Abstract
Dipeptidyl peptidases 4 (DPP4) inhibitors are a new class of oral anti-hyperglycemic drugs for the treatment of type 2 diabetes (T2DM). They are also called “incretins” because they act by inhibiting the degradation of endogenous incretin hormones, in particular GLP-1, that mediates their main metabolic effects. DPP4 is an ubiquitous protease that regulates not only glucose and lipid metabolism, but also exhibits several systemic effects at different site levels. DPP4 inhibition improves endothelial function, reduces the pro-oxidative and the pro-inflammatory state, and exerts renal effects. These actions are mediated by different DPP4 ligands, such as cytokines, growth factors, neuotransmitters etc. Clinical and experimental studies have demonstrated that DPP4 inhibitors are efficient in protecting cardiac, renal and vascular systems, through antiatherosclerotic and vasculoprotective mechanisms. For these reasons DDP4 inhibitors are thought to be “cardiovascular protective” as well as anti-diabetic drugs. Clinical trials aimed to demonstrate the efficacy of DPP4 inhibitors in reducing cardiovascular events, independent of their anti-hyperglycemic action, are ongoing. These trials will also give necessary information on their safety.
Keywords: Dipeptidyl peptidases, diabetes mellitus, cardiovascular system, incretins.
INTRODUCTION
A novel class of oral anti-hyperglycemic drugs has recently become available for the treatment of T2DM. These drugs, also termed incretins, are inhibitors of the dipeptidyl peptidases 4 (DPP4), ubiquitous enzymes that degrade several substrates among which there are the so-called entero-hormones, such as Glucagon Like Peptide-1 (GLP-1) and Glucose-dependent Insulinotropic Paptide (GIP). Thus incretins allow a more prolonged and more effective action of endogenous GLP-1 that not only regulates pancreatic hormone metabolism, but also mediates other important effects, mainly in the nervous and cardiovascular systems and in the kidney. The pleiotropic effects of DPP4 inhibitors, along with the control of glucose plasma concentrations, represent important mechanisms of action of these drugs, among which a potential cardiovascular protection activity appears to be of major importance, especially in T2DM. Specific cardiovascular effects of DPP-4 inhibitors will be discussed in detail in this review.
DPP4 INHIBITOR MECHANISMS OF ACTION
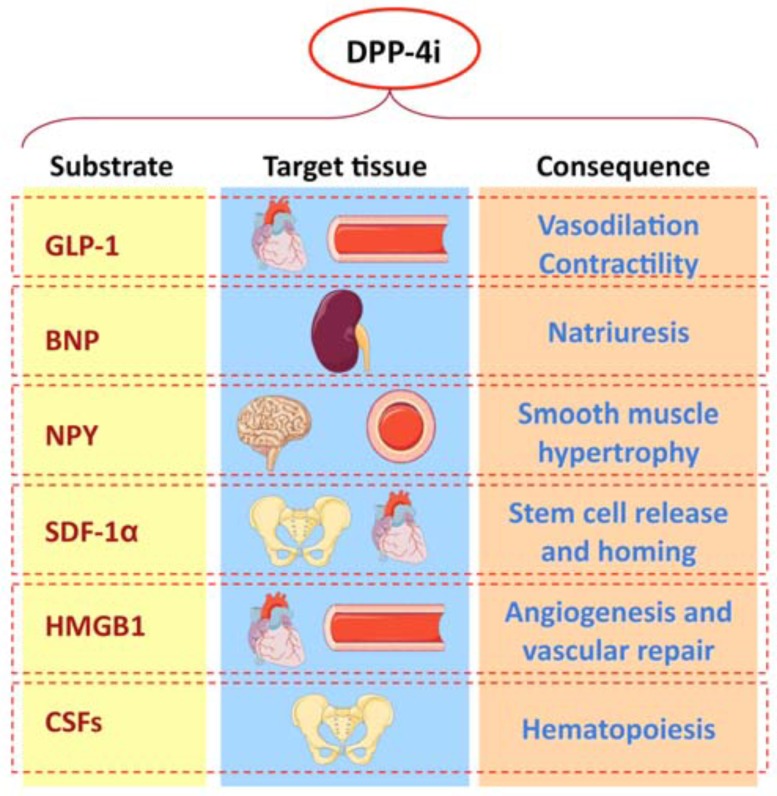
Dipeptidyl peptidases (DPP) 4 (CD26) is an ubiquitous multifunctional glycoproteic protease that plays important roles in metabolism, immunology and nutrition [1]. Its natural ligands includes adenosine-deaminase, renal Na+/H+ countertransport and fibronectin. DPP4 degrade some entero-hormones, the glucagon-like peptide (GLP)-1 and the glucose insulinotropic peptide (GIP), also called incretins, within few minutes. Incretins stimulate insulin, while inhibiting glucagon secretion. Specific DPP4 inhibitors can be administered to avoid incretin degradations: the inhibition of this protease prevents in particular GLP-1 degradation, and allows a more sustained biological activity of this entero-hormone, that enhances glucose-dependent insulin secretion, inhibits glucagon secretion and slows gastric empty. Marguet and coll. confirmed the important role of DPP4 in regulating glucose metabolism. They demonstrated that CD26 knock-out mice show reduced glycaemic spikes, and increased glucose-dependent insulin levels after an oral glucose load [2]. DPP4 inhibitors can reduce glycated haemoglobin of 0.5-0.9%, inducing a progressive but mild reduction of body weight [3]. Many DPP4 inhibitors are now available in several countries: sitagliptin, vildagliptin, saxagliptin, linagliptin, alogliptin, while others are under testing. DPP-4 inhibitors have been approved for the treatment of T2DM, both in monotherapy and in association with metformin, glitazones, sulfonylureas and insulin. Several studies have demonstrated that DPP4 inhibitors display positive effects on cardiovascular system, both by increasing GLP-1 levels and by inhibiting the degradation of other substrates involved in the cardiovascular omeostasis, beside the effects on glucose metabolism (Fig. 1). A soluble dimeric form of DPP4 circulates in plasma; it is of ubiquitous derivation, but is mainly produced by lymphocytes, endothelial cells, kidney and seminal liquid. DPP4 plasma activity is significantly increased in both type 1 and type 2 diabetes, and strongly correlates with HbA1c values; this enhanced activity is further significantly increased in presence of HbA1c values > 8.5%, in type 2 diabetic patients [4]. This phenomenon can contribute to a greater GLP-1 catabolism, and consequently to a reduced insulin secretion, especially in T2DM.
Fig. (1).
Fig. (1).
DPP4 INHIBITORS, ENDOTHELIAL DYSFUNCTION AND ATHEROGENESIS
DPP4 inhibitors have a protective effect on the endothelium. This protection is particularly useful because endothelial cells exposed to high glucose exhibit enhanced DPP4 activity, independent of hyperosmolarity [5]. The loss of DPP4 activity makes the human coronary endothelium less prothrombotic, suggesting that DDP4 should play an important role in the interaction between endothelium and platelets [6]. It has been demonstrated that sitagliptin induces vasodilatation through nitric oxide (NO) production [7]. Matsubara et al. have also demonstrated that DPP4 inhibition with des-fluro-sitagliptin in apoE knock-out mice ameliorates endothelial function, reduces c-jun-N-terminal kinase and extracellular regulated kinase (ERK)1 / 2 phosphorilation, the translocation of nuclear factor kB and the production of pro-inflammatory cytokines. Another group found that sitagliptin administration for 2 weeks in spontaneously hypertensive rats improves endothelium-dependent vasodilatation of renal arteries, renormalizes renal blood flow and reduces systolic blood pressure. The same researchers demonstrated that these actions are mediated by GLP-1 [8]. In obese Zucker rats saxagliptin increased NO synthesis, and reduced peroxynitrite (ONOO-) production; this effect was observed before the hypoglycaemic action [9]. Moreover, saxagliptin was able to stimulate NO release from isolated aorta rings, of about 18%, with a contemporary peroxynitrite reduction. The NO/ONOO- rate raised of about 40%. A further experimental study has recently demonstrated that saxagliptin administration to ApoE−/− high fat diet fed mice reduced macrophage infiltration in the plaque, and matrix metalloproteinase (MMP)-9 production, in comparison to controls. DPP4 inhibition increased collagen content in the atherosclerotic plaque and reduced monocyte migration in response to MCP-1 [10]. The effects of DPP4 inhibitors on the endothelium-dependent response, in humans, have been evaluated by Van Poppel and coll., who found an improvement of vasodilatation in type 2 diabetic subjects [11]. Acetylcholine infusion, after administration of vildagliptin, 50 mg b.i.d. determined a significantly increased vasodilatation, in comparison with acarbose, while non endothelium-dependent vasodilatation was unaffected.
Another contributor of the vascular damage in diabetes is determined by the overproduction of advanced glycation end products (AGEs) that bind to their specific receptors (RAGE) thus inducing oxidative stress generation and inflammatory and thrombogenic reactions [12]. Matsui et al. have studied the effects of vildagliptin, in thoracic aorta of Otsuka Long-Evans Tokushima Fatty (OLETF) rats, an animal model of type 2 diabetes with obesity [13]. Vildagliptin treatment inhibited levels of AGEs, RAGE, mRNA and protein expression of some markers of oxidative stress; remarkably they observed a significant reduction of both mRNA and protein levels of monocyte chemoattractant protein-1, vascular cell adhesion molecule-1 and plasminogen activator inhibitor-1. Ishibashi et al have investigated the effects of sitagliptin, on the AGE-RAGE-induced endothelial cell damage, in vitro [14]. Sitagliptin alone (0.5 μM) modestly inhibited RAGE gene and protein expression, but, in combination with GLP-1 (10 pM), completely blocked the AGE-induced increase in RAGE mRNA and protein levels, inhibiting subsequent reactive oxygen species generation and endothelial nitric oxide synthase (eNOS) mRNA level, in human umbilical vein endothelial cells (HUVEC). These data suggest that DPP-4 inhibitors could play a protective role against vascular injury in diabetes, partly by attenuating the deleterious effects of AGEs-RAGE axis. Finally, a direct vascular protective effect of 2 week vildagliptin treatment was demonstrated by Maeda et al. in Sprague Dawley rats, with streptozotcin-induced diabetes. Reduced oxidative stress generation and suppressed ICAM-1, TGF-β, and PAI-1 gene expression were observed in the thoracic aorta of treated animals; these effects seem independent of a better glucose control, since fasting plasma glucose levels were unaffected by the drug [15]. In conclusion, the inhibition of DPP4 seems to play a protective role not only on the endothelial function, but also on cellular processes involved in the atherosclerotic plaque formation.
MYOCARDIAL EFFECTS
Experimental Studies
Cardiac structure and function have been evaluated in DPP4 knockout (DPP-4 -/-) mice, after left anterior descending coronary artery ligation-induced acute myocardial infarction (AMI) [16]. DPP-4 -/- mice showed normal parameters of cardiac structure and function compared to wild-type littermate controls, while post-AMI survival was slightly better; mice without the genetic deletion, but treated with sitagliptin, showed reduced mortality rate after AMI. Sitagliptin also significantly improved cardiac functional recovery after ischemia/reperfusion (I/R). At the molecular level, the DPP4 genetic deletion was associated with increased expression of Akt, heme-oxygenase and atrial natriuretic peptide. It was also observed that, in obese Wistar rats, the treatment with an analogue of vildagliptin limited the infarct size, after I/R [17]. Chinda et al. have administered to pigs either vildagliptin 50 mg or placebo, 90 min before ligation of left anterior descending coronary artery, followed by 120 min of I/R [18]. With respect to control group, pigs treated with vildagliptin showed a significantly smaller infarct size and a lower myocardial reactive oxygen specie production. Male Wistar rats treated with linagliptin, undergone to left anterior descending coronary artery ligation presented a smaller infarct size after 7 days, persisting after 8 weeks. Immunohistochemical evaluation evidenced a greater number of stromal-derived factor (SDF)-1 positive cells and of their receptor CXCR4 [19]. It has also been reported that sitagliptin, administered for 3 and 14 days, limits the infarct zone, in non diabetic mice: this effect is associated with an increased intramyocardial activity both of cyclic AMP and of protein kinase A [20]. In the same study, it was also observed, in animals treated with sitagliptin, a rise of Akt phosphorilation at Ser-473 and Thr-308, and of eNOS phosphorilation at Ser-633 and Ser-1177. In conclusion, all these experimental studies demonstrate a protective effect of DPP4 inhibition toward I/R damage.
Clinical Studies
Two clinical studies have tested cardioprotective effects of DPP4 inhibition, obtained with sitagliptin. In the first study, 14 patients with ischemic cardiac disease and preserved left ventricular function, eligible for revascularization were evaluated [21]. After a single sitagliptin dose of 100 mg or placebo, an echo-stress with dobutamine at rest, at stress peak and after 30 min was performed. Sitagliptin treatment significantly improved ejection fraction, compared to placebo (64% to 73%; p < 0.0001). Similar encouraging results have been observed in a phase 3 clinical trial [22], where 100 patients with acute myocardial infarction, undergone effective revascularization, have been randomized either to placebo or granulocyte colony-stimulating factor (G-CSF) for 5 days plus sitagliptin 100 mg/day for 28 days. The aim of this study was to evaluate the effects of G-CSF add-on therapy to sitagliptin on myocardial regeneration, myocardial progenitor cell homing and myocardial function. The myocardial homing process is based on the ability of DPP4 inhibitors to inhibit the degradation of SDF-1 and of its receptor CXCR-4, a pathway of utmost importance for myocardial progenitor cell recruitment. The combined therapy sitagliptin/G-CSF effectively stimulated myocardial regeneration, during the first 5 weeks of treatment. Some meta-analyses confirmed the safety and efficacy profile of DPP4-inhibitors. A more recent meta-analysis of 18 clinical randomized controlled trials, for a total of 4,998 patients randomized to DPP4 inhibitors and 3,546 patients randomized to other active therapy, evaluated the cardiovascular safety of these drugs, during a period superior to 24 weeks [23]. The relative risk (RR) for each adverse cardiovascular outcome during DPP4 treatment was 0.48 (0.31 to 0.75; p < 0.001), and the RR for non fatal acute myocardial infarction or acute coronary syndrome was 0.40 (0.18 to 0.88; p < 0.02). In agreement with this observation, Monami and coll. in a further meta-analysis including 42 clinical trials, concluded that DPP4 inhibitor treatment is associated with a risk of cardiovascular major events of 0.689 [0.528-0.899], independent of trial duration, DPP4 inhibitor type and active comparator [24]. Protective actions of DPP4 inhibitors on the cardiovascular system do not seem dependent on DPP4 genetic polymorphisms [25]. Finally, it was also shown that DPP4 inhibition may also positively affect the myocardial metabolism: Witteles and colleagues have demonstrated that, in non-diabetic humans, sitagliptin significantly increased myocardial glucose uptake assessed by positron emission tomography [26].
POTENTIAL POSITIVE INDIRECT EFFECTS ON CARDIOVASCULAR FUNCTION
The DPP4 is also present in omental adipose tissue [27], where, through the degradation of neuropeptide Y (NPY) released by adrenergic fibres innerving this tissue, can influence lipid metabolism itself, by modulating pre-adipocyte proliferation and differentiation. Mice treated with des-fluoro-sitagliptin showed a lesser weight increase, and an enhanced insulin sensitivity when fed with a fat rich diet [28]. Rosmaninho-Salgado et al., exposing a pre-adipocyte murine line to vildagliptin, found that this drug was able to suppress DPP4-activated adipogenesis, without observing any effect on lipolysis [29]. The neutralization of NPY or of its receptor was also associated with a reduced lipid storage, suggesting an important role of DPP4 in the adipogenesis process. Furthermore, vildagliptin was able to suppress PPAR-gamma activity and to block pre-adipocyte conversion to mature adipocyte. These data suggest that DPP4 inhibitors can positively influence the cardiovascular risk profile, reducing intra-adipocyte lipid accumulation, either by adrenergic signal modulation (NPY) or by reducing PPAR-gamma activity. To investigate the relationship between NPY and lipolysis, visceral adipose tissue, obtained ex-vivo from women undergoing elective surgery, has been treated with human recombinant NPY (rhNPY) (1-100 nM), in the presence and absence of DPP4 inhibitor [27]. rhNPY blunted glycerol release, an effect that was further potentiated by DPP4 inhibition. In an interesting paper, Boschmann et al. have demonstrated that vildagliptin increased post-prandial lipid mobilization and oxidation, in T2DM patients, effects mainly attributable to the activation of the adrenergic system, rather than to a peculiar metabolic action [30]. In a similar mouse model, vildagliptin administration was able not only to ameliorate the insulin resistance condition, but also to determine cardioprotection. Apaijai et al. have shown, in insulin-resistant high-fat fed mice that treatment with DPP4 inhibitor can restore cardiac rate variability and improve cardiac and mitochondrial dysfunction induced by the high-fat diet, similarly to the control group, treated with metformin [31]. Finally, Kern et al. found that linagliptin, when administered to obese mice, was able to reduce liver fat storage, to improve insulin sensitivity and consequently to suppress hepatic glucose production [32].
Some studies, designed to evaluate lipid metabolism in animal, found that GLP-1 can reduce triglyceride (TG) absorption and apolipoprotein synthesis, while GIP enhance chilomicron clearance and post-load TG levels [33,34]. In man, DPP4 inhibitors seem to exert a positive effect on lipid metabolism, and in particular on cholesterol concentrations: in a meta-analysis of 17 studies, the treatment with DPP4 inhibitors determined a significant reduction of total cholesterol of about 7 mg/100 ml [I.C. -11.2 to -2.5]. Moreover, a fair number of studies highlighted a wider spectrum of actions of these drugs on lipid metabolism. In a double-blind study in type 2 diabetic patients, vildagliptin 50 mg b.i.d. significantly reduced the area under the curve (AUC) of both TG and chilomicrons, 8 hours after a fat-reach meal ingestion. Moreover, the treatment with vildagliptin, for 4 weeks, significantly reduced the synthesis of apoB-100 [35]. On the other hand, Trembley et al. evaluated the efficacy of sitagliptin in reducing post-prandial lipoprotein concentrations, in 36 T2DM patients [36]. Six weeks of sitagliptin therapy significantly decreased the AUC of apolipoprotein (apo)B, apoB-48, TG, VLDL-cholesterol and free fatty acids. Eventually, Eliasson et al., in a double-blind randomized trial, observed that 16 week alogliptin treatment decreased TG and apo-B48 post-prandial levels, at the same extent of pioglitazone [37]. Vildagliptin, administered to Zucker fatty rats for 6-weeks in association with nateglinide, was able not only to reduce triglyceride contents in the liver, but also increased hepatic levels of phosphorylated forkhead box protein 1A (FOXO1A), an important regulator of glucose and lipid metabolism [38]. Both these mechanisms contribute to ameliorate the condition of insulin resistance, and post-prandial metabolism. Altogether, these data agree with an improvement of lipid profile and insulin-resistance, especially in the post-prandial period, after treatment with DDP4 inhibitors.
RELATIONSHIP BETWEEN DPP4 INHIBITORS AND BLOOD PRESSURE LEVELS
DPP4 converts the neuropeptide Y1-36 released by sympathetic renal fibres and agonist of Y1 receptor, to neuropeptide Y3-36, selective agonist of Y2 receptor. Since Y1 receptor potentiates renovascular response to angiotensin II, it has been postulated that DPP4 inhibitors should increase NPY 1-36 capacity to enhance the hypertensive response to angiotensin II [39]. This interaction has been investigated in a small group of subjects with metabolic syndrome, treated with enalapril 5 mg or 10 mg/die, associated for 5 days with sitagliptin 100 mg/die. Marney et al. observed that sitagliptin blunted the hypotensive response at maximal enalapril dose [40]. This finding has not been confirmed by other studies, and needs to be verified, while other studies reported small decrements of pressure values during therapy with DPP4 inhibitors. Rather interesting are the relationships between DPP4 inhibition, blood pressure, and kidney function. In a study, renal complication was evaluated in a streptozotocin (STZ)-induced model of diabetes, both in wild-type and in DPP4-deficient rats. Plasma DPP4 activity increased after STZ treatment, positively correlating to blood glucose. DPP4-deficient rats were resistant to developing diabetes, while susceptible to dyslipidaemia and reduction of glomerular filtration rate by STZ [41]. About 70% of excreted Na+ is reabsorbed in the proximal tubule, via a Na/H (NHE3) exchanger: DPP4 forms a complex with NHE3 at the level of the brush membrane [42]. DPP4 inhibitor administration could interfere with this Na reabsorption mechanism, and consequently significantly increase natriuresis, reducing blood pressure levels [43]. A more recent experimental study has demonstrated that sitagliptin reduced blood pressure levels related to renal sodium/hydrogen co-transport, in spontaneously hypertensive rats [44]. Other DPP4 potential substrates can play an important role in natriuresis regulation. The brain-derived natriuretic peptide (BNP), produced by cardiac ventricles, is involved in natriuresis, in vasodilatation and in adrenergic activity suppression. DPP4 converts the active form of BNP(1-33) in a form inactive on natriuresis, but still active on cGMP production, in cardiomyocites [45,46]. Therefore, DPP4 inhibitor administration should act through 2 mechanisms rather independent, both able to induce natriuresis: one at the renal level, by inhibiting, sodium/hydrogen exchange, the other at cardiac level, by inhibiting BNP degradation. Mistry et al. assessed the effect of sitagliptin, after 5 days, on blood pressure levels, in human [47]. Compared to placebo, sitagliptin induced mean daily systolic blood pressure reduction of 2.0 mm Hg at the dose of 50 mg b.i.d, and of 2.2 mm Hg at the dose of 100 mg b.i.d; diastolic blood pressure was reduced respectively of 1.8 mm Hg and 1.6 mm Hg, at the 2 different doses. In the Diabetes therapy Utilization: Researching changes in A1c, weight and other factors Through Intervention with exenatide Once weekly (DURATION)-2 Study, sitagliptin determined a significant reduction of systolic blood pressure, in patients with arterial hypertension at enrolment [48]. In a retrospective study in 5,861 patients, treatment with sitagliptin in monotherapy or in association with insulin was able to decrease blood pressure levels [49]. A more recent study failed to find significant differences both in arterial blood pressure and stiffness, in patients treated with sitagliptin [50]. In a meta-analysis including all main phase III studies, saxagliptin in monotherapy or in association reduced systolic and diastolic blood pressure values of about 4 mm Hg [51]. Finally, in a randomized, controlled, double-blind study on tolerability and safety, the therapy with linagliptin did not imply significant modifications of blood pressure values [52]. We can conclude that the treatment with DPP4 inhibitors induces a small reduction of arterial blood pressure; nonetheless very few studies considered the reduction of blood pressure as a primary end-point.
DPP4 AND MICROANGIOPATHY
Microangiopathy is an important risk factor for macroangiopathy. Microalbuminuria, among other microangiopathic complications, predicts either cardiovascular events or cardiovascular death. The role of DPP4 in the kidney is of particular relevance for two reasons: first, because the exposure of glomerular endothelial cells to high glucose significantly increases the activity of DPP4 [53], second because DPP4 inhibitors accumulate especially in the kidney [54]. To strengthen these data, chronic administration of sitagliptin to diabetic Zucker rats, for 6 weeks, was accompanied by an improvement of glomerular, tubulo-interstitial and vascular lesions, and by a lower degree of oxidative stress [55]. Some data exist demonstrating a protective effect of DPP4 inhibitors also at the retinal level. Goncalves and coll. have shown, in Zucker Diabetic Fatty (ZDF) rats, treated with sitagliptin, that this drug not only improved glucose control, as expected, but also reduced nitrosative stress in the retina, reduced the apoptosis and ameliorated the adhesion capacity of endothelial progenitor cells (EPC) to retinal vessels [56]. In conclusion, although further data are needed, these results could suggest a direct protective effect of DPP4 inhibitors both on renal and retinal microcirculation.
DPP4 INHIBITORS AND SUBCLINICAL INFLAMMATION
In the last 10 years, several clinical and experimental studies have shown that obesity is characterized by a chronic, low-grade inflammation that contributes to the onset of insulin resistance and cardiovascular disease [57]. DPP4 inhibitors play an effective role in the suppression of this pro-inflammatory state. DPP4 is also involved in the immune system: in vitro this protein expression is stimulated after T lymphocyte activation and proliferation; an elevated expression of CD26 correlates with cytokine synthesis, such as interferon-gamma, and is implied in the transformation of B lymphocyte to plasmacells. Eventually, CD26 plays a pivotal role in the interaction antigen-presenting cells (APC) and in T lymphocyte activation [58]. It is therefore clear the important role of DPP4 inhibition on immune and pro-inflammatory processes, especially in the adipose tissue. In this regard, Lamers et al. have recently suggested DPP4 can be considered as a new adipokine, released by human adipocytes, with autocrine and paracrine effects, that induce insulin-resistance; moreover, DPP4 expression is particularly high in visceral fat of obese subjects and correlates with all metabolic syndrome components [59]. These authors conclude DPP4 should be a trait d’union between insulin-resistance and metabolic syndrome. Other experimental studies have demonstrated the key role of DPP4 in inducing a pro-inflammatory condition. In diabetic Zucker rats, the chronic administration of sitagliptin, for 6 weeks, reduced C-reactive protein and IL-1β levels, along with the oxidative stress [60]. Dobrian et al. have studied the effect of sitagliptin on subacute inflammation of the adipose tissue, in a mouse model fed with a high fat diet, for 12 weeks [61]. Sitagliptin reduced the proinflammatory milieu, along with macrophage infiltration, and genic transcription of MCP-1, IL-6, IL-12(p40), and IL-12, in the adipose tissue (p35). Shirakawa et al. determined the effects of DPP4 inhibition on hepatosteatosis and on proinflammatory condition, in an aplo-insufficient for glucokinase (Gck+/2) diabetic mouse model, exposed to high fat diet. DPP4 inhibition prevented adipose tissue infiltration of T CD8+ lymphocyte and M1 macrophages; the production of cytokines from activated T cells was not affected by DPP4 inhibition. These authors also observed that DPP4 inhibition could prevent hepatic steatosis [62]. Anti-inflammatory effects of DPP4 have been demonstrated in the atherosclerotic plaque. Ta et al. found that alogliptin treatment, in ApoE2/2 diabetic mice, induced a significant reduction of atherosclerotic lesions and a concomitant reduction of IL-6 and IL-1beta, especially in diabetic mice [63]. Moreover, alogliptin inhibited mononuclear cell IL-6 production, both basal and after stimulus with lypopolisaccaride, in a dose-dependent manner. In another LDL receptor knock-out mouse model, fed with high fat diet, Shah et al. demonstrated alogliptin was able to lessen macrophage content in visceral fat and the expression of proinflammatory genes. In addition, alogliptin showed a positive effect on the aortic plaque size, and a remarkable reduction of intraplaque macrophages [64]. DPP4 inhibition prevented macrophage and TNF-alfa-induced monocyte migration. In human, Rizzo et al. evaluated the efficacy of DPP4 inhibitors sitagliptin and vildagliptin in reducing the oxidative and proinflammatory stress, in type 2 diabetic patients not adequately treated with metformin alone, in a clinical prospective trial [65]. These authors observed that vildagliptin determined a nitrotirosine, IL-6 and IL-18 reduction, better than sitagliptin and that these reductions were correlated to a lessen glycemic variability. The group of Dandona treated 22 type 2 diabetic patients with sitagliptin 100 mg die or placebo, for 12 weeks [66]. Besides a significant improvement of HbA1c (-0.7%), these researchers observed a significant reduction of proinflammatory cytokines, TNF-alpha, endotoxin receptor, Toll-like receptor (TLR)-4, TLR-2, nuclear factor-kB, and of C-reactive protein and IL-6 concentrations. More recently, Satoh-Asahara and colleagues showed that sitagliptin 50 mg q.i.d. for 3 months decreased serum levels of serum amyloid A-LDL, C reactive protein, and TNF-alpha [67]. In conclusion, experimental animal and human studies have demonstrated that DPP4 inhibition is able not only to reduce the proinflammatory condition linked to obesity/insulin-resistance, but also the immune processes linked to atherosclerotic plaque development.
DPP4 AND PROGENITORS
In 1997, a new class of circulating cells derived from the bone marrow and able to differentiate in the phenotype of endothelial cells were identified; these cells showed the capacity to repair the damaged endothelium, integrating with the vascular structures [68]. They were called Endothelial Progenitor Cells (EPC). The link between diabetes, with its increased risk of cardiovascular disease, and EPC has been extensively studied. Solid studies evidenced diabetes associates with quantitative and qualitative EPC dysfunction [69]. This observation allowed to hypothesize EPC dysfunction can play a role in pathophysiology of micro- and macrovascular diabetes complications. Ischemia is a potent stimulus for EPC mobilization from the bone marrow: this effect is due to a reduced release of VEGF and SDF-1α, essential for EPC mobilization [70]. SDF-1 α, a substrate for DPP4, plays a crucial role in the activation of circulating progenitor cells and is activated by DPP4 itself. SDF-1α acts after interaction with its CXC chemokine receptor type 4 [CXCR4]/Janus kinase (JAK) 2. SDF-1α/CXCR4/JAK-2 is essential in vascular re-endothelization and in the modulation of progenitor homing in the marrow [71]. SDF-1 alpha is also effectively involved in wound healing, and in the response to tissue hypoxia. Therefore, DPP4 inhibitors, by inhibiting SDF-1 α, indirectly recall progenitor cells in the ischemia site. Zaruba et al. have demonstrated, in mice, that genetic or pharmacologic DPP4 deletion is able to increase CXCR4+ EPCs homing in the infarcted myocardium, to reduce myocardial remodelling and cardiac function [72]. This study gives evidence for a possible use of DPP4 inhibition in vascular repair, both in diabetic and in non diabetic patients. In this regard, we have demonstrated that sitagliptin administration for 4 weeks induces a significant increase of EPC, of SDF-1 α and a reduction of MCP-1 [73]. Interestingly, Shigeta and colleagues have found that DPP4 inhibition reverses left ventricular dysfunction via membrane-bound DPP4/stromal cell-derived factor 1 alpha-dependent actions on both angiogenesis and circulating DPP4/GLP-1 mediated inotropic actions [74]. Jungraithmayr et al. observed that systemic administration of a DPP4 inhibitor reduces the I/R damage, in lung transplant, in mice [75]. More recently, it has been reported that sitagliptin administration in a transgenic mouse model increases not only EPC levels, but also their angiogenic potential [76]. The cytokine High Mobility Group Box 1 (HMGB1), involved in processes of vascular regeneration and angiogenesis is itself a substrate for DPP4: this enzyme seems to inhibit angiogenic activity of HMGB1 [77]. Recently, Broxmeyer et al. have proved that DPP4 interacts with colony stimulating factor (CSF), and showed that the inhibition or the deficiency of DPP4 in vivo enhanced recovery of hematopoiesis after stress [78]. Our group has shown that stem and proangiogenic cell mobilization in response to hrG-CSF is impaired in T2DM, possibly because of maladaptive CD26/DPP-4 regulation [79]. We have also shown that disturbed compartmentalization of CD34+ cells is associated with aging and cardiovascular risk factors, especially diabetes. High activity of DPP-4 is associated with altered stem cell compartmentalization. We have also demonstrated that diabetes differentially affects DPP-4 activity in blood and in bone marrow and impairs stem/progenitor cell mobilization after ischemia or G-CSF administration [80]. Therefore, the treatment with DPP4 inhibitors seems to increase angiogenetic properties of this cytokine, especially in type 2 diabetes. In conclusion, DPP4 inhibitors give important therapeutic opportunity for the treatment of ischemic tissues through qualitative and quantitative amelioration of progenitor cells.
CONCLUSIONS
Evidence exists that DPP4 inhibitors is not only an anti-hyperglycaemic class drug able to reduce fasting and post-prandial hyperglycaemia, but also exerts several protective effects on cardiovascular system. Many clinical and experimental evidence so far summarized, regarding the cardiovascular protective actions of DPP4 inhibitors (Table 1), represent the rationale for the study design of several ongoing large intervention trials. These trials should demonstrate whether DPP4 inhibitors are able to offer to the diabetic patients not only a good glycaemic control, but also a cardiovascular protection. Hopefully, they should also provide us a definite answer about their safety.
Table 1.
Main protective actions of DPP4 inhibitors on cardiovascular system.
| Site | Mechanism | Effect |
|---|---|---|
| Endothelium | c-JUN N-terminal Kinase Extracellular-regulated Kinase Nuclear factor kB Nitric oxide Peroxynitrite |
Vasodilatation |
| Vessels | Macrophage infiltration Matrix metallo-proteinase Interleukin 6 Interleukin 1 beta |
Plaque reduction |
| Heart | Akt Stromal-derived factor 1 Cyclic AMP eNOS serine phosphorilation Brain natriuretic peptide Progenitor homing |
Protection of Ischemia/reperfusion damage |
| Adipose tissue | Insulin-sensitivity PPAR gamma Lipid oxidation MCP-1 Interleukin 6 Interleukin 1 beta Macrophage infiltration |
Reduction of pro-inflammatory state |
| Liver | Lipid accumulation Cholesterol synthesis VLDL synthesis Hepatic glucose production C reactive protein |
Reduction of circulating lipid level |
| Kidney | Natriuresis Countertransport Na+/H+ |
Blood pressure reduction |
| Bone Marrow | Stromal-derived factor 1 High Mobility Group Box colony-stimulating factor |
Increased angiogenesis |
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ABBREVIATIONS
- AGEs
= Advanced glycation end products
- AMI
= Acute myocardial infarction
- AMP
= Adenosine monophosphate
- APC
= Antigen-presenting cell
- AUC
= Area under the curve
- BNP
= Brain-derived natriuretic peptide
- cGMP
= Cyclic guanosine monophosphate
- CXCR4
= Chemokine receptor type 4
- DPP4
= Dipeptidyl peptidases 4
- EPC
= Endothelial progenitor cells
- eNOS
= Endothelial nitric oxide synthase
- ERK
= Extracellular regulated kinase
- FOXO1A
= Forkhead box protein 1A
- Gck
= Glucokinase
- G-CSF
= Granulocyte colony-stimulating factor
- GLP-1
= Glucagon-like peptide-1
- GIP
= Glucose insulinotropic peptide
- HbA1c
= Haemoglobin A1c
- HMGB1
= High mobility group box 1
- HUVEC
= Human umbilical vein endothelial cells
- ICAM-1
= Intercellular adhesion molecule-1
- IL
= Interleukin
- I/R
= Ischemia/reperfusion
- JAK
= Janus kinase
- LDL
= Low density lipoprotein
- MCP-1
= Monocyte chemoattractant protein-1
- MMP
= Matrix metalloproteinase
- mRNA
= Messenger ribonucleic acid
- NHE3
= Sodium/hydrogen exchanger-3
- NO
= Nitric oxide
- NPY
= Neuropeptide Y
- OLETF rats
= Otsuka Long-Evans Tokushima fatty rats
- ONOO
= Peroxynitrite
- PAI-1
= Plasminogen activator inhibitor-1
- PPAR
= Peroxisome proliferator-activated receptor
- RAGE
= Receptor for advanced glycation end products
- RR
= Relative risk
- SDF-1
= Stromal-derived factor-1
- STZ
= Streptozotocin
- T2DM
= Type 2 diabetes mellitus
- TGF-β
= Transforming growth factor-β
- TLR
= Toll-like receptor
- TG
= Triglyceride
- TNF-alfa
= Tumor necrosis factor-alfa
- VEGF
= Vascular endothelial growth factor
- VLDL
= Very low density lipoprotein
- ZDF
= Zucker diabetic fatty rat
REFERENCES
- 1.Gorrell MD. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clinical Science. 2005;108:277–92. doi: 10.1042/CS20040302. [DOI] [PubMed] [Google Scholar]
- 2.Marguet D, Baggio L, Kobayashi T , et al. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. PNAS. 2000;97:6874–79. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drucker DJ. Dipeptidyl peptidase-4 inhibitors and the treatment of type 2 diabetes. Diabetes Care. 2007;30:1335–43. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]
- 4.Mannucci E, Pala L, Ciani S , et al. Hyperglycaemia increases dipeptidyl peptidase IV activity in diabetes mellitus. Diabetologia. 2005;48:1168–72. doi: 10.1007/s00125-005-1749-8. [DOI] [PubMed] [Google Scholar]
- 5.Pala L, Pezzatini A, Dicembrini I , et al. Different modulation of dipeptidyl peptidase-4 activity between microvascular and macrovascular human endothelial cells. Acta Diabetol . doi: 10.1007/s00592-010-0195-3. [DOI] [PubMed] [Google Scholar]
- 6.Anagnostis P, Athyros VG, Adamidou F , et al. Glucagon-like peptide-1 based therapies and cardiovascular disease: looking beyond glycemic control. Diabetes Obes Metab. 2011;13:302–12. doi: 10.1111/j.1463-1326.2010.01345.x. [DOI] [PubMed] [Google Scholar]
- 7.Matsubara J, Sugiyama S, Sugamura K , et al. A dipeptidyl peptidase-4 inhibitor. des-fluoro-sitaglitin.improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J Am Coll Cardiol. 2012; 59:265–76. doi: 10.1016/j.jacc.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Liu J, Wong WTR. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like Peptide 1-dependent mechanism. Hypertension. 2012;60:833–41. doi: 10.1161/HYPERTENSIONAHA.112.195115. [DOI] [PubMed] [Google Scholar]
- 9.Mason P, Jacob RF, Kubant R , et al. Effect of Enhanced Glycemic Control with Saxagliptin on Endothelial Nitric Oxide Release and CD40 Levels in Obese Rats. J Atheroscler Thromb. 2011;18:774–83. doi: 10.5551/jat.7666. [DOI] [PubMed] [Google Scholar]
- 10.Vittone F, Liberman A, Vasic D. Sitagliptin reduces plaque macrophage content and stabilises arteriosclerotic lesions in Apoe-/- mice. Diabetologia. 2012;55:2267–75. doi: 10.1007/s00125-012-2582-5. [DOI] [PubMed] [Google Scholar]
- 11.Van Poppel PCM, Smits P, Netea M, Tack CE. Vildagliptin Improves Endothelium-Dependent Vasodilatation in Type 2 Diabetes. Diabetes Care. 2011;34:2072–77. doi: 10.2337/dc10-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. 2010;106:842–53. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsui T, Nishino Y, Takeuchi M, Yamagishi S. Vildagliptin blocks vascular injury in thoracic aorta of diabetic rats by suppressing advanced glycation end product-receptor axis. Pharmacol Res. 2011;63:383–388. doi: 10.1016/j.phrs.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Sitagliptin augments protective effects of GLP-1 against advanced glycation end product receptor axis in endothelial cells. Horm Metab Res. 2011;Sep43(10):731–4. doi: 10.1055/s-0031-1284383. [DOI] [PubMed] [Google Scholar]
- 15.Maeda S, Matsui T, Yamagishi S. Vildagliptin inhibits oxidative stress and vascular damage in streptozotocin-induced diabetic rats. Int J Cardiol. 2012;158:171–172. doi: 10.1016/j.ijcard.2012.04.087. [DOI] [PubMed] [Google Scholar]
- 16.Sauve M, Ban K, Momen MA , et al. Genetic deletion or pharmacologic inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after acute myocardial infarction in mice. Diabetes. 2010;59:1063–73. doi: 10.2337/db09-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huisamen B, Genis A, Marais E , et al. Pretreatment with DPP-4 inhibitor is infarct sparing in hearts from obese. prediabetic rats. . Cardiovasc Drugs Ther. 2011;25:13–20. doi: 10.1007/s10557-010-6271-7. [DOI] [PubMed] [Google Scholar]
- 18.Chinda K, Palee S, Surinkaew S , et al. Cardioprotective effect of dipeptidyl peptidase-4 inhibitor during ischemia-reperfusion injury. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Hocher B, Sharkovska Y, Mark M, Klein T, Pfab T. The novel DPP-4 inhibitors linagliptin and BI 14361 reduce infarct size after myocardial ischemia/reperfusion in rats. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Ye Y, Keyes KT, Zhang C, Perez-Polo JR, Lin Y, Birnbaum Y. The myocardial infarct size-limiting effect of sitagliptin is PKA-dependent. whereas the protective effect of pioglitazone is partially dependent on PKA. Am J Physiol Heart Circ Physiol. 2010;298:H1454–H1465. doi: 10.1152/ajpheart.00867.2009. [DOI] [PubMed] [Google Scholar]
- 21.Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardivasc Imaging. 2010;3:195–201. doi: 10.1161/CIRCIMAGING.109.899377. [DOI] [PubMed] [Google Scholar]
- 22.Theiss HD, Brenner C, Engelman MG , et al. Safety and efficacy of SITAgliptin plus Granulocyte-colony-stimulating factor in patients suffering from Acute Myocardial Infarction (SITAGRAMI-Trial)-rationale. design and first analysis. . Int J Cardiol. 2010;145:282–84. doi: 10.1016/j.ijcard.2009.09.555. [DOI] [PubMed] [Google Scholar]
- 23.Patil HR, Al Badarin FJ, Al Shami HA , et al. Meta-Analysis of Effect of Dipeptidyl Peptidase-4 Inhibitors on Cardiovascular Risk in Type 2 Diabetes Mellitus. Am J Cardiol. 2012;Jun 14 doi: 10.1016/j.amjcard.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 24.Monami M, Dicembrini I, Martelli D, Mannucci E. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Current Medical Research & Opinion. 2011;27 S3:57–64. doi: 10.1185/03007995.2011.602964. [DOI] [PubMed] [Google Scholar]
- 25.Bouchard L, Faucher G, Tchernof A , et al. Comprehensive genetic analysis of the dipeptidyl peptidase-4 gene and cardiovascular disease risk factors in obese individuals. Acta Diabetol. 2009;46:13–21. doi: 10.1007/s00592-008-0049-4. [DOI] [PubMed] [Google Scholar]
- 26.Witteles RM, Visith Keu K , et al. Dipeptidyl Peptidase 4 inhibition increases myocardial glucose uptake in nonischemic cardiomyoptahy. J. Card Fail. 2012;18:804–809. doi: 10.1016/j.cardfail.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Kos K, Baker AR, Jernas M , et al. DPP-IV inhibition enhances the antilipolytic action of NPY in human adipose tissue. Diabetes Obes Metab. 2009;11:285–92. doi: 10.1111/j.1463-1326.2008.00909.x. [DOI] [PubMed] [Google Scholar]
- 28.Lamont BJ, Drucker DJ. Differential antidiabetic efficacy of incretin agonists versus DPP-4 inhibition in high fat fed mice. Diabetes. 2008;57:190–8. doi: 10.2337/db07-1202. [DOI] [PubMed] [Google Scholar]
- 29.Rosmaninho-Salgado J, Marques AP, Estrada M , et al. Dipeptidyl-peptidase-IV by cleaving neuropeptide Y induces lipid accumulation and PPAR-? expression. Peptides. 2012;37:49–54. doi: 10.1016/j.peptides.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Boschmann M, Engeli S, Dobberstein K , et al. Dipeptidyl-Peptidase-IV Inhibition Augments Postprandial Lipid Mobilization and Oxidation in Type 2 Diabetic Patients. J Clin Endocrinol Metab. 2009;94:846–52. doi: 10.1210/jc.2008-1400. [DOI] [PubMed] [Google Scholar]
- 31.Apaijai N, Pintana H, Chattipakorn SC, Chattipakorn N. Vildagliptin in Adult Rats with Insulin Resistance Induced by a High-Fat Diet. Endocrinology. 2012;153:3878–85. doi: 10.1210/en.2012-1262. [DOI] [PubMed] [Google Scholar]
- 32.Kern M, Kloting N, Niessen HG , et al. Linagliptin Improves Insulin Sensitivity and Hepatic Steatosis in Diet-Induced Obesity. PLoS One. 2012;7:e38744. doi: 10.1371/journal.pone.0038744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wasada T, McCorkle K, Harris V , et al. Effect of gastric inhibitory polypeptide on plasma levels of chylomicron triglycerides in dogs. J Clin Invest. 1981;68:1106–07. doi: 10.1172/JCI110335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ebert R, Nauck M, Creutzfeldt W. Effect of exogenous or endogenous gastric inhibitory polypeptide (GIP) on plasma triglyceride responses in rats. Horm Metab Res. 1991;23:517–21. doi: 10.1055/s-2007-1003745. [DOI] [PubMed] [Google Scholar]
- 35.Matikainen N, Ma¨ntta¨ri S, Schweizer A , et al. Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia. 2006;49:2049–57. doi: 10.1007/s00125-006-0340-2. [DOI] [PubMed] [Google Scholar]
- 36.Tremblay AJ, Lamarche B, Deacon CF, Weisnagel SJ, Couture P. Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabtes.Obesity and Metabolism. 2011; 13:366–73. doi: 10.1111/j.1463-1326.2011.01362.x. [DOI] [PubMed] [Google Scholar]
- 37.Eliasson B, Mo¨ller-Goede D, Eeg-Olofsson K , et al. Lowering of postprandial lipids in individuals with type 2 diabetes treated with alogliptin and/or pioglitazone: a randomised double-blind placebo-controlled study. Diabetologia. 2012;55:915–25. doi: 10.1007/s00125-011-2447-3. [DOI] [PubMed] [Google Scholar]
- 38.Miura K, Kitahara Y, Yamagishi S. Combination therapy with nateglinide and vildagliptin improves postprandial metabolic derangements in Zucker fatty rats. Horm Metab Res. 2010;42:731–735. doi: 10.1055/s-0030-1261929. [DOI] [PubMed] [Google Scholar]
- 39.Jackson EK, Zhang M, Liu W, Mi Z. Inhibition of renal dipeptidyl peptidase IV enhances peptide YY1-36-induced potentiation of angiotensin II-mediated renal vasoconstriction in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2007;323:431–37. doi: 10.1124/jpet.107.126847. [DOI] [PubMed] [Google Scholar]
- 40.Marney A, Kunchakarra S, Byrne L, Brown NJ. Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibitionin humans. Hypertension. 2010;56:728–33. doi: 10.1161/HYPERTENSIONAHA.110.156554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirino Y, Sato Y, Kamimoto T , et al. Interrelationship of dipeptidyl peptidase IV (DPP4) with the development of diabetes. dyslipidaemia and nephropathy: a streptozotocin-induced model using wild-type and DPP4-deficient rats. Journal of Endocrinology. 2009;200:53–61. doi: 10.1677/JOE-08-0424. [DOI] [PubMed] [Google Scholar]
- 42.Girardi AC, Degray BC, Nagy T, Biemesderfer D, Aronson PS. Association of Na(+)-H(+) exchanger isoform NHE3 and dipeptidyl peptidase IV in the renal proximal tubule. J Biol Chem. 2001;276:46671–77. doi: 10.1074/jbc.M106897200. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka T, Nangaku M, Nishiyama A. The role of incretins in salt-sensitive hypertension: the potential use of dipeptidyl peptidase-IV inhibitors. Current Opinion in Nephrology and Hypertension. 2011;20:476–48. doi: 10.1097/MNH.0b013e328349af9d. [DOI] [PubMed] [Google Scholar]
- 44.Pacheco BP, Crajoinas RO, Couto GK , et al. Dipeptidyl peptidase IV inhibition attenuates blood pressure rising in young spontaneously hypertensive rats. J Hypertens. 2011;29:520–8. doi: 10.1097/HJH.0b013e328341939d. [DOI] [PubMed] [Google Scholar]
- 45.Boerrigter G, Costello-Boerrigter LC, Harty GJ, Lapp H, Burnett JC Jr. Des-serine-proline brain natriuretic peptide 3-32 in cardiorenal regulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R897–R901. doi: 10.1152/ajpregu.00569.2006. [DOI] [PubMed] [Google Scholar]
- 46.Heublein DM, Huntley BK, Boerrigter G , et al. Immunoreactivity and guanosine 30 .50 -cyclic monophosphate activating actions of various molecular forms of human B-type natriuretic peptide. Hypertension. 2007;49:1114–19. doi: 10.1161/HYPERTENSIONAHA.106.081083. [DOI] [PubMed] [Google Scholar]
- 47.Mistry GC, Maes AL, Lasseter KC , et al. Effect of sitagliptin. a dipeptidyl peptidase-4 inhibtor.on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol. 2008;48:592–8. doi: 10.1177/0091270008316885. [DOI] [PubMed] [Google Scholar]
- 48.Bergenstal RM, Wysham C, Macconell L , et al. for the DURATION-2 Study Group.Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. . Lancet. 2010;376:431–9. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 49.Horton ES, Silberman C, Davis KL, Berria R. Weight loss. glycemic conrol.and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33:1759–65. doi: 10.2337/dc09-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koren S, Shemesh-Bar L, Tirosh A , et al. The effect of sitagliptin versus glibenclamide on arterial stiffness. blood presurelipids., and inflammation in type 2 diabetes mellitus patients.. Diabetes Technol Ther. 2012;14:561–7. doi: 10.1089/dia.2011.0296. [DOI] [PubMed] [Google Scholar]
- 51.Cobble ME, Frederich R. Saxagliptin for the treatment of type 2 diabetes mellitus: assessing cardiovascular data. Cardiovasc Diabetol. 2012;16:11–6. doi: 10.1186/1475-2840-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horie Y, Kanada S, Watada H , et al. Pharmacokinetic. pharmacodynmic.and tolerability profiles of the dipeptidyl peptidase-4 inhibitor linagliptina 4 week multicenter. Clin Ther. 2011;33:973–89. doi: 10.1016/j.clinthera.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Pala L, Mannucci E, Pezzatini A , et al. Dipeptidyl peptidase-IV expression and activity in human glomerular endothelial cells. Biochemical and Biophysical Research Communications. 2003;310:28–31. doi: 10.1016/j.bbrc.2003.08.111. [DOI] [PubMed] [Google Scholar]
- 54.Fuchs H, Binder R, Greischel A. Tissue Distribution of the Novel DPP-4 Inhibitor BI 1356 is Dominated by Saturable Binding to its Target in Rats. Biopharm Drug Dispos. 2009;30:229–40. doi: 10.1002/bdd.662. [DOI] [PubMed] [Google Scholar]
- 55.Mega C, de Lemos ET, Vala H , et al. Diabetic Nephropathy Amelioration by a Low-Dose Sitagliptin in an Animal Model of Type 2 Diabetes (Zucker Diabetic Fatty Rat). Exp Diabetes Res. 2011;2011:162092. doi: 10.1155/2011/162092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goncalves A, Leal E, Paiva A , et al. Protective effects of the dipeptidyl peptidase IV inhibitor sitagliptin in the blood-retinal barrier in a type 2 diabetes animal model. Diabtes.Obesity and Metabolism . 2012; 14:454–63. doi: 10.1111/j.1463-1326.2011.01548.x. [DOI] [PubMed] [Google Scholar]
- 57.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohnuma K, Dang NH, Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 2008;29:295–301. doi: 10.1016/j.it.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 59.Lamers D, Famulla S, Wronkowitz Netal. Dipeptidyl Peptidase 4 Is a Novel Adipokine Potentially Linking Obesity to the Metabolic Syndrome. Diabetes. 2011;60:1917–25. doi: 10.2337/db10-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferreira L, Teixeira-de-Lemos E, Pinto F , et al. Effects of Sitagliptin Treatment on Dysmetabolism Inflammation and Oxidative Stress in an Animal Model of Type 2 Diabetes (ZDF Rat). Mediators Inflamm. 2010;2010:592760. doi: 10.1155/2010/592760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dobrian AD, Ma Q, Lindsay JW , et al. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab. 2011;300:E410–E421. doi: 10.1152/ajpendo.00463.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shirakawa J, Fujii H, Ohnuma Ketal. Diet-Induced Adipose Tissue Inflammation and Liver Steatosis Are Prevented by DPP-4 Inhibition in Diabetic Mice. Diabetes. 2011;60:1246–57. doi: 10.2337/db10-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ta NN, Schuyler CA, Li Y, Lopes-Virella MF, Huang Y. DPP-4 (CD26) Inhibitor Alogliptin Inhibits Atherosclerosis in Diabetic Apolipoprotein E-Deficient Mice. J Cardiovasc Pharmacol. 2011;58:157–66. doi: 10.1097/FJC.0b013e31821e5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah Z, Kampfrath T, Deiuliis JAetal. Long-Term Dipeptidyl-Peptidase 4 Inhibition Reduces Atherosclerosis and Inflammation via Effects on Monocyte Recruitment and Chemotaxis. Circulation. 2011;124:2338–49. doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rizzo MR, Barbieri M, Marfella R, Paolisso G. Reduction of Oxidative Stress and Inflammation by Blunting Daily Acute Glucose Fluctuations in Patients With Type 2 Diabetes. Diabetes Care. 2012;35:2076–82. doi: 10.2337/dc12-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Makdissi A, Ghanim H, Vora Metal. Sitagliptin Exerts an Antinflammatory Action. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sato-Asahara N, Sasaki Y, Wada H , et al. A dipeptydil peptidase-4 inhibitor. sitaglitin.exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism. 2012; 09 doi: 10.1016/j.metabol.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Asahara T, Murohara T, Sullivan A , et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 69.Fadini GP, Miorin M, Facco M , et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–57. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 70.Fadini GP, Sartore S, Schiavon M , et al. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia. 2006;49:3075–84. doi: 10.1007/s00125-006-0401-6. [DOI] [PubMed] [Google Scholar]
- 71.Christopherson KW 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–3. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 72.Zaruba MM, Theiss HD, Vallaster M , et al. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313–23. doi: 10.1016/j.stem.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 73.Fadini GP, Boscaro E, Albiero M , et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes mellitus.Possible role of stromal derived factor-1{alpha}. Diabetes Care. 2010;33:1607–09. doi: 10.2337/dc10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shigeta T, Aoyama M, Bando YK , et al. Dipeptydil petidase-4 modulates left ventricular dysfunction in cheronic heart failure via angiogenesis-dependent and -independent actions. Circulation. 2012;126:1838–1851. doi: 10.1161/CIRCULATIONAHA.112.096479. [DOI] [PubMed] [Google Scholar]
- 75.Jungraithmayr W, De Meester I, Matheeussen V , et al. CD26/DPP-4 inhibition recruits regenerative stem cells via stromal cell-derived factor-1 and beneficially influences ischaemia-reperfusion injury in mouse lung transplantation. Eur J Cardiothorac Surg. 2012;41:1166–73. doi: 10.1093/ejcts/ezr180. [DOI] [PubMed] [Google Scholar]
- 76.Huang CY, Shih CM, Tsao NW, et al. Dipeptidyl peptidase-4 inhibitor improves neovascularization by increasing circulating endothelial progenitor cells. Br J Pharmacol. 2012 doi: 10.1111/j.1476-5381.2012.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marchetti C, Di Carlo A, Facchiano F , et al. High mobility group box 1 is a novel substrate of dipeptidyl peptidase-IV. Diabetologia. 2012;55:236–44. doi: 10.1007/s00125-011-2213-6. [DOI] [PubMed] [Google Scholar]
- 78.Broxmeyer HE, Hoggatt J, O’Leary HA , et al. Dipeptydilpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoyesis. Nat Med. 2012 doi: 10.1038/nm.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fadini GP, Albiero M, Vigili de Kreutzenberg Setal. Diabetes Impairs Stem Cell and Proangiogenic Cell Mobilization in Humans. Diabetes Care. 2012;30 doi: 10.2337/dc12-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fadini GP, Albiero M, Seeger F , et al. Stem cell compartmentalization in diabetes and high cardiovascular risk reveals the role of DPP-4 in diabetic stem cell mobilopathy. Basic Res Cardiol. 2013 doi: 10.1007/s00395-012-0313-1. [DOI] [PubMed] [Google Scholar]