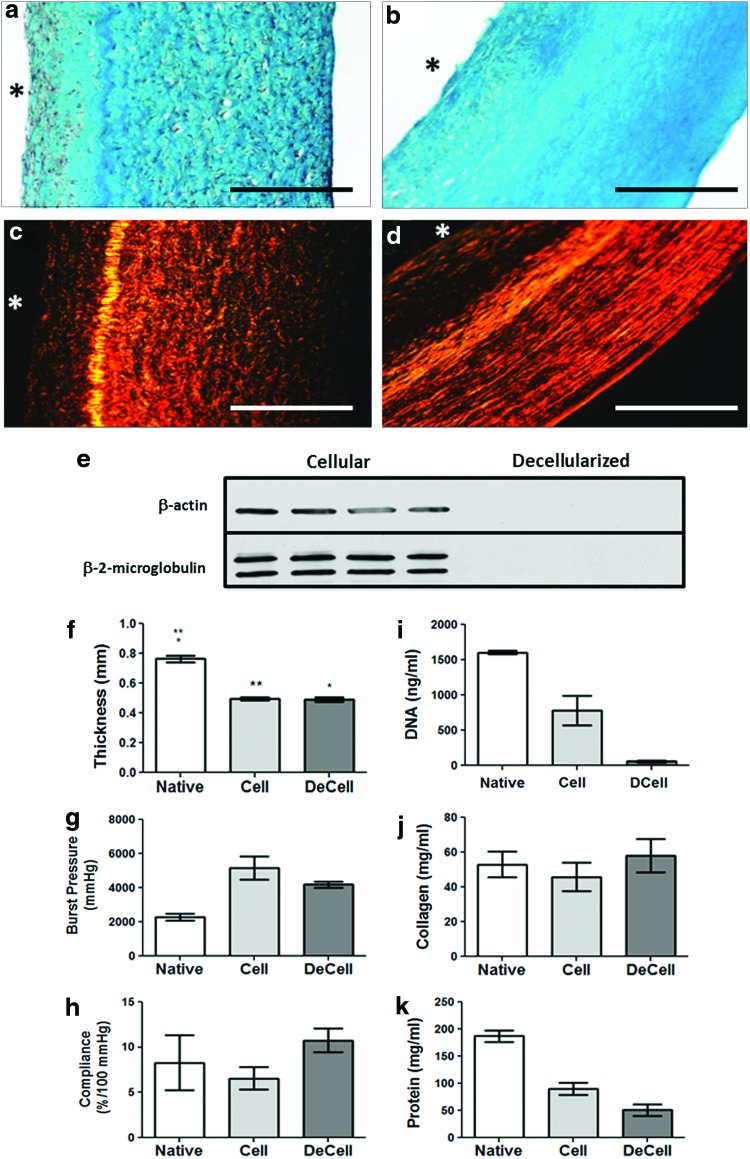
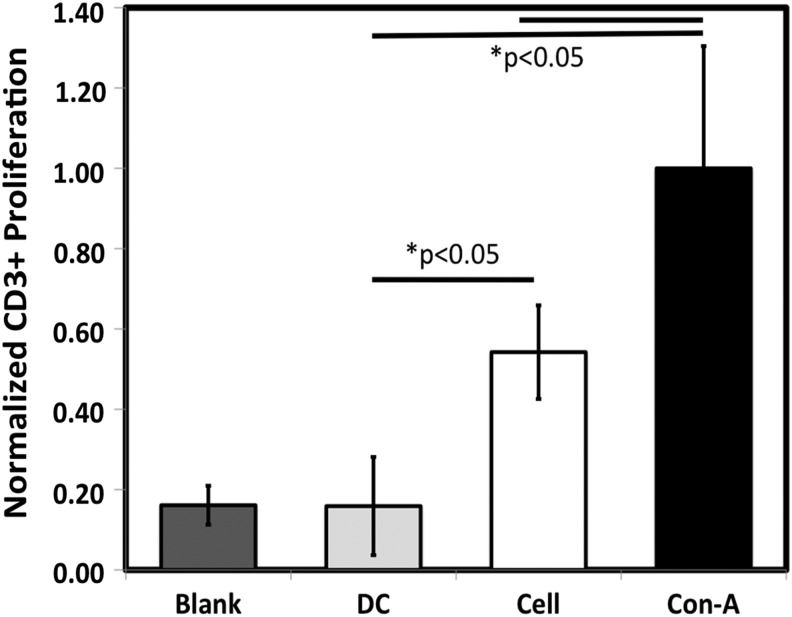
Following maturation, grafts were decellularized and evaluated for biomechanical properties and changes in DNA, and content of collagen and total protein. Lillie's Trichrome and PSR staining showed that the decellularized grafts presented some visible differences in extracellular matrix organization, with less texture in the staining patterns evident after decellularization (Fig. 1a–d). Cellular membrane-associated protein β-2-microglobulin and intracellular protein β-actin were detected by western blot prior to decellularization, but neither were detectable following decellularization (Fig. 1e). The average graft thickness was unchanged following decellularization (0.49±0.01 mm before and 0.49±0.02 mm following decellularization; Fig. 1f). Burst pressures were 5160±680 mmHg before and 4200±180 mmHg after decellularization, with no change in compliance (Fig. 1g, h). Suture retention strength was 0.58±0.04 N (n=3) and 0.60±0.05 N (n=3) before and after decellularization, respectively. The DNA content was reduced 93%±2% after decellularization with no change in total collagen concentration (Fig. 1i, j). The protein content of the grafts was reduced 44% after decellularization, reflecting a reduction of cellular proteins (Fig. 1k). The decellularized grafts were cultured with allogeneic, ovine blood mononuclear cells for 48 h and lymphocyte proliferation was evaluated with segments of an allogeneic, cellular engineered vascular graft for comparison and concanavalin A was used as a positive control. Lymphocyte activation was not seen with the decellularized graft compared with the negative (blank) and positive controls while the amount of induced lymphocyte proliferation in the presence of a cellular graft was significantly elevated above that observed for a decellularized construct (Fig. 2). All occurrences of “graft” hereafter imply the decellularized graft.
FIG. 1.
In vitro TEVG characterization. Trichrome and picrosirius red staining images comparing cellular (a, c) and decellularized (b, d). (e) Immunoblot analysis of decellularization effectiveness through identification of residual β-actin and β-2-microglobulin in cellular and decellularized graft samples (n=4). The symbol * indicates lumenal surface in histology images. Mechanical testing and composition of TEVG in vitro. (f) Thickness, (g) burst pressure, (h) compliance, (i) DNA content, (j) collagen concentration, and (k) total protein concentration of cellular TEVG (Cell, n=3) and decellularized TEVG (DeCell, n=3) in comparison to the ovine femoral artery (Native, n=3). Paired symbols are used to designate statistically significant differences at p<0.05 in bar plots. TEVG, tissue-engineered vascular graft. Color images available online at www.liebertpub.com/tea
FIG. 2.
Lymphocyte proliferation count for BrdU+/CD3+ cells with concanavalin A (ConA), control MNCs (Blank), decellularized TEVG (DC), and segments of a cellular TEVG (Cell).
Ultrasonography and angiography measurements
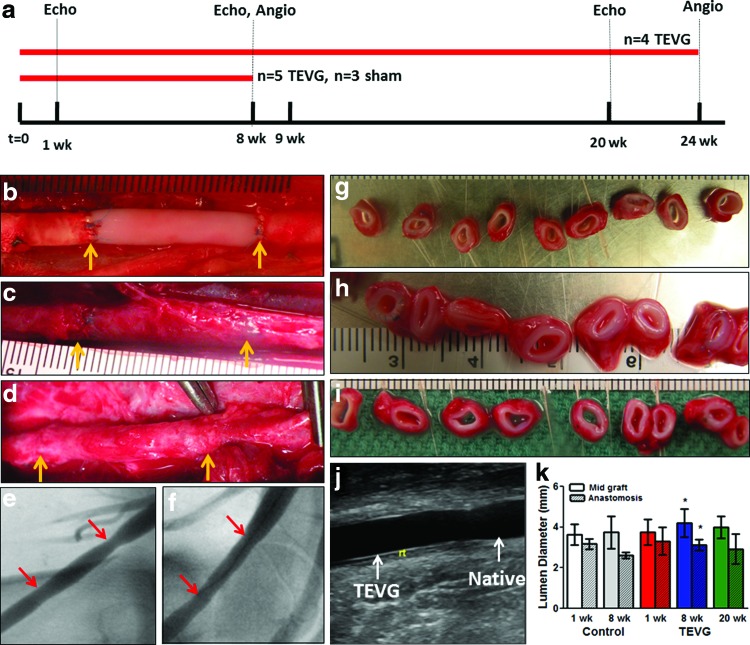
The implantation time-line for all grafts is shown in Figure 3a with implant durations, “n” values, and anticoagulation regimen. Postimplantation, grafts were evaluated using ultrasonography at 1, 8, and 20 weeks and angiogram at the time of explant (i.e., 8 and 24 weeks). The wall thickness and lumen diameter along the graft lengths were calculated from ultrasound data. Representative images of the grafts immediately after implantation, and at weeks 8 and 24 prior to explant are shown in Figure 3b–d. Cross-sectional rings from the explanted grafts showed no qualitative differences along the length of the grafts and grossly resemble the sham control (Fig. 3g–i). Angiography at 8 and 24 weeks (Fig. 3e, f, respectively) indicated no dilatation. At 8 weeks, the lumen diameter based on ultrasonography was slightly narrowed at the anastomoses compared with mid-graft, with a similar trend evident in the sham controls, although no statistical difference existed at 24 weeks (Fig. 3j, k). A composite of ultrasounds acquired at 8 and 20 weeks is shown in Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/tea).
FIG. 3.
TEVG implantation, in vivo imaging, and macroscopic observations at explantation. (a) Timeline summary of the implants performed, anticoagulation utilized therein, and in vivo monitoring used throughout the experimental duration. Acellular TEVG images (b) immediately postimplant, (c) 8 weeks, and (d) 24 weeks with yellow arrows indicating anastomoses. Angiogram images at (e) 8 weeks and (f) 24 weeks, with red arrows pointing to anastomoses. At explantation, (g) sham control (8 weeks) and TEVG were cut into rings at (h) 8 weeks and (i) 24 weeks. Grafts were monitored with ultrasonography for diameter, wall thickness, and flow characteristics at 1, 8, and 20 weeks, with (j) image of anastomotic site; (k) the measured diameter of sham control (Control, n=3) and grafts (TEVG, n=8) are shown near mid-graft (solid) and anastomoses (hashed). Statistical differences at p<0.05 are shown with paired symbols. Color images available online at www.liebertpub.com/tea
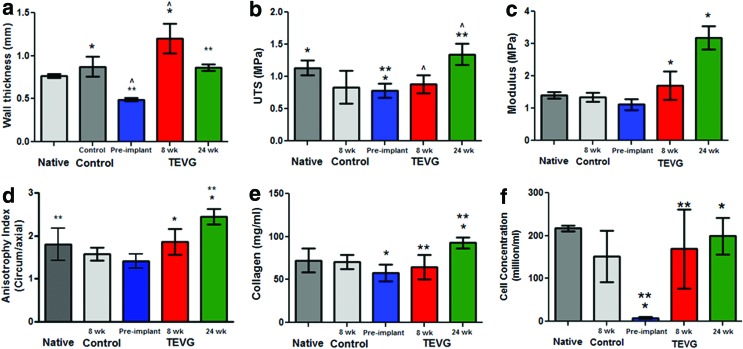
TEVG mechanical properties after 8 and 24 weeks
Graft thickness increased after implantation, measuring 1.2±0.2 mm at 8 weeks and 0.87±0.1 mm at 24 weeks (Fig. 4a), with the value at 24 weeks indicating no difference from the femoral artery value (0.87±0.2 mm). The ultimate tensile strength (UTS) of the grafts at implant was lower than the native value but there was no difference in stiffness as measured by the modulus (Fig. 4b, c). No difference in UTS or modulus between implanted and explanted grafts was observed at 8 weeks. At 24 weeks, both UTS and modulus increased over preimplant graft values, with no difference between native and graft UTS. The values of UTS and modulus when multiplied by graft thickness (i.e., the maximum tension and membrane stiffness, respectively), which removes any artifact due to variable amounts of loose connective tissue left on the explants, of explanted grafts at 8 and 24 weeks showed no difference (Supplementary Fig. S2a, b). The anisotropy index, measured as the ratio of modulus in the circumferential and radial directions, showed that the grafts increased in their anisotropic tensile properties during in vivo remodeling (Fig. 4d).
FIG. 4.
TEVG mechanical properties and composition after 8 and 24 weeks. Comparison of (a) wall thickness, (b) ultimate tensile strength (UTS), (c) modulus, (d) anisotropy index, (e) collagen concentration, and (f) cell concentration in fresh femoral artery (Native, n=3) and 8-week sham control (Control, n=3) in comparison to TEVG before implantation (Preimplant, n=3) and after 8 weeks ( n=5) and 24 weeks (n=3) of implantation. Statistical difference at p<0.05 between properties is shown with paired symbols. Color images available online at www.liebertpub.com/tea
TEVG composition analyses after 8 and 24 weeks
Collagen concentration of the grafts was increased at 24 weeks compared with preimplant and 8-week values (Fig. 4e). The collagen concentration of graft at 24 weeks (93±17 mg/mL) was not different from the femoral artery (72±14 mg/mL). Total protein and elastin concentrations also increased over the duration of implantation with 24-week values at 72% and 8.8% compared with the femoral artery, respectively (Supplementary Fig. S2c–d). The graft recellularized extensively during implantation, achieving cellularity comparable to the femoral artery at 8 and 24 weeks (Fig. 4f).
In vivo TEVG remodeling, cellular invasion, and endothelialization
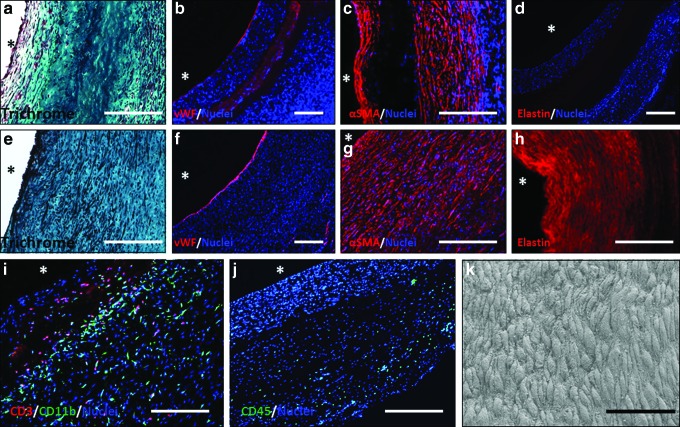
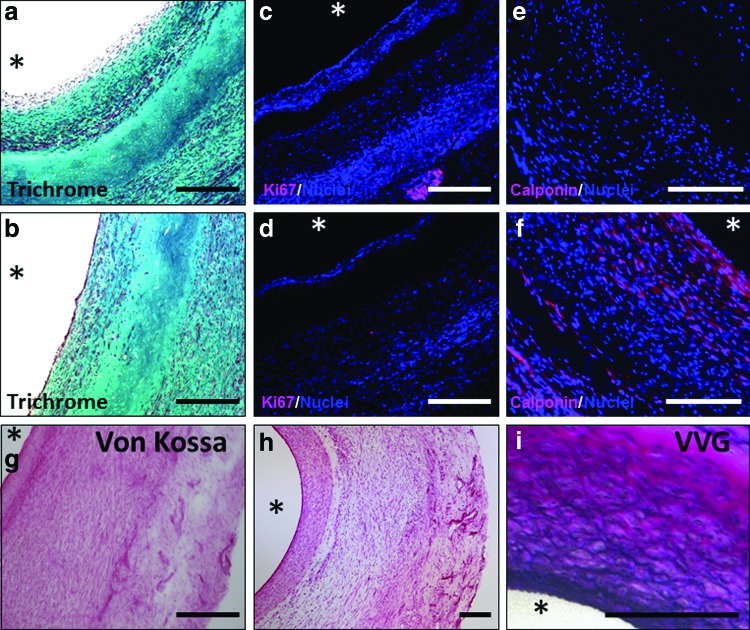
Grafts exhibited cellular invasion from both the lumenal and ablumenal surfaces (Fig. 5a, c). The cellular region along the lumen was thicker near the anastomoses compared with mid-graft at 8 weeks (Fig. 6a, b). The measured thickness of the central acellular region at 8 weeks was ∼0.2 mm, much less than the implanted thickness of 0.49±0.02 mm. At 24 weeks, no acellular region was observed, with circumferentially aligned cells populating the entire thickness of the graft (Fig. 5e, g). The grafts endothelialized during implantation, with partial endothelialization at 8 weeks mid-graft (Fig. 5b) and complete endothelialization toward the anastomoses (Supplementary Fig. S3f). After 24 weeks, endothelialization was complete throughout the length of the graft (Fig. 5f and Supplementary Fig. S3f), and the ECs were aligned with the direction of blood flow and exhibited tight junctions as observed with SEM imaging of the surface (Fig. 5k). The majority of the invaded cells in the graft were αSMA+ (Fig. 5c, g), with some αSMA+ cells also positive for calponin after 24 weeks, but not at 8 weeks (Fig. 6e, f). Evidence of inflammatory and immune cells by CD45 staining was also observed in the grafts (Supplementary Fig. S3e). The grafts at 8 weeks stained positive for subpopulations of both CD3+ and CD11b+ cells, indicating the presence of both T-lymphocyte and macrophages, respectively; the majority of these cells were located on the ablumenal side of the acellular region (Fig. 5i). Based on the low frequency of CD45+ cells at 24 weeks (Fig. 5j), there were far fewer leukocytes of all types in the graft compared with 8 weeks. Proliferative cells were labeled with Ki67, which showed negligible presence of proliferative cells within the grafts (Fig. 6c, d). While grafts possessed negligible elastin at implantation and after 8 weeks, organized elastin was deposited throughout the graft after 24 weeks (Fig. 5d, h), more so near the anastomoses compared with the mid-graft regions. Staining of 24-week explants with VVG indicated that the presence of elastic fibers concentrated toward the graft lumens (Fig. 6i). Additionally, all grafts stained negative for mineralization using Von Kossa stain (Fig. 6g, h). A comparison of staining conducted on sections from the anastomoses and mid-graft regions shows qualitative similarity at both 8 and 24 weeks (Supplementary Fig. S3).
FIG. 5.
Histological and immunohistochemical TEVG characterization at explantation. Explants at 8 weeks (top panel) and 24 weeks (middle panel) were assessed with Trichrome (a, e), and immunostaining for vWF (b, f), αSMA (c, g), and elastin (d, h). Grafts at 8 weeks were costained for CD3 (red) and CD11b (green) (i) and at 24 weeks for CD45 (green) (j). (k) scanning electron microscopy (SEM) image of graft lumenal surface at 24 weeks. In all immunostained sections, cell nuclei are counterstained with Hoechst 33342 (blue, except in “h” where nuclear staining is removed for clarity). The symbol * indicates lumenal surface. White scale bar=200 μm, black scale bar=50 μm. Color images available online at www.liebertpub.com/tea
FIG. 6.
Explant histology and immunogenicity evaluation of unseeded TEVG. Trichrome (a, b) and Ki67 (c, d) images of 8-week grafts with anastomotic region (a, c) and mid-graft region (b, d). Calponin-stained sections of acellular grafts implanted for 8 weeks (e) and 24 weeks (f). Von Kossa staining of 8-week graft (g) and 24-week graft (h) shows the absence of mineralization. Verhoeff-Van Gieson (VVG) staining of 6-month explants shows elastic fibers (i). Scale bars=200 μm and the symbol * indicates lumenal surface. Color images available online at www.liebertpub.com/tea
Discussion
A major obstacle to commercialization of TEVG is the cost and time associated with developing an autologous TEVG.22 Recently, the decellularization process has been utilized to develop acellular TEVG and circumvent this obstacle.6 The nonimmunogenic properties of allogeneic extracellular matrix have been characterized in multiple animal models.6,23,24 In this study, we utilized the ovine femoral artery model to evaluate completely biological, acellular TEVG made from fibrin remodeling by allogeneic fibroblasts in vitro as a potential solution for the clinical need of small-diameter arterial grafts. No successful arterial implant studies have yet been reported using a biopolymer-based TEVG since Weinberg and Bell proposed this approach more than 25 years ago.25
A variety of approaches have been presented for decellularization of native and engineered tissue with successful removal of cellular material.26 In this study, sequential SDS and Triton X-100 detergent treatments were used for decellularization.27 This engineered tissue required 6 h for extensive removal of cellular components and did not elicit an immune response based on a lymphocyte proliferation assay. Quint et al. reported that cellular DNA was reduced by 86% with a 2-h detergent treatment of a TEVG fabricated from a synthetic polymer tube, with a negligible drop in burst pressure.9 In comparison, a native artery required 44 h of detergent treatment to completely remove cellular material.28
Herein, decellularized fibrin-based TEVGs were implanted interpositionally in the sheep femoral artery with ultrasonography performed at 1, 8, and 20 weeks. Anastomotic stenosis corresponding with a thicker cellular region on the lumenal side of the graft was evident at 8 weeks, which was also suggested in the sham control, but this was not statistically significant at 24 weeks. There was no evidence of aneurysm formation in any grafts (9/9 grafts), nor any evidence of mineralization. The recellularization of the grafts from both the lumenal and ablumenal surfaces is similar to that reported for other TEVG implants to date.6,8 After 24 weeks, all grafts (4/4) were completely recellularized. In contrast, the presence of an acellular region up to 1 year has been reported.6 This could reflect a difference in the animal species used as well as in the nature of the graft.
The invading cells were predominantly positive for αSMA at 8 and 24 weeks, with calponin expression, which is indicative of SMC maturation, evident at 24 weeks. The αSMA-positive cells appeared to be invading from both the ablumenal and lumenal surfaces of the grafts. In the former case, these cells were presumably derived from adjacent tissue. In the latter case, the cells could have been of circulating origin such as fibrocytes29 or resulted from endothelial-to-mesenchymal transition30 of endothelial cells that migrated from the anastomoses or progenitor cells that adhered from the blood.
Immunostaining for elastin showed fiber structures present at 24 weeks throughout the length of the construct; biochemical assays revealed that the elastin content attained 8.8% of the femoral artery value. Other indications of graft remodeling by invading cells were increases in collagen concentration and mechanical anisotropy observed at 24 weeks.
At 8 weeks, macrophages and T-lymphocytes were present in the grafts, particularly within the acellular region. Macrophages are likely associated with the early stages of inflammation-mediated TEVG remodeling leading to recruitment of αSMA+ cells via paracrine mechanisms as in the mouse model.31,32 At 24 weeks, there was complete recellularization with αSMA+ cells and only rare CD45+ cells, consistent with the hypothesis that favorable remodeling coincides with the resolution of inflammation and an absence of immunogenicity of these grafts.
Short TEVGs (2–3 cm) that were implanted interpositionally were used in these studies to evaluate graft remodeling as efficiently as possible. Substantially longer TEVGs made from human dermal fibroblasts are needed to be clinically relevant as coronary artery bypass grafts and they must be evaluated as bypass grafts; using the same methods as described herein, we have fabricated 10-cm human grafts with burst pressure exceeding 2500 mmHg following decellularization as proof-of-principle (unpublished studies). Also, creating a hemocompatible surface will be necessary. We report elsewhere studies of these grafts preseeded with endothelial cells.33
It is well known that predominate circumferential alignment of the media in the native artery confers its unique biomechanical properties.34 The method used to create the fibrin-based TEVG—cell-induced gel compaction on a nonadhesive mandrel, which allows for axial shortening, but with mechanically constrained circumferential and radial compaction—yields strong circumferential alignment.35,36 While axial constraint of gel compaction can be used to create fibrin-based TEVG without circumferential alignment, this leads to reduced circumferential stiffness and strength as well as loss of mechanical anisotropy characteristic of the native artery and thus was not considered for these studies. Compared to the femoral artery, the pre-implant fibrin-based TEVG had a thinner wall, and similar collagen content, but higher burst pressure. As one possible explanation, unlike the native artery, where collagen in the media is strongly aligned circumferentially but adventitial collagen is not aligned, all of the collagen appears highly aligned in our TEVG. The crosslinking density in the TEVG may also be higher.
In conclusion, this study demonstrates the first long-term function of a completely biological engineered arterial graft fabricated in vitro that possesses the circumferential alignment and mechanical anisotropy characteristic of native arteries.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the University of Minnesota Experimental Surgical Service staff for surgical assistance, Jim Berry for ultrasonography measurements, and Pat Schaeffer for histological sectioning/staining. The funding for these studies was provided by NIH R01 HL-083880 (to R.T.T.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Conte M.S.The ideal small arterial substitute: a search for the Holy Grail? FASEB J 12,43, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Kannan R.Y., Salacinski H.J., Butler P.E., Hamilton G., and Seifalian A.M.Current status of prosthetic bypass grafts: a review. J Biomed Mater Res Part B Appl Biomater 74,570, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Peck M., Gebhart D., Dusserre N., McAllister T.N., and L'Heureux N.The evolution of vascular tissue engineering and current state of the art. Cells Tissues Organs 195,144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niklason L.E., Gao J., Abbott W.M., Hirschi K.K., Houser S., Marini R., et al. Functional arteries grown in vitro. Science 284,489, 1999 [DOI] [PubMed] [Google Scholar]
- 5.L'Heureux N., Paquet S., Labbe R., Germain L., and Auger F.A.A completely biological tissue-engineered human blood vessel. FASEB J 12,47, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Dahl S.L., Kypson A.P., Lawson J.H., Blum J.L., Strader J.T., Li Y., et al. Readily available tissue-engineered vascular grafts. Sci Transl Med 3,68ra9 2011 [DOI] [PubMed] [Google Scholar]
- 7.L'Heureux N., McAllister T.N., and de la Fuente L.M.Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med 357,1451, 2007 [DOI] [PubMed] [Google Scholar]
- 8.L'Heureux N., Dusserre N., Konig G., Victor B., Keire P., Wight T.N., et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med 12,361, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quint C., Kondo Y., Manson R.J., Lawson J.H., Dardik A., and Niklason L.E.Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A 108,9214, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wystrychowski W., McAllister T.N., Zagalski K., Dusserre N., Cierpka L., and L'Heureux N.First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J Vasc Surg pii:, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Grassl E.D., Oegema T.R., and Tranquillo R.T.Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. J Biomed Mater Res 60,607, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Gleason R.L., Wilson E., and Humphrey J.D.Biaxial biomechanical adaptations of mouse carotid arteries cultured at altered axial extension. J Biomech 40,766, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Syedain Z.H., Meier L.A., Bjork J.W., Lee A., and Tranquillo R.T.Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials 32,714, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallegos R.P., Nockel P.J., Rivard A.L., and Bianco R.W.The current state of in-vivo pre-clinical animal models for heart valve evaluation. J Heart Valve Dis 14,423, 2005 [PubMed] [Google Scholar]
- 15.Byrom M.J., Bannon P.G., White G.H., and Ng M.K.Animal models for the assessment of novel vascular conduits. J Vasc Surg 52,176, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Syedain Z.H., Meier L.A., Lahti M.T., Bianco R.W., Kren S., Taylor D.A., and Tranquillo R.T.In vivo remodeling of fibrin-based tissue-engineered arterial grafts. Regenerative Medicine: Innovations for Clinical Applications. Hilton Head, SC, 2012 [Google Scholar]
- 17.Bjork J.W., Meier L.A., Johnson S.L., Syedain Z.H., and Tranquillo R.T.Hypoxic culture and insulin yield improvements to fibrin-based engineered tissue. Tissue Eng Part A 18,785, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stegemann H., and Stalder K.Determination of hydroxyproline. Clin Chim Acta 18,267, 1967 [DOI] [PubMed] [Google Scholar]
- 19.Starcher B.C., and Galione M.J.Purification and comparison of elastins from different animal species. Anal Biochem 74,441, 1976 [DOI] [PubMed] [Google Scholar]
- 20.Kim Y.J., Sah R.L., Doong J.Y., and Grodzinsky A.J.Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem 174,168, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Bjork J.W., Johnson S.L., and Tranquillo R.T.Ruthenium-catalyzed photo cross-linking of fibrin-based engineered tissue. Biomaterials 32,2479, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAllister T.N., Dusserre N., Maruszewski M., and L'Heureux N.Cell-based therapeutics from an economic perspective: primed for a commercial success or a research sinkhole? Regen Med 3,925, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Lehr E.J., Rayat G.R., Chiu B., Churchill T., McGann L.E., Coe J.Y., et al. Decellularization reduces immunogenicity of sheep pulmonary artery vascular patches. J Thorac Cardiovasc Surg 141,1056, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Muratov R., Britikov D., Sachkov A., Akatov V., Soloviev V., Fadeeva I., et al. New approach to reduce allograft tissue immunogenicity. Experimental data. Interact Cardiovasc Thorac Surg 10,408, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Weinberg C.B., and Bell E.A blood vessel model constructed from collagen and cultured vascular cells. Science 231,397, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Gilbert T.W., Sellaro T.L., and Badylak S.F.Decellularization of tissues and organs. Biomaterials 27,3675, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Ott H.C., Matthiesen T.S., Goh S.K., Black L.D., Kren S.M., Netoff T.I., et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14,213, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Gui L., Chan S.A., Breuer C.K., and Niklason L.E.Novel utilization of serum in tissue decellularization. Tissue Eng Part C Methods 16,173, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quan T.E., Cowper S.E., and Bucala R.The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep 8,145, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Piera-Velazquez S., Li Z., and Jimenez S.A.Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol 179,1074, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hibino N., Yi T., Duncan D.R., Rathore A., Dean E., Naito Y., et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J 25,4253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roh J.D., Sawh-Martinez R., Brennan M.P., Jay S.M., Devine L., Rao D.A., et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A 107,4669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meier L.A., Syedain Z.H., Lahti M.T., Johnson S.S., Chen M.H., Hebbel R.P., et al.Blood outgrowth endothelial cells alter remodeling of completely biological engineered grafts implanted into the sheep femoral artery. J Cardiovasc Transl Res 2014. [Epub ahead of print]; DOI: 10.1007/s12265-013-9539-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.JD H.Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. New York: Springer-Verlag, 2002 [Google Scholar]
- 35.Barocas V.H., Girton T.S., and Tranquillo R.T.Engineered alignment in media equivalents: magnetic prealignment and mandrel compaction. J Biomech Eng 120,660, 1998 [DOI] [PubMed] [Google Scholar]
- 36.L'Heureux N., Germain L., Labbe R., and Auger F.A.In vitro construction of a human blood vessel from cultured vascular cells: a morphologic study. J Vasc Surg 17,499, 1993 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.