SUMMARY
To date, little is known about the dynamics of vertical transmission of Toxoplasma gondii in Australian marsupials. Studies in mice demonstrate that vertical transmission of T. gondii is common and that chronically infected mice can transmit T. gondii to successive generations. In this study, PCR and immunohistochemistry were used to detect T. gondii in chronically infected marsupial dams and their offspring. T. gondii was detected in the unfurred pouch young of 2 out of 10 chronically infected western grey kangaroos (Macropus fuliginosus) and in the unfurred pouch young of a brush-tailed bettong (Bettongia penicillata). Results of the study suggest vertical transmission of T. gondii can occur in chronically infected Australian marsupials.
Keywords: Toxoplasma gondii, Marsupial, Vertical transmission, Australia, Congenital, Toxoplasmosis
INTRODUCTION
Vertical (transplacental or transmammary) transmission of Toxoplasma gondii and its influence on the maintenance of T. gondii in natural populations has been a matter of debate (Johnson 1997). Although it has long been known that congenital infection with T. gondii in mice and guinea pigs can occur while the dam is chronically infected (Remington et al. 1961), more recent studies have ignited debate as to whether vertical transmission occurs in other chronically infected animals. Recent studies have verified the high frequency of congenital transmission of T. gondii in chronically infected mice, and it was proposed congenital transmission in chronically infected mice can maintain T. gondii infection in wild mouse populations (Owen and Trees, 1998; Marshall et al. 2004). Recent data also suggests T. gondii can be transmitted via successive vertical transmission within families of sheep (Morley et al. 2005).
Evidence for vertical transmission in marsupials to date is anecdotal (Boorman et al. 1977; Dubey et al. 1988), and the dynamics of vertical transmission in marsupials is poorly understood. However, considering the potential impact of toxoplasmosis in marsupials and the current efforts associated with wildlife conservation, it is important to examine the causes of infection of Australian marsupials with T. gondii. Australian marsupials are highly susceptible hosts for T. gondii, and the parasite causes both chronic and acutely fatal infection (Beveridge 1993). Information on the occurrence of vertical transmission in chronically infected marsupials may benefit captive breeding programmes of Australian marsupials by ensuring only T. gondii-free animals are bred, thereby improving animal health and assisting animal conservation and management.
In order to better understand T. gondii transmission in marsupials, we tested the dam and pouch young of western grey kangaroos (Macropus fuliginosus) and a woylie (Bettongia penicillata) for evidence of T. gondii infection. The MAT (modified agglutination test) and DAT (direct agglutination test) were used to test for anti-T. gondii IgM in sera of western grey kangaroo dams. Experimental studies in eastern grey kangaroos demonstrate that differences in titre between MAT and DAT are indicative of an IgM response and acute T. gondii infection (Johnson et al. 1989). Subsequent studies in a range of marsupial species have successfully used the MAT and DAT to diagnose acute T. gondii infection (Bettiol et al. 2000; Hartley 2006; Lynch et al. 1993; Skerratt et al. 1997). All pouch young were tested before the time of first pouch exit. While within the pouch, young are protected from the external environment and so are extremely unlikely to be exposed to T. gondii oocysts. Marsupial young are born at a very immature state (less than 1gram neonatal weight) at which time they enter the pouch. Young first exit the pouch after a long period of permanent residence (Tyndale-Biscoe and Renfree 1987). Immunohistochemistry and PCR were used to detect T. gondii in the tissue of a woylie dam and western grey kangaroo dams and their corresponding pouch young.
MATERIALS AND METHODS
Western grey kangaroo sera and tissues were obtained from kangaroos culled during Department of Environment and Conservation (DEC) population control programmes in Perth, Western Australia. Kangaroos were culled from a large reserve due to overpopulation. Sixty two dams were obtained, from which samples of blood, tongue and brain were collected. Blood was collected using intracardiac puncture. Each dam’s head was removed and labelled. Brain and tongue samples were collected after heads were transported to a laboratory and stored at 4 °C for a maximum of 3 days prior to processing. Samples of brain and tongue were dissected, placed in sterile containers and frozen at -20°C. The young present in each dam’s pouch were killed in line with population control measures, via blunt trauma to the head in field. Pouch young were labelled and stored at 4°C for a maximum of 3 days prior to processing in the laboratory. Each pouch young was weighed and measured to estimate its age (Poole et al. 1982) after which blood was collected via intracardiac puncture. All sera collected were separated via centrifugation and stored at -20 °C. Pouch young were dissected under sterile conditions to obtain samples of brain, heart, skeletal muscle, liver, lung, small intestine, kidney and spleen. Some portions of tissues were frozen at -20°C for DNA extraction while other portions were placed in 10% buffered formalin for histology.
A female woylie with young in pouch from a wild population near Manjimup, Western Australia, was submitted to Murdoch University Veterinary Hospital for necropsy with a history of neurological signs. Tissue samples were placed in 10% buffered formalin and consisted of brain, heart, skeletal muscle, lung, liver, spleen and mammary tissue. In addition, brain, heart and mammary gland tissue samples were set aside in 70% ethanol for DNA extraction. The furless pouch young present in the pouch of the necropsied woylie had samples of brain, heart, skeletal muscle, lung and liver removed and placed in 70% ethanol for DNA extraction. The lack of fur in the woylie pouch young sampled indicated it was too immature to have ever left the pouch.
The 62 western grey kangaroo dams and the corresponding 62 pouch young were all screened for T. gondii antibodies using an ELISA (Parameswaran et al. 2009) to detect IgG antibodies to T. gondii in macropod marsupials. Sera samples of seropositive dams were sent to the Animal Health Laboratory, Tasmania to obtain MAT (modified agglutination test) and DAT (direct agglutination test) titres in order to determine the presence of T. gondii IgM. No serum samples were taken from the adult woylie or its pouch young.
The brain and tongue of seropositive and seronegative western grey kangaroo dams and a range of tissues of their offspring underwent DNA extraction. In addition, the brain, heart and mammary gland tissue of a woylie dam and the brain, heart, skeletal muscle, lung and liver of its pouch young underwent DNA extraction. Three methods of DNA extraction were used for each sample. DNA samples were extracted using a MasterPure DNA purification kit (Epicentre Biotechnologies, Madison, USA). A method of phenol-chloroform DNA extraction was also used (Miller et al. 2008). In addition, extraction using QIAamp DNA MiniKit (QIAGEN Hilden, Germany) was used. In each DNA extraction 25 mg of homogenised tissue was used. Tissues of seronegative dams and seronegative pouch young were used as DNA extraction negative controls.
Samples of DNA extracted from tissue specimens underwent nested PCR amplification for T. gondii using nested primers at the ITS1 locus (Miller et al. 2001) and B1 gene, (Bretagne et al. 1993; Grigg and Boothroyd 2001). Each sample of DNA was tested once using each set of primers. DNA from the T. gondii RH strain (Type I strain) was used as a positive PCR control and PCR negative controls consisted of distilled water. PCR products were visualized using 0.8% agarose gels stained with ethidium bromide. To confirm infection with T. gondii, ITS1 and B1 PCR products were gel-purified from agarose gels using the UltraClean GelSpin DNA Extraction Kit (MO BIO Laboratories Inc, Carlsbad, USA) prior to DNA sequencing. Sequencing reactions were performed using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Scoresby, Australia) according to the manufacturer’s directions using the internal primers used to PCR amplify the ITS1 and B1 gene sequences. Reactions were electrophoresed through an ABI 3730 automatic sequencer and sequencing profiles analysed using FinchTV version 1.4 (Geospiza, Seattle, USA). Sequences were subjected to BlastN analysis using the GenBank nr-nt database at NCBI to confirm that the PCR-amplified sequences were that of T. gondii.
Formalin-fixed tissue samples were trimmed and processed before being embedded in paraffin wax, sectioned and stained with haematoxylin and eosin. Paraffin embedded tissues were also sectioned and immunohistochemically stained with rabbit polyclonal antibodies to T. gondii, as previously described by Lindsay and Dubey (1989). Stored paraffin embedded brain tissue from a T. gondii infected cat was used as a positive control for immunohistochemistry. Brain tissue was not included in histological analysis of western grey kangaroo pouch young due to autolysis, associated with post-mortem changes. (Lindsay and Dubey 1989)
RESULTS AND DISCUSSION
Of 62 Western Kangaroo dams that were screened for T. gondii antibodies using an ELISA, 10 dams were seropositive for T. gondii and 7 of these dams had corresponding seropositive pouch young (Table 1). The remaining 52 seronegative dams all had corresponding seronegative pouch young. All 10 ELISA seropositive dams were also MAT and DAT positive. MAT and DAT titres were identical in all western grey kangaroo dams, which indicated a lack of IgM in all dam sera samples (Table 2). The lack of IgM in the sera samples is suggestive of chronic T. gondii infection in the western grey kangaroos.
Table 1.
Results of various PCRs for western grey kangaroo dams and their pouch young.
| PCR Dams | PCR PY | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Dam ID | ELISA Dam | ELISA PY | Age PY (days) | Brain | Tongue | Brain | Heart | Sk musc | Lung | Liver | Kidney | Spleen | Sm Intest |
| C14 | Positive | Positive | 145 | B1, ITS1 | N | N | B1 | N | N | N | nd | N | N |
| C9 | Positive | Positive | 114 | B1, ITS1 | ITS1 | N | N | N | N | N | nd | N | N |
| J6 | Positive | Positive | 124 | B1, ITS1 | ITS1 | N | N | N | nd | nd | nd | nd | N |
| J10 | Positive | Positive | 125 | nd | B1, ITS1 | N | N | N | N | N | N | N | N |
| R7 | Positive | Positive | 90 | B1, ITS1 | N | N | N | N | nd | nd | nd | nd | nd |
| Q1 | Positive | Positive | 154 | N | B1, ITS1 | N | N | N | nd | nd | nd | nd | nd |
| G21 | Positive | Negative | 58 | B1 | ITS1 | N | N | N | nd | nd | nd | nd | nd |
| F19 | Positive | Negative | 132 | ITS1 | N | N | N | N | nd | nd | nd | nd | nd |
| R19 | Positive | Negative | 142 | ITS1 | N | N | B1 | N | nd | nd | nd | nd | nd |
| 15B1 | Positive | Positive | 246 | nd | nd | N | N | N | N | N | N | N | N |
| R4 | Negative | Negative | 89 | nd | nd | N | N | nd | nd | nd | nd | nd | nd |
| F8 | Negative | Negative | 98 | N | N | N | N | nd | nd | nd | nd | nd | nd |
| H14 | Negative | Negative | 84 | N | N | N | N | N | nd | nd | nd | nd | nd |
| I14 | Negative | Negative | 75 | N | N | N | N | nd | nd | nd | nd | nd | nd |
| Q20 | Negative | Negative | 129 | nd | nd | N | N | nd | nd | nd | nd | nd | nd |
| 15B2 | Negative | Negative | 233 | nd | nd | N | N | N | N | N | N | N | N |
Abbreviation key:
B1- Positive B1 PCR (Bretagne et al., 1993)
B1- Positive B1 PCR (Grigg and Boothroyd, 2001)
ITS1- Positive ITS1 PCR (Miller et al, 2001)
N- Negative on all PCRs
nd- No tests undertaken
Sk musc- Skeletal muscle
Sm intest- Small intestine
Table 2.
T. gondii DAT and MAT titres of seropositive western grey kangaroo dams
| Dam ID | DAT | MAT |
|---|---|---|
| C14 | 256000 | 256000 |
| C9 | 256000 | 256000 |
| J6 | 4096 | 4096 |
| J10 | 4096 | 4096 |
| R7 | 64000 | 64000 |
| Q1 | 4096 | 4096 |
| G21 | 64000 | 64000 |
| F19 | 4096 | 4096 |
| R19 | 4096 | 4096 |
| 15B1 | 64000 | 64000 |
To confirm the ELISA results, DNA extracted from 9 kangaroo dam tissues was tested for the presence of T. gondii DNA by PCR. One seropositive kangaroo dam was not tested due to the absence of tissue samples. All 9 seropositive western grey kangaroo dams were PCR positive for the presence of T. gondii DNA using primers at the ITS1 locus and B1 gene, confirming the serology results (Table 1). Negative controls included DNA extracted from tissues of three seronegative dams and 6 pouch young from seronegative dams. All negative controls were PCR negative for T. gondii. Serology results in adult kangaroos had a high correlation with PCR results, suggesting the PCR methods used were sensitive and specific.
For the 10 chronically infected western grey kangaroos, T. gondii DNA was detected in the heart tissue of two pouch young from the seropositive dams. One of the T. gondii PCR-positive pouch young was seronegative by the ELISA assay. No T. gondii specific DNA was detected in tissues from the remaining 8 pouch young. All ITS-1 and B1 PCR bands amplified were sequenced and were confirmed to be that of T. gondii (data not shown). As PCR results had a high correlation with serology results in adult kangaroos, it is likely that the 6 seropositive, PCR negative kangaroo pouch young were not infected with T. gondii and were only seropositive due to the passive transfer of antibodies from the dam.
PCR using primers for B1 and ITS1 detected T. gondii DNA in the mammary gland of the one woylie tested and in the brain of its corresponding pouch young. All other woylie tissue tested was negative for T. gondii DNA. Identification of T. gondii DNA in the mammary gland of the woylie dam suggests infection of the woylie pouch young was from suckling breast milk. Clean PCR amplification products were observed for both the B1 gene (Figure 1) and ITS1 locus (Figure 2). Both ITS1 and B1 PCR bands sequenced were that of T. gondii (data not shown).
Figure 1.
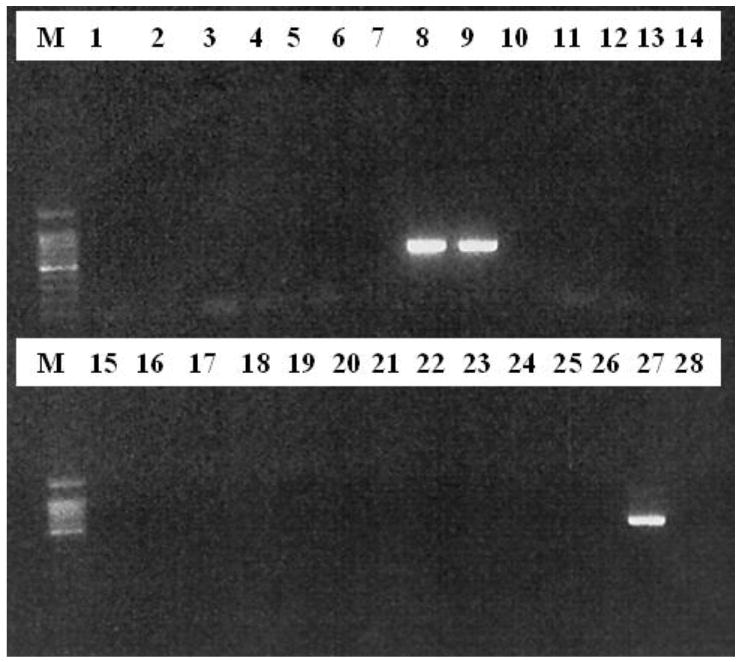
Agarose gel of nested PCR for the B1 gene (Grigg and Boothroyd, 2001) Lane M, 100bp DNA ladder; Lane 1, PYG21, brain; Lane 2, PYG21, heart; Lane 3, PYG21, skeletal muscle; Lane 4, PYF19, brain; Lane 5, PYF19, heart; Lane 6, PYF19, skeletal muscle; Lane 7, PYR19, brain; Lane 8, PYR19, heart; Lane 9, PYR19 heart 1:10 dilution; Lane 10, PYR19, skeletal muscle; Lane 11, PY15B1, brain; Lane 12, PY15B1, heart; Lane 13, PY15B1, skeletal muscle; Lane 14, PY15B1, lung; Lane 15, PY15B1, liver; Lane 16, PY15B1, kidney; Lane 17, PY15B1, spleen; Lane 18, PY15B1, small intestine; Lane 19, PYR4, brain; Lane 20, PYR4, heart; Lane 21, PYF8, brain; Lane 22, PYF8 heart; Lane 23, PYH14, brain; Lane 24, PYH14, heart; Lane 25, PYI14, brain; Lane 25, PYI14, heart; Lane 27, T. gondii RH strain positive control; Lane 28, T. gondii negative control.
Figure 2.
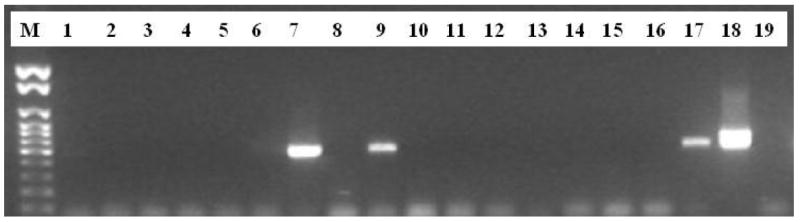
Agarose gel of nested PCR for the ITS1 locus (Miller et al, 2001) Lane M, 100bp DNA ladder; Lane 1, PYR4, brain. Lane 2, PYR4, heart. Lane 3, PYF8, brain. Lane 4, PYF8, heart. Lane 5, PYH14, brain. Lane 6, PYH14, heart. Lane 7, R7, brain. Lane 8, R7, tongue. Lane 9, Q1, brain. Lane 10, Q1, tongue. Lane 11, PYI14, brain. Lane 12, PYI14, heart. Lane 13, PYQ20, brain. Lane 14, PYQ20, heart, Lane 15, PY15B2, brain, Lane 16, PY15B2, heart. Lane 17, C14, brain. Lane 18, T. gondii positive control. Lane 19, T. gondii negative control.
It is highly unlikely that the pouch young tested in this study were exposed to T. gondii oocysts from the external environment. Marsupial young first exit the pouch after a long period of permanent residence and while within the pouch, young are protected from the external environment (Tyndale-Biscoe and Renfree 1987). All western grey kangaroo pouch young in this study were tested before the time of first pouch exit, which is ~ 298 days for this species (Tyndale-Biscoe and Renfree 1987). In addition, the woylie pouch young in this study was unfurred and therefore too young to have left the pouch.
Generalised congestion and oedema of the lung was observed in all western grey kangaroo pouch young. No other significant lesions were observed in histological sections from all other pouch young tested from seropositive and seronegative dams. Upon immunohistochemistry, no T. gondii tachyzoites or cysts were found in the pouch young tested. The presence of T. gondii was, however, detected in the positive control tissues analysed at the same time. T. gondii was not detected upon histology or immunohistochemistry in the adult woylie. Histologically there was acute diffuse pulmonary congestion and oedema, and autolysis of the intestines. In addition, there was a small haematoma and focus of inflammation associated with one mammary gland.
The detection of T. gondii DNA in only the heart muscle of the 2 western grey kangaroo pouch young is not surprising; T. gondii has been detected previously in the heart muscle of a black-faced kangaroo (Macropus fuliginosus melanops) pouch young (Dubey et al. 1988) and two juvenile common wombats (Vombatus ursinus) (Hartley 2006), and is known to commonly infect the heart of adult macropod marsupials (Canfield et al. 1990). However, as no pathology or T. gondii organisms was observed upon pathologic and immunohistological examination of tissue sections from the two PCR-positive pouch young in this study, this suggests that neither of the PCR positive pouch young had clinical toxoplasmosis.
This is the first evidence of vertical transmission of T. gondii in chronically infected marsupials and points to further research regarding the frequency of vertical transmission of T. gondii in Australian marsupials. Information on the frequency of vertical transmission of T. gondii in chronically infected Australian marsupials would benefit captive breeding programmes of endangered Australian marsupial species and contribute to the management of wild populations.
Acknowledgments
We wish to thank Glen Goudie and his team for their immense help with collection of western grey kangaroo samples. This research was supported by the Australian Research Council, Department of Environment and Conservation, Western Australia and in part by the Intramural Research Program of the NIH and NIAID (MEG). MEG is a Scholar of the Canadian Institute for Advanced Research (CIFAR) Integrated Microbial Biodiversity Program.
References
- Bettiol SS, Obendorf DL, Nowarkowski M, Goldsmid JM. Pathology of experimental toxoplasmosis in eastern barred bandicoots in Tasmania. J Wildl Dis. 2000;36:141–144. doi: 10.7589/0090-3558-36.1.141. [DOI] [PubMed] [Google Scholar]
- Beveridge I. Marsupial parasitic diseases. In: Fowler M, editor. Zoo & wild animal medicine : current therapy. W.B. Saunders; Phildelphia: 1993. pp. 288–293. [Google Scholar]
- Boorman GA, Kollias GV, Taylor RF. An outbreak of toxoplasmosis in wallaroos (Macropus robustus) in a California zoo. J Wildl Dis. 1977;13:64–68. doi: 10.7589/0090-3558-13.1.64. [DOI] [PubMed] [Google Scholar]
- Bretagne S, Costa JM, Vidaud M, Tran J, Nhieu V, Fleury-Feith J. Detection of Toxoplasma gondii by competitive DNA amplification of bronchoalveolar lavage samples. J Infect Dis. 1993;168:1585–1588. doi: 10.1093/infdis/168.6.1585. [DOI] [PubMed] [Google Scholar]
- Canfield PJ, Hartley WJ, Dubey JP. Lesions of toxoplasmosis in Australian marsupials. J Comp Pathol. 1990;103:159–167. doi: 10.1016/s0021-9975(08)80172-7. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Ott-Joslin J, Torgerson RW, Topper MJ, Sundberg JP. Toxoplasmosis in black-faced kangaroos (Macropus fuliginosus melanops) Vet Parasitol. 1988;30:97–105. doi: 10.1016/0304-4017(88)90156-2. [DOI] [PubMed] [Google Scholar]
- Grigg ME, Boothroyd JC. Rapid identification of virulent type I strains of the protozoan pathogen Toxoplasma gondii by PCR-restriction fragment length polymorphism analysis at the B1 gene. J Clin Microbiol. 2001;39:398–400. doi: 10.1128/JCM.39.1.398-400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley MP. Toxoplasma gondii infection in two common wombats (Vombatus ursinus) Aust Vet J. 2006;84:107–109. doi: 10.1111/j.1751-0813.2006.tb12242.x. [DOI] [PubMed] [Google Scholar]
- Johnson AM. Speculation on possible life cycles for the clonal lineages in the genus toxoplasma. Parasitol Today. 1997;13:393–397. doi: 10.1016/s0169-4758(97)01129-0. [DOI] [PubMed] [Google Scholar]
- Johnson AM, Roberts H, Statham P, Munday BL. Serodiagnosis of acute toxoplasmosis in macropods. Vet Parasitol. 1989;34:25–33. doi: 10.1016/0304-4017(89)90160-x. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Dubey JP. Immunohistochemical diagnosis of Neospora caninum in tissue sections. Am J Vet Res. 1989;50:1981–1983. [PubMed] [Google Scholar]
- Lynch MJ, Obendorf DL, Statham P, Reddacliff GL. An evaluation of a live Toxoplasma gondii vaccine in Tammar wallabies (Macropus eugenii) Aust Vet J. 1993;70:352–353. doi: 10.1111/j.1751-0813.1993.tb00884.x. [DOI] [PubMed] [Google Scholar]
- Marshall PA, Hughes JM, Williams RH, Smith JE, Murphy RG, Hide G. Detection of high levels of congenital transmission of Toxoplasma gondii in natural urban populations of Mus domesticus. Parasitology. 2004;128:39–42. doi: 10.1017/s0031182003004189. [DOI] [PubMed] [Google Scholar]
- Miller M, Sverlow K, Crosbie P, Barr B, Lowenstine L, Gulland F, Packham A, Conrad P. Isolation and characterization of two parasitic protozoa from a Pacific harbor seal (Phoca vitulina richardsi) with meningoencephalomyelitis. J Parasitol. 2001;87:816–822. doi: 10.1645/0022-3395(2001)087[0816:IACOTP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Miller MA, Miller WA, Conrad PA, James ER, Melli AC, Leutenegger CM, Dabritz HA, Packham AE, Paradies D, Harris M, Ames J, Jessup DA, Worcester K, Grigg ME. Type X Toxoplasma gondii in a wild mussel and terrestrial carnivores from coastal California: New linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters. Int J Parasitol. 2008 doi: 10.1016/j.ijpara.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Morley EK, Williams RH, Hughes JM, Terry RS, Duncanson P, Smith JE, Hide G. Significant familial differences in the frequency of abortion and Toxoplasma gondii infection within a flock of Charollais sheep. Parasitology. 2005;131:181–185. doi: 10.1017/s0031182005007614. [DOI] [PubMed] [Google Scholar]
- Owen MR, Trees AJ. Vertical transmission of Toxoplasma gondii from chronically infected house (Mus musculus) and field (Apodemus sylvaticus) mice determined by polymerase chain reaction. Parasitology. 1998;116(Pt 4):299–304. doi: 10.1017/s003118209700231x. [DOI] [PubMed] [Google Scholar]
- Parameswaran N, O’handley R, Grigg ME, Fenwick SG, Thompson RCA. Seroprevalence of Toxoplasma gondii in wild kangaroos using an ELISA. Parasitology International. 2009 doi: 10.1016/j.parint.2009.01.008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole WE, Carpenter SM, Wood JT. Growth of Grey Kangaroos and the Reliability of Age Determination from Body Measurements. II. The Western Grey Kangaroos, Macropus fuliginosus, M. f. melanops and M. f. ocydromus. Aust Wildl Res. 1982;9:203–212. [Google Scholar]
- Remington JS, Jacobs L, Melton ML. Congenital transmission of toxoplasmosis from mother animals with acute and chronic infections. J Infect Dis. 1961;108:163–173. doi: 10.1093/infdis/108.2.163. [DOI] [PubMed] [Google Scholar]
- Skerratt LF, Phelan J, Mcfarlane R, Speare R. Serodiagnosis of toxoplasmosis in a common wombat. J Wildl Dis. 1997;33:346–351. doi: 10.7589/0090-3558-33.2.346. [DOI] [PubMed] [Google Scholar]
- Tyndale-Biscoe H, Renfree MB. Reproductive physiology of marsupials. Cambridge; Melbourne: 1987. [Google Scholar]


