Abstract
Objective
To determine associations between pre-treatment characteristics and treatment selection in patients presenting with clinical stage I (cT1) renal masses.
Methods
Using institutional data, patients presenting with cT1 (≤7cm) renal tumors managed with active surveillance (AS), tumor ablation (ABL), partial nephrectomy (PN), or radical nephrectomy (RN) from 2005-2011 were identified. The associations between pre-treatment characteristics and selected treatment strategy were assessed using multinomial regression models using RN as the reference group.
Results
969 patients (mean age 61.9±12.8 years) with 1,034 cT1 lesions (mean tumor size 3.3±1.5 cm) met inclusion criteria. Patients were initially managed with RN (29.4%), PN (38.8%), ABL (6.1%), and AS (25.7%) respectively. Traditionally captured covariates including older age (PN: OR 0.96 [CI 0.94-0.99]) and decreasing tumor size (PN: OR 0.2 [CI 0.1-0.4], ABL: OR 0.01 [CI 0.0-0.1], AS: OR 0.2 [CI 0.1-0.3]) were associated with alternative treatment types when compared to RN. However, characteristics associated with treatment type that are not included in traditional registry or administrative data included presence of a solitary kidney (PN: OR 11.9 [CI 2.9-48.9], ABL: OR 15.5 [CI 2.5-98.1], AS: OR 7.1 [CI 1.3-39.3]) and high complexity NS (PN: OR 0.1 [CI 0.1-0.3], ABL: OR 0.1 [CI 0.0-0.6], AS: OR 0.1 [CI 0.03-0.3]).
Conclusions
Pre-treatment characteristics associated with treatment type in our series, including presence of a solitary kidney and anatomic complexity, are poorly captured using administrative and registry data. Observational studies investigating variation in practice patterns for stage I renal masses require improved integration of clinical and tumor characteristics to reduce selection biases.
Keywords: Renal Cell Carcinoma, localized, treatment, practice patterns, variation
Introduction
It has been estimated that more than 64,000 patients will be diagnosed with cancer of the kidney or renal pelvis in 20121, and the increased use of abdominal imaging over the past decade has resulted in a stage and size migration towards localized small renal masses (SRM)2-4. Although there is conflicting evidence regarding an overall survival benefit5, partial nephrectomy (PN) has supplanted radical nephrectomy (RN) as the recommended treatment for amenable lesions6,7 to reduce the risk of adverse outcomes associated with the development of chronic kidney disease (CKD)8. While use of open or minimally invasive nephron sparing surgery (NSS) has slowly but steadily increased over the last decade9-12, tumor ablative techniques (ABL) and active surveillance (AS) have been simultaneously integrated into treatment algorithms despite a lack of meaningful long term data6. The rapid adoption of these treatments has outpaced the understanding of the marginal benefits of each respective therapy, confounding management decisions as well as assessment of national practice patterns.
To better understand the gradual uptake of NSS in community practice, significant attention has been directed towards identifying characteristics associated with treatment type using observational data9-16. However, clinical characteristics that more accurately reflect case mix may impact surgical management decisions for localized renal tumors but are poorly captured using administrative and registry data. This may result in selection and omitted variable biases and limit the reliability of study findings17. Further, the relationship between pre-treatment characteristics and utilization of ABL or AS remains poorly studied14,18-20. In this retrospective cohort study, our objective was to determine the relationship between pre-treatment patient characteristics and selected management strategy in patients presenting with clinical stage I renal masses to a large tertiary care cancer center and to compare these characteristics to those captured in administrative and registry datasets.
Methods
Following Institutional Review Board approval, our prospectively maintained kidney tumor database was queried to identify all patients presenting with a clinically localized stage I (≤7cm) renal mass who elected to pursue treatment at our institution from 2005-2011. Patients were stratified by initial treatment strategy employed (PN, RN, ABL, AS). As treatment type included non-extirpative management strategies, differences between open versus minimally invasive surgery were not evaluated. Patients undergoing cryo- or radiofrequency ABL via either a percutaneous or laparoscopic approach were collapsed into a single ABL category. Patients who initially were managed expectantly (for a minimum of 6 months) and later progressed to definitive therapy were captured only in the AS group.
Pre-treatment variables evaluated included those easily measured using administrative claims data (age, gender, race, comorbidities, history of previous malignancy, history of previous abdominal/retroperitoneal surgery, year of diagnosis), those captured using tumor registry data (tumor size, bilateral disease at presentation, pathologic characteristics), and clinical data most accessible through chart abstraction (Eastern Cooperative Oncology Group (ECOG) performance status, Body Mass Index (BMI), serum creatinine (Cr), Glomerular Filtration Rate (GFR), Chronic Kidney Disease (CKD) stage, and tumor Nephrometry Score (NS)). Clinical severity was determined by Charlson Comorbidity Index (CCI)21. Anatomic severity was determined by NS22, and patients were stratified by low (NS 4-6), intermediate (NS 7-9), and high (NS 10-12) anatomic complexity groups. GFR was calculated using the Modification of Diet in Renal Disease Equation and patients were further stratified by CKD stage (I-V) using established GFR thresholds.
Statistical Analyses
Patient and tumor characteristics at the tumor level were compared between treatment groups using analysis of variance and Pearson chi square analyses. Trends in procedures performance were compared between 2005 and 2011 using Fisher Exact and chi square tests. The associations between pre-treatment characteristics (at the tumor level) and treatment strategy employed were assessed using multinomial logistic regression models using RN as the reference group. Covariates meeting a p<0.10 level of significance were included for model development, and our final model was adjusted for age, gender, CCI, ECOG performance status, CKD stage, previous history of abdominal or retroperitoneal surgery, history of prior malignancy, presence of solitary kidney, bilateral tumors at presentation, tumor size, NS complexity group, hilar designation, and year of diagnosis. Sensitivity analyses were performed to assess the impact of missing data. All analyses were performed using Stata v10 (StataCorp., College Station, TX), all hypothesis tests were 2-sided, and the criterion for statistical significance was p<0.05.
Results:
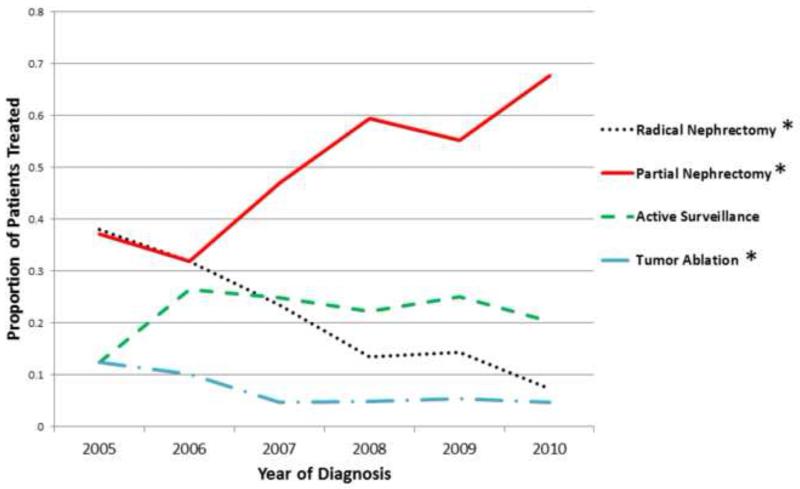
A total of 969 patients (mean age 61.9±12.8 years; 62.2% male, 87% Caucasian) presenting with 1,034 localized stage I renal tumors (mean tumor size 3.3±1.5cm, mean NS sum 7.5±1.9) met final inclusion criteria (Table 1). The initial treatment strategy employed for each tumor was RN (n=304, 29.4%), PN (n=401, 38.8%), ABL (n=63, 6.1%), and AS (n=266, 25.7%) respectively. Comparing the proportion of patients treated in 2005 and 2010 (last year with complete data), the use of PN significantly increased (37 versus 68%, p<0.0001), utilization of AS did not significantly change (12 versus 20%, p=0.09), while the use of RN (38 versus 7%, p=0.02) and ABL (12 versus 5%, p<0.001) significantly decreased over time (Figure 1). Note that all patients including those with missing data were included (n=1,021) to accurately portray evolving practice patterns at our institution over the length of the study period, and these proportions are reflective of treatment at the patient and not tumor level. Pathology and histology data were available for 75.2% (n=729) and 92.3% (n=894) of the sample respectively. Pathologic examination revealed predominantly pT1a (56.4%) and pT1b (22.6) disease in patients undergoing definitive resection (including patients managed initially with AS who progressed to intervention). Of patients with available histologic type (including biopsy data), clear cell (59.5%) and papillary (14.8%) were the most common RCC phenotypes, while 15.5% and 2.7% of lesions were benign or non-RCC malignancies respectively.
Table 1.
Pre-treatment characteristics of the study population
| Characteristics | mean±SD (median; range), N (%) |
|---|---|
| Patient Level (N=969) | |
| Age (years) | 61.9±12.8 (63; 24-92) |
| Gender | |
| Male | 603 (62.2) |
| Female | 366 (37.8) |
| Race | |
| Caucasian | 847 (87.4) |
| AA | 84 (8.7) |
| Other | 27 (2.8) |
| Missing | 11 (1.1) |
| ECOG | |
| 0 | 891 (92) |
| 1 | 63 (6.5) |
| 2 | 12 (1.2) |
| 3 | 3 (0.3) |
| Body Mass Index (kg/m2) | 30.2±6.7 (29.1; 10.9-58.9) |
| Charlson Comorbidity Index | 1.4 (2; 0-11) |
| Pre op Serum Creatinine | 1.1 ±0.6 (1; 0.3-8) |
| Pre op GFR (mL/min/1.73m2) | 75.8±25.4 (74.9; 6.5-318.8) |
| missing | 138 (14.3) |
| Bilateral tumors | 72 (7.4) |
| Solitary kidney | 41 (4.9) |
| Previous surgery | |
| abdominal | 488 (56.9) |
| retroperitoneal | 63 (6.5) |
| missing | 12 (1.2) |
| Previous malignancy | |
| Yes | 253 (26.1) |
| missing | 16 (1.7) |
| Tumor Level (N=1,034) | |
| Clinical Stage | |
| cT1a | 748 (72.3) |
| cT1b | 286 (27.7) |
| Maximum tumor size (cm) | 3.3±1.5 (3; 0.7-7) |
| Mean Nephrometry score sum | 7.5±1.9 (8; 4-12) |
| Nephrometry score complexity groupings | |
| Low (NS 4-6) | 235 (22.7) |
| Intermediate (NS 7-9) | 414 (40) |
| High (10-12) | 126 (12.2) |
| missing | 259 (25.1) |
| H designation (hilar tumor) | 103 (10) |
Figure 1.
Trends in treatment strategy employed over time. (*) represent significant differences (p<0.05) in the proportion of total procedures performed from 2005 to 2010 (last year with complete data).
Treatment groups significantly differed by patient age (p<0.0001), gender (p=0.03), CCI (p<0.0001), CKD stage (p<0.0001), history of abdominal or retroperitoneal surgery (P<0.0001), history of prior malignancy (P<0.0001), presence of solitary kidney (P<0.0001), bilateral tumors at presentation (p<0.0001), tumor size (p<0.0001), NS complexity group (p<0.0001), and hilar designation (p<0.01) (Table 2). In contrast, no differences were observed with respect to race, ECOG performance status, or BMI between groups.
Table 2.
Pre-treatment characteristics stratified by treatment type and data accessibility. Data are presented at the tumor level as proportions excluding patients with missing data.
| Characteristic | All | RN | PN | ABL | AS | P Value |
|---|---|---|---|---|---|---|
| N | 1034 | 304 | 401 | 63 | 266 | |
| Median (range) or N (%) | ||||||
| Characteristics accessible using administrative (claims) data | ||||||
| Age (years) | 63 (24-92) |
63 (24-89) |
59 (25-83) |
70 (41-88) |
71 (26-92) |
<0.0001 |
| Gender | ||||||
| Female | 377 (36.5) |
125 (41.1) |
146 (36.4) |
14 (22.2) |
92 (34.6) |
0.0326 |
| Male | 657 (63.5) |
179 (58.9) |
255 (63.6) |
49 (77.8) |
174 (65.4) |
|
| Race | ||||||
| Caucasian | 900 (88.0) |
265 (90.1) |
345 (86.0) |
55 (88.7) |
235 (88.4) |
0.2127 |
| Black | 96 (9.4) |
23 (10.5) |
39 (9.7) |
6 (9.7) |
28 (10.5) |
|
| Other | 27 (2.6) |
6 (2.0) |
17 (4.2) |
1 (1.6) |
3 (1.1) |
|
| CCI | <0.0001 | |||||
| 0 | 408 (39.7) |
140 (46.2) |
219 (54.6) |
13 (20.6) |
36 (13.7) |
|
| 1-2 | 343 (33.3) |
105 (34.7) |
128 (31.9) |
27 (42.9) |
83 (31.7) |
|
| ≥3 | 283 (27.0) |
58 (19.1) |
54 (13.5) |
23 (36.5) |
143 (54.6) |
|
| Hx of ABD/RP surgery |
561 (54.9) |
149 (50.5) |
201 (50.1) |
40 (63.5) |
171 (65.0) |
<0.0001 |
| Hx of prior malignancy |
273 (26.7) |
69 (23.5) |
82 (20.5) |
96 (36.5) |
26 (41.3) |
<0.0001 |
| Characteristics accessible using national cancer registry data | ||||||
| Tumor size (cm) | 3 (0.7-7) |
4.4 (1.1-7) |
3.0 (0.9-7) |
2 (1-5.2) |
2.6 (0.7-7) |
<0.0001 |
| Bilateral tumors | 113 (10.9) |
8 (2.6) |
54 (13.5) |
14 (22.2) |
37 (13.9) |
<0.0001 |
| Characteristics accessible using clinical chart abstraction | ||||||
| ECOG status | ||||||
| 0 | 949 (91.8) |
290 (95.4) |
364 (90.8) |
60 (95.2) |
235 (88.4) |
0.053 |
| 1 | 69 (6.7) |
12 (4.0) |
32 (8.0) |
2 (3.2) |
23 (8.7) |
|
| 2 | 12 (1.2) |
1 (0.3) |
5 (1.3) |
1 (1.6) |
5 (1.9) |
|
| 3 | 4 (0.4) |
1 (0.3) |
0 | 0 | 3 (1.1) |
|
| BMI (kg/m2) | 0.2737 | |||||
| <25 | 153 (20.2) |
40 (23.8) |
69 (17.3) |
10 (23.8) |
34 (23.0) |
|
| ≥25 to <30 | 269 (35.5) |
57 (33.9) |
152 (38.0) |
15 (35.7) |
45 (30.4) |
|
| ≥30 to <35 | 203 (26.8) |
39 (23.2) |
106 (26.5) |
11 (26.2) |
47 (31.8) |
|
| ≥35 to <40 | 69 (9.1) |
22 (13.1) |
34 (8.5) |
2 (4.8) |
11 (7.4) |
|
| ≥40 | 64 (8.4) |
10 (6.0) |
39 (9.8) |
4 (9.5) |
11 (7.4) |
|
| CKD stage | <0.0001 | |||||
| I | 239 (26.9) |
64 (23.6) |
140 (36.9) |
5 (10.0) |
30 (16.0) |
|
| II | 423 (47.6) |
152 (56.1) |
189 (49.9) |
23 (46.0) |
59 (31.4) |
|
| III | 194 (21.9) |
49 (18.1) |
50 (13.2) |
15 (30.0) |
80 (42.6) |
|
| IV | 19 (2.1) |
2 (0.7) |
0 | 3 (6.0) |
14 (7.5) |
|
| V | 13 (1.5) |
4 (1.5) |
0 | 4 (8.0) |
5 (2.7) |
|
| Solitary Kidney | 50 (5.6) |
3 (1.0) |
20 (7.0) |
9 (14.3) |
18 (7.4) |
<0.0001 |
| NS complexity group | <0.0001 | |||||
| Low (NS 4-6) | 235 (30.3) |
9 (9.2) |
120 (30.2) |
13 (40.6) |
93 (35.0) |
|
| Intermediate (NS 7- 9) |
414 (53.4) |
41 (41.8) |
229 (57.7) |
16 (50.0) |
128 (48.1) |
|
| Complex (NS 10- 12) |
126 (16.3) |
48 (48.9) |
48 (12.1) |
3 (9.4) |
27 (10.2) |
|
| Hilar (h) designation | 103 (16.2) |
26 (26.5) |
57 (14.2) |
1 (3.6) |
19 (17.3) |
0.0065 |
RN – radical nephrectomy, PN – partial nephrectomy, ABL – ablation, AS – active surveillance, cCi – Charlson Comorbidity Index, Hx – history, ABD – abdominal, RP – retroperitoneal, ECOG – Eastern Cooperative Oncology Group, BMI – body mass index, CKD – chronic kidney disease, NS – Nephrometry Score
After adjusting for age, gender, CCI, ECOG performance status, CKD stage, previous history of abdominal or retroperitoneal surgery, history of prior malignancy, presence of solitary kidney, bilateral tumors at presentation, tumor size, NS complexity group, hilar designation, and year of diagnosis (Table 3), patients with ECOG performance status ≥1 (OR 2.8 [CI 1.1-7.4]), solitary kidney (OR 11.9 [CI 2.9-48.9]), bilateral disease (OR 6.2 [CI 2.0-18.9]), and more recent year of diagnosis (OR 1.8 [CI 1.5-2.1]) were more likely to undergo PN, while patients with increased age (OR 0.96 [CI 0.94-0.99]), CKD stage V (OR 0.0 [CI 0.0-0.0]), increasing tumor size (OR 0.2 [CI 0.1-0.4]), and high NS complexity (OR 0.1 [CI 0.1-0.3]) were less likely to undergo PN. Factors associated with ABL treatment included CCI 1-2 (OR 2.7 [CI 1.1-6.8]), CCI ≥3 OR 3.7 [CI 1.2-12.0]), solitary kidney (OR 15.5 [CI 2.5-98.1]), bilateral disease (OR 5.3 [CI 1.2-24.1]), and more recent year of diagnosis (OR 1.2 [CI 1.1-1.5]), while patients with increasing tumor size (OR 0.01 [CI 0.0-0.1]) and high NS complexity (OR 0.1 [CI 0.0-0.6]) were less likely to undergo ABL. Characteristics associated with use of AS included CCI 1-2 (OR 2.2 [CI 1.1-4.5]) and ≥3 (OR 6.9 [CI 2.5-18.9]), solitary kidney (OR 7.1 [CI 1.3-39.3]), and bilateral disease (OR 7.6 [CI 2.2-26.0]), while patients with CKD stage V (OR 0.1 [CI 0.01-0.7]), history of prior malignancy (OR 0.5 [CI 0.2-0.9]), increasing tumor size (OR 0.2 [CI 0.1-0.3]), and high NS anatomic complexity (OR 0.1 [CI 0.03-0.3]) were less likely to undergo expectant management. When repeating analyses restricted to tumors with documented NS (n=775) or CKD stage (n=896), few differences were noted in specific clinical characteristics associated with each treatment modality. As these analyses minimally impacted our findings these data were not reported.
Table III.
Multinomial logistic regression adjusting for demographic characteristics, clinical severity, anatomic complexity, and year of diagnosis using radical nephrectomy as the reference group
| Characteristic | Partial Nephrectomy | Ablation | Active Surveillance | |||
|---|---|---|---|---|---|---|
| OR [CI] |
P Value | OR [CI] |
P Value | OR [CI] |
P Value | |
| Characteristics accessible using administrative (claims) data | ||||||
| Year of diagnosis | 1.8 [1.5-2.1] |
<0.001 | 1.2 [1.1-1.5] |
0.01 | 1.0 [0.9-1.1] |
|
| Age | 0.96 [0.94-0.99] |
0.002 | 1.02 [0.99-1.1] |
1.02 [0.99-1.1] |
||
| Gender | ||||||
| female | 1.0 | 1.0 | 1.0 | |||
| male | 1.2 [0.7-2.0] |
1.9 [0.9-4.2] |
1.1 [0.6-2.0] |
|||
| CCI | ||||||
| 0 | 1.0 | 1.0 | 1.0 | |||
| 1-2 | 1.0 [0.6-1.8] |
2.7 [1.1-6.8] |
0.037 | 2.2 [1.1-4.5] |
0.03 | |
| ≥3 | 1.0 [0.4-2.7] |
3.7 [1.2-12.0] |
0.028 | 6.9 [2.5-18.9] |
<0.001 | |
| ABD/RP surgery | ||||||
| No | 1.0 | 1.0 | 1.0 | |||
| Yes | 0.8 [0.5-1.4] |
1.3 [0.6-2.9] |
1.3 [0.7-2.4] |
|||
| Prior malignancy | ||||||
| No | 1.0 | 1.0 | 1.0 | |||
| Yes | 1.0 [0.5-1.9] |
0.8 [0.4-1.9] |
0.5 [0.2-0.9] |
0.035 | ||
| Characteristics accessible using national cancer registry data | ||||||
| Tumor size | 0.2 [0.1-0.4] |
<0.001 | 0.01 [0.0-0.1] |
<0.001 | 0.2 [0.1-0.3] |
<0.001 |
| Bilateral tumors | ||||||
| No | 1.0 | 1.0 | 1.0 | |||
| Yes | 6.2 [2.0-18.9] |
0.001 | 5.3 [1.2-24.1] |
0.031 | 7.6 [2.2-26.0] |
0.001 |
| Characteristics accessible using clinical chart abstraction | ||||||
| ECOG status | ||||||
| 0 | 1.0 | 1.0 | 1.0 | |||
| ≥1 | 2.8 [1.1-7.4] |
0.037 | 0.9 [0.2-4.2] |
1.3 [0.5-3.5] |
||
| CKD stage | ||||||
| I-II | 1.0 | 1.0 | 1.0 | |||
| III-IV | 1.1 [0.5-2.6] |
0.9 [0.3-2.6] |
0.75 [0.3-1.7] |
|||
| V | 0.0 [0.0-0.0] |
<0.001 | 1.1 [0.1-11.2] |
0.1 [0.01-0.7] |
0.02 | |
| Solitary kidney | ||||||
| No | 1.0 | 1.0 | 1.0 | |||
| Yes | 11.9 [2.9-48.9] |
0.001 | 15.5 [2.5-98.1] |
0.004 | 7.1 [1.3-39.3] |
0.024 |
| NS complexity group | ||||||
| Low | 1.0 | 1.0 | 1.0 | |||
| Intermediate | 0.8 [0.4-1.8] |
0.9 [0.3-2.6] |
0.7 [0.3-1.7] |
|||
| High | 0.1 [0.1-0.3] |
<0.001 | 0.1 [0.0-0.6] |
0.009 | 0.1 [0.03-0.3] |
<0.001 |
| Hilar designation | ||||||
| No | 1.0 | 1.0 | 1.0 | |||
| Yes | 0.7 [0.3-1.4] |
0.2 [0.0-1.8] |
1.4 [0.6-3.1] |
|||
Discussion
With increasing evidence that RN is over utilized in the treatment of RCC13, significant attention has been directed towards studying practice patterns for localized renal tumors using observational data from cancer and population registries as well as administrative data sets. These studies consistently report that use of NSS is gradually increasing9,10,13, and a recent study using the National Cancer Database (NCDB) demonstrated that performance of radical nephrectomy for localized stage I RCC declined from 88% in 1993 to 58% in 200712. Importantly, these studies also demonstrate that access disparities and regional variation in practice patterns exist, highlighting a potential quality of care issue10-12. Trends in the surgical management of localized renal tumors at our institution (Figure 1) are consistent with these national trends, as use of RN decreased from 38% to 7% from 2005 to 2010. Accordingly, use of NSS increased from 37% to 68% over the same time period, and a PN was performed in 90% of patients undergoing surgery for a stage I renal mass in 2010.
However, while observational studies have consistently demonstrated that hospital and patient demographic characteristics are associated with treatment type, minimal research has been directed towards identifying clinical characteristics that may influence the adoption of PN or alternative management strategies such as ABL or AS18,19. Highlighting this deficiency in the literature, our findings suggest that surgeon preference for performing RN for highly complex lesions, and maximizing nephron preservation (via PN, ABL, or AS) in the case of bilateral disease or a solitary functioning renal unit, may account for some of the variation in practice patterns that have been previously described due to differences in case mix9,10,12,13.
Treatment decisions for patients presenting with localized SRMs are complex, and the risks of PN may outweigh a marginal survival benefit in select elderly or comorbid patients5,23. Current best practice guidelines recommend that NSS should be considered in all patients with a clinical T1 renal mass presuming that oncologic control can be achieved, but that RN is a viable option based on tumor size, anatomy, and location. Further, ABL and AS represent acceptable management options in patients at high surgical risk due to advanced age or comorbidity6,7. Nonetheless, the vast majority of studies evaluating practice patterns focus on hospital and patients characteristics associated with performance of PN relative to RN, while few evaluate utilization of AS or ABL12,14. Due to concerns regarding biological efficacy and the need for long term surveillance, performance of ABL as primary treatment at our center has decreased from 12% to 5% since 2005, and is now most commonly performed for lesions exhibiting rapid growth kinetics during a period of AS in those deemed poor surgical candidates for definitive resection. In contrast, AS was the primary management strategy employed in 22% of patients with stage I tumors at our institution, a trend that remained consistent over the length of the study period.
Administrative and national registry data offer the ability to assess trends in procedure performance at the regional and national level, and can be more generalizable to care received in the community setting compared to institutional samples. Studies assessing contemporary renal surgery practice patterns have demonstrated associations between hospital (volume, teaching status, region, site), patient (race, gender, marriage status, income, insurance type), and tumor (size, year of treatment) characteristics and receipt of NSS9,11-16. However, administrative datasets are limited by use of claims to derive clinical severity and the lack of tumor specific information. Cancer registries offer more robust tumor characteristics, and can be linked to claims data for select patient populations24. However, measures of clinical severity using claims are limited to demographics and comorbidity measured using secondary diagnosis codes, and a number of prognostic indicators thought to influence physician treatment decisions such as baseline renal function, performance status, and tumor complexity cannot be reliably determined25.
Our study identified a number of pre-treatment clinical characteristics associated with performance of PN that are consistent with those reported in previous secondary data analyses, such as decreasing age and more recent year of diagnosis/treatment. However, we did not observe a relationship between gender and treatment type as previously described12,15. Similar to tumor registry analyses, decreasing tumor size and presence of bilateral disease were associated with each alternative treatment type other than RN9,12,20. Not assessed in prior administrative or registry reports, burden of comorbidity (CCI 1-2 (ABL: OR 2.7 [CI 1.1-6.8]; AS: OR 2.2 [1.1-4.5]) and CCI ≥3 (ABL: OR 3.7 [CI 1.2-12.0]; AS: OR 6.9 [CI 2.5-18.9]), was associated with receipt of AS and ABL. More notably, presence of a solitary kidney (PN: OR 11.9 [CI 2.9-48.9]; ABL: OR 15.5 [CI 2.5-98.1]; and AS: OR 7.1 [CI 1.3-39.3]) and high anatomic complexity (PN: OR 0.1 [CI 0.1-0.3]; ABL: OR 0.1 [CI 2.5-98.1]; AS: OR 0.1 [CI 0.03-0.3]) were independent predictors of each alternative treatment relative to RN in our cohort. Associations between low tumor complexity measured by NS and treatment type (PN26, AS19) have been reported in small institutional cohort studies, and in the absence of tumor specific characteristics other than size, anatomic complexity is challenging to measure using claims based or registry data. Although diagnosis codes exist suggestive of a solitary functional unit (ICD-9 753.0 renal agenesis and dysgenesis, V4573 acquired absence of kidney), the reliability of documenting the presence of a solitary kidney is limited due to lack of physician recognition and variable coding practice in response to changes in reimbursement25.
While surprising that baseline renal function was not an independent predictor of NSS in our sample, this may reflect that while important, alternative factors such as competing risks to mortality or feasibility of resection contributed more to physician treatment choice in our cohort. Our findings that patients with a previous cancer diagnosis were less likely to undergo AS and patients with a poor performance status were more likely to undergo PN are difficult to place into context and is likely that these trends are either spurious in nature due to low prevalence or a reflection of our institutional case mix.
Our single center cohort study is limited by its retrospective methodology and our intention is not to imply that our findings from a high volume academic cancer center are generalizable to the general population. In addition, imaging to determine NS was not available in 25% of lesions, the majority of which were tumors that underwent RN early in the study period. To address this limitation, sensitivity analyses were performed excluding subjects with missing data, which minimally impacted our main findings. Further, we did not assess physician characteristics, such as time interval from the completion of residency, receipt of fellowship training, and familiarity with and access to available technology, all factors that likely influence treatment preferences27,28.
To accurately assess variation in practice patterns and identify barriers to the delivery of quality care, measurement of hospital, patient, and physician level characteristics in conjunction with robust clinical data is needed. In February of 2009, the American Recovery and Reinvestment Act (ARRA) was signed into law, setting aside $19 billion dollars for the development and implementation of health information technology (HIT)29. Although the adoption of HIT has been slower than anticipated, an integrated electronic medical record (EMR) will greatly improve the ability to assess variations in the quality of healthcare delivery and adherence to best practice guidelines, first at the healthcare system and ultimately the population level30.
Our study highlights that clinical and tumor characteristics, including presence of a solitary kidney and anatomic complexity determined by NS, are independent predictors of alternative treatment type relative to radical nephrectomy in our institutional cohort. However, these variables are poorly captured using traditional administrative or registry data that is frequently used to evaluate variation in practice patterns. Until integrated data systems incorporating more robust clinical data are available, practice pattern assessment for the stage I SRM using observational data will remain prone to significant selection biases.
Acknowledgment statement
The authors acknowledge Debra Kister and Michelle Collins for their expertise and support of the Fox Chase Kidney Cancer Database.
Funding: This publication was supported in part by grant number P30 CA006927 from the National Cancer Institute (RGU). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The authors were supported in part through the Department of Defense, Physician Research Training Award (AK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Kane CJ, Mallin K, Ritchey J, et al. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113:78–83. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Mallin K, Ritchey J, et al. Decreasing size at diagnosis of stage 1 renal cell carcinoma: analysis from the National Cancer Data Base, 1993 to 2004. J Urol. 2008;179:2131–2135. doi: 10.1016/j.juro.2008.01.097. [DOI] [PubMed] [Google Scholar]
- 4.Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 5.Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59:543–552. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398–406. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 9.Dulabon LM, Lowrance WT, Russo P, et al. Trends in renal tumor surgery delivery within the United States. Cancer. 2010;116:2316–2321. doi: 10.1002/cncr.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SP, Shah ND, Weight CJ, et al. Contemporary trends in nephrectomy for renal cell carcinoma in the United States: results from a population based cohort. J Urol. 2011;186:1779–1785. doi: 10.1016/j.juro.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 11.Patel SG, Penson DF, Pabla B, et al. National trends in the use of partial nephrectomy: a rising tide that has not lifted all boats. J Urol. 2012;187:816–821. doi: 10.1016/j.juro.2011.10.173. [DOI] [PubMed] [Google Scholar]
- 12.Cooperberg MR, Mallin K, Kane CJ, et al. Treatment trends for stage I renal cell carcinoma. J Urol. 2011;186:394–399. doi: 10.1016/j.juro.2011.03.130. [DOI] [PubMed] [Google Scholar]
- 13.Hollenbeck BK, Taub DA, Miller DC, et al. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology. 2006;67:254–259. doi: 10.1016/j.urology.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 14.Yang G, Villalta JD, Meng MV, et al. Evolving practice patterns for the management of small renal masses in the USA. BJU Int. 2012 doi: 10.1111/j.1464-410X.2012.10969.x. [DOI] [PubMed] [Google Scholar]
- 15.Porter MP, Lin DW. Trends in renal cancer surgery and patient provider characteristics associated with partial nephrectomy in the United States. Urol Oncol. 2007;25:298–302. doi: 10.1016/j.urolonc.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Miller DC, Hollingsworth JM, Hafez KS, et al. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol. 2006;175:853–857. doi: 10.1016/S0022-5347(05)00422-2. discussion 858. [DOI] [PubMed] [Google Scholar]
- 17.Giordano SH, Kuo YF, Duan Z, et al. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112:2456–2466. doi: 10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs BL, Tan HJ, Montgomery JS, et al. Understanding criteria for surveillance of patients with a small renal mass. Urology. 2012;79:1027–1032. doi: 10.1016/j.urology.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canter D, Kutikov A, Manley B, et al. Utility of the R.E.N.A.L. nephrometry scoring system in objectifying treatment decision-making of the enhancing renal mass. Urology. 2011;78:1089–1094. doi: 10.1016/j.urology.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choueiri TK, Schutz FA, Hevelone ND, et al. Thermal ablation vs surgery for localized kidney cancer: a Surveillance, Epidemiology, and End Results (SEER) database analysis. Urology. 2011;78:93–98. doi: 10.1016/j.urology.2011.01.068. [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 23.Smaldone MC, Egleston B, Uzzo R, et al. Does partial nephrectomy result in a durable overall survival benefit in the elderly? J Urol. 2012 doi: 10.1016/j.juro.2012.07.099. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV–3. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 25.Sarrazin MS, Rosenthal GE. Finding pure and simple truths with administrative data. JAMA. 2012;307:1433–1435. doi: 10.1001/jama.2012.404. [DOI] [PubMed] [Google Scholar]
- 26.Broughton GJ, Clark PE, Barocas DA, et al. Tumour size, tumour complexity, and surgical approach are associated with nephrectomy type in small renal cortical tumours treated electively. BJU Int. 2012;109:1607–1613. doi: 10.1111/j.1464-410X.2011.10607.x. [DOI] [PubMed] [Google Scholar]
- 27.Filson CP, Banerjee M, Wolf JS, Jr., et al. Surgeon characteristics and long-term trends in the adoption of laparoscopic radical nephrectomy. J Urol. 2011;185:2072–2077. doi: 10.1016/j.juro.2011.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weight CJ, Crispen PL, Breau RH, et al. Practice-setting and surgeon characteristics heavily influence the decision to perform partial nephrectomy among American Urologic Association surgeons. BJU Int. 2012 doi: 10.1111/j.1464-410X.2012.11112.x. [DOI] [PubMed] [Google Scholar]
- 29.Blumenthal D. Wiring the health system--origins and provisions of a new federal program. N Engl J Med. 2011;365:2323–2329. doi: 10.1056/NEJMsr1110507. [DOI] [PubMed] [Google Scholar]
- 30.O’Malley AS. Tapping the unmet potential of health information technology. N Engl J Med. 2011;364:1090–1091. doi: 10.1056/NEJMp1011227. [DOI] [PubMed] [Google Scholar]