Abstract
As part of a program to explore the biological activity of analogues of the natural schweinfurthins, a set of compounds has been prepared where an indole system can be viewed as a substitution for the resorcinol substructure of the schweinfurthin’s D-ring. Twelve of these schweinfurthin indoles have been prepared and evaluated in the 60 cell line screen of the National Cancer Institute. While a range of activity has been observed, it is now clear that schweinfurthin indoles can demonstrate the intriguing pattern of activity associated with the natural stilbenes. In the best cases, these indole analogues display both potency and differential activity across the various cell lines comparable to the best resorcinol analogues.
Keywords: Schweinfurthin, indole, stilbene analogues, SAR
1. Introduction
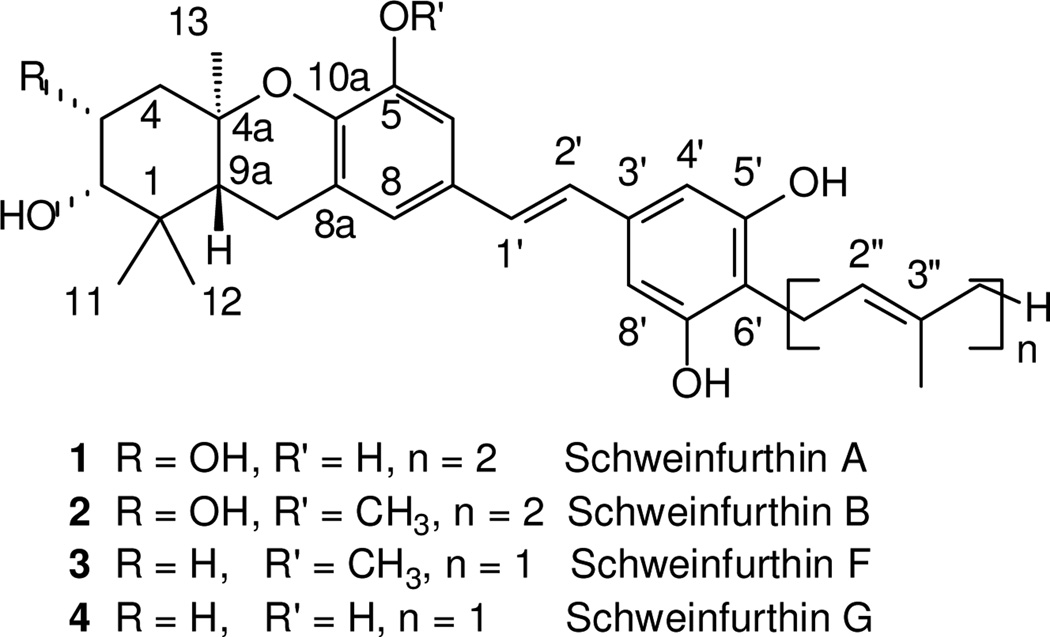
Extracts of the plant Macaranga schweinfurthii1–3 and other Macaranga species4–6 have yielded a small set of natural stilbenes referred to collectively as the schweinfurthins. The tetracyclic members of this family, represented by schweinfurthins A (1), B (2), F (3), and G (4) (Figure 1), have shown intriguing results in the National Cancer Institute’s 60 cell line screen, including potent anti-proliferative effects, good differential activity, and a pattern of activity against different cell types that suggests a novel mechanism of action. While efforts to secure more material from the natural source have met with limited success, a program to synthesize these natural products has been more rewarding. Our synthesis efforts have determined the absolute stereochemistry of the natural products,7 provided access to most of the tetracyclic examples in the correct natural stereochemistry,8–11 and afforded a host of analogues designed to gather information on structure-activity relationships12–15 and to afford fluorescent16 or biotinylated17 probes. From the results of these studies it is now clear that: a) the C-3 hydroxyl group is not essential for activity; b) compounds with a free phenol at C-5 show greater activity than the corresponding methoxy compounds, although both are active; c) methylation of one D-ring phenol improves chemical stability but does not diminish activity greatly; and, d) the para position on the D-ring is tolerant of a variety of substituents. Integration of all of these factors led us to an interest in compounds where the entire D-ring was replaced with an indole motif.
Figure 1.
Some natural schweinfurthins.
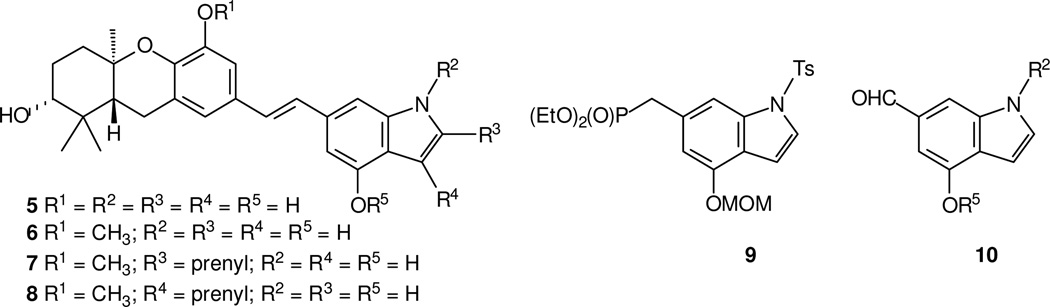
The indole system is found in an enormous variety of natural products and many bioactive agents, and it is sometimes considered to be a privileged scaffold for drug development.18,19 If one were to view the indole nitrogen as a replacement for one D-ring phenol, analogues to schweinfurthin F (or G) could take on the general structure 5 (or 6, Figure 2). Given the inherent flexibility for substituents at the para position noted above, isoprenoid substituents might be appended at either of the two positions on the 5-membered ring (as shown in structures 7 and 8). If such target compounds were to be prepared through a late-stage formation of the central olefin, as has been the case with so many of the schweinfurthins, then advanced intermediates bearing the substitution pattern of structures 9 or 10 would be attractive. While there are many routes to various indoles,20 strategies for preparation of the desired substitution pattern are much more limited. We recently reported preparation21,22 of compound 9 through a variation of the procedures refined by Vedejs.23 This specific intermediate was used to assemble indoles 5 and 6, and similar phosphonate intermediates were used to make compounds 7 and 8.21,22 In this manuscript, we report the preparation of complementary aldehydes of the general structure 10, synthesis of a variety of methylated analogues, and the biological activity of twelve of these schweinfurthin indoles in the NCI’s 60 cell line screen.
Figure 2.
Intermediates for assembly of schweinfurthin indoles.
2. Chemical Synthesis
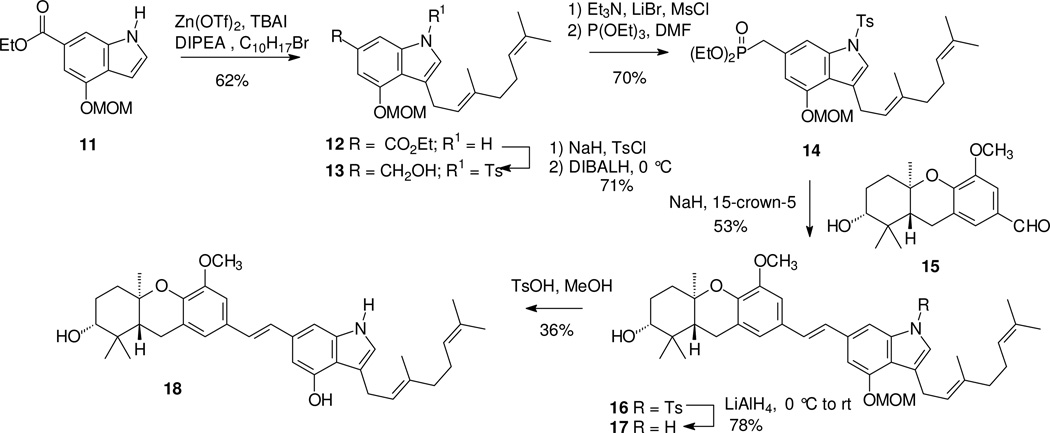
Most of the new compounds prepared here can be viewed as analogues of schweinfurthin F (3)5 or 3-deoxyschweinfurthin B14 which carries a geranyl group on the D-ring in place of the prenyl group of schweinfurthin F. This choice was made as a compromise between synthetic expediency and the known activity of these parent compounds. The first of the new compounds prepared was the geranylated indole 18 (Scheme 1). Reaction of the MOM-protected compound 1121 with geranyl bromide gave the 3-geranyl derivative 12, as expected from the parallel reaction of prenyl bromide.22 After protection of the indole nitrogen (12) through reaction with tosyl chloride, reduction with DIBALH gave the primary alcohol 13, and conversion to the phosphonate 14 proceeded under standard conditions. An HWE condensation with aldehyde 1514,8 gave the desired stilbene 16 as a single E-olefin isomer,24 and removal of the tosyl group by treatment with LiAlH4 gave the free indole 17. Hydrolysis of the MOM group gave the target compound 18 in modest yield.
Scheme 1.
Synthesis of a geranylated schweinfurthin indole.
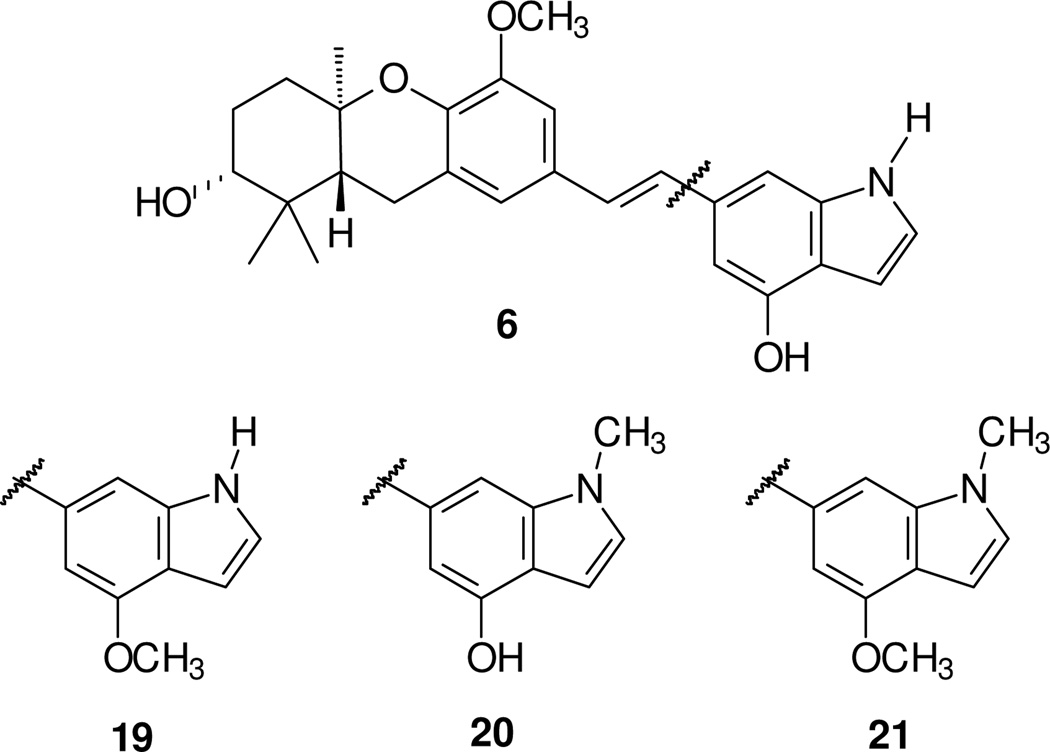
Because methylation of one D-ring phenol improves the chemical stability of the schweinfurthins, determination of the impact of methylation in the indole subsystem was the next objective. With compound 6 already in hand, the two isomeric monomethyl compounds 19 and 20, as well as the dimethyl compound 21, became the next targets (Figure 3).
Figure 3.
Mono- and dimethylated schweinfurthin indole targets.
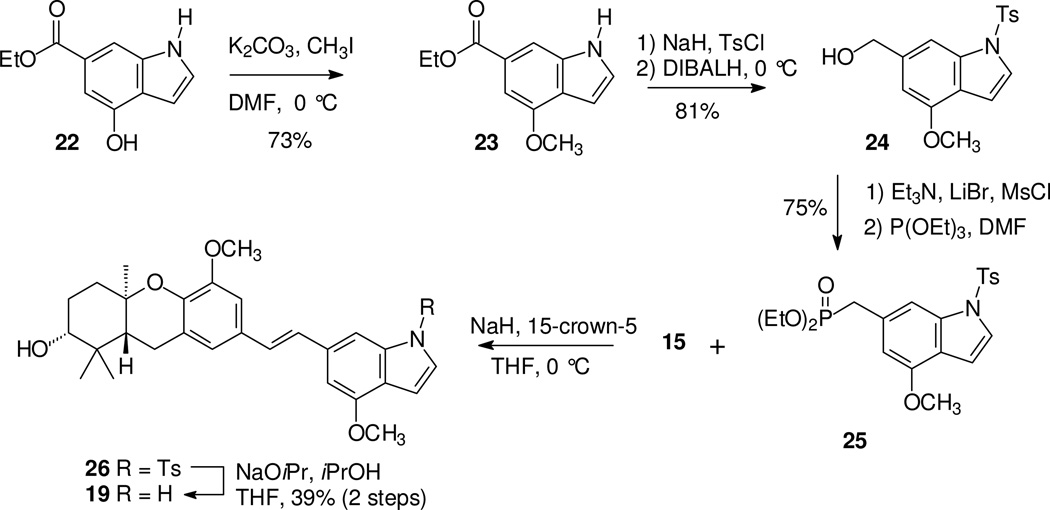
Preparation of the O-methyl compound 19 began with treatment of compound 22 with K2CO3 and limited methyl iodide to obtain the methyl ether 23 (Scheme 2). Protection of the indole nitrogen and reduction gave the expected alcohol 24 smoothly, and this product could be converted to the phosphonate 25 under standard conditions. An HWE condensation with aldehyde 15 and in situ cleavage of the tosyl group gave the first of the methylated analogues, compound 19.
Scheme 2.
Synthesis of an O-methyl schweinfurthin indole.
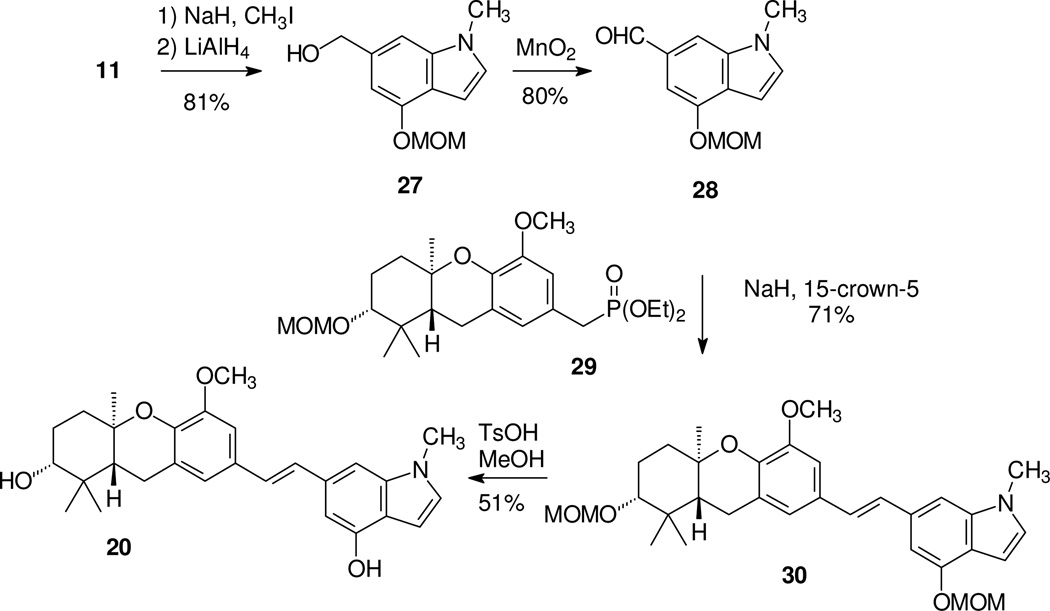
Preparation of the N-methyl compound 20 began in a similar fashion (Scheme 3). Treatment of the MOM protected indole 11 with base and methyl iodide gave the expected product 27. However, after attempted conversion of compound 27 to the corresponding phosphonate proved difficult, the complementary HWE condensation was pursued. Therefore compound 27 was oxidized to the corresponding aldehyde 28 and phosphonate 297 was used to assemble the central olefin. This HWE condensation proceeded smoothly to afford the desired stilbene 30. Treatment of this compound with TsOH in methanol under standard conditions gave the desired stilbene 20.
Scheme 3.
Synthesis of an N-methyl schweinfurthin indole.
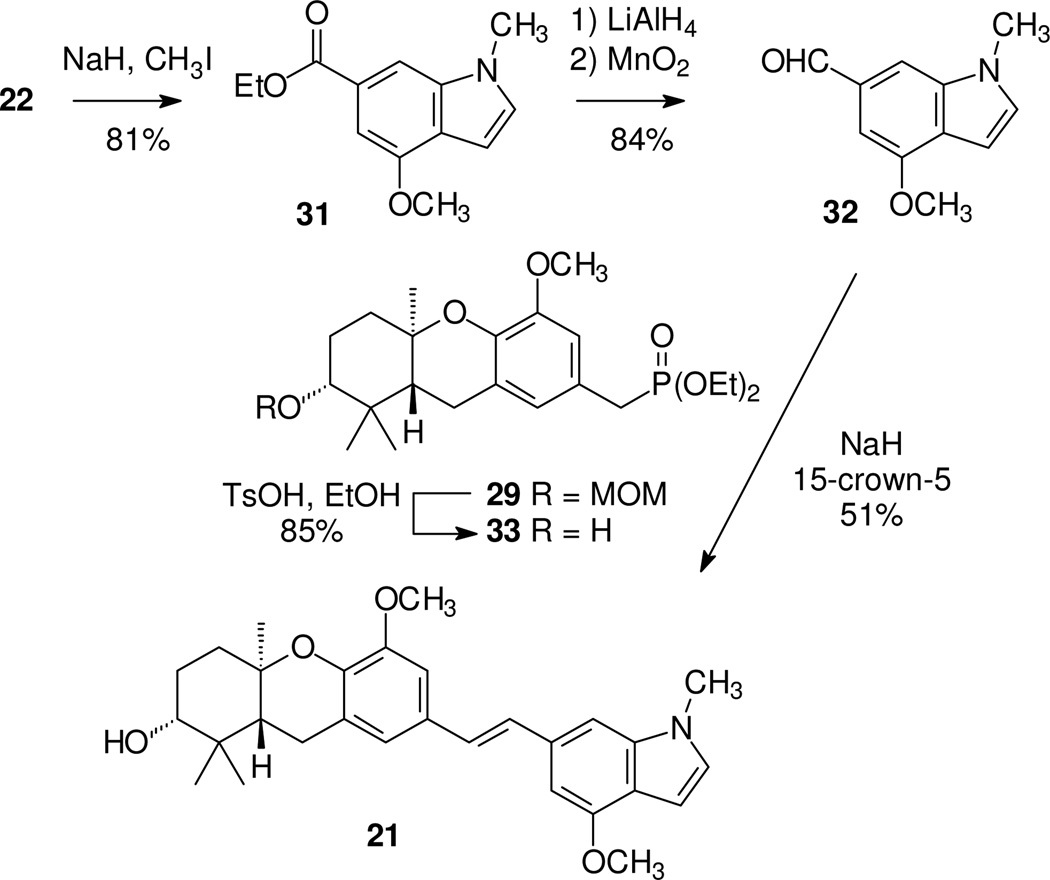
A similar strategy was employed to prepare the dimethyl compound 21 (Scheme 4). Complete methylation of indole 22 was followed by a standard reduction-oxidation sequence on indole 31 to obtain aldehyde 32. Because hydrolysis of the MOM group from the C-2 alcohol had proven slow with compound 30, phosphonate 29 was treated with TsOH/EtOH to remove the MOM group prior to the HWE condensation. Reaction of aldehyde 32 with phosphonate 33 proceeded in modest yield, but gave the desired target 21 directly.
Scheme 4.
Synthesis of an N,O-dimethyl schweinfurthin indole.
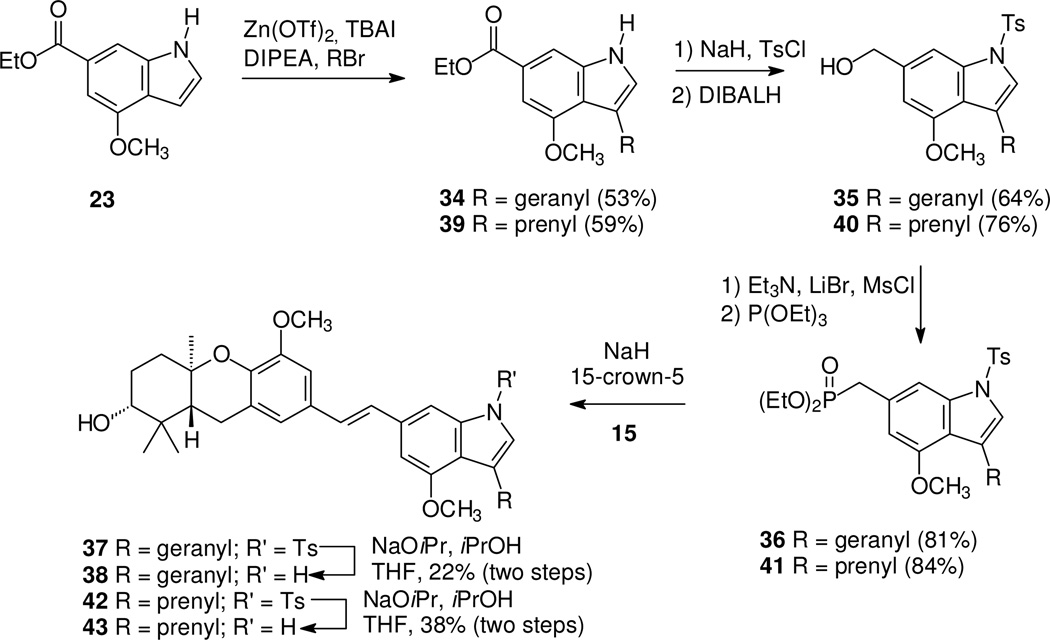
To allow a second comparison of the impact of hydrogen (5), prenyl, and geranyl substituents in an O-methyl series, compounds 38 and 43 were prepared (Scheme 5). For the geranyl series, reaction of indole 23 with geranyl bromide in the presence of Zn(OTf)225 gave the geranylated indole 34. After introduction of the tosyl protecting group, reduction of the ester gave the primary alcohol 35. In this series, formation of the phosphonate 36 proceeded smoothly under standard conditions, and condensation with aldehyde 15 gave the desired stilbene 37. Final deprotection gave the geranylated target 38 in modest yield. Preparation of the prenyl analogue proceeded through a parallel series of reactions in comparable yields, through intermediates 39, 40, and 41. In this case, after the HWE condensation of compounds 41 and 15, deprotection was conducted without isolation of the intermediate 42, to afford the prenyl compound 43.
Scheme 5.
Synthesis of geranyl and prenyl-substituted schweinfurthin indoles.
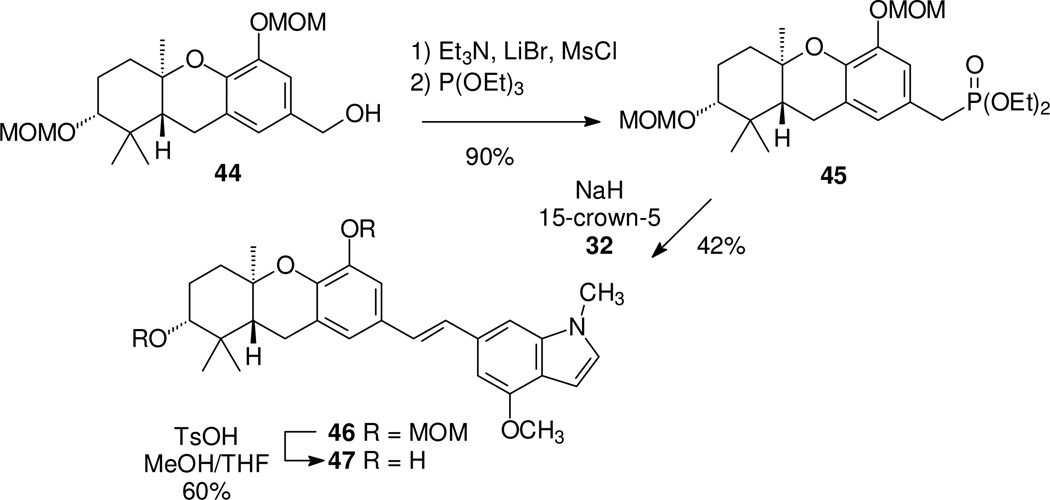
Finally, because the C-ring phenols tend to be more active that the corresponding methoxy compounds in other schweinfurthin studies, two analogues of schweinfurthin G (4) were prepared (47 and 50). For the first of these targets (Scheme 6), the tricyclic alcohol 44 was converted to the phosphonate 45.8 Condensation with aldehyde 32 gave the desired stilbene, and final hydrolysis of the A-ring MOM group gave the desired phenol 47.
Scheme 6.
Synthesis of a C-5 hydroxy schweinfurthin indole.
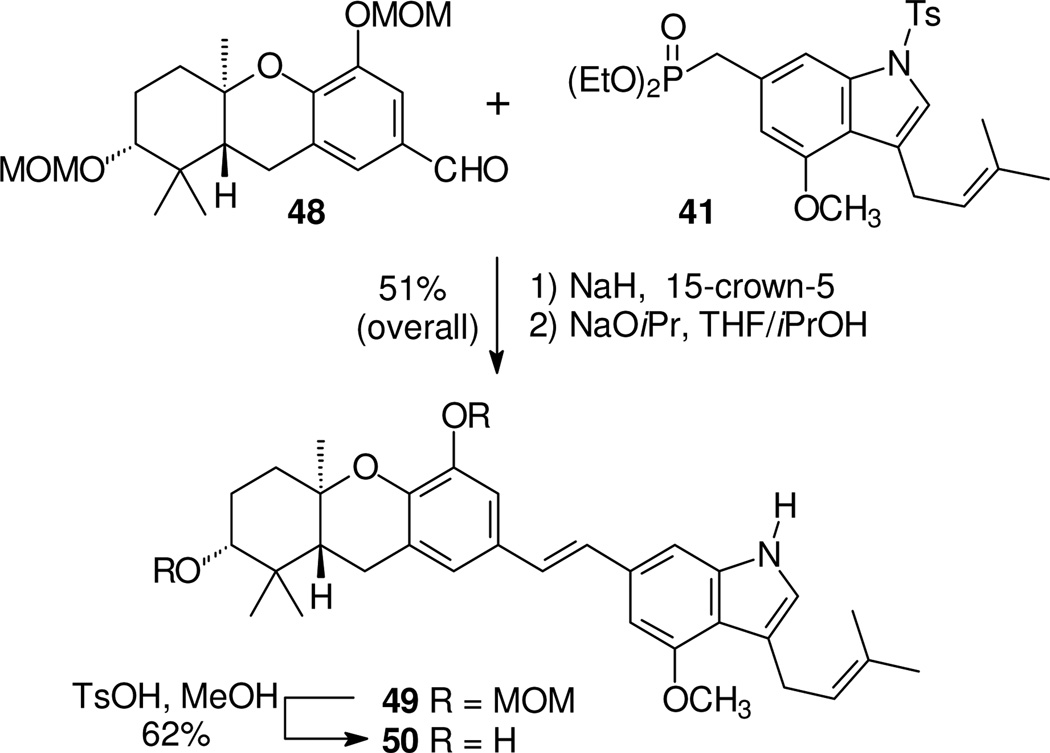
Because phosphonate 41 already was in hand, compound 50 was prepared from the right half phosphonate and a left half aldehyde (Scheme 7). To exclude the possibility of tosyl transfer to an A-ring hydroxyl group,21 the protected aldehyde 4811 was used. This HWE condensation proceeded smoothly and the resulting stilbene was treated with base in situ to remove the tosyl group and afford compound 49. Final hydrolysis of the MOM protecting groups gave the desired analogue 50.
Scheme 7.
Synthesis of a C-5 hydroxy E-ring prenyl schweinfurthin indole.
With this set of new compounds complete, these eight schweinfurthin indoles (18–21, 38, 43, 47, and 50), together with the four previously prepared (5–8), were examined for their biological activity in the 60 cell line assay.
3. Biological Results and Discussion
Twelve indole schweinfurthins were tested in the 60 cell line bioassay. Each compound maintains the hexahydroxanthene A-B-C ring system found in the natural products schweinfurthin F (3) and G (4), along with the R,R,R-stereochemistry, to focus on the impact of the indole substructure on bioactivity. Key aspects of the data generated through these assays are summarized in Table 1. Because the set of assay data is quite substantial, any number of comparisons might be made, but some central conclusions are summarized below.
Table 1.
Activity of schweinfurthin indoles in the NCI 60 cell line screen.
| cmpd | NSC# | R1 | R2 | R3 | R4 | R5 | Mean GI50 (nM) |
GI50Range (log10 units) |
n | SF-295 GI50 (nM) |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 750658 | H | H | H | H | H | 570 | 3.29 | 2a | 89 |
| 6 | 750655 | CH3 | H | H | H | H | 1020 | 2.02 | 2a | 230 |
| 7 | 752116 | CH3 | H | prenyl | H | H | 3160 | 3.08 | 2a | 290 |
| 8 | 752816 | CH3 | H | H | prenyl | H | 390 | 3.00 | 2a | <21b |
| 18 | 750656 | CH3 | H | H | geranyl | H | 1620 | 3.19 | 1 | 110 |
| 19 | 751060 | CH3 | H | H | H | CH3 | 380 | 2.41 | 1 | 55 |
| 20 | 752115 | CH3 | CH3 | H | H | H | 1260 | 2.63 | 2a | 760 |
| 21 | 752114 | CH3 | CH3 | H | H | CH3 | 1260 | 2.61 | 2a | 170 |
| 38 | 751061 | CH3 | H | H | geranyl | CH3 | 1070 | 2.54 | 1 | 200 |
| 43 | 754492 | CH3 | H | H | prenyl | CH3 | 430 | 2.38 | 2a | 48 |
| 47 | 752817 | H | CH3 | H | H | CH3 | 450 | 3.00 | 2a | <12b |
| 50 | 754493 | H | H | H | prenyl | CH3 | 580 | 3.26 | 2a | <23b |
| 3 | 740544 | Schweinfurthin F | 780 | 2.79 | 2a | <36b | ||||
| 4 | 744343 | Schweinfurthin G | 110 | 3.12 | 2a | 10 | ||||
Data represents the average of two independent experiments.
In one experiment the GI50 was below the lowest concentration tested (10 nM).
Compound 5 can be considered parallel to schweinfurthin G with a 4-hydroxyindole system replacing the resorcinol unit found in the natural material, while compound 6 bears the same relationship to schweinfurthin F. As shown in Table 1, both compounds showed significant activity in the 60 cell line screen, with mean GI50’s of 570 and 1020 nM (vs. 110 and 780 nM for schweinfurthin G and F, respectively) as well as differential activity of 3.29 and 2.02 log10 units (vs. 3.12 and 2.79). Visual inspection of the activity profiles was consistent with schweinfurthin-like activity, given that the CNS and leukemia subpanels were particularly sensitive while the ovarian subpanel was relatively resistant. Perhaps more importantly, comparison of the mean GI50 data from all the tested cell lines with a Pearson correlation shows good correlations to the natural schweinfurthins. The activity of compound 5, which carries a C-ring hydroxyl group, correlates very well with schweinfurthin G (0.83), while the activity of compound 6, which bears a C-ring methoxy group, correlates nearly as well with schweinfurthin F (0.61). This confirms that the new indoles demonstrate schweinfurthin-like behavior, and indicates that modification of the right-half resorcinol to an indole motif is a well-tolerated change.
The early results summarized above encouraged efforts to prepare additional analogues, including some with isoprenoid side chains that more closely resemble natural schweinfurthins. Compound 7 bears a prenyl substituent at C-2 of the indole ring, while compound 8 carries a prenyl substituent at C-3 and compound 18 carries a geranyl substituent at the same site. All three compounds showed differential activity of three log10 units or more, and their activity correlated well with that of the natural schweinfurthins. However, compound 8 showed the greatest potency, with a mean GI50 of 390 nM.
In past studies we have shown that monomethylation of the D-ring resorcinol increased chemical stability with a minimal impact on potency.7,13 Therefore, we prepared the O- and N-methylindoles as shown in compounds 19 and 20, as well as the N-,O-dimethyl compound 21. All three compounds showed activity in the 60 cell line screen, but only compound 19 had a mean GI50 below 1 µM. To explore this activity further, both the 3-geranyl (38) and 3-prenyl (43) derivatives of compound 19 were prepared and tested for their activity. Both of these compounds showed activity but only the prenyl compound 43 displayed a sub-micromolar mean GI50.
Finally, because the C-ring hydroxyl group of schweinfurthin G appears to confer somewhat greater activity than the C-ring methoxy group of schweinfurthin F, and this pattern was observed again with compounds 5 and 6, the C-ring phenols 47 and 50 were prepared. Both compounds showed sub-micromolar mean GI50’s and differential activity of more than three orders of magnitude. Addition of the C-3 prenyl substituent to the indole system in this case does not appear to enhance substantially either the potency or the differential activity in this pairwise comparison.
Mean GI50 values provide a useful comparison of these indole schweinfurthins, and differential activity is a useful indication of selective activity. However, activity in a single cell line also is a valid comparator and Table 1 includes the GI50 values for the SF-295 cell line, as run within the NCI 60 screen. This is a human-derived glioblastoma cell line, and one of those most sensitive to the schweinfurthins. In this set of 12 compounds, four (8, 43, 47, and 50) displayed mean GI50 values of 50 nM or less against this specific cell line, values that are comparable to those of schweinfurthins F and G.
4. Conclusions
A number of new schweinfurthin indoles have been prepared, including compounds that bear geranyl or prenyl groups at C-2 or C-3 of the indole system, as well as compounds that have been selectively methylated on the indole nitrogen or a phenolic hydroxyl group. Twelve such compounds have been tested for biological activity in the NCI’s 60 cell line screen and all of them showed activity. The best compounds display GI50s comparable to those of the natural schweinfurthins, show a range of activity of ~3 orders of magnitude between the most sensitive and the most resistant cell lines, and display a schweinfurthin-like pattern of activity that suggest they possess a similar mechanism of action. Taken together, these data suggest that an indole moiety is a viable replacement for the resorcinol-based D-ring of the natural schweinfurthins.
5. Experimental Section
5.1 General Experimental Conditions
Tetrahydrofuran was freshly distilled from sodium/benzophenone, while CH2Cl2 and Et3N were freshly distilled from CaH2. All reactions in non-aqueous solvents were conducted in oven dried glassware under a positive pressure of argon with magnetic stirring. All commercial reagents were used without further purification unless otherwise stated. NMR spectra were recorded at 300 MHz for 1H, and 75 MHz for 13C or higher with CDCl3 as solvent and (CH3)4Si (1H, 0.00 ppm) or CDCl3 (13C, 77.0 ppm) as internal standards unless otherwise noted. High resolution mass spectra were run with magnet detection unless another method is noted. Elemental analyses were performed by a commercial facility.
5. 2 Geranylated indole 12
To indole 1121 (1.11 g, 4.44 mmol), TBAI (820 mg, 2.22 mmol), and Zn(OTf)2 (968 mg, 2.66 mmol) in toluene (15 mL) at rt was added DIPEA (0.86 mL, 4.88 mmol) and the reaction mixture was allowed to stir for 15 min. Geranyl bromide (481 mg, 2.22 mmol) was added dropwise, the reaction was allowed to proceed for 3 h and then quenched by addition of NH4Cl (satd.) and extracted with Et2O. The combined organic layers were washed with H2O, dried (Na2SO4), concentrated, and purified by column chromatography (17.5 to 20% EtOAc in hexanes) to afford geranylated indole 12 (524 mg, 62%) as a colorless oil along with recovered starting material 11 (553 mg): 1H NMR δ 8.72 (br s, 1H), 7.81 (d, J = 0.9 Hz, 1H), 7.35 (d, J = 0.9 Hz, 1H), 6.96–6.95 (m, 1H), 5.51–5.46 (m, 1H), 5.35 (s, 2H), 5.14–5.10 (m, 1H), 4.37 (q, J = 7.1 Hz, 2H), 3.67 (d, J = 7.1 Hz, 2H), 3.53 (s, 3H), 2.16–2.03 (m, 4H), 1.72 (s, 3H), 1.68 (s, 3H), 1.60 (s, 3H) 1.38 (t, J = 7.1 Hz, 3H); 13C NMR δ 167.7, 151.3, 137.3, 135.1, 131.2, 124.3, 124.3, 123.9, 123.5, 121.2, 116.4, 108.3, 102.6, 94.1, 60.7, 56.1, 39.6, 26.6, 25.6, 25.2, 17.6, 15.9, 14.3. Anal. Calcd for C23H31NO4: C, 71.66; H, 8.11; N, 3.63. Found: C, 71.43; H, 8.19, N, 3.77.
5.3 Alcohol 13
To indole 12 (397 mg, 1.04 mmol) in THF at 0 °C was added NaH (55 mg, 1.38 mmol, 60% dispersion in oil) and the solution was allowed to stir for 10 min. Tosyl chloride (235 mg, 1.23 mmol) was added and the solution was allowed to warm to rt. When the reaction was judged complete by TLC analysis the reaction mixture was cooled to 0 °C, then DIBALH (0.54 mL, 2.63 mmol) was added. After 30 min the solution was quenched by addition of NH4Cl (satd.), poured into EtOAc, acidified, and extracted with EtOAc. The combined organic extracts were washed with Na2CO3 (satd.), brine, dried (MgSO4), and filtered, and then concentrated in vacuo. Final purification by flash column chromatography (35% EtOAc in hexanes) afforded benzylic alcohol 13 (366 mg, 71%) as an oil: 1H NMR δ 7.70 (d, J = 8.2 Hz, 2H), 7.61 (s, 1H), 7.15 (d, J = 8.2 Hz, 2H), 7.13 (s, 1H), 6.86 (s, 1H), 5.44–5.39 (m, 1H), 5.22 (s, 2H), 5.14–5.10 (m, 1H), 4.71 (s, 2H), 3.53 (d, J = 7.0 Hz, 2H), 3.47 (s, 3H), 2.31–2.29 (m, 4H), 2.14–2.05 (m, 4H), 1.69–1.68 (m, 6H), 1.61 (s, 3H); 13C NMR δ 151.9, 144.6, 139.1, 137.1, 136.6, 135.3, 131.5, 129.7 (2C), 126.6 (2C), 124.1, 122.7, 121.9, 121.7, 120.3, 106.0, 105.8, 94.2, 65.5, 56.1, 39.6, 26.6, 25.6, 25.5, 21.4, 17.6, 16.1. Anal. Calcd for C28H35NO5S: C, 67.58; H, 7.09; N, 2.81. Found: C, 67.81; H, 7.19, N, 2.83.
5.4 Phosphonate 14
To alcohol 13 (366 mg, 0.74 mmol) in THF (20 mL) was added LiBr (510 mg 5.87 mmol) and Et3N (0.41 mL, 2.94 mmol) and the solution was cooled to 0 °C. After 20 min, MsCl (0.14 mL, 1.8 mmol) was added dropwise. The reaction was allowed to stir and slowly warm to rt. When complete by TLC analysis it was quenched by addition of NaHCO3 (satd.) and extracted with Et2O. The organic layers were washed with brine, dried (MgSO4), and filtered, and the filtrate was concentrated in vacuo. The resulting residue was dissolved in DMF (3 mL) and P(OEt)3 (0.4 mL) was added and the solution was heated at reflux overnight. The next day the solution was allowed to cool to rt, then poured into water, and extracted with EtOAc. The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo. Final purification by flash column chromatography (2.5% EtOH in Et2O) afforded phosphonate 14 (320 mg, 70%) as a light yellow oil: 1H NMR δ 7.74 (d, J = 8.3 Hz, 2H), 7.59–7.58 (m, 1H), 7.20 (d, J = 8.4 Hz, 2H), 7.09 (d, J = 1.1 Hz, 1H), 6.82–6.80 (m, 1H), 5.43–5.38 (m, 1H), 5.23 (s, 2H), 5.15–5.11 (m, 1H), 4.06–3.95 (m, 4H), 3.52 (d, J = 7.2 Hz, 2H), 3.47 (s, 3H), 3.22 (d, JPH = 21.5 Hz, 2H), 2.32 (s, 3H), 2.14–2.07 (m, 4H), 1.70 (s, 3H), 1.68 (s, 3H), 1.62 (s, 3H) 1.25 (t, J = 7.1 Hz, 6H); 13C NMR δ 151.6 (d, JCP = 2.8 Hz) 144.4, 137.1 (d, JCP = 2.9 Hz), 136.5, 135.3, 131.4, 129.6 (2C), 129.2 (d, JCP = 9.3 Hz), 126.7 (2C), 124.0, 122.7 (d, JCP = 1.6 Hz), 121.7, 121.6, 119.7 (d, JCP = 3.2 Hz), 108.9 (d, JCP = 5.6 Hz), 108.7 (d, JCP = 7.7 Hz), 94.3, 61.9 (d, JCP = 6.7 Hz, 2C), 56.0, 39.5, 34.1 (d, JCP = 138.1 Hz), 26.5, 25.5, 25.4, 21.3, 17.5, 16.2 (d, JCP = 6.0 Hz, 2C), 15.9; 31P NMR δ 26.9; HRMS (EI) calcd for C32H44NO7PS (M+) 617.2576; found 617.2562.
5.5 Indole 16
To phosphonate 14 (84 mg, 0.14 mmol) and aldehyde 158 (32 mg, 0.10 mmol) in THF (5 mL) at rt was added NaH (60 mg, 1.50 mmol, 60% dispersion in oil) followed by 15-crown-5 (3 drops). The reaction mixture was allowed to stir for 90 min, then quenched by addition of Na2CO3 (satd.), and extracted with EtOAc. The combined organic extracts were washed with brine, dried (MgSO4), and filtered, and then concentrated in vacuo. Final purification by flash column chromatography (35 to 40% EtOAc in hexanes) afforded compound 16 (53%, 43 mg): 1H NMR δ 7.75–7.73 (m, 3H), 7.21 (d, J = 8.0 Hz, 2H), 7.11 (s, 1H), 7.04–7.02 (m, 3H), 6.94–6.93 (m, 1H), 6.91–6.90 (m, 1H), 5.44–5.40 (m, 1H), 5.28, (s, 2H), 5.15–5.11 (m, 1H), 3.93 (s, 3H), 3.53–3.51 (m, 2H), 3.51 (s, 3H), 3.46–3.42 (m, 1H), 2.75–2.73 (m, 2H), 2.33 (s, 3H), 2.17–2.08 (m, 5H), 1.92–1.60 (m, 5H), 1.70 (s, 3H), 1.69 (s, 3H), 1.62 (s, 3H), 1.27 (s, 3H), 1.12 (s, 3H), 0.91 (s, 3H); 13C NMR δ 152.0, 149.0, 144.6, 142.7, 137.5, 136.7, 135.7, 135.3, 131.5, 129.7 (2C), 128.9, 128.5, 126.7, 126.7 (2C), 124.1, 123.1, 122.6, 122.1, 121.7, 120.5, 120.3, 106.9, 105.9, 104.8, 94.3, 78.0, 77.0, 56.2, 56.0, 46.8, 39.7, 38.4, 37.7, 28.3, 27.3, 26.6, 25.7, 25.6, 23.1, 21.5, 19.8, 17.7, 16.1, 14.2; HRMS (EI) calcd for C46H57NO7S (M+) 767.3856; found 767.3853.
5.6 Indole 17
To the schweinfurthin analogue 16 (43 mg, 0.056 mmol) in THF (5 mL) at 0 °C was added LiAlH4 (45 mg, 1.06 mmol) and then the mixture was allowed to warm to rt. The following day, the reaction mixture was quenched by addition of NH4Cl (satd.), poured into H2O, and extracted with EtOAc. The combined organic extracts were washed with water and brine, dried (MgSO4), and filtered and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (30 to 40% EtOAc in hexanes) afforded indole 17 (27 mg, 78%) as a light yellow oil: 1H NMR δ 7.92 (br s, 1H), 7.07–7.06 (m, 1H), 7.02 (d, J = 16.2 Hz, 1H), 6.95 (d, J = 16.3 Hz, 1H), 6.92, (d, J = 0.7 Hz, 1H), 6.90 (d, J = 1.7 Hz, 1H), 6.87 (d, J = 1.4 Hz, 1H), 6.81–6.80 (m, 1H), 5.53–5.47 (m, 1H), 5.36 (s, 2H), 5.16–5.10 (m, 1H), 3.90 (s, 3H), 3.64 (d, J = 7.3 Hz, 2H) 3.57 (s, 3H), 3.45–3.40 (m, 1H), 2.74–2.71 (m, 2H), 2.11–2.04, (m, 6H), 1.89–1.84 (m, 2H), 1.72 (d, J = 0.6 Hz, 3H), 1.72–1.57 (m, 2H), 1.69 (d, J = 0.8 Hz, 3H), 1.60 (s, 3H), 1.26 (s, 3H), 1.11 (s, 3H), 0.89 (s, 3H); 13C NMR δ 152.2, 148.9, 142.3, 138.6, 135.0, 133.0, 131.3, 129.4, 127.6, 126.7, 124.4, 123.8, 122.6, 121.0, 120.2, 117.6, 116.7, 106.9, 103.8, 100.9, 94.3, 78.1, 77.0, 56.0, 56.0, 46.8, 39.8, 38.4, 37.7, 28.3, 27.3, 26.7, 25.7, 25.5, 23.2, 19.8, 17.7, 16.0, 14.3; HRMS (EI) calcd for C39H51NO5 (M+) 613.3767; found 613.3754.
5.7 Analogue 18
To the protected indole 17 (21 mg, 0.034 mmol) in MeOH (2 mL) was added TsOH (40 mg, 0.21 mmol) in two proportions 3 h apart, and the solution was allowed to stir. The next day the solution was quenched by addition of NaHCO3 (satd.), diluted with H2O and extracted with EtOAc. The combined organics extracts were washed with brine, dried (MgSO4), and filtered, and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (50% EtOAc in hexanes) afforded schweinfurthin analogue 18 (7 mg, 36%) as a light yellow oil: 1H NMR (CD3OD) δ 6.96–6.93 (m, 3H), 6.92–6.91 (m, 1H), 6.85–6.84 (m, 1H), 6.73 (d, J = 0.9 Hz, 1H), 6.61–6.60 (m, 1H), 5.54–5.49 (m, 1H), 5.16–5.11 (m, 1H), 3.61 (d, J = 7.1 Hz, 2H), 3.38–3.33 (m, 1H), 3.25 (s, 3H), 2.74–2.71 (m, 2H), 2.15–2.01 (m, 5H), 1.83–1.59 (m, 4H), 1.72 (s, 3H), 1.68 (s, 3H), 1.61 (s, 3H), 1.22 (s, 3H), 1.09 (s, 3H), 0.87 (s, 3H); 13C NMR (CD3OD) δ 153.2, 150.1, 143.2, 140.7, 135.4, 133.6, 132.1, 131.4, 129.2, 126.9, 125.8, 125.6, 124.0, 121.8, 121.4, 118.0, 116.6, 108.0, 103.8, 101.4, 78.7, 77.1, 56.4, ~49*, 40.9, 39.5, 38.9, 29.0, 27.9, 27.7, 26.4, 26.0, 24.1, 20.2, 17.8, 16.1, 14.9; HRMS (EI) calcd for C37H47NO4 (M+) 569.3505 found 569.3504. *Obscured by solvent
5.8 Preparation of the methylated indole 23 and the dimethylated indole 31
Following Vedejs23 procedure, to phenol 22 (1.05 g, 5.12 mmol) in DMF (28 mL) was added K2CO3 (2.12 g, 15.4 mmol), the reaction mixture was cooled to 0 °C, and after 20 min MeI (0.32 mL, 5.12 mmol) was added dropwise. The reaction was maintained at 0 °C overnight, then quenched by addition of 1M HCl and extracted with Et2O. The combined organic extracts were washed with brine, dried (MgSO4), filtered, and then concentrated in vacuo. Final purification by flash column chromatography (20 to 25% EtOAc in hexanes) afforded methoxyindole 23 (824 mg, 73%) as a white solid: 1H NMR δ 8.66 (br s, 1H), 7.85 (t, J = 0.9 Hz, 1H), 7.26 (dd, J = 3.3, 2.5 Hz, 1H), 7.22 (d, J = 1.0 Hz, 1H), 6.70–6.68 (m, 1H), 4.40 (q, J = 7.1 Hz, 2H), 4.00 (s, 3H), 1.41 (t, J = 7.1 Hz, 3H); 13C NMR δ 167.8, 152.7, 136.2, 125.9, 124.9, 122.3, 107.5, 100.2, 99.9, 60.8, 55.4, 14.4; HRMS (EI) calcd for C12H13NO3 (M+) 219.0895; found 219.0904.
Also recovered was the dimethylated indole 31 (31 mg, 3%) as a white solid: 1H NMR δ 7.77 (t, J = 0.9 Hz, 1H), 7.21 (d, J = 1.0 Hz, 1H), 7.11 (d, J = 3.0 Hz, 1H), 6.60 (dd, J = 3.1, 0.8 Hz, 1H), 4.41 (q, J = 7.1 Hz, 2H), 4.01 (s, 3H), 3.83 (s, 3H), 1.43 (t, J = 7.1 Hz, 3H); 13C NMR δ 167.7, 152.7, 137.1, 130.3, 124.4, 122.7, 105.8, 99.6, 98.7, 60.8, 55.4, 33.2, 14.5; HRMS (EI) calcd for C13H15NO3 (M+) 233.1052; found 233.1056.
5.9 Benzylic indole alcohol 24
To indole 23 (436 mg, 1.99 mmol) in THF (10 mL) at 0 °C was added NaH (100 mg, 2.5 mmol, 60% dispersion in oil), followed by TsCl (430 mg, 2.25 mmol). Once the reaction was judged complete by TLC analysis, DIBALH (1.07 mL, 6.0 mmol) was added dropwise. After 1 h the reaction mixture was quenched by addition of NH4Cl (satd.), diluted with EtOAc, acidified with 1M HCl to dissolve the solids, and extracted with EtOAc. The combined organic extracts were washed with NaHCO3 (satd.), brine, dried (MgSO4), and filtered, and then the filtrate was concentrated in vacuo. Final purification by flash column chromatography (35% EtOAc in hexanes) afforded alcohol 24 (533 mg, 81%) as a white solid: 1H NMR δ 7.72 (d, J = 8.4, Hz, 2H), 7.57 (s, 1H), 7.43 (d, J = 3.6 Hz, 1H), 7.16 (d, J = 8.5 Hz, 2H), 6.72 (dd, J = 3.7, 0.8 Hz, 1H), 6.67 (s, 1H), 4.74 (s, 2H), 3.85 (s, 3H), 2.32 (br s, 1H), 2.29 (s, 3H); 13C NMR δ 153.0, 144.9, 139.2, 135.8, 135.0, 129.8 (2C), 126.7 (2C), 124.9, 120.4, 105.9, 104.7, 102.7, 65.8, 55.3, 21.4; HRMS (EI) calcd for C17H17NO4S (M+) 331.0878; found 331.0873.
5.10 Phosphonate 25
To benzylic alcohol 24 (517 mg, 1.56 mmol) in THF (15 mL) was added LiBr (1.09 g, 12.5 mmol) and the solution was cooled to 0 °C. Next Et3N (0.87 mL, 6.2 mmol) and, after 10 min, MsCl (0.36 mL, 1.73 mmol) were added and the reaction mixture was allowed to stir for 2.5 h. The reaction mixture was quenched by addition of NH4Cl (satd.) and extracted with Et2O. The combined organic layers were washed with brine, dried (MgSO4), and filtered, and then the filtrate was concentrated in vacuo. After the resulting oil was dissolved in DMF (6mL) and P(OEt)3 (2 mL) was added, the solution was heated at reflux. The next day the reaction was allowed to cool to rt and poured into Et2O, washed with H2O, brine, dried (MgSO4), and filtered and then the filtrate was concentrated in vacuo. Final purification by flash column chromatography (2.5 to 3% MeOH in Et2O) afforded phosphonate 25 (529 mg, 75%) as a light yellow solid: 1H NMR δ 7.75 (d, J = 8.3 Hz, 2H), 7.54 (d, J = 2.9 Hz, 1H), 7.42 (dd, J = 3.7, 1.3 Hz, 1H), 7.21 (d, J = 8.4 Hz, 2H), 6.72 (d, J = 3.6 Hz, 1H), 6.65 (s, 1H), 4.03–3.93 (m, 4H), 3.88 (s, 3H), 3.25 (d, JHP = 21.5 Hz, 2H), 2.33 (s, 3H), 1.23 (t, J = 7.1 Hz, 6H); 13C NMR δ 152.8 (d, JCP = 2.8 Hz), 144.8, 136.0 (d, JCP = 3.1 Hz), 135.3, 129.7 (2C), 129.4 (d, JCP = 9.2 Hz), 126.7 (2C), 124.8 (d, JCP = 1.7 Hz), 120.0 (d, JCP = 3.3 Hz), 107.7 (d, JCP = 7.9 Hz), 106.0 (d, JCP = 1.7 Hz), 105.6 (d, JCP = 5.7 Hz), 62.1 (d, JCP = 6.7 Hz, 2C), 55.4, 34.4 (d, JCP = 138.3 Hz), 21.4, 16.3 (d, JCP = 5.9 Hz, 2C); 13P δ 26.2; HRMS (EI) calcd for C21H26NO6PS (M+) 451.1218; found 451.1216.
5.11 Schweinfurthin analogue 19
To aldehyde 15 (40 mg, 0.13 mmol) and phosphonate 25 (72 mg, 0.16 mmol) in THF (1.2 mL) was added NaH (50 mg, 1.25 mmol, 60% dispersion in oil) and 15-crown-5 (3 drops). After 1 h the reaction was quenched by addition of NH4Cl (satd.) and extracted with EtOAc. The combined organic extracts were washed with brine, dried (MgSO4), filtered, and then concentrated in vacuo. Purification by flash column chromatography (35% EtOAc in hexanes) afforded a mixture of tosyl protected indole 26 and the free indole 19 (36 mg). The resulting mixture in THF (5 mL) was added dropwise to a solution of NaH (115 mg, 2.88 mmol, 60% dispersion in oil) in 2-propanol (2 mL). The solution was allowed to stir overnight and then quenched by addition of H2O and extracted with EtOAc. The combined organic layers were washed with brine, dried (MgSO4), and filtered, and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (40% EtOAc in hexanes) afforded schweinfurthin analogue 19 (23 mg, 39% for 2 steps) as a colorless oil: 1H NMR δ 8.17 (br s, 1H), 7.12–7.10 (m, 2H), 7.06 (d, J = 16.3, Hz, 1H), 6.99 (d, J = 16.3 Hz, 1H), 6.91–6.89 (m, 2H), 6.74 (s, 1H), 6.63 (t, J = 2.4 Hz, 1H), 4.02 (s, 3H), 3.91 (s, 3H), 3.44 (dd, J = 11.6, 4.0 Hz, 1H), 2.76–2.73 (m, 2H), 2.16–2.11 (m, 1H), 1.90–1.56 (m, 5H), 1.27 (s, 3H), 1.11 (s, 3H), 0.90 (s, 3H); 13C NMR δ 153.5, 149.1, 142.5, 137.6, 133.3, 129.6, 128.0, 127.0, 123.3, 122.8, 120.4, 118.7, 107.1, 103.5, 100.4, 97.9, 78.2, 56.2, 55.5, 47.0, 38.6, 37.8, 28.5, 27.5, 23.4, 20.0, 14.4; HRMS (EI) calcd for C28H33NO4 (M+) 447.2410; found 447.2413.
5.12 Alcohol 27
To indole 11 (202 mg, 0.81 mmol) in THF (10 mL) at 0 °C was added NaH (49 mg, 1.2 mmol, 60% dispersion in mineral oil) followed after 5 min by MeI (0.06 mL, 0.96 mmol), and the reaction mixture was allowed to stir for 2 h. After LiAlH4 (92 mg, 2.42 mmol) was added, the reaction mixture was allowed to stir for 1 h and then quenched with NH4Cl (satd.) and extracted with EtOAc. The combined organic extracts were washed with brine, dried (MgSO4), and filtered, and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (40% EtOAc in hexanes) afforded benzylic alcohol 27 (146 mg, 81%, 2 steps) as a light yellow solid: 1H NMR δ 6.96 (s, 1H), 6.91 (d, J = 3.2 Hz, 1H), 6.70 (s, 1H), 6.53 (d, J = 3.1 Hz, 1H), 5.27 (s, 2H), 4.69 (s, 2H), 3.65 (s, 3H), 3.48 (s, 3H), 2.68 (br s 1H); 13C NMR δ 150.3, 138.1, 135.7, 127.8, 118.9, 102.6, 102.2, 97.9, 94.4, 65.8, 56.0, 32.8; HRMS (EI) calcd for C12H15NO3 (M+) 221.1052; found 221.1042.
5.13 Aldehyde 28
To alcohol 27 (73 mg, 0.33 mmol) in CH2Cl2 (10 mL) at rt was added MnO2 (430 mg, 4.9 mmol) and the resulting mixture was allowed to stir for 4 h, then filtered through celite, and washed with EtOAc. The solvent was removed in vacuo to afford aldehyde 28 (58 mg, 80%) as a light yellow solid: 1H NMR δ 9.98 (s, 1H), 7.55 (s, 1H), 7.27 (s, 1H), 7.19 (d, J = 2.9 Hz, 1H), 6.65 (d, J = 2.8 Hz, 1H), 5.38, (s, 2H), 3.85 (s, 3H), 3.54 (s, 3H); 13C NMR δ 192.2, 150.7, 137.4, 131.8, 131.7, 124.8, 108.5, 102.0, 99.2, 94.5, 56.2, 33.2; HRMS (EI) calcd for C12H13NO3 (M+) 219.0895; found 219.0889.
5.14 Stilbene 30
To aldehyde 28 (11 mg, 0.05 mmol) and phosphonate 29 (27 mg, 0.06 mmol) in THF (1.5 mL) at rt was added NaH (40 mg, 1.0 mmol, 60% dispersion in oil). After the reaction mixture was allowed to stir for 6 h, it was quenched by addition of NH4Cl (satd.) and then extracted with EtOAc. The combined organic layers were washed with brine, dried (MgSO4), filtered, and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (50% Et2O in hexanes) afforded stilbene 30 (19 mg, 71%) as a light yellow oil: 1H NMR δ 7.11 (d, J = 16.1 Hz, 1H), 7.10 (s, 1H), 7.01 (d, J = 16.2 Hz, 1H), 7.00 (s, 1H), 6.98 (d, J = 3.1 Hz, 1H), 6.92 (s, 1H), 6.89 (s, 1H), 6.56 (d, J = 3.0 Hz, 1H), 5.39 (s, 2H), 4.78 (d, J = 6.9 Hz, 1H), 4.66 (d, J = 6.9 Hz, 1H), 3.92 (s, 3H), 3.72 (s, 3H), 3.57 (s, 3H), 3.42 (s, 3H), 3.29 (dd, J = 11.5, 4.0 Hz, 1H), 2.74–2.71 (m, 2H), 2.17–2.12 (m, 1H), 1.87–1.57 (m, 4H), 1.25 (s, 3H), 1.10 (s, 3H), 0.92 (s, 3H); 13C NMR δ 150.7, 148.9, 142.3, 138.4, 132.7, 129.3, 128.2, 127.7, 126.8, 122.6, 120.2, 119.5, 106.7, 102.3, 101.5, 98.4, 96.1, 94.8, 84.0, 76.9, 56.1, 55.7, 55.6, 47.0, 38.2, 37.6, 33.0, 29.7, 25.3, 23.1, 19.8, 15.1; HRMS (EI) calcd for C32H41NO6 (M+) 535.2934; found 535.2919.
5.15 Indole 20
To stilbene 30 (19 mg 0.035 mmol) in a 1:1 mixture of THF and MeOH (2 mL) was added TsOH (30 mg, 0.16 mmol) and the resulting solution was allowed to stir at rt overnight. It was then quenched by addition of NaHCO3 (satd.) and extracted with EtOAc. The combined organic layers were washed with brine, dried (MgSO4), and filtered and then concentrated in vacuo. Final purification by flash column chromatography (40% EtOAc in hexanes) afforded analogue 20 (8 mg, 51%) as a light yellow oil: 1H NMR δ 7.05 (d, J = 16.1 Hz, 1H), 7.00 (s, 1H), 6.98 (d, J = 3.1 Hz, 1H), 6.97 (d, J = 16.5 Hz, 1H), 6.91 (s, 1H), 6.87 (s, 1H), 6.77 (s, 1H), 6.50 (d, J = 3.1 Hz, 1H), 5.24 (br s, 1H), 3.91 (s, 3H), 3.79 (s, 3H), 3.44 (dd, J = 11.6, 3.8 Hz, 1H), 2.75–2.72 (m, 2H), 2.17–2.11 (m 1H), 1.90–1.55 (m, 5H), 1.25 (s, 3H), 1.11 (s, 3H), 0.89 (s, 3H); 13C NMR δ 148.9, 148.9, 142.3, 138.8, 132.9, 129.3, 128.2, 127.5, 127.0, 122.6, 120.3, 117.8, 106.6, 101.6, 101.5, 97.4, 78.0, 56.0, 46.7, 38.4, 37.6, 33.1, 29.7, 28.2, 27.3, 19.8, 14.3; HRMS (EI) calcd for C28H33NO4 (M+) 447.2410; found 447.2422.
5.16 N, O-Dimethylindole 31
To indole 22 (500 mg, 2.43 mmol) in a mixture of THF and DMF (5:1) at 0 °C was added NaH (224 mg, 5.6 mmol, as a 60% dispersion in oil), followed after 20 min by MeI (0.34 mL, 5.35 mmol). The reaction was allowed to stir for 3 h, then quenched by addition of NH4Cl (satd.), and finally extracted with EtOAc. The combined organic extracts were washed with brine, dried (MgSO4), filtered and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (20% EtOAc in hexanes) afforded indole 31 (460 mg, 81%) as a white solid with 1H and 13C NMR spectra identical to those of material obtained above.
5.17 Aldehyde 32
To indole 31 (54 mg, 0.24 mmol) in THF (5 mL) at 0 °C was added LiAlH4 (28 mg, 0.73 mmol), and the reaction was allowed to warm to rt over 50 min. It was then quenched by addition by NH4Cl (satd.) and extracted with EtOAc. The combined organic layers were washed with brine, dried (MgSO4), and filtered, and the solvent was removed in vacuo. The resulting residue was then dissolved in CH2Cl2 (10 mL) and MnO2 (315 mg, 3.62 mmol) was added. After the reaction mixture was allowed to stir for 4 h, it was filtered through celite and the solvent was removed in vacuo to afford aldehyde 32 (38 mg, 84%, for 2 steps) as a light yellow solid: 1H NMR δ 9.98 (s, 1H), 7.48 (s, 1H), 7.17 (d, J = 2.9 Hz, 1H), 7.05 (s, 1H), 6.64 (d, J = 2.7 Hz, 1H), 4.00 (s, 3H), 3.85 (s, 3H); 13C NMR δ 192.2, 153.5, 137.0, 132.0, 131.4, 124.2, 109.3, 99.4, 97.1, 55.4, 33.2; HRMS (EI) calcd for C11H11NO2 (M+)189.0790; found 189.0787.
5.18 Phosphonate 33
To phosphonate 298 (81 mg, 0.17 mmol) in EtOH (3 mL) was added TsOH (80 mg, 0.42 mmol) and the reaction flask was wrapped in foil. The reaction mixture was allowed to stir for 2 days, then quenched by addition of NaHCO3 (satd.), and extracted with EtOAc. The combined organic layers were washed with brine, dried (MgSO4), and filtered, and then concentrated in vacuo. Final purification by flash column chromatography (3% EtOH in Et2O) afforded phosphonate 33 (62 mg, 85%) as a colorless oil: 1H NMR δ 6.66–6.65 (m, 1H), 6.64–6.62 (m, 1H), 4.07–3.99 (m, 4H), 3.83 (s, 3H), 3.39 (dd, J = 11.5, 3.9 Hz, 1H), 3.05 (d, JHP = 21.1 Hz, 2H), 2.69–2.66 (m, 2H), 2.13–2.09 (m, 1H), 1.90–1.59 (m, 5H), 1.26 (t, J = 7.0 Hz, 3H), 1.26 (t, J = 7.0 Hz, 3H), 1.22 (s, 3H), 1.08 (s, 3H), 0.86 (s, 3H); 13C NMR δ 148.6 (d, JCP = 3.1 Hz), 141.5 (d, JCP = 3.6 Hz), 122.7 (d, JCP = 7.5 Hz), 122.6 (d, JCP = 3.1 Hz), 122.0 (d, JCP = 9.2 Hz), 111.8 (d, JCP = 5.9 Hz), 77.9, 77.7, 62.1 (d, JCP = 6.8 Hz), 62.0 (d, JCP = 6.7 Hz), 46.6, 38.3, 37.6, 33.1 (d, JCP = 138.1 Hz), 28.2, 27.3, 23.0, 19.7, 16.4 (d, JCP = 6.1 Hz), 16.4 (d, JCP = 6.2 Hz), 14.2; 31P NMR δ 27.1; HRMS (EI) calcd for C22H35O6P (M+) 426.2171 found 426.2177.
5.19 Analogue 21
To phosphonate 33 (31 mg, 0.073 mmol) and aldehyde 32 (12 mg, 0.063 mmol) in THF (1 mL) was added NaH (40 mg, 1.0 mmol, 60% dispersion in oil) and 15-crown-5 (1 drop). The solution was allowed to stir overnight, then quenched by addition of NH4Cl (satd.) and finally extracted with EtOAc. The combined organic extracts were washed with brine, dried (MgSO4), and filtered, and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (45% EtOAc in hexanes) afforded analogue 21 (15 mg, 51%) as a yellow oil: 1H NMR δ 7.11 (d, J = 16.1 Hz, 1H), 7.04–6.99 (m, 2H), 6.96 (d, J = 3.2 Hz, 1H), 6.93 (d, J = 1.6 Hz, 1H), 6.90 (d, J = 1.5 Hz, 1H), 6.75 (s, 1H), 6.55 (d, J = 2.9 Hz, 1H), 4.02 (s, 3H), 3.92 (s, 3H), 3.79 (s, 3H), 3.44 (dd, J = 11.7, 4.0 Hz, 1H), 2.75–2.72 (m, 2H), 2.17–2.12 (m, 1H), 1.90–1.60 (m, 5H), 1.27 (s, 3H), 1.11 (s, 3H), 0.90 (s, 3H); 13C NMR δ 153.3, 148.9, 142.3, 138.3, 132.6, 129.4, 128.0, 127.9, 126.6, 122.6, 120.2, 118.8, 106.7, 101.8, 98.5, 97.2, 78.0, 77.0, 56.0, 55.3, 46.7, 38.4, 37.6, 33.1, 28.3, 27.4, 23.2, 19.8, 14.3; HRMS (EI) calcd for C29H35NO4 (M+) 461.2566; found 461.2569.
5.20 Geranyl indole 34
According to the procedure of Zhu and Ganesan,25 to indole 23 (824 mg, 3.76 mmol), TBAI (663 mg, 1.80 mmol), and Zn(OTf)2 (745 mg, 2.05 mmol) were allowed to react in a mixture of toluene (16 mL), and CH2Cl2 (2 mL) and DIPEA (0.66 mL, 3.76 mmol). After stirring for 15 min, geranyl bromide (371 mg, 1.71 mmol) was added dropwise and after an additional 2.5 h the reaction mixture was quenched by addition of NH4Cl (satd.) and finally extracted with EtOAc. The combined organic layers were washed with brine, dried (MgSO4), and filtered and then concentrated in vacuo. Final purification by flash column chromatography (17% EtOAc in hexanes) afforded the geranylated indole 34 (325 mg, 53%) as a colorless oil along with recovered starting indole (436 mg): 1H NMR δ 8.18 (br s, 1H), 7.74 (d, J = 0.9 Hz, 1H), 7.15 (d, J = 1.0 Hz, 1H), 6.94–6.93 (m, 1H), 5.49–5.44 (m, 1H), 5.15–5.10 (m, 1H), 4.39 (q, J = 7.2 Hz, 2H), 3.96 (s, 3H), 3.64 (d, J = 7.2 Hz, 2H), 2.14–2.04 (m, 4H), 1.71 (s, 3H), 1.68 (s, 3H), 1.60 (s, 3H), 1.41 (t, J = 7.1 Hz, 3H); 13C NMR δ 167.7, 154.5, 137.1, 135.3, 131.3, 124.7, 124.7, 123.5, 123.2, 120.9, 117.2, 107.4, 99.8, 60.7, 55.3, 39.7, 26.7, 25.7, 25.3, 17.7, 16.0, 14.4; HRMS (EI) calcd for C22H29NO3 (M+) 355.2147; found 355.2152.
5.21 Benzylic alcohol 35
To indole 34 (325 mg, 0.91 mmol) in THF (10 mL) at 0 °C was added NaH (44 mg, 1.1 mmol, 60% dispersion in oil) followed by TsCl (183 mg, 0.96 mmol). When the reaction was judged complete by TLC, DIBALH (0.53 mL, 2.97 mmol) was added dropwise. After an additional h the solution was quenched by addition of NH4Cl (satd.), diluted with EtOAc, acidified with 1M HCl to dissolve the solids, and finally extracted with EtOAc. The combined organic layers were washed with NaHCO3 (satd.), brine, dried (MgSO4), and filtered and then concentrated in vacuo. Final purification by flash column chromatography (35% EtOAc in hexanes) afforded alcohol 35 (273 mg, 64%) as a colorless oil: 1H NMR δ 7.68 (d, J = 8.4 Hz, 2H), 7.54 (s, 1H), 7.12 (d, J = 8.2 Hz, 2H), 7.09 (s, 1H), 6.62 (s, 1H), 5.41–5.37 (m, 1H), 5.15–5.11 (m, 1H), 4.71 (s, 2H), 3.79 (s, 3H), 3.50 (d, J = 7.0 Hz, 2H), 2.74 (br s, 1H), 2.26 (s, 3H), 2.14–2.04 (m, 4H), 1.67 (s, 3H), 1.67 (s, 3H), 1.61 (s, 3H); 13C NMR δ 154.6, 144.5, 139.0, 136.9, 136.5, 135.2, 131.4, 129.6 (2C), 126.5 (2C), 124.0, 123.1, 121.7, 121.4, 119.8, 104.8, 102.8, 65.6, 55.1, 39.6, 26.5, 25.6, 25.5, 21.3, 17.6, 15.9; HRMS (EI) calcd for C27H33NO4S (M+) 467.2130; found 467.2135.
5.22 Phosphonate 36
To benzylic alcohol 35 (269 mg, 0.58 mmol) in THF (10 mL) was added LiBr (400 mg, 4.60 mmol) and the solution was cooled to 0 °C. Next Et3N (0.32 mL, 2.3 mmol) followed after 10 min by MsCl (0.13 mL, 1.73 mmol) were added, and the reaction mixture was allowed to stir for 2 h. The reaction mixture was then quenched by addition of NH4Cl (satd.) and extracted with Et2O. The combined organic extracts were washed with brine, dried (MgSO4), and filtered, and the filtrate was concentrated in vacuo. The resulting oil was dissolved in P(OEt)3 (2 mL) and heated at reflux. The next day the reaction was allowed to cool to rt, then poured into Et2O, washed with H2O, brine, dried (MgSO4), and filtered, and finally the filtrate was concentrated in vacuo. Final purification by flash column chromatography (2.5 to 3% MeOH in Et2O) afforded phosphonate 36 (273 mg, 81%) as a colorless oil; 1H NMR δ 7.71 (d, J = 8.4 Hz, 2H), 7.50 (dd, J = 3.0, 1.1 Hz, 1H), 7.19 (d, J = 8.4 Hz, 2H), 7.07–7.06 (m, 1H), 6.62–6.61 (m, 1H), 5.41–5.36 (m, 1H), 5.15–5.11 (m, 1H), 4.02–3.96 (m, 4H), 3.84 (s, 3H), 3.49 (d, J = 7.2 Hz, 2H), 3.23 (d, JPH = 21.4 Hz, 2H), 2.33 (s, 3H), 2.14–2.05 (m, 4H), 1.70 (d, J = 0.8 Hz, 3H), 1.67 (d, J = 1.0 Hz, 3H), 1.62 (s, 3H), 1.24 (t, J = 7.1 Hz, 6H); 13C NMR δ 154.3 (d, JCP = 3.0 Hz), 144.4, 137.0 (d, JCP = 3.1 Hz), 136.5, 135.4, 131.4, 129.6 (2C), 129.1 (d, JCP = 9.1 Hz), 126.6 (2C), 124.1, 123.1 (d, JCP = 1.6 Hz), 121.6, 121.4 (d, JCP = 1.3 Hz), 119.3 (d, JCP = 3.2 Hz), 107.8 (d, JCP = 7.9 Hz), 105.8 (d, JCP = 5.5 Hz), 62.1 (d, JCP = 6.6 Hz, 2C), 55.2, 39.6, 34.3 (d, JCP = 138.1 Hz), 26.6, 25.6, 25.5, 21.4, 17.6, 16.3 (d, JCP = 5.9 Hz, 2C), 16.0; 13P NMR δ 26.2; HRMS (EI) calcd for C31H42NO6PS (M+) 587.2470; found 587.2481.
5.23 Geranylated stilbene 37
To phosphonate 36 (100 mg, 0.17 mmol) and aldehyde 15 (40 mg, 0.13 mmol) in THF (1.0 mL) at 0 °C was added NaH (60 mg, 1.0 mmol, 60% oil dispersion) and 15-crown-5 (1 drop). When the aldehyde had disappeared as judged by TLC, the reaction was quenched by addition of NH4Cl (satd.), and extracted with Et2O. The combined organic extracts were dried (MgSO4), and filtered, and then concentrated in vacuo. Final purification by flash column chromatography (30% EtOAc in hexanes) afforded analogue 37 (37 mg, 38%) as an oil which was used directly in the next step: 1H NMR δ 7.73 (d, J = 8.3 Hz, 2H), 7.68 (d, J = 0.9 Hz, 1H), 7.20 (d, J = 8.1 Hz, 2H), 7.09 (t, J = 1.1 Hz, 1H), 7.04–7.03 (m, 2H), 6.94–6.93 (m, 1H), 6.91–6.90 (m, 1H), 6.79 (s, 1H), 5.42–5.37 (m, 1H), 5.15–5.11 (m, 1H), 3.93 (s, 3H), 3.90, (s, 3H), 3.51–3.42 (m, 3H), 2.76–2.73 (m, 2H), 2.17–2.04 (m, 5H), 1.91–1.82 (m, 3H), 1.75–1.59 (m, 11H), 1.27 (s, 3H), 1.11 (s, 3H), 0.90 (s, 3H); 13C NMR δ 154.6, 149.0, 144.6, 142.7, 137.5, 136.6, 135.7, 135.4, 131.5, 129.7 (2C), 128.9, 128.2, 127.0, 126.7 (2C), 124.2, 123.5, 122.7, 121.8, 121.7, 120.5, 120.0, 106.9, 105.4, 101.4, 78.0, 77.1, 56.0, 55.2, 46.8, 39.7, 38.4, 37.7, 28.3, 27.3, 26.6, 25.7, 25.6, 23.2, 21.5, 19.8, 17.7, 16.0, 14.3.
5.24 Analogue 38
Stilbene 37 (37 mg, 0.05 mmol) in THF (2 mL) was added to a solution of NaH (150 mg, 3.75 mmol, 60% dispersion in oil) in 2-propanol (2 mL) and the solution was allowed to stir overnight. The reaction mixture was then quenched by addition of H2O and extracted with EtOAc. The combined organic extracts were washed with brine, dried (MgSO4), filtered, and then concentrated in vacuo. Final purification by flash column chromatography (45% EtOAc in hexanes) afforded schweinfurthin analogue 38 (17 mg, 58%, or 22% over two steps from compound 36) as a colorless oil: 1H NMR δ 7.91 (br s, 1H), 7.02–6.99 (m, 3H), 6.91–6.90 (m, 1H). 6.88–6.87 (m, 1H), 6.78 (s, 1H), 6.68 (s, 1H), 5.50–5.46 (m, 1H), 5.16–5.12 (m, 1H), 3.97 (s, 3H), 3.90 (s, 3H), 3.63 (d, J = 7.2 Hz, 2H), 3.46–3.41 (m, 1H), 2.75–2.71 (m, 2H), 2.16–2.06 (m, 5H), 1.90–1.61 (m, 14H), 1.26 (s, 3H), 1.11 (s, 3H), 0.89 (s, 3H); 13C NMR δ 155.0, 148.9, 142.3, 138.4, 135.0, 132.9, 131.3, 129.5, 127.9, 126.5, 124.5, 123.8, 122.6, 120.6, 120.2, 117.2, 117.0, 106.9, 103.3, 97.4, 78.0, 56.0, 55.1, 46.8, 39.8, 38.4, 37.7, 28.3, 27.4, 26.8, 25.7, 25.5, 23.2, 19.8, 17.7, 16.0, 14.3; HRMS (EI) calcd for C38H49NO4 (M+) 583.3662; found 587.2672.
5.25 Prenylated indole 39
To indole 23 (388 mg, 1.77 mmol), TBAI (360 mg, 0.98 mmol), and Zn(OTf)2 (436 mg, 1.2 mmol) in a 5:1 mixture of toluene and CH2Cl2 (12 mL) at rt was added DIPEA (0.38 mL, 2.2 mmol) and the reaction mixture was allowed to stir for 10 min. Prenyl bromide (126 mg, 0.88 mmol) was added dropwise. After 2 h the reaction mixture was quenched by addition of NH4Cl (satd.) and extracted with EtOAc. The combined organic extracts were washed with H2O, dried (MgSO4), and filtered, and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (10 to 15% EtOAc in hexanes) afforded prenylated indole 39 (209 mg, 59%) along with recovered indole 23 (149 mg) as expected based on the literature precedent:25 1H NMR δ 8.31 (br s, 1H), 7.74 (d, J = 1.0 Hz, 1H), 7.15 (d, J = 0.5 Hz, 1H), 6.94–6.93 (m, 1H), 5.47–5.42 (m, 1H), 4.39 (q, J = 7.1 Hz, 2H), 3.95 (s, 3H), 3.63 (d, J = 7.2 Hz, 2H), 1.75 (s, 3H), 1.72 (s, 3H), 1.40 (t, J = 7.2 Hz, 3H); 13C NMR δ 167.8, 154.4, 137.1, 131.5, 124.6, 123.7, 123.2, 120.8, 117.1, 107.4, 99.8, 60.7, 55.3, 25.7, 25.4, 17.7, 14.4; HRMS (EI) calcd for C17H21NO3 (M+) 287.1521; found 287.1523.
5.26 Alcohol 40
To a solution of indole 39 (18 mg, 0.06 mmol) in THF (3 mL) at rt was added NaH (5 mg, 0.13 mmol, 60% dispersion oil) and the reaction mixture was allowed to stir for 10 min. After TsCl (15 mg, 0.08 mmol) was added, the solution was stirred for 2 h and then DIBALH (0.05 mL, 0.44 mmol) was added dropwise. After an additional 30 min, the reaction was quenched with NH4Cl (satd.), poured into EtOAc, acidified with 1M HCl, and extracted with EtOAc. The combined organic extracts were washed with NaHCO3 (satd.), and brine, dried (MgSO4), and filtered, and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (35% EtOAc in hexanes) afforded the benzylic alcohol 40 (19 mg, 76%): 1H NMR δ 7.70 (d, J = 8.1 Hz, 2H), 7.53 (s, 1H), 7.15 (d, J = 8.3 Hz, 2H), 7.10 (s, 1H), 6.64 (s, 1H), 5.39–5.35 (m, 1H), 4.72 (s, 2H), 3.82 (s, 3H), 3.49 (d, J = 7.1 Hz, 2H), 2.29 (s, 3H), 1.76 (s, 3H), 1.68 (s, 3H); 13C NMR δ 154.7, 144.6, 139.0, 136.9, 135.2, 132.9, 129.7 (2C), 126.6 (2C), 123.1, 121.8, 121.5, 119.8, 104.9, 102.9, 65.7, 55.2, 25.7, 25.6, 21.4, 17.7; HRMS (EI) calcd for C22H25NO4S (M+) 399.1504; found 399.1508.
5.27 Phosphonate 41
To alcohol 40 (102 mg, 0.25 mmol) in THF (5 mL) at 0 °C was added LiBr (133 mg, 1.53 mmol) and Et3N (0.11 mL, 0.79 mmol). The solution was stirred for 5 min, MsCl (0.05 mL, 0.65 mmol) was added dropwise, and the reaction was allowed to warm to rt. After 2 h it was quenched by addition of NH4Cl (satd.), extracted with Et2O, dried (MgSO4), and filtered, and the filtrate was concentrated in vacuo. To the resulting residue was added P(OEt)3 (2 mL) and the solution was heated to 130 °C and allowed to stir overnight. The next day the solution was allowed to cool to rt and the solvent was removed in vacuo. Final purification by flash column chromatography (2% EtOH in Et2O) afforded indole phosphonate 41 (111 mg, 84%) as a colorless oil: 1H NMR δ 7.72 (d, J = 8.4 Hz, 2H), 7.50 (d, JHP = 2.3 Hz, 1H), 7.19 (d, J = 8.2 Hz, 2H), 7.07 (d, J = 1.1 Hz, 1H), 6.62 (s, 1H), 5.40–5.34 (m, 1H), 4.06–3.92 (m, 4H), 3.84 (s, 3H), 3.48 (d, J = 7.1 Hz, 2H), 3.23 (d, JHP = 21.5 Hz, 2H), 2.32 (s, 3H), 1.76 (s, 3H), 1.67 (s, 3H), 1.24 (t, J = 7.1 Hz, 6H); 13C NMR δ 154.3 (d, JCP = 2.9 Hz), 144.4, 136.9 (JCP = 2.9 Hz), 135.2, 132.9, 129.7 (2C), 129.1 (d, JCP = 9.8 Hz), 126.7 (2C), 123.1 (d, JCP = 1.7 Hz), 121.7, 121.3 (d, JCP = 1.6 Hz), 119.3 (d, JCP = 3.2 Hz), 107.8 (d, JCP = 7.8 Hz), 105.8 (d, JCP = 5.6 Hz), 62.0 (d, JCP = 2.9 Hz, 2C), 55.2, 34.2 (d, JCP = 138.2 Hz), 25.7, 25.6, 21.4, 17.7, 16.3 (d, JCP = 6.0 Hz, 2C); 31P NMR δ 26.2; HRMS (EI) calcd for C26H34NO6PS (M+) 519.1844; found 519.1843.
5.28 Analogue 43
To phosphonate 41 (45 mg, 0.089 mmol) and aldehyde 15 (21 mg, 0.069 mmol) in THF (1mL) at 0 °C was added NaH (40 mg, 1.0 mmol, 60% dispersion oil) and 15-crown-5 (2 drops). The reaction mixture was allowed to stir for 45 min, then quenched by addition of NH4Cl (satd.), and extracted with EtOAc. The combined organic extracts were washed with brine, dried (MgSO4), and filtered, and then the filtrate was concentrated in vacuo. Purification by flash column chromatography (20 to 50% EtOAc in hexanes) afforded a mixture of protected and unprotected indoles (26 mg). This mixture was treated with NaOi-Pr in THF (3 mL), generated in situ from NaH (160 mg, 4 mol, 60% dispersion oil) and i-PrOH, and the reaction mixture was allowed to stir overnight. The next day the reaction mixture was quenched by addition of H2O and extracted with EtOAc. The combined organic extracts were washed with water and brine, dried (MgSO4), and filtered, and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (45% EtOAc in hexanes) afforded indole 43 (13.5 mg, 38% for two steps) as a light yellow oil: 1H NMR δ 7.90 (br s, 1H), 7.03 (d, J = 16.0 Hz, 1H), 7.01 (s, 1H), 6.96 (d, J = 16.2 Hz, 1H), 6.91–6.90 (m, 1H), 6.88–6.87 (m, 1H), 6.79–6.78 (m, 1H), 6.68 (s, 1H) 5.49–5.44 (m, 1H), 3.97 (s, 3H), 3.90 (s, 3H), 3.61 (d, J = 7.2 Hz, 2H), 3.46–3.41 (m, 1H), 2.75–2.72 (m, 2H), 2.17–2.10 (m, 1H), 1.91–1.59 (m, 5H), 1.75 (s, 3H), 1.73 (s, 3H), 1.26 (s, 3H), 1.11 (s, 3H), 0.89 (s, 3H); 13C NMR δ 155.0, 148.9, 142.3, 138.3, 132.9, 131.2, 129.5, 127.9, 126.5, 124.0, 122.6, 120.5, 120.2, 117.2, 117.0, 106.9, 103.3, 97.4, 78.1, 77.0, 56.0, 55.1, 46.8, 38.3, 37.7, 28.3, 27.3, 25.8, 25.6, 23.3, 19.8, 17.7, 14.3; HRMS (EI) calcd for C33H41NO4 (M+) 515.3036; found 515.3040.
5.29 Phosphonate 45
Through a variation of our earlier procedure, alcohol 448 (204 mg, 0.66 mmol) in THF (10 mL) at 0 °C was treated with LiBr (400 mg, 4.6 mmol) and Et3N (0.37 mL, 2.65 mmol), and then after 5 min MsCl (0.13 mL, 1.65 mmol) was added. After 90 min, the reaction mixture was quenched by addition of water and extracted with Et2O. The combined organic extracts were washed with brine, dried (MgSO4), and filtered, and the filtrate was concentrated in vacuo. The resulting residue was dissolved in P(OEt)3 (3 mL) and the solution was heated to reflux overnight. The next day the solution was allowed to cool to rt and then concentrated in vacuo. Final purification by flash column chromatography (2% EtOH in Et2O) afforded phosphonate 45 (297 mg, 90%) as an oil whose 1H and 13C NMR spectra were consistent with those from previously prepared materials.8
5.30 Stilbene 46
To aldehyde 32 (15 mg, 0.08 mmol) and phosphonate 45 (48 mg, 0.10 mmol) in THF (3 mL) at 0 °C was added NaH (40 mg, 1.0 mmol, 60% dispersion oil) and 15-crown-5 (2 drops) and the reaction mixture was allowed to warm to rt. The following day the reaction mixture was quenched by addition of NH4Cl (satd.) and extracted with EtOAc. The combined organic extracts were washed with brine, dried (MgSO4), and filtered, and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (20% EtOAc in hexanes) afforded stilbene 46 (18 mg, 42%) as a light yellow oil: 1H NMR δ 7.17 (d, J = 1.7 Hz, 1H), 7.08 (d, J = 16.8 Hz, 1H), 7.03 (s, 1H), 6.99 (d, J = 16.4 Hz, 1H), 6.98 (d, J = 1.4 Hz, 1H), 6.94 (d, J = 3.1 Hz, 1H), 6.73 (s, 1H), 6.54 (d, J = 2.9 Hz, 1H), 5.25 (d, J = 6.7 Hz, 1H), 5.21 (d, J = 6.5 Hz, 1H), 4.78 (d, J = 6.9 Hz, 1H), 4.65 (d, J = 6.8 Hz, 1H), 4.01 (s, 3H), 3.78 (s, 3H), 3.55 (s, 3H), 3.41 (s, 3H), 3.29 (dd, J = 11.5, 3.9 Hz, 1H), 2.75–2.72 (m, 2H), 2.13–1.97 (m, 2H), 1.80–1.57 (m, 3H), 1.26 (s, 3H), 1.10 (s, 3H), 0.91 (s, 3H); 13C NMR δ 153.3, 146.2, 143.6, 138.3, 132.7, 129.6, 128.2, 127.8, 126.4, 123.2, 121.7, 118.9, 113.4, 101.8, 98.5, 97.3, 96.2, 95.9, 84.0, 76.9, 56.2, 55.6, 55.3, 47.1, 38.3, 37.7, 33.0, 27.3, 25.3, 23.2, 19.9, 15.1; HRMS (EI) calcd for C32H41NO6 (M+) 535.2934; found 535.2933.
5.31 Analogue 47
To the MOM-protected analogue 46 (18 mg, 0.034 mmol) in 1:1 MeOH:THF (0.8 mL) protected from ambient light was added TsOH (50 mg, excess) and the resulting solution was allowed to stir overnight. The reaction mixture was quenched by addition of NH4Cl (satd.) and extracted with EtOAc. The combined organic layers were washed with brine, dried (MgSO4), and filtered and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (50% EtOAc in hexanes) afforded analogue 47 (9 mg, 60%) as a light green oil: 1H NMR δ 7.08 (d, J = 16.2 Hz, 1H), 7.02 (s, 1H), 7.00–6.95 (m, 2H), 6.94 (d, J = 3.0 Hz, 1H), 6.82 (d, J = 1.5 Hz, 1H), 6.73 (s, 1H), 6.54 (d, J = 2.6 Hz, 1H), 5.46 (br s, 1 OH), 4.01 (s, 3H), 3.78 (s, 3H), 3.45 (dd, J = 11.3, 4.0 Hz, 1H), 2.74–2.70 (m, 2H), 2.06–2.01 (m, 1H), 1.91–1.60 (m, 4H), 1.55 (br s, 1 OH), 1.26 (s, 3H), 1.12 (s, 3H), 0.90 (s, 3H); 13C NMR δ 153.3, 145.2, 139.7, 138.4, 132.7, 130.3, 128.3, 127.9, 126.5, 122.0, 119.2, 118.9, 119.4, 101.8, 98.5, 97.3, 77.9, 77.9, 55.3, 47.2, 38.5, 37.7, 33.0, 28.2, 27.3, 22.7, 20.2, 14.3; HRMS (EI) calcd for C28H33NO4 (M+) 447.2410; found 447.2404.
5.32 Stilbene 49
To phosphonate 41 (45 mg, 0.089 mmol) and aldehyde 4811 (25.7 mg, 0.068 mmol) in THF (3mL) at 0 °C was added NaH (50 mg, 1.25 mmol, 60% dispersion oil) and 15-crown-5 (2 drops). The reaction was allowed to warm to rt and then allowed to stir for 4 h. To the reaction mixture was added 2-propanol (3 mL) and NaH (40 mg, 1.0 mmol, 60% dispersion oil) and the solution was allowed to stir. After 20 h the reaction was quenched by addition of NaHCO3 (satd.) and extracted with EtOAc. The combined organic extracts were washed with brine, dried (MgSO4), and filtered, and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (40% EtOAc in hexanes) afforded indole 49 (20 mg, 51% for 2 steps) as a light yellow oil: 1H NMR δ 7.89 (br s, 1H), 7.15 (d, J = 1.4 Hz, 1H), 7.00 (s, 1H), 6.99 (s, 1H), 6.98–6.97 (m, 2H), 6.78 (d, J = 1.1 Hz, 1H), 6.68 (s, 1H), 5.49–5.44 (m, 1H), 5.25 (d, J = 6.6 Hz, 1H), 5.20 (d, J = 6.6 Hz, 1H), 4.78 (d, J = 6.9 Hz, 1H), 4.65 (d, J = 6.9 Hz, 1H), 3.97 (s, 3H), 3.61 (d, J = 7.2 Hz, 2H), 3.55 (s, 3H), 3.41 (s, 3H), 3.29 (dd, J = 11.6, 3.9 Hz, 1H), 2.75–2.71 (m, 2H), 2.13–1.94 (m, 2H), 1.75–1.55 (m, 3H), 1.75 (s, 3H), 1.73 (s, 3H), 1.26 (s, 3H), 1.10 (s, 3H), 0.91 (s, 3H); 13C NMR δ 155.0, 146.2, 143.6, 138.3, 132.9, 131.2, 129.6, 128.0, 126.3, 124.7, 123.3, 121.7, 120.5, 117.2, 117.0, 113.4, 103.4, 97.4, 96.2, 95.9, 84.0, 76.9, 56.2, 55.6, 55.1, 47.1, 38.3, 37.7, 27.4, 25.8, 25.6, 25.3, 23.2, 19.9, 17.7, 14.3; HRMS (EI) calcd for C36H47NO6 (M+) 589.3403; found 589.3416.
5.33 Analogue 50
To protected analogue 49 (12.2 mg, 0.021 mmol) was added 1:1 THF/MeOH (2 mL) and TsOH (35 mg, 0.18 mmol) and the reaction mixture was allowed to stir overnight. The next day the reaction mixture was quenched by addition of NH4Cl (satd.) and extracted with EtOAc. The combined organic extracts were washed with brine, dried (MgSO4), and filtered, and the filtrate was concentrated in vacuo. Final purification by flash column chromatography (25 to 50% EtOAc in hexanes) afforded analogue 50 (6.4 mg, 62%) as an oil: 1H NMR δ 7.89 (br s, 1H), 7.01 (d, J = 16.1 Hz, 1H), 7.00 (s, 1H), 6.97 (d, J = 1.9 Hz, 1H), 6.93 (d, J = 16.2 Hz, 1H), 6.80–6.78 (m, 2H), 6.67 (s, 1H), 5.49–5.44 (m, 2H), 3.97 (s, 3H), 3.61 (d, J = 7.2 Hz, 2H), 3.45 (dd, J = 11.2, 4.0 Hz, 1H), 2.78–2.63 (m, 2H), 2.06–2.00 (m, 1H), 1.93–1.58 (m, 5H), 1.75 (s, 3H), 1.73 (s, 3H), 1.25 (s, 3H), 1.12 (s, 3H), 0.89 (s, 3H); 13C NMR δ 155.0, 145.2, 139.7, 138.3, 132.9, 131.2, 130.3, 128.2, 126.4, 124.1, 122.0, 120.5, 119.2, 117.2, 117.1, 109.4, 103.4, 97.4, 77.9, 77.8, 55.1, 47.2, 38.5, 37.7, 28.2, 27.3, 25.8, 25.6, 22.7, 20.2, 17.7, 14.3; HRMS (EI) calcd for C32H39NO4 (M+) 501.2879; found 501.2881.
Supplementary Material
Acknowledgements
We thank the UI Graduate College for a Presidential Fellowship (to J.G.K.). This research was supported in part by the Intramural Research Program of NIH, National Cancer Institute, Center for Cancer Research. Financial support from the Roy J. Carver Charitable Trust as a Research Program of Excellence is gratefully acknowledged. We thank the Developmental Therapeutics Program, NCI, for the 60-cell testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data (including NMR spectra and complete bioassay data) associated with this article can be found, in the online version, at
References and notes
- 1.Beutler JA, Shoemaker RH, Johnson T, Boyd MR. J. Nat. Prod. 1998;61:1509–1512. doi: 10.1021/np980208m. [DOI] [PubMed] [Google Scholar]
- 2.Beutler JA, Jato J, Cragg GM, Boyd MR. Natural Product Letters. 2000;14:399–404. [Google Scholar]
- 3.Klausmeyer P, Van QN, Jato J, McCloud TG, Beutler JA. J. Nat. Prod. 2010;73:479–481. doi: 10.1021/np9006348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thoison O, Hnawia E, Guerittevoegelein F, Sevenet T. Phytochemistry. 1992;31:1439–1442. [Google Scholar]
- 5.Yoder BJ, Cao SG, Norris A, Miller JS, Ratovoson F, Razafitsalama J, Andriantsiferana R, Rasamison VE, Kingston DGI. J. Nat. Prod. 2007;70:342–346. doi: 10.1021/np060484y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Kaaden JE, Hemscheidt TK, Mooberry SL. J. Nat. Prod. 2001;64:103–105. doi: 10.1021/np000265r. [DOI] [PubMed] [Google Scholar]
- 7.Mente NR, Wiemer AJ, Neighbors JD, Beutler JA, Hohl RJ, Wiemer DF. Bioorg. Med. Chem. Lett. 2007;17:911–915. doi: 10.1016/j.bmcl.2006.11.096. [DOI] [PubMed] [Google Scholar]
- 8.Mente NR, Neighbors JD, Wiemer DF. J. Org. Chem. 2008;73:7963–7970. doi: 10.1021/jo800951q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topczewski JJ, Neighbors JD, Wiemer DF. J. Org. Chem. 2009;74:6965–6972. doi: 10.1021/jo901161m. [DOI] [PubMed] [Google Scholar]
- Topczewski JJ, Kodet JG, Wiemer DF. J. Org. Chem. 2011;76:909–919. doi: 10.1021/jo1022102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topczewski JJ, Wiemer DF. Tetrahedron Lett. 2011;52:1628–1630. doi: 10.1016/j.tetlet.2011.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neighbors JD, Topczewski JJ, Swenson DC, Wiemer DF. Tetrahedron Lett. 2009;50:3881–3884. [Google Scholar]
- 13.Topczewski JJ, Callahan MP, Kodet JG, Inbarasu JD, Mente NR, Beutler JA, Wiemer DF. Bioorg. Med. Chem. 2011;19:7570–7581. doi: 10.1016/j.bmc.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neighbors JD, Beutler JA, Wiemer DF. J. Org. Chem. 2005;70:925–931. doi: 10.1021/jo048444r. [DOI] [PubMed] [Google Scholar]
- 15.Ulrich NC, Kodet JG, Mente NR, Kuder CH, Beutler JA, Hohl RJ, Wiemer DF. Bioorg. Med. Chem. 2010;18:1676–1683. doi: 10.1016/j.bmc.2009.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topczewski JJ, Kuder CH, Neighbors JD, Hohl RJ, Wiemer DF. Bioorg. Med. Chem. 2010;18:6734–6741. doi: 10.1016/j.bmc.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulrich NC, Kuder CH, Hohl RJ, Wiemer DF. Bioorg. Med. Chem. Lett. 2010;20:6716–6720. doi: 10.1016/j.bmcl.2010.08.143. [DOI] [PubMed] [Google Scholar]
- 18.Nandy JP, Prakesch M, Khadem S, Reddy PT, Sharma U, Arya P. Chem. Rev. 2009;109:1999–2060. doi: 10.1021/cr800188v. [DOI] [PubMed] [Google Scholar]
- 19.Humphrey GR, Kuethe JT. Chem. Rev. 2006;106:2875–2911. doi: 10.1021/cr0505270. [DOI] [PubMed] [Google Scholar]
- 20.Taber DF, Tirunahari PK. Tetrahedron. 2011;67:7195–7210. doi: 10.1016/j.tet.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kodet JG, Wiemer DF. J. Org. Chem. 2013;78:9291–9302. doi: 10.1021/jo4014244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kodet JG. Ph. D. Thesis. University of Iowa; 2010. [Google Scholar]
- 23.Kim M, Vedejs E. J. Org. Chem. 2004;69:6945–6948. doi: 10.1021/jo040191e. [DOI] [PubMed] [Google Scholar]
- 24.Treadwell EM, Cermak SC, Wiemer DF. J. Org. Chem. 1999;64:8718–8723. [Google Scholar]
- 25.Zhu XW, Ganesan A. J. Org. Chem. 2002;67:2705–2708. doi: 10.1021/jo010996b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.