Abstract
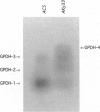
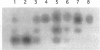
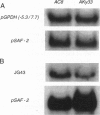
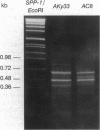
The insertion of the blood retrotransposon into the untranslated region of exon 7 of the sn-glycerol-3-phosphate dehydrogenase-encoding gene (Gpdh) in Drosophila melanogaster induces a GPDH isozyme-GPDH-4-and alters the pattern of expression of the three normal isozymes-GPDH-1 to GPDH-3. The process of transcript terminus formation inside the retrotransposon insertion reduces the level of the Gpdh transcript that contains exon 8 and increases the level of the transcript that contains exons 1-7. The induced GPDH-4 isozyme is a translation product of the three transcripts that contain fragments of the blood retrotransposon. The mechanism of mutagenesis by the blood insertion is postulated to involve the pause or termination of transcription within the blood sequence, which in turn is caused by the interference of a DNA-binding protein with the RNA polymerase. Thus, we show the formation of a new functional GPDH protein by the insertion of a transposable element and discuss the evolutionary significance of this phenomenon.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banki K., Halladay D., Perl A. Cloning and expression of the human gene for transaldolase. A novel highly repetitive element constitutes an integral part of the coding sequence. J Biol Chem. 1994 Jan 28;269(4):2847–2851. [PubMed] [Google Scholar]
- Bewley G. C., Cook J. L., Kusakabe S., Mukai T., Rigby D. L., Chambers G. K. Sequence, structure and evolution of the gene coding for sn-glycerol-3-phosphate dehydrogenase in Drosophila melanogaster. Nucleic Acids Res. 1989 Nov 11;17(21):8553–8567. doi: 10.1093/nar/17.21.8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. M., Chapman C. H. Evidence that white-blood is a novel type of temperature-sensitive mutation resulting from temperature-dependent effects of a transposon insertion on formation of white transcripts. EMBO J. 1986 Dec 1;5(12):3343–3351. doi: 10.1002/j.1460-2075.1986.tb04649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biémont C. Population genetics of transposable DNA elements. A Drosophila point of view. Genetica. 1992;86(1-3):67–84. doi: 10.1007/BF00133712. [DOI] [PubMed] [Google Scholar]
- Chia W., Savakis C., Karp R., Pelham H., Ashburner M. Mutation of the Adh gene of Drosophila melanogaster containing an internal tandem duplication. J Mol Biol. 1985 Dec 20;186(4):679–688. doi: 10.1016/0022-2836(85)90388-2. [DOI] [PubMed] [Google Scholar]
- Cook J. L., Bewley G. C., Shaffer J. B. Drosophila sn-glycerol-3-phosphate dehydrogenase isozymes are generated by alternate pathways of RNA processing resulting in different carboxyl-terminal amino acid sequences. J Biol Chem. 1988 Aug 5;263(22):10858–10864. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsett D. Potentiation of a polyadenylylation site by a downstream protein-DNA interaction. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4373–4377. doi: 10.1073/pnas.87.11.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsett D., Viglianti G. A., Rutledge B. J., Meselson M. Alteration of hsp82 gene expression by the gypsy transposon and suppressor genes in Drosophila melanogaster. Genes Dev. 1989 Apr;3(4):454–468. doi: 10.1101/gad.3.4.454. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J. Eukaryotic transposable elements and genome evolution. Trends Genet. 1989 Apr;5(4):103–107. doi: 10.1016/0168-9525(89)90039-5. [DOI] [PubMed] [Google Scholar]
- Flavell A. J. Ty1-copia group retrotransposons and the evolution of retroelements in the eukaryotes. Genetica. 1992;86(1-3):203–214. doi: 10.1007/BF00133721. [DOI] [PubMed] [Google Scholar]
- Freeth A. L., Gibson J. B., Wilks A. V. Transcription analysis of alcohol dehydrogenase null alleles from natural populations of Drosophila melanogaster. Genome. 1988 Feb;30(1):25–30. doi: 10.1139/g88-005. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. B., Cao A., Symonds J., Reed D. Low activity sn-glycerol-3-phosphate dehydrogenase variants in natural populations of Drosophila melanogaster. Heredity (Edinb) 1991 Feb;66(Pt 1):75–82. doi: 10.1038/hdy.1991.10. [DOI] [PubMed] [Google Scholar]
- Gibson J. B., Wilks A. V., Agrotis A. Molecular relationships between alcohol dehydrogenase null-activity alleles from natural populations of Drosophila melanogaster. Mol Biol Evol. 1992 Mar;9(2):250–260. doi: 10.1093/oxfordjournals.molbev.a040717. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild N. L., Wilkinson D. A., Mager D. L. A human endogenous long terminal repeat provides a polyadenylation signal to a novel, alternatively spliced transcript in normal placenta. Gene. 1992 Nov 16;121(2):287–294. doi: 10.1016/0378-1119(92)90133-a. [DOI] [PubMed] [Google Scholar]
- O'Brien S. J., Macintyre R. J. The -glycerophosphate in Drosophila melanogaster. II. Genetic aspects. Genetics. 1972 May;71(1):127–138. doi: 10.1093/genetics/71.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K., Murphy C., Levis R., Rubin G. M. DNA sequence of the white locus of Drosophila melanogaster. J Mol Biol. 1984 Dec 15;180(3):437–455. doi: 10.1016/0022-2836(84)90021-4. [DOI] [PubMed] [Google Scholar]
- Reed D. S., Gibson J. B. Defective P element insertions affect the expression of sn-glycerol-3-phosphate dehydrogenase alleles in natural populations of Drosophila melanogaster. Proc Biol Sci. 1993 Jan 22;251(1330):39–45. doi: 10.1098/rspb.1993.0006. [DOI] [PubMed] [Google Scholar]
- Reed D. S., Gibson J. B. Molecular heterogeneity of naturally occurring sn-glycerol-3-phosphate dehydrogenase low-activity variants in Drosophila melanogaster. Biochem Genet. 1994 Jun;32(5-6):161–179. doi: 10.1007/BF00554620. [DOI] [PubMed] [Google Scholar]
- Smith P. A., Corces V. G. Drosophila transposable elements: mechanisms of mutagenesis and interactions with the host genome. Adv Genet. 1991;29:229–300. doi: 10.1016/s0065-2660(08)60109-1. [DOI] [PubMed] [Google Scholar]
- Symonds J. E., Gibson J. B. Restriction site variation, gene duplication, and the activity of sn-glycerol-3-phosphate dehydrogenase in Drosophila melanogaster. Biochem Genet. 1992 Apr;30(3-4):169–188. doi: 10.1007/BF02399707. [DOI] [PubMed] [Google Scholar]
- Triglia T., Peterson M. G., Kemp D. J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988 Aug 25;16(16):8186–8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D. A., Shaw C. R. Genetics and ontogeny of alpha-glycerophosphate dehydrogenase isozymes in Drosophila melanogaster. Biochem Genet. 1969 Aug;3(4):343–353. doi: 10.1007/BF00485718. [DOI] [PubMed] [Google Scholar]
- von Kalm L., Weaver J., DeMarco J., MacIntyre R. J., Sullivan D. T. Structural characterization of the alpha-glycerol-3-phosphate dehydrogenase-encoding gene of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5020–5024. doi: 10.1073/pnas.86.13.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]