ABSTRACT
Differentiation of single cells along filaments of cyanobacteria constitutes one of the simplest developmental patterns in nature. In response to nitrogen deficiency, certain cells located in a semiregular pattern along filaments differentiate into specialized nitrogen-fixing cells called heterocysts. The process involves the sequential activation of many genes whose expression takes place, either exclusively or at least more strongly, in those cells undergoing differentiation. In the model cyanobacterium Anabaena (Nostoc) sp. strain PCC 7120, increased transcription of hetR, considered the earliest detectable heterocyst-specific transcript, has been reported to occur in pairs or even in clusters of cells, thus making it difficult to identify prospective heterocysts during the early stages of differentiation, before any morphological change is detectable. The promoter of nsiR1 (nitrogen stress inducible RNA1), a heterocyst-specific small RNA, constitutes a minimal sequence promoting heterocyst-specific transcription. Using confocal fluorescence microscopy, I have analyzed expression of a gfp reporter transcriptionally fused to PnsiR1. The combined analysis of green fluorescence (reporting transcriptional activity from PnsiR1) and red fluorescence (an indication of progress in the differentiation of individual cells) shows that expression of PnsiR1 takes place in single cells located in a semiregular pattern before any other morphological or fluorescence signature of differentiation can be observed, thus providing an early marker for cells undergoing differentiation.
IMPORTANCE
Cyanobacterial filaments containing heterocysts constitute an example of bacterial division of labor. When using atmospheric nitrogen, these filaments behave as multicellular organisms in which two different cell types (vegetative cells and nitrogen-fixing heterocysts) coexist and cooperate to achieve growth of the filament as a whole. The molecular basis governing the differentiation of individual vegetative cells, and thus the establishment of a one-dimensional pattern from cells that are apparently the same, remains one of the most intriguing aspects of this differentiation process. Recent evidence suggests that, at any given time, some cells in the filaments are more likely than others to become heterocysts when nitrogen limitation is encountered. The robust heterocyst-specific nsiR1 promoter, which is induced very early during differentiation, provides a valuable tool to analyze issues such as early candidacy or the possible role of transcriptional noise in determining the fate of specific cells in cyanobacterial filaments.
Observation
Heterocystous cyanobacteria are filamentous photosynthetic organisms that, in response to nitrogen deficiency, are able to differentiate a semiregular pattern of specialized cells devoted to fixation of atmospheric nitrogen. Differentiation of individual vegetative cells into nitrogen-fixing heterocysts requires the sequential activation of genes whose products are specifically involved in operating the transformation of an oxygen-producing photosynthetic vegetative cell into a nitrogen-fixing cell. Such transformation results in a significant shift in metabolic capabilities as well as extensive morphological changes, including the rearrangement of photosynthetic membranes and the deposition of a distinctive envelope outside the outer membrane (1–4). Most genes involved in heterocyst differentiation and/or function are upregulated in cells undergoing differentiation. This is, for instance, the case of the two regulators controlling the process, NtcA (a CRP/FNR family transcriptional regulator exerting global nitrogen control) and HetR (a regulator specifically required for cellular differentiation) (5, 6).
Heterocysts differentiate from certain vegetative cells and provide fixed nitrogen to the rest of the nondifferentiated intercalary cells. When nitrogen limitation is first encountered, a series of heterocysts differentiates synchronously at semiregular intervals along the filaments. Once diazotrophic growth is established, and as intercalary cells continue to divide, the distance between heterocysts from the first generation increases and new heterocysts differentiate between two preexisting, older ones. The mechanisms underlying the establishment of a one-dimensional pattern “de novo” must involve selection of certain cells from a filament in which, in principle, all cells are simultaneously exposed to the same nutritional limitation. A two-stage model of heterocyst differentiation involving biased initiation followed by competitive resolution of clusters has been proposed (7). Recent evidence showing biased inheritance of the protein PatN in the presence of combined nitrogen suggests that at any given time, only some cells are initially competent to become heterocysts when nitrogen limitation is encountered (8). The term “candidacy” has also been used in this context (9). Maintenance of the pattern is ultimately controlled by gradients of an inhibitor(s) of differentiation produced by mature heterocysts (10).
Because the progression of heterocyst differentiation involves alterations in the content of light-harvesting complexes (phycobilisomes), this process has been classically observed by fluorescence microscopy through imaging of chlorophyll-derived red fluorescence. As the heterocysts become mature, phycobilisome-induced red fluorescence completely disappears, so the degree of maturation of a specific (pro)heterocyst can be correlated to both the disappearance of red fluorescence and a change in size, shape, and envelopes with respect to adjacent vegetative cells (9, 11). The initial stages of differentiation, however, involve a transitory increase in red fluorescence that is interpreted to reflect disintegration of the phycobilisome structure as a result of a decrease in photosystem II in the differentiating cell (9). Such an increase in fluorescence, actually involving a shift to shorter wavelengths (11), has been suggested as the first detectable sign of differentiation and therefore an early marker of cell differentiation.
Small noncoding RNAs (sRNAs) are transcribed in all groups of bacteria and are involved in every bacterial response to environmental cues. In the case of the model filamentous cyanobacterium Anabaena (Nostoc) sp. strain PCC 7120, several sRNAs whose expression is dramatically induced upon removal of combined nitrogen, including some HetR-dependent transcripts, have been identified (12). In the course of ongoing studies concerning heterocyst-specific sRNAs, I have noticed that induction of some of their promoters is very strong in individual cells at very short times after nitrogen step-down. I therefore aimed at determining whether expression from one of these promoters could be used as an early marker of the differentiation of specific cells in the cyanobacterial filaments into heterocysts.
Results.
NsiR1 (nitrogen stress inducible RNA1) is a 60-nucleotide, HetR-dependent sRNA whose transcription is induced in cells that are differentiating as heterocysts (13). All available genomes for heterocystous cyanobacteria appear to carry several copies of nsiR1 arranged in tandem (12 copies in the case of Anabaena sp. PCC 7120, each of them bearing its own promoter) in the vicinity of hetF, a gene involved in differentiation of heterocysts. Although the molecular mechanisms governing heterocyst-specific transcription are not completely understood, a group of heterocyst-specific promoters, including that of nsiR1, contain a strongly conserved sequence that appears to be associated with heterocyst-specific transcription and has been named the DIF motif (5′ TCCGGA 3′, located around position −35 with respect to the transcriptional start site) (12). The natural nsiR1 promoter (only 70 nucleotides) is the minimal sequence described to confer heterocyst-specific transcription of a gfp reporter (12).
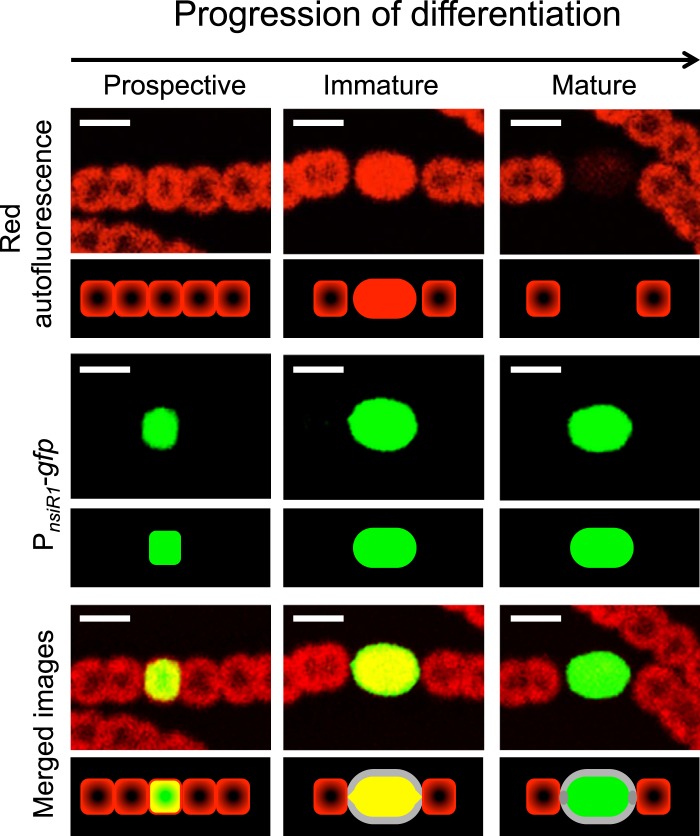
The use of confocal fluorescence microscopy allows a precise localization of fluorescence signals in cyanobacterial cells (Fig. 1). Phycobilisome-induced red fluorescence of vegetative cells (Fig. 1, upper left panel) is associated with thylakoid membranes and is observed mostly in a circular arrangement at the periphery of the cytoplasm. Therefore, images of confocal fluorescence microscopy show the center of the vegetative cells as less fluorescent than the periphery. In contrast, and as a consequence of phycobilisome detachment from membranes, the transient increase in fluorescence that takes place in immature heterocysts is no longer observed in a circular arrangement but covers the entire cell area (Fig. 1, upper middle panel). This shift in cellular localization of chlorophyll-derived fluorescence can be considered a detectable sign of early differentiation of a particular cell. As differentiation progresses, the mature heterocysts are imaged as dark areas with very low red fluorescence (Fig. 1, upper right panel).
FIG 1 .
Expression of PnsiR1-gfp compared to red fluorescence along three stages of heterocyst differentiation. Pictured are three segments containing a prospective heterocyst (showing no distinctive change at the red fluorescence level) (left panels), an immature heterocyst (showing increased red fluorescence) (middle panels), and a mature heterocyst (right panels). A schematic of the fluorescence signals observed is included below each panel. The nonfluorescent thick envelope of the heterocysts (accounting for the black region between fluorescence signals from vegetative cells and heterocysts) is shown in gray. Scale bars, 5 µm.
I have analyzed expression of a fusion between the heterocyst-specific promoter of nsiR1 (genome coordinates 4272627 to 4272556) and the gfp gene encoding green fluorescent protein (GFP) (Fig. 1 and see Fig. S1 in the supplemental material). The combined analysis of both green and red fluorescence shows that during growth at the expense of atmospheric nitrogen, expression from PnsiR1 takes place not only in morphologically distinct heterocysts at intermediate (Fig. 1, middle panels) or advanced (Fig. 1, right panels) maturation stages but also in prospective heterocysts still showing the circular arrangement of red fluorescence that is characteristic of vegetative cells (Fig. 1, left panels). This circular arrangement, together with a size that is similar to that of adjacent vegetative cells, makes prospective heterocysts indistinguishable on the basis of red fluorescence and/or morphology. This observation indicates that in contrast to other heterocyst-related genes whose initial induction takes place in pairs or clusters of cells, expression of PnsiR1 is already localized to single cells before any early change in red fluorescence (indicative of differentiation) takes place. On the other hand, expression of PnsiR1 in filaments growing in the presence of combined nitrogen (ammonium or nitrate) is very low and unpatterned (data not shown). Therefore, the promoter for this small RNA might provide an early marker for prospective heterocysts.
Sustained growth at the expense of atmospheric N2 requires differentiation of new heterocysts as the filaments elongate and mature heterocysts become distant. In order to assess whether expression of PnsiR1 could be used as an indicator of candidacy to become a differentiated cell, we analyzed filaments growing at the expense of atmospheric nitrogen on top of solid medium. This experimental setting has the advantage of preserving the integrity of the filaments, so that several heterocysts (and the corresponding intervals of vegetative cells between them) can be analyzed in one single filament. In addition, because differentiation during sustained growth is not synchronous, heterocysts with different degrees of maturation can be analyzed in relation to their closest neighbors. In this way, the relative age of each heterocyst can be inferred from its degree of maturation and its relative position in the filament.
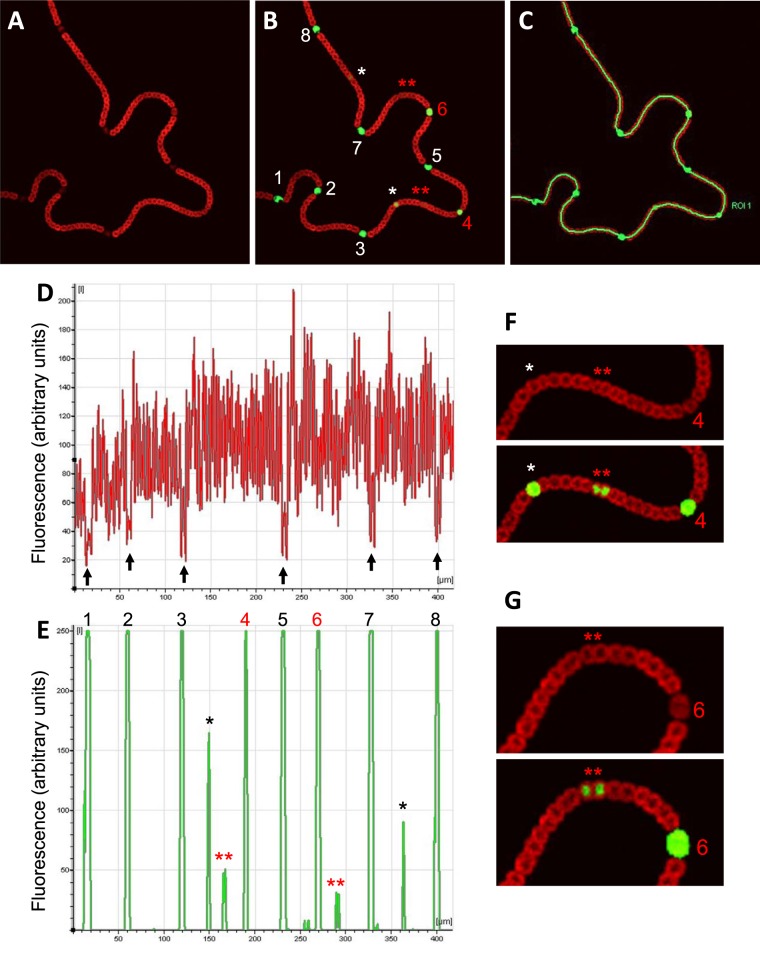
Figure 2A and B show that during growth at the expense of atmospheric nitrogen, the observation of cell size and red fluorescence along the filaments allows identification of mature heterocysts (numbered 1, 2, 3, 5, 7, and 8 in Fig. 2B; black arrows in Fig. 2D) as well as immature heterocysts (numbered 4 and 6 in Fig. 2B). However, the analysis of green fluorescence associated with transcription of gfp from the PnsiR1 also identifies single prospective heterocysts (Fig. 2B, single white asterisks) that cannot be distinguished from their adjacent vegetative cells in terms of size or red fluorescence. Quantification of fluorescence along the filament (Fig. 2C) is shown for both red (Fig. 2D) and green (Fig. 2E) channels. Observation of signals in the red channel alone confirms the location of mature heterocysts in areas with very low signal (Fig. 2D, black arrows). However, the quantification of signals in the green channel shows a semiregular pattern of peaks that correspond to six mature (Fig. 2E, numbered in black above the green peaks), two immature (numbered in red above the green peaks), and two single prospective (marked with single black asterisks) heterocysts located about midway in the two longer intervals [those flanked by heterocysts 3 and 4 and by heterocysts 7 and 8]. Interestingly, in the filament shown, two much less intense peaks of green fluorescence appear approximately midway between higher peaks (Fig. 2E, double red asterisks). Both regions (depicted with the green signals enhanced for clarity in Fig. 2F and G) correspond to pairs of vegetative cells. The observation of many filaments containing several heterocysts shows that in most cases, the intervals between two similarly mature heterocysts contain one centrally located prospective heterocyst (e.g., see interval 7-8 in Fig. 2; see several examples in Fig. S2 and S3 in the supplemental material). However, it is also fairly common to find intervals between two similarly mature heterocysts in which two evenly spaced (instead of one centrally located) immature heterocysts differentiate (see one case in each of the filaments shown in Fig. S3 in the supplemental material). According to the level of green fluorescence and the relative position of cells expressing NsiR1, it seems likely that pairs of vegetative cells showing green fluorescence (Fig. 2, double red asterisks) are resolved to single prospective heterocysts and, later, mature heterocysts as differentiation progresses. Taken together, these observations suggest that following the expression of PnsiR1 might be a useful tool to analyze the dynamics of the appearance of new heterocysts in nitrogen-fixing filaments.
FIG 2 .
Expression of PnsiR1-gfp along nitrogen-fixing filaments. Confocal fluorescence images of a filament growing on top of nitrogen-free medium are shown for the red (A) and red plus green (B) channels, together with the region of interest (ROI) drawn to quantify signals along the filament (C). Quantification of the signals is shown for the red (D) and green (E) channels. Black arrows in panel D indicate the positions of mature heterocysts. Two regions of the filament are enlarged and enhanced for better observation of green fluorescence in panels F and G. Mature heterocysts (numbered 1, 2, 3, 5, 7, and 8) are indicated in white (B) or black (E). Immature heterocysts (numbered 4 and 6) are indicated in red. Prospective heterocysts are indicated by single asterisks. Double red asterisks indicate pairs of cells showing distinct green fluorescence above background.
Conclusion and implications.
The mechanisms governing selection of cells that differentiate as heterocysts from apparently equivalent vegetative cells are not completely understood. Biased inheritance of the protein PatN might determine the fate of cells in the filament so that when nitrogen limitation is encountered, some cells are more likely than others to become heterocysts (8). It is also possible that some heterocyst-specific genes exhibit a high degree of transcriptional noise and that expression of regulatory elements above a critical threshold could provide a distinct signal triggering differentiation of a given cell (14). The possibility of early identification of prospective heterocysts might shed light on aspects such as the rules governing candidacy among vegetative cells or the possible role of transcriptional noise in determining which cell(s) enters the differentiation program. Most experimental approaches used thus far involve promoters whose expression is localized to single cells only after several hours of nitrogen starvation, when heterocysts are already distinguishable from vegetative cells by means of morphological criteria, such as larger size or reduced red fluorescence. For instance, expression of the heterocyst-specific promoter of hetR takes place in pairs or even in clusters of cells that are later resolved to single cells as heterocyst differentiation progresses (6). In addition hetR shows patterned expression (clusters) in the presence of nitrate, an observation interpreted as the filaments being maintained in an “intermediate state” in the presence of a nitrogen source that does not fully repress heterocyst differentiation (6). Therefore, on the basis of hetR expression, it seems difficult to identify early single cells that will eventually differentiate as heterocysts. In contrast, as shown here, strong expression from PnsiR1 is already localized to single cells before any other morphological criteria can be applied, therefore providing a valuable marker for cells undergoing early steps of differentiation.
Strain and methods.
The Anabaena sp. strain PCC 7120 derivative that was analyzed contains the PnsiR1-gfp fusion in pSAM264 (12) placed in a neutral site located in the alpha megaplasmid by using pCSEL24 as described previously (5). Cyanobacterial filaments were cultivated using BG11 medium (15) without nitrate. Filaments that had been growing in the presence of 2.5 mM ammonium were streaked on agar-solidified nitrogen-free medium and allowed to grow for 5 days under white light. Samples were analyzed using a Leica HCX PLAN-APO 63× 1.4 NA oil immersion objective attached to a Leica TCS SP2 confocal laser-scanning microscope. Green fluorescence and red fluorescence were imaged using the 488-nm line supplied by an argon ion laser. Fluorescent emissions were monitored by collection across windows of 500 to 538 nm (GFP imaging) and 630 to 700 nm (chlorophyll fluorescence). Images were treated with ImageJ 1.45s software (W. S. Rasband, U.S. National Institutes of Health, Bethesda, MD; http://imagej.nih.gov/ij/).
SUPPLEMENTAL MATERIAL
Expression of PnsiR1-gfp along several stages of heterocyst maturation. Ten single cells in which expression of PnsiR1-gfp is detected are arranged according to morphological and fluorescence criteria. Cell 1 is a prospective heterocyst. Cell 10 is a morphologically mature heterocyst. Cells 1, 3, and 10 are shown in Fig. 1. Scale bars, 5 µm. Download
Location of prospective heterocysts approximately midway between two mature heterocysts. Pairs of siblings produced after division of intercalary vegetative cells are numbered starting at the two mature heterocysts flanking the intervals. One pair of siblings in which only one of the two cells shows strong expression of PnsiR1-gfp is framed in each case. Download
Location of prospective heterocysts in the intervals between mature or immature heterocysts. Red autofluorescence (upper panel in each case) and merged signals corresponding to PnsiR1-gfp and red fluorescence (lower panel in each case) are shown for two filaments containing morphologically distinguishable mature (white triangles) and immature (red triangles) heterocysts. Prospective heterocysts are indicated by white asterisks. Download
ACKNOWLEDGMENTS
This work was supported by grant BFU2010-14821 from the Spanish Government (Ministerio de Ciencia e Innovación), cofinanced by FEDER, and by Junta de Andalucia (BIO215).
I thank Alicia Orea for help with fluorescence quantification and Iris Maldener and Agustín Vioque for their comments on the manuscript.
Footnotes
Citation Muro-Pastor AM. 2014. The heterocyst-specific NsiR1 small RNA is an early marker of cell differentiation in cyanobacterial filaments. mBio 5(3):e01079-14. doi:10.1128/mBio.01079-14.
REFERENCES
- 1. Zhao J, Wolk CP. 2008. Developmental biology of heterocysts, 2006, p 397–418 In Whitworth DE, Myxobacteria: multicellularity and differentiation. ASM Press, Washington, DC [Google Scholar]
- 2. Muro-Pastor AM, Hess WR. 2012. Heterocyst differentiation: from single mutants to global approaches. Trends Microbiol. 20:548–557. 10.1016/j.tim.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 3. Maldener I, Muro-Pastor A. October 2010. Cyanobacterial heterocysts. In eLS. John Wiley & Sons, Ltd., Chichester, United Kingdom. 10.1002/9780470015902.a0000306.pub2 [DOI] [Google Scholar]
- 4. Flores E, Herrero A. 2010. Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol. 8:39–50. 10.1038/nrmicro2242 [DOI] [PubMed] [Google Scholar]
- 5. Olmedo-Verd E, Muro-Pastor AM, Flores E, Herrero A. 2006. Localized induction of the ntcA regulatory gene in developing heterocysts of Anabaena sp. strain PCC 7120. J. Bacteriol. 188:6694–6699. 10.1128/JB.00509-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rajagopalan R, Callahan SM. 2010. Temporal and spatial regulation of the four transcription start sites of hetR from Anabaena sp. strain PCC 7120. J. Bacteriol. 192:1088–1096. 10.1128/JB.01297-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meeks JC, Elhai J. 2002. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol. Mol. Biol. Rev. 66:94–121. 10.1128/MMBR.66.1.94-121.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Risser DD, Wong FC, Meeks JC. 2012. Biased inheritance of the protein PatN frees vegetative cells to initiate patterned heterocyst differentiation. Proc. Natl. Acad. Sci. U. S. A. 109:15342–15347. 10.1073/pnas.1207530109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toyoshima M, Sasaki NV, Fujiwara M, Ehira S, Ohmori M, Sato N. 2010. Early candidacy for differentiation into heterocysts in the filamentous cyanobacterium Anabaena sp. PCC 7120. Arch. Microbiol. 192:23–31. 10.1007/s00203-009-0525-4 [DOI] [PubMed] [Google Scholar]
- 10. Risser DD, Callahan SM. 2009. Genetic and cytological evidence that heterocyst patterning is regulated by inhibitor gradients that promote activator decay. Proc. Natl. Acad. Sci. U. S. A. 106:19884–19888. 10.1073/pnas.0909152106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ke S, Haselkorn R. 2013. Fluorescence spectroscopy study of heterocyst differentiation in Anabaena PCC 7120 filaments. Microbiology 159:253–258. 10.1099/mic.0.064220-0 [DOI] [PubMed] [Google Scholar]
- 12. Mitschke J, Vioque A, Haas F, Hess WR, Muro-Pastor AM. 2011. Dynamics of transcriptional start site selection during nitrogen stress-induced cell differentiation in Anabaena sp. PCC7120. Proc. Natl. Acad. Sci. U. S. A. 108:20130–20135. 10.1073/pnas.1112724108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ionescu D, Voss B, Oren A, Hess WR, Muro-Pastor AM. 2010. Heterocyst-specific transcription of NsiR1, a non-coding RNA encoded in a tandem array of direct repeats in cyanobacteria. J. Mol. Biol. 398:177–188. 10.1016/j.jmb.2010.03.010 [DOI] [PubMed] [Google Scholar]
- 14. Eldar A, Elowitz MB. 2010. Functional roles for noise in genetic circuits. Nature 467:167–173. 10.1038/nature09326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. 1979. Generic assignments, strain stories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1–61. 10.1099/00221287-111-1-1 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of PnsiR1-gfp along several stages of heterocyst maturation. Ten single cells in which expression of PnsiR1-gfp is detected are arranged according to morphological and fluorescence criteria. Cell 1 is a prospective heterocyst. Cell 10 is a morphologically mature heterocyst. Cells 1, 3, and 10 are shown in Fig. 1. Scale bars, 5 µm. Download
Location of prospective heterocysts approximately midway between two mature heterocysts. Pairs of siblings produced after division of intercalary vegetative cells are numbered starting at the two mature heterocysts flanking the intervals. One pair of siblings in which only one of the two cells shows strong expression of PnsiR1-gfp is framed in each case. Download
Location of prospective heterocysts in the intervals between mature or immature heterocysts. Red autofluorescence (upper panel in each case) and merged signals corresponding to PnsiR1-gfp and red fluorescence (lower panel in each case) are shown for two filaments containing morphologically distinguishable mature (white triangles) and immature (red triangles) heterocysts. Prospective heterocysts are indicated by white asterisks. Download