ABSTRACT
Linezolid resistance is uncommon among staphylococci, but approximately 2% of clinical isolates of coagulase-negative staphylococci (CoNS) may exhibit resistance to linezolid (MIC, ≥8 µg/ml). We performed whole-genome sequencing (WGS) to characterize the resistance mechanisms and genetic backgrounds of 28 linezolid-resistant CoNS (21 Staphylococcus epidermidis isolates and 7 Staphylococcus haemolyticus isolates) obtained from blood cultures at a large teaching health system in California between 2007 and 2012. The following well-characterized mutations associated with linezolid resistance were identified in the 23S rRNA: G2576U, G2447U, and U2504A, along with the mutation C2534U. Mutations in the L3 and L4 riboproteins, at sites previously associated with linezolid resistance, were also identified in 20 isolates. The majority of isolates harbored more than one mutation in the 23S rRNA and L3 and L4 genes. In addition, the cfr methylase gene was found in almost half (48%) of S. epidermidis isolates. cfr had been only rarely identified in staphylococci in the United States prior to this study. Isolates of the same sequence type were identified with unique mutations associated with linezolid resistance, suggesting independent acquisition of linezolid resistance in each isolate.
IMPORTANCE
Linezolid is one of a limited number of antimicrobials available to treat drug-resistant Gram-positive bacteria, but resistance has begun to emerge. We evaluated the genomes of 28 linezolid-resistant staphylococci isolated from patients. Multiple mutations in the rRNA and associated proteins previously associated with linezolid resistance were found in the isolates investigated, underscoring the multifocal nature of resistance to linezolid in Staphylococcus. Importantly, almost half the S. epidermidis isolates studied harbored a plasmid-borne cfr RNA methylase gene, suggesting that the incidence of cfr may be higher in the United States than previously documented. This finding has important implications for infection control practices in the United States. Further, cfr is commonly detected in bacteria isolated from livestock, where the use of phenicols, lincosamides, and pleuromutilins in veterinary medicine may provide selective pressure and lead to maintenance of this gene in animal bacteria.
INTRODUCTION
Linezolid is an oxazolidinone with activity against Gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant coagulase-negative staphylococci (CoNS) (1). Fourteen years after its approval for clinical use in the United States, linezolid remains highly active against clinical isolates of S. aureus and CoNS, with >99% and 98% susceptibility reported from surveillance studies, respectively (2). Nonetheless, linezolid-resistant staphylococci have been reported worldwide (2).
Linezolid exerts bacteriostatic activity via protein synthesis inhibition, by reversibly binding and blocking the ribosomal peptidyl transferase center (PTC) (3). Modification of the ribosome at the PTC, commonly by mutation of the V domain of the 23S rRNA, is associated with resistance in clinical isolates of staphylococci (4, 5). The most common mutation at this site is G2576U (2, 6, 7). Other mutations, such as G2447U, C2461U, U2500A, G2534U, G2603U, and U2504A, have also been identified in linezolid-resistant staphylococci (2). A dosage effect has been described for mutations in the 23S rRNA, whereby the number of mutated rRNA copies is directly related to linezolid MIC (8). Linezolid resistance has also been associated with mutations in the ribosomal L3 and L4 proteins (3). In addition, acquisition of the 23S rRNA methyltransferase gene cfr can also provide resistance via modification of A2503 in domain V of the 23S rRNA, thereby impeding binding of linezolid along with the phenicol, lincosamide, pleuromutilin, and streptogramin A and the 16-member ring macrolides to the ribosome (9–11). Isolates that carry cfr may test linezolid susceptible (12) or resistant.
In this study, we investigated the incidence of linezolid resistance among CoNS isolated from blood at a large tertiary-care hospital and an acute-care hospital in the United States. A collection of linezolid-resistant CoNS (LRCoNS) from 28 patients were evaluated for mechanisms of linezolid resistance and evidence of clonal spread.
RESULTS
Characterization of LRCoNS isolated from blood over a 5-year period.
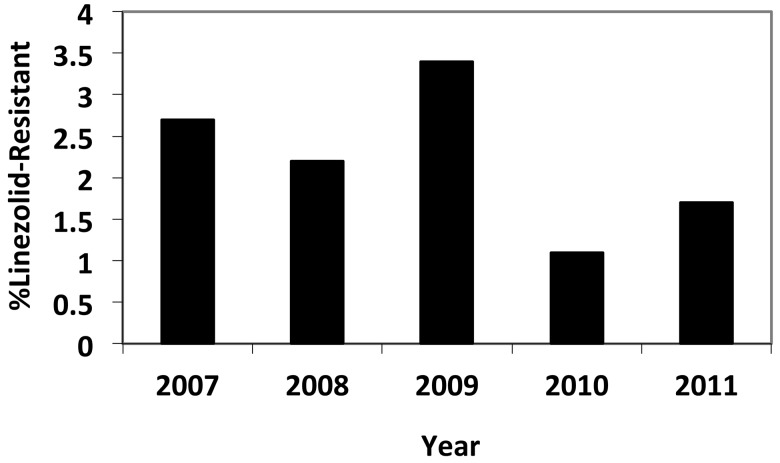
During the study period of 2007 to 2012, we identified 47 patients with LRCoNS isolated from blood cultures, yielding an annual incidence of linezolid resistance between 1.2 and 3.2% (Fig. 1). No temporal trend was noted. Twenty-eight LRCoNS were available for further characterization, and chart reviews were performed for these patients. Twenty-one isolates (75%) were identified as Staphylococcus epidermidis and 7 (25%) as Staphylococcus haemolyticus.
FIG 1 .
Incidence of linezolid resistance among clinically significant coagulase-negative staphylococci isolated from blood. Data represent one isolate per patient.
The median patient age was 65 years (range, 17 to 97), and 21 (75%) were female. Prior infection with a multidrug-resistant organism (e.g., MRSA, extended-spectrum-beta-lactamase-expressing Enterobacteriaceae, or vancomycin-resistant Enterococcus) was noted in 25 (89%) patients. Comorbidities included renal disease (n = 8; 29%), diabetes (n = 6; 21.4%), organ transplantation (n = 5; 17.9%), and liver disease (n = 3; 10.7%). All patients except for one (patient 17) were hospitalized, with a mean duration of 38.5 days (range, 0 to 135 days) prior to the isolation of LRCoNS (Table 1). For six patients, LRCoNS was isolated on the first day of hospitalization (Table 1). Linezolid was administered to 27 patients (96.4%) in the 3 months prior to isolation of LRCoNS (Table 1), with a mean treatment duration of 16 days (range, 0 to 42 days). Patient 13 (Table 1) had no documented exposure to linezolid. In all cases, linezolid use was parenteral.
TABLE 1 .
Linezolid use and linezolid MICs
| Case | Date LRCoNS was isolated (mo/yr) | Linezolid MIC (µg/ml) |
Hospital day LRCoNS was isolated |
No. of days of prior linezolid exposure |
|---|---|---|---|---|
| S. epidermidis | ||||
| 1 | 1/2010 | 128 | 19 | 8 |
| 2 | 2/2011 | 256 | 7 | 7 |
| 3 | 3/2011 | >256 | 37 | 9 |
| 4 | 6/2011 | 32 | 120 | 28 |
| 5 | 7/2011 | 8 | 1 | 20 |
| 6 | 9/2011 | 128 | 1 | 6 |
| 7 | 5/2007 | 16 | 20 | 6 |
| 13 | 11/2007 | 8 | 12 | 17 |
| 14 | 4/2008 | >256 | 1 | 14 |
| 15 | 6/2008 | >256 | Outpatient | 14 |
| 17 | 6/2008 | 32 | 84 | 24 |
| 18 | 8/2008 | 32 | 5 | 24 |
| 19 | 8/2008 | 32 | 135 | 7 |
| 20 | 10/2008 | 128 | 23 | 21 |
| 21 | 11/2008 | 32 | 97 | 22 |
| 22 | 11/2008 | 16 | 21 | 80 |
| 23 | 12/2008 | >256 | 41 | 19 |
| 25 | 9/2009 | >256 | 19 | 3 |
| 26 | 10/2009 | 64 | 64 | 31 |
| 27 | 9/2009 | 32 | 25 | 9 |
| 28 | 10/2009 | 128 | 9 | 3 |
| S. haemolyticus | ||||
| 8 | 5/2007 | 64 | 64 | 42 |
| 9 | 4/2007 | 64 | 32 | 9 |
| 10 | 10/2007 | 64 | 1 | 17 |
| 11 | 10/2007 | 64 | 1 | 0 |
| 12 | 10/2007 | 128 | 57 | 30 |
| 16 | 7/2008 | 64 | 1 | NDa |
| 24 | 7/2009 | 64 | 123 | 23 |
| Avg (SD) | 38.5 (41.7) | 16 (10) |
ND, no data.
Genetic backgrounds of LRCoNS.
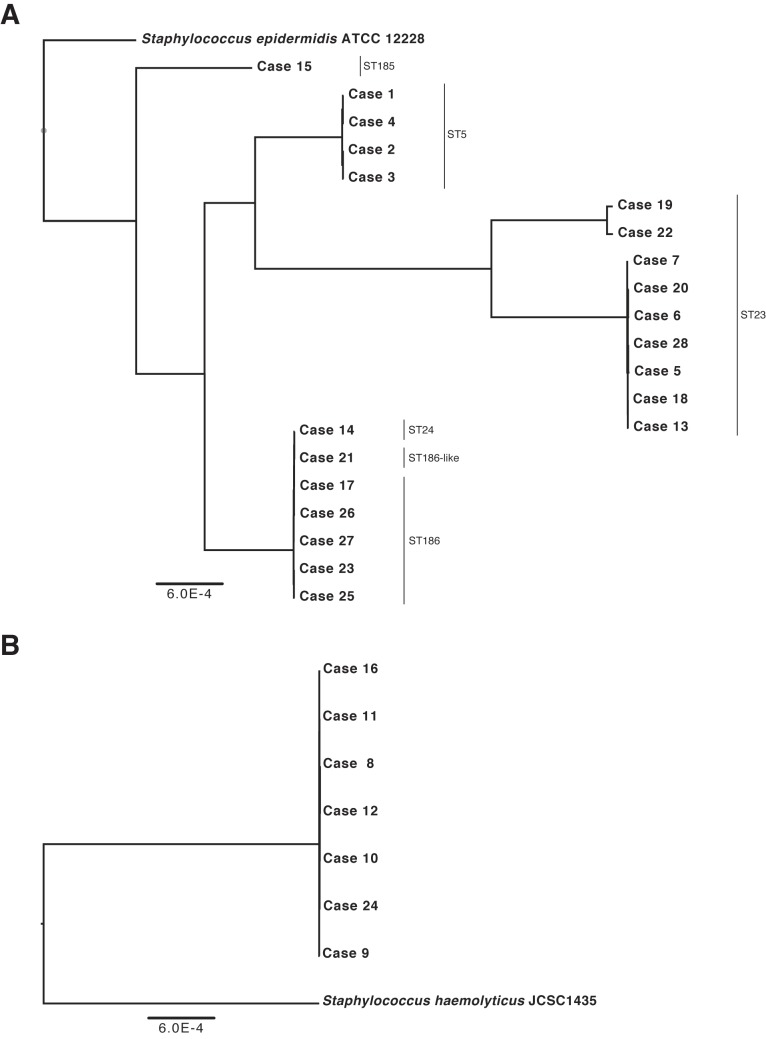
Six MLST sequence types (ST) were identified for the S. epidermidis isolates, including ST5 (4 isolates), ST23 (9 isolates), ST24 (1 isolate), ST185 (1 isolate), ST186 (5 isolates), and one 186-like ST (1 isolate) (Fig. 2A). Evaluation of the WGS data showed fine-resolution heterogeneity within sequence types; isolates within the same sequence type had an average of 33 single-nucleotide variants (SNVs) relative to other isolates in that ST (range, 12 to 52 SNVs). In contrast, across sequence types, isolates differed by an average of 6,229 SNVs (range, 10 to 8,812) (Fig. 2A). Isolates in ST5 were all recovered from patients in the same calendar year, whereas ST23 was isolated over a 3- to 5-year period (Table 2). However, no given ST predominated in any given study year (Table 2).
FIG 2 .
Dendrogram of WGS results for 21 linezolid-resistant S. epidermidis isolates (A) and 7 linezolid-resistant S. haemolyticus isolates (B). Phylogenetic trees were constructed by aligning the core coding genome of both the S. epidermidis and S. haemolyticus isolates separately.
TABLE 2 .
Genetic characteristics of LRCoNS (n = 28)
| Case | Species | ST | cfr |
cfr plasmid |
% of 23S rRNA copies harboring SNV |
L3 mutation(s) |
L4 mutation |
Linezolid MIC (µg/ml)a |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G2576U | C2534U | G2447U | U2504A | ||||||||
| 1 | S. epidermidis | 5 | 0 | 20 | 0 | 0 | H146Q, V154L, A157R | 71 N | 128 | ||
| 2 | 5 | Present | Smallb | 0 | 20 | 0 | 0 | H146Q, V154L, A157R | 71 N | 256 | |
| 3 | 5 | Present | Small | 0 | 20 | 0 | 0 | H146Q, V154L, A157R | 71 N | >256 | |
| 4 | 5 | 0 | 20 | 0 | 0 | H146Q, V154L, A157R | 71 N | 32 | |||
| 6 | 23 | Present | Largec | 0 | 0 | 0 | 0 | D159L | 128 | ||
| 7 | 23 | 0 | 0 | 0 | 0 | D159L | K68N | 16 | |||
| 19 | 23 | 100 | 100 | 0 | 0 | 32 | |||||
| 20 | 23 | Present | Large | 0 | 0 | 0 | 0 | D159L | 128 | ||
| 5 | 23 | 0 | 0 | 0 | 0 | D159L | 8 | ||||
| 13 | 23 | Present | Large | 0 | 0 | 0 | 0 | D159L | 8 | ||
| 18 | 23 | Present | Large | 0 | 0 | 0 | 0 | D159L | 32 | ||
| 28 | 23 | Present | Large | 0 | 0 | 0 | 0 | D159L | 128 | ||
| 22 | 23 | 100 | 0 | 0 | 0 | 16 | |||||
| 14 | 24 | Present | Small | 0 | 40 | 0 | 0 | H146Q, V154L, A157R | 71 N | >256 | |
| 15 | 185 | 0 | 0 | 100 | 0 | A157R | >256 | ||||
| 27 | 186 | 0 | 40 | 0 | 0 | H146Q, V154L, A157R | 71 N | 32 | |||
| 23 | 186 | Present | Small | 0 | 40 | 0 | 0 | V154L, A157R | 71 N | >256 | |
| 17 | 186 | 0 | 40 | 0 | 0 | H146Q, V154L, A157R | 71 N | 32 | |||
| 25 | 186 | Present | Small | 0 | 40 | 0 | 0 | H146Q, V154L, A157R | 71 N | >256 | |
| 26 | 186 | 0 | 40 | 0 | 0 | H146Q, V154L, A157R | 71 N | 64 | |||
| 21 | 186-like | 0 | 40 | 0 | 0 | H146Q, V154L, A157R | 71 N | 32 | |||
| 8 | S. haemolyticus | 100 | 100 | 100 | 100 | 64 | |||||
| 10 | 100 | 100 | 100 | 100 | 64 | ||||||
| 24 | 100 | 100 | 100 | 100 | 64 | ||||||
| 11 | 100 | 100 | 100 | 100 | 64 | ||||||
| 12 | 100 | 100 | 100 | 100 | 128 | ||||||
| 16 | 100 | 100 | 100 | 100 | G139R | 64 | |||||
| 9 | 100 | 100 | 100 | 100 | 64 | ||||||
Determined by broth microdilution.
Small (2.8- to 3.8-kb) plasmid with homology to the cfr-carrying plasmid identified in Staphylococcus cohnii (14).
Large (17-kb) plasmid with 70% homology to the cfr-carrying plasmid pSCFS1, identified in Staphylococcus sciuri (13).
As no MLST database exists at present for S. haemolyticus, isolates were evaluated by WGS data alone, which indicated that all seven of the S. haemolyticus isolates were nearly identical (Fig. 2B). These isolates were predominantly isolated in 2007 (Table 2), with one isolate recovered in 2008 and one isolate recovered in 2009.
Resistance mechanism of LRCoNS isolates.
WGS was used to evaluate the putative resistance mechanisms present in the 28 LRCoNS isolates. Polymorphisms to the domain V region of 23S rRNA were identified in 14 (62%) of S. epidermidis and 7 (100%) of S. haemolyticus isolates. The polymorphisms identified included G2576U (9/28 isolates; 32%), G2447U (8/28 isolates; 28%) and U2504A (7/28 isolates; 25%); these polymorphisms have all been clearly associated with linezolid resistance (3). The mutation C2534U was also identified in 68% of the isolates studied (19/28 isolates; 68%). While C2534U has been reported by others in linezolid-resistant staphylococci (3), the role of this polymorphism in linezolid resistance is not well characterized, and it is typically found in isolates that harbor other mutations, either in the V domain or to the L3 and L4 ribosomal proteins, as was the case in our study (Table 2). All S. haemolyticus isolates harbored all 4 23S mutations (G2576U, G2447U, U2504A, and C2534U) in all copies of the 23S rRNA (Table 2). Only three S. epidermidis isolates harbored 23S rRNA mutations known to be associated with linezolid resistance: isolates 19 and 22 had the G2576U mutation in all copies of the 23S rRNA, and isolate 15 had the G2447U mutation in all copies. In contrast, 20 to 100% of 23S rRNA copies harbored the C2534U mutation in 12 S. epidermidis isolates with this mutation (Table 2).
rplC, rplD, and rplV sequences were investigated for predicted mutations in L3, L4, and L22 ribosomal proteins, respectively. Alterations to L3 and/or L4 were identified in all but 2 S. epidermidis isolates but in only one S. haemolyticus isolate (Table 2). Only synonymous mutations were identified in rplV among the isolates investigated (data not shown). Single-nucleotide variants predicted to encode the following mutations were identified in rplC (Table 2): H146Q (10 isolates), V154L (11 isolates), A157R (12 isolates), D159L (7 isolates), and G139R (1 isolate). Two mutations were identified in rplD: insertion of an asparagine at position 71 (11 isolates) and K68N (1 isolate) (Table 2). These sites are in close proximity to the linezolid binding site in the ribosome, and mutations at these sites have been described in other studies of linezolid-resistant staphylococci (3). However, site-directed mutagenesis experiments have not been performed that conclusively attribute linezolid resistance to these mutations.
The presence of a cfr gene was found in 10 S. epidermidis isolates (Table 2). Evaluation of WGS data indicated the cfr gene was present on two different plasmids in our sample collection, a large (17-kb) plasmid with 70% homology to the cfr-carrying plasmid, pSCFS1, identified in Staphylococcus sciuri (13), and a small (2.8- to 3.8-kb) plasmid with homology to the cfr-carrying plasmid identified in Staphylococcus cohnii (14). The large plasmid was >99.9% identical between isolates 8, 15, 21, 23, and 31, and the small plasmid was 100% identical between isolates 3, 4, 17, 26, and 28. The large plasmid was identified only in ST23, and each isolate was estimated to have 2 to 10 copies of the plasmid per cell, based on WGS read coverage data. In contrast, the small plasmid was found in ST5, ST24, and ST186 (Table 2). The copy number of the small plasmid was estimated to be 1 to 4, based on coverage data. We cannot exclude the possibility of a chromosomal integration of this plasmid in isolate 2 from the WGS data, as it carries multiple transposases complicating de novo assembly, and integration of the cfr plasmid in S. aureus has been reported (15). Regardless, linezolid MICs were significantly higher for isolates that harbored cfr than for those that did not carry this gene (Table 2) (modal MIC, >256 µg/ml versus 64 µg/ml; P < 0.01). Interestingly, linezolid MICs were also significantly higher among isolates that carried the small cfr plasmid than among those that carried the large cfr plasmid (modal MIC, >256 µg/ml versus 128 µg/ml; P < 0.001). All isolates with the small cfr plasmid also harbored ribosomal mutations putatively associated with linezolid resistance: C2534T, an asparagine inserted at position 71 in L4, and the mutations V154L and A157R in L3. The mutation H146Q in L3 was additionally present in four of these isolates (Table 2). These same ribosomal mutations were identified in five S. epidermidis isolates in the absence of cfr (isolates 1, 4, 21, 26 and 27) (Table 2), and lower linezolid MICs were noted in these isolates (modal MIC, 32 versus >256 µg/ml; P < 0.001). These findings reinforce the additive nature of resistance to linezolid, via these three mechanisms.
Isolates 13, 18, and 28, all of ST23, carried cfr on the large plasmid and harbored identical alterations to the 23S, L3, and L4 genes (Table 2). No epidemiological link could be found between the three patients from whom these isolates were recovered. The patients were located at two different facilities, our acute-care hospital and our tertiary-care facility. Interestingly, linezolid MICs for these isolates were 32, 8, and 128 µg/ml, respectively. No mutations in or proximal to the cfr gene were identified in isolates 13 or 18 compared to isolate 28, nor was there an obvious difference in plasmid copy number between the isolates, suggesting that additional factors may impact linezolid MIC in these isolates. Evaluation of the WGS data did not reveal any apparent differences at the level that might result in these disparate MICs. All MICs for these isolates were confirmed in duplicate.
All seven of the S. haemolyticus isolates were nearly identical, based on WGS data, with the core genes having between 7 and 36 SNVs during cross-comparisons. All isolates harbored identical mutations associated with linezolid resistance. Five of the isolates were recovered in 2007, with three (patients 10, 11, and 12) isolated over an 11-day time period. All five patients were at the same facility but in different wards of the hospital. The sixth and seventh S. haemolyticus isolates (from patients 16 and 24) were isolated one and two years later, respectively, at the acute-care hospital.
No chromosomal mutations in efflux genes capable of exporting linezolid or their upstream regions were identified in any of the isolates investigated. Others have reported such mutations associated with increased expression and linezolid resistance, in Streptococcus pneumoniae (16) and S. aureus (17). The presence of vgaA and vgaB was investigated in the whole-genome data, and two isolates (19 and 22) harbored vgaB.
DISCUSSION
CoNS are common causes of various types of infections, including bloodstream infections, some of which are health care associated (18, 19). Increasing antibiotic resistance, in S. epidermidis and S. haemolyticus in particular, has led to limited therapeutic options for treatment of infections caused by these organisms (20). In addition, S. haemolyticus can frequently be non-vancomycin susceptible, further limiting available treatments. We identified an overall 1.2 to 3.2% annual incidence of linezolid resistance in blood isolates of CoNS. This incidence rate parallels that reported by surveillance studies in the United States (21–24). Importantly, linezolid resistance may emerge in CoNS after only short treatment courses with linezolid, in the range of days. In contrast, linezolid resistance in S. aureus occurs only after months of therapy (2). In our study, patients were exposed to linezolid for an average of 16.9 days prior to the isolation of a resistant isolate (Table 1). This rapid acquisition of linezolid resistance may relate to the highly plastic nature of the S. epidermidis genome, which is driven largely by insertion sequences and other mobile genetic elements (25). Over the 5-year study period, the laboratory identified only a single linezolid-resistant S. aureus isolate (data not shown).
Linezolid-resistant isolates of the same ST with identical linezolid resistance mechanisms (i.e., isolates 13, 18, and 28) were isolated from patients over a 3-year period and across two geographically distant hospitals, indicating that LRCoNS may persist within and across the health care system for extended periods of time. Such persistence of LRCoNS has been reported by others (21, 26). As infection control practices for MRSA, such as contact precautions, are not used for CoNS, even MDR isolates, LRCoNS may go unnoticed in various health care settings.
Unlike prior studies, where ST2 was predominant among linezolid-resistant S. epidermidis strains (27), 42% of the linezolid-resistant S. epidermidis isolates investigated herein were ST23, and no isolates were ST2 (Table 2). The majority of isolates investigated harbored one or more SNVs in the domain V region of the 23S rRNA. The most common mutation identified was C2534T, which has been observed in other LRCoNS in the United States (27) and is not clearly associated with linezolid resistance. In contrast, only two S. epidermidis isolates harbored the G2576T mutation, which is frequently reported among linezolid-resistant staphylococci (2). Interestingly, mutations in the 23S rRNA were more frequent among S. haemolyticus isolates than S. epidermidis isolates, in terms of both the number of SNVs identified and the percentage of copies affected (Table 2). 23S rRNA mutations in domain V are known to confer growth defects in other Gram-positive bacteria, and it is possible that these mutations are less costly, from a fitness perspective, to S. haemolyticus than S. epidermidis. Alternatively, one or more of the 4 mutations in the 23S rRNA identified in the S. haemolyticus isolates here may compensate for growth defects caused by others. However, as the S. haemolyticus isolates in this study appear to be a single clone, this finding needs to be validated across a larger number of isolates. Nonetheless, the fact that an S. haemolyticus strain was isolated 2 years after the cluster of isolates identified in 2007 suggests persistence of the clone and stable incorporation of linezolid resistance determinants over extended periods of time. In contrast, nearly all (19/21 isolates) S. epidermidis isolates harbored one or more mutations in the L3 and/or L4 proteins, whereas only one S. haemolyticus isolate harbored an L3 mutation (Table 2). An L3 mutation was shown to compensate for growth defects associated with 23S mutations in Streptococcus pneumoniae (28). As only 2 S. epidermidis isolates harbored 23S mutations in the absence of an L3 mutation, this may similarly indicate a cost disadvantage to 23S mutations in S. epidermidis. Importantly, others have shown that linezolid-susceptible S. epidermidis isolates do not exhibit L3/L4 mutations, suggesting that the observed alterations are not naturally occurring polymorphisms and may not provide any additional benefits in this highly variable bacterium (27).
While mutations in the 23S rRNA are the most common mechanism of resistance, mutations in the L3 and L4 proteins are increasingly associated with linezolid resistance. In this study, we identified several strains with mutations in the ribosomal proteins putatively associated with linezolid resistance; two linezolid-resistant isolates harbored only a mutation in L3 and/or L4. Isolate 7 harbored a single L3 mutation, D159L, and isolate 9 harbored mutations only in L3 (D159L) and L4 (K68N). While the linezolid MICs for these isolates were low, 8 and 16 µg/ml, respectively (Table 2), compared to other LRCoNS investigated herein, L3 and L4 mutations in the absence of mutations of the primary target site of linezolid (23S V domain) may confer resistance.
Unlike a prior study, where cfr was not identified in 40 linezolid-resistant S. epidermidis isolates obtained in the United States between 2004 and 2007 (27), nearly half (48%) of the S. epidermidis isolates investigated in our study harbored a cfr gene. This was an important finding, in the context that only a single other cfr-positive strain (an S. aureus isolate) has been identified to our knowledge in the same geographical area as our study (29). cfr has been identified in a number of both Gram-positive and Gram-negative bacteria isolated from clinical, veterinary, and environmental Gram-positive and Gram-negative bacteria (30). This spread is attributed to the low fitness cost of the gene in Gram-positive bacteria, coupled with not only selective pressure of antimicrobials used in human medicine but also the use of phenicols, lincosamides, and pleuromutilins in veterinary medicine. Animal isolates of Staphylococcus may be an important reservoir of cfr. cfr was associated with the highest linezolid MICs, with the exception of isolates 13 and 18, which had linezolid MICs of 8 and 32 µg/ml, respectively (Table 2). These two isolates also had only one ribosomal mutation, D159L, in L3. Indeed, only two S. epidermidis isolates without cfr, isolates 1 and 15, had a linezolid MIC of >64 µg/ml. Isolate 1 had mutations in both L3 (H146Q, V154L, and A157R) and L4 (71N), along with the C2534U mutation in the 23S rRNA. This combination of mutations was found in 5 other isolates in our study and was associated with linezolid MICs ranging from 32 to 64 µg/ml in these other isolates (Table 1). Similarly, others have reported such mutations in S. epidermidis in isolates with linezolid MICs of 16 µg/ml by (31), and thus it is unclear why isolate 1 had a much higher MIC. In contrast, isolate 15 harbored a 23S G2447U mutation and L3A157R. These two mutations were detected previously in S. epidermidis strain 1653059, which similarly has a documented linezolid MIC of 256 µg/ml (32). Both mutations are thought to directly interfere with the key bases of the PTC that are targeted by linezolid.
Not all clonal isolates harbored identical mutations associated with linezolid resistance; for instance, ST23 isolates harbored 4 different ribosome-associated SNVs, plus cfr in only selected isolates (Table 2). This suggests the occurrence of independent mutational events, presumably following exposure to linezolid, rather than a single mutation event and subsequent spread throughout our health system that was maintained by widespread linezolid consumption in the environment.
In conclusion, we describe clinical and microbiological data for a series of LRCoNS from a tertiary-care and an acute-care center. These data contribute to the expanding knowledge of oxazolidone resistance mechanisms and incidence of linezolid resistance among CoNS. Furthermore, these findings underscore the need to develop strategies to prevent the emergence of linezolid resistance.
MATERIALS AND METHODS
Bacterial isolates and patient characteristics.
Linezolid-resistant coagulase-negative staphylococci (LRCoNS) were identified through retrospective review of laboratory records between January 2007 and January 2012 for CoNS isolated from blood with a linezolid MIC of ≥8 µg/ml. Linezolid susceptibility testing was routinely performed by the clinical laboratory on CoNS isolated from patients with ≥2 blood culture sets or upon physician request for CoNS isolated from a single blood culture set. Incidence of LRCoNS in blood cultures was calculated using the total number of single-patient CoNS bloodstream isolates with susceptibility test results as the denominator. Isolates were stored at room temperature on tryptic soy agar slants (BD, Sparks, MD) with mineral oil overlay for up to 2 years. All isolates were subcultured twice on sheep blood agar (BD) prior to testing in this study. Patient medical records were reviewed to determine patient demographics and linezolid exposures. This study was approved by the UCLA Institutional Review Board with exemption of informed consent, as this was a review of existing data.
Bacterial identification and antimicrobial susceptibility testing.
Isolates were identified to the species level using Vitek2 GP cards (bioMérieux, Durham, NC).
Antimicrobial susceptibility testing was performed at the time of CoNS isolation from blood cultures by the reference broth microdilution (BMD) method using panels prepared in house according to Clinical and Laboratory Standards Institute (CLSI) standards (33). All MICs were confirmed following storage using BMD with 2-fold dilutions of linezolid at concentrations ranging from 1 to 256 µg/ml. Linezolid resistance was defined as an MIC of ≥8 µg/ml. Quality control was assessed using the strains S. aureus ATCC 29213 and Enterococcus faecalis ATCC 29212 (33).
Whole-genome sequencing.
DNA preparation and sequencing were performed as previously described (34). Briefly, DNA was isolated from overnight cultures in tryptic soy broth (BD, Franklin Lakes, NJ) and extracted with a BIOstic DNA isolation kit (MO BIO Laboratories, Carlsbad, CA). Genomic DNA was sheared to an average size of 300 bp using a Covaris S2 instrument (Covaris, Woburn, MA). Illumina library creation was performed using a NEBNext master mix set (New England Biolabs, Ipswich, MA) with “bead carryover” between reactions as previously described (35). Equimolar concentrations of uniquely bar-coded samples were pooled and size selected on a 2% NuSieve GTG agarose gel (Lonza, Rockland, ME) followed by 4 cycles of PCR enrichment with Phusion polymerase. Library quantification was performed using a Qubit fluorometer (Life Technologies, Carlsbad, CA) and a Bioanalyzer 2100 instrument (Agilent Technologies, Santa Clara, CA) prior to sequencing on an Illumina HiSeq 2000 sequencer with v3 chemistry using 100-bp paired-end reads at a raw cluster density of ~550,000 clusters/mm2.
Approximately 500 Mb of sequence data was obtained for each isolate. To determine the presence and absence of genes, de novo assembly was performed using the overlap consensus assembler Edena v3 using an overlap of 65 and a coverage cutoff at contig ends of 10 reads or greater (36). Assembled contigs are available upon request.
Identification of cfr was performed with BLAT by aligning the Cfr protein (accession no. A5HBL2) to the six de novo contigs, that were frame translated. For the identification of point mutations and small indels, reads were mapped with Stampy (37) using either S. epidermidis ATCC 12228 or S. haemolyticus JCSC 1435 as a reference. Mutations in rplC, rplD, and rplV were determined using SAMtools and custom Perl scripts, as previously described (34). For mutations in the 23S subunit, allele counts were summed across the five copies found in ATCC 12228. Copy number was estimated by calculating 5*A/(A + B) and rounding to the nearest integer, where A and B represent the number of reads corresponding to the alternate and reference allele, respectively. For the purpose of these calculations, the 23S operon copy number was assumed to be 5, although some strains of S. epidermidis may harbor 6 copies of the operon (38). Throughout the study, the numbering convention for the 23S mutations was based on Escherichia coli numbering, whereas L3 and L4 mutations were based on staphylococcal numbering.
Phylogenetic tree reconstruction.
Phylogenetic trees were constructed by aligning the core coding genome of both the S. epidermidis and S. haemolyticus isolates separately. The annotated reference genomes S. epidermidis ATCC 12228 and S. haemolyticus JCSC 1435 were used as the starting seed for each respective species. Coding sequences were aligned to each of the draft assemblies using BWASW (39). Alignments were accepted if the gene was nonrepetitive (mapping score greater than or equal to 125) and had a sequence identity of >90%. A total of 2,093 genes in the S. epidermidis and 2,219 in the S. haemolyticus met this criterion. Acceptable genes were concatenated end to end, producing a single continuous sequence for each isolate. Concatenated sequences were then aligned against each other using Fast statistical analysis (FSA), resulting in a multiple sequence alignment of the core genome (40). Phylogenetic trees were estimated using RAxML using 100 bootstraps and final trees drawn in FigTree (41).
Strain typing.
S. epidermidis multilocus sequence typing (MLST) was performed by mapping all reported sequences deposited at http://sepidermidis.mlst.net/ against the de novo contigs. Perfect matches were identified, and profiles were queried against the existing database for sequence type identification. The single S. epidermidis isolate labeled 186-like aligned with six of the seven loci of 186 in the database.
Nucleotide sequence accession number.
Raw reads for all genomes have been deposited in the NCBI Sequence Read Archive, under accession number SRP039360.
Footnotes
Citation Tewhey R, Gu B, Kelesidis T, Charlton C, Bobenchik A, Hindler J, Schork NJ, Humphries RM. 2014. Mechanisms of linezolid resistance among coagulase-negative staphylococci determined by whole-genome sequencing. mBio 5(3):e00894-14. doi:10.1128/mBio.00894-14.
REFERENCES
- 1. Shinabarger D. 1999. Mechanism of action of the oxazolidinone antibacterial agents. Expert Opin. Investig. Drugs 8:1195–1202. 10.1517/13543784.8.8.1195 [DOI] [PubMed] [Google Scholar]
- 2. Gu B, Kelesidis T, Tsiodras S, Hindler J, Humphries RM. 2013. The emerging problem of linezolid-resistant Staphylococcus. J. Antimicrob. Chemother. 68:4–11. 10.1093/jac/dks354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Long KS, Vester B. 2012. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob. Agents Chemother. 56:603–612. 10.1128/AAC.05702-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsiodras S, Gold HS, Sakoulas G, Eliopoulos GM, Wennersten C, Venkataraman L, Moellering RC, Ferraro MJ. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207–208. 10.1016/S0140-6736(01)05410-1 [DOI] [PubMed] [Google Scholar]
- 5. Mazzariol A, Lo Cascio G, Kocsis E, Maccacaro L, Fontana R, Cornaglia G. 2012. Outbreak of linezolid-resistant Staphylococcus haemolyticus in an Italian intensive care unit. Eur. J. Clin. Microbiol. Infect. Dis. 31:523–527. 10.1007/s10096-011-1343-6 [DOI] [PubMed] [Google Scholar]
- 6. Mendes RE, Deshpande LM, Farrell DJ, Spanu T, Fadda G, Jones RN. 2010. Assessment of linezolid resistance mechanisms among Staphylococcus epidermidis causing bacteraemia in Rome, Italy. J. Antimicrob. Chemother. 65:2329–2335. 10.1093/jac/dkq331 [DOI] [PubMed] [Google Scholar]
- 7. Gales AC, Sader HS, Andrade SS, Lutz L, Machado A, Barth AL. 2006. Emergence of linezolid-resistant Staphylococcus aureus during treatment of pulmonary infection in a patient with cystic fibrosis. Int. J. Antimicrob. Agents 27:300–302. 10.1016/j.ijantimicag.2005.11.008 [DOI] [PubMed] [Google Scholar]
- 8. Besier S, Ludwig A, Zander J, Brade V, Wichelhaus TA. 2008. Linezolid resistance in Staphylococcus aureus: gene dosage effect, stability, fitness costs, and cross-resistances. Antimicrob. Agents Chemother. 52:1570–1572. 10.1128/AAC.01098-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toh SM, Xiong L, Arias CA, Villegas MV, Lolans K, Quinn J, Mankin AS. 2007. Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic linezolid. Mol. Microbiol. 64:1506–1514. 10.1111/j.1365-2958.2007.05744.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kehrenberg C, Schwarz S, Jacobsen L, Hansen LH, Vester B. 2005. A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503. Mol. Microbiol. 57:1064–1073. 10.1111/j.1365-2958.2005.04754.x [DOI] [PubMed] [Google Scholar]
- 11. Smith LK, Mankin AS. 2008. Transcriptional and translational control of the mlr operon, which confers resistance to seven classes of protein synthesis inhibitors. Antimicrob. Agents Chemother. 52:1703–1712. 10.1128/AAC.01583-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flamm RK, Farrell DJ, Mendes RE, Ross JE, Sader HS, Jones RN. 2012. LEADER surveillance program results for 2010: an activity and spectrum analysis of linezolid using 6801 clinical isolates from the United States (61 medical centers). Diagn. Microbiol. Infect. Dis. 74:54–61. 10.1016/j.diagmicrobio.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 13. Schwarz S, Werckenthin C, Kehrenberg C. 2000. Identification of a plasmid-borne chloramphenicol-florfenicol resistance gene in Staphylococcus sciuri. Antimicrob. Agents Chemother. 44:2530–2533. 10.1128/AAC.44.9.2530-2533.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mendes RE, Deshpande L, Rodriguez-Noriega E, Ross JE, Jones RN, Morfin-Otero R. 2010. First report of Staphylococcal clinical isolates in Mexico with linezolid resistance caused by cfr: evidence of in vivo cfr mobilization. J. Clin. Microbiol. 48:3041–3043. 10.1128/JCM.00880-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Locke JB, Rahawi S, Lamarre J, Mankin AS, Shaw KJ. 2012. Genetic environment and stability of cfr in methicillin-resistant Staphylococcus aureus CM05. Antimicrob. Agents Chemother. 56:332–340. 10.1128/AAC.05420-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng J, Lupien A, Gingras H, Wasserscheid J, Dewar K, Légaré D, Ouellette M. 2009. Genome sequencing of linezolid-resistant Streptococcus pneumoniae mutants reveals novel mechanisms of resistance. Genome Res. 19:1214–1223. 10.1101/gr.089342.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Floyd JL, Smith KP, Kumar SH, Floyd JT, Varela MF. 2010. LmrS is a multidrug efflux pump of the major facilitator superfamily from Staphylococcus aureus. Antimicrob. Agents Chemother. 54:5406–5412. 10.1128/AAC.00580-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calderón-Jaimes E, Espinosa de los Monteros LE, Avila-Beltrán R. 2002. Epidemiology of drug resistance: the case of Staphylococcus aureus and coagulase-negative staphylococci infections. Salud Publica Mex. 44:108–112. 10.1590/S0036-36342002000200004 [DOI] [PubMed] [Google Scholar]
- 19. Potoski BA, Adams J, Clarke L, Shutt K, Linden PK, Baxter C, Pasculle AW, Capitano B, Peleg AY, Szabo D, Paterson DL. 2006. Epidemiological profile of linezolid-resistant coagulase-negative staphylococci. Clin. Infect. Dis. 43:165–171. 10.1086/505114 [DOI] [PubMed] [Google Scholar]
- 20. Tegnell A, Grabowska K, Jacobsson A, Andersson M, Giesecke J, Ohman L. 2003. Study of developed resistance due to antibiotic treatment of coagulase-negative staphylococci. Microb. Drug Resist. 9:1–6. 10.1089/107662903322541838 [DOI] [PubMed] [Google Scholar]
- 21. Farrell DJ, Mendes RE, Ross JE, Jones RN. 2009. Linezolid surveillance program results for 2008 (LEADER Program for 2008). Diagn. Microbiol. Infect. Dis. 65:392–403. 10.1016/j.diagmicrobio.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 22. Farrell DJ, Mendes RE, Ross JE, Sader HS, Jones RN. 2011. LEADER Program results for 2009: an activity and spectrum analysis of linezolid using 6,414 clinical isolates from 56 medical centers in the United States. Antimicrob. Agents Chemother. 55:3684–3690. 10.1128/AAC.01729-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones RN, Ross JE, Castanheira M, Mendes RE. 2008. United States resistance surveillance results for linezolid (LEADER Program for 2007). Diagn. Microbiol. Infect. Dis. 62:416–426. 10.1016/j.diagmicrobio.2008.10.010 [DOI] [PubMed] [Google Scholar]
- 24. Pillar CM, Draghi DC, Sheehan DJ, Sahm DF. 2008. Prevalence of multidrug-resistant, methicillin-resistant Staphylococcus aureus in the United States: findings of the stratified analysis of the 2004 to 2005 LEADER Surveillance Programs. Diagn. Microbiol. Infect. Dis. 60:221–224. 10.1016/j.diagmicrobio.2007.08.007 [DOI] [PubMed] [Google Scholar]
- 25. Schoenfelder SM, Lange C, Eckart M, Hennig S, Kozytska S, Ziebuhr W. 2010. Success through diversity—how Staphylococcus epidermidis establishes as a nosocomial pathogen. Int. J. Med. Microbiol. 300:380–386. 10.1016/j.ijmm.2010.04.011 [DOI] [PubMed] [Google Scholar]
- 26. Liakopoulos A, Spiliopoulou I, Damani A, Kanellopoulou M, Schoina S, Papafragas E, Marangos M, Fligou F, Zakynthinos E, Makris D, Protonotariou E, Tsiapara F, Filos K, Diza E, Anastassiou ED, Petinaki E. 2010. Dissemination of two international linezolid-resistant Staphylococcus epidermidis clones in Greek hospitals. J. Antimicrob. Chemother. 65:1070–1071. 10.1093/jac/dkq065 [DOI] [PubMed] [Google Scholar]
- 27. Wong A, Reddy SP, Smyth DS, Aguero-Rosenfeld ME, Sakoulas G, Robinson DA. 2010. Polyphyletic emergence of linezolid-resistant staphylococci in the United States. Antimicrob. Agents Chemother. 54:742–748. 10.1128/AAC.00621-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Billal DS, Feng J, Leprohon P, Légaré D, Ouellette M. 2011. Whole genome analysis of linezolid resistance in Streptococcus pneumoniae reveals resistance and compensatory mutations. BMC Genomics 12:512. 10.1186/1471-2164-12-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Flamm RK, Mendes RE, Ross JE, Sader HS, Jones RN. 2013. Linezolid surveillance results for the United States: LEADER surveillance program 2011. Antimicrob. Agents Chemother. 57:1077–1081. 10.1128/AAC.02112-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen J, Wang Y, Schwarz S. 2013. Presence and dissemination of the multiresistance gene cfr in Gram-positive and gram-negative bacteria. J. Antimicrob. Chemother. 68:1697–1706. 10.1093/jac/dkt092 [DOI] [PubMed] [Google Scholar]
- 31. Mendes RE, Deshpande LM, Costello AJ, Farrell DJ. 2012. Molecular epidemiology of Staphylococcus epidermidis clinical isolates from U.S. hospitals. Antimicrob. Agents Chemother. 56:4656–4661. 10.1128/AAC.00279-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Locke JB, Hilgers M, Shaw KJ. 2009. Mutations in ribosomal protein L3 are associated with oxazolidinone resistance in staphylococci of clinical origin. Antimicrob. Agents Chemother. 53:5275–5278. 10.1128/AAC.01032-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clinical and Laboratory Standards Institute 2012. Methods for dilution antimicrobial susceptibility tests for Bacteria that grow aerobically; approved standard, 9th ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 34. Humphries RM, Kelesidis T, Tewhey R, Rose WE, Schork N, Nizet V, Sakoulas G. 2012. Genotypic and phenotypic evaluation of the evolution of high-level daptomycin nonsusceptibility in vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 56:6051–6053. 10.1128/AAC.01318-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fisher S, Barry A, Abreu J, Minie B, Nolan J, Delorey TM, Young G, Fennell TJ, Allen A, Ambrogio L, Berlin AM, Blumenstiel B, Cibulskis K, Friedrich D, Johnson R, Juhn F, Reilly B, Shammas R, Stalker J, Sykes SM, Thompson J, Walsh J, Zimmer A, Zwirko Z, Gabriel S, Nicol R, Nusbaum C. 2011. A scalable, fully automated process for construction of sequence-ready human exome targeted capture libraries. Genome Biol. 12:R1. 10.1186/gb-2011-12-S1-P1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hernandez D, François P, Farinelli L, Osterås M, Schrenzel J. 2008. De novo bacterial genome sequencing: millions of very short reads assembled on a desktop computer. Genome Res. 18:802–809. 10.1101/gr.072033.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lunter G, Goodson M. 2011. Stampy: a statistical algorithm for sensitive and fast mapping of Illumina sequence reads. Genome Res. 21:936–939. 10.1101/gr.111120.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liakopoulos A, Neocleous C, Klapsa D, Kanellopoulou M, Spiliopoulou I, Mathiopoulos KD, Papafrangas E, Petinaki E. 2009. A T2504A mutation in the 23S rRNA gene responsible for high-level resistance to linezolid of Staphylococcus epidermidis. J. Antimicrob. Chemother. 64:206–207. 10.1093/jac/dkp167 [DOI] [PubMed] [Google Scholar]
- 39. Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bradley RK, Roberts A, Smoot M, Juvekar S, Do J, Dewey C, Holmes I, Pachter L. 2009. Fast statistical alignment. PLoS Comput. Biol. 5:e1000392. 10.1371/journal.pcbi.1000392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stamatakis A, Ludwig T, Meier H. 2005. RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics 21:456–463. 10.1093/bioinformatics/bti191 [DOI] [PubMed] [Google Scholar]