Abstract
Failures in fracture healing after conventional autologous and allogenic bone grafting are mainly due to poor vascularization. To meet the clinical demand, recent attentions in the regeneration and repair of bone have been focused on the use of stem cells such as bone marrow mesenchymal stem cells and circulating skeletal stem cells. Circulating stem cells are currently paid a lot of attention due to their ease of clinical setting and high potential for osteogenesis and angiogenesis. In this report, we focus on the first proof-of-principle experiments demonstrating the collaborative characteristics of circulating CD34+ cells, known as endothelial and hematopoietic progenitor cell-rich population, which are capable to differentiate into both endothelial cells and osteoblasts. Transplantation of circulating CD34+ cells provides a favorable environment for fracture healing via angiogenesis/vasculogenesis and osteogenesis, finally leading to functional recovery from fracture. Based on a series of basic studies, we performed a phase 1/2 clinical trial of autologous CD34+ cell transplantation in patients with tibial or femoral nonunions and reported the safety and efficacy of this novel therapy. In this review, the current concepts and strategies in circulating CD34+ cell-based therapy and its potential applications for bone repair will be highlighted.
Introduction
Despite embryonic stem cell potential differentiating into many cell types in the blastocyst stage, most adult stem cells had known inherent limited potential for postnatal tissue and organ regeneration. Among the sources of phenotypically characterized adult stem/progenitor cells,1–3 the hematopoietic system has traditionally been known as an organized, hierarchical system spearheaded by multipotent and self-renewing stem cells at the top, followed by lineage-committed progenitor cells in the middle, and finally, lineage-restricted precursor cells at the bottom, which give rise to terminally differentiated cells.4 However, another stem cell population, adult human circulating/peripheral blood (PB) CD34+ cells, has been added to this schematic. Following the discovery of bone marrow (BM)-derived and circulating endothelial progenitor cells (EPCs) in adults, CD34+ cells are reported to include EPCs and hematopoietic stem/progenitor cells (HSCs/HPCs)5 and promote embryonic vasculogenesis.5–8
The identification of various stimuli that direct stem cell activity toward tissue regeneration is a fundamental issue in tissue engineering research. Thus, recent studies have demonstrated that tissue ischemia triggered mobilization of EPCs from the BM into the PB with cytokines upregulation, ultimately migrating and incorporating EPCs to regions of neovascularization/vasculogenesis.9 Based on these findings, a lot of studies have shown the therapeutic potential of EPCs for neovascularization in animal models of limb ischemia, myocardial infarction, and liver disorders10–16 (Table 1). Several studies using an immunodeficient rat model of acute myocardial infarction have demonstrated effective transplantation of either human CD34+ cells or ex vivo-expanded EPCs into the site of myocardial neovascularization. Following transplantation, these cells could differentiate into mature endothelial cells, augment capillary density, inhibit myocardial fibrosis and apoptosis, and finally preserve left ventricular function.10,12,13,17 Based on these promising results, clinical trials using PB CD34+ cells have been performed with initial good clinical outcomes.18–20
Table 1.
Therapeutic Application of CD34+ Cells/EPCs for Various Disorders
| Author | Species | Model | Targed materials |
|---|---|---|---|
| Iwasaki et al.10 | Nude rat | Intramyocardial transplantation to myocardial ischemic model | Human circulating CD34+ cells |
| Kalka et al.11 | Nude rat | Local transplantation to mindlimb ischemic model | Ex vivo expanded EPCs |
| Kawamoto et al.12 | Nude rat | Systemic transplantation to myocardial ischemic model | Ex vivo expanded EPCs |
| Kawamoto et al.13 | Swine | Autologous intramyocardial transplantation to myocardial ischemic model | Ex vivo expanded EPCs |
| Murohara et al.14 | Nude rat | Local transplantation to hindlimb ischemic model | Human cord blood-derived CD34+ cells |
| Lemoli et al.15 | Human | Liver transplantation Live resection |
Mobilization of CD34+ cells |
| Kollet et al.16 | Immunocompromised mice | Systemic transplantation for liver injury model | Human circulating CD34+ cells |
| Taguchi et al.20 | Immunocompromised mice | Systemic transplantation for stroke model | Human cord blood-derived CD34+ cells |
| Sivan-Loukianova et al.21 | Diabetic mouse | Local transplantation to skin wound | Human circulating CD34+ cells |
| Kijima et al.23 | Nude rat | Local transplantation to sciatic nerve injury model | Human circulating CD133+ cells |
| Kamei et al.24 | Immunocompromised mice | Local transplantation to spinal cord injury model | Ex vivo expanded CD133+ cells |
| Tei et al.25 | Nude rat | Local transplantation to ligament injury | Human circulating CD34+ cells |
EPCs, endothelial progenitor cells.
In various areas of regenerative medicine, a lot of investigations on the promotion of tissue neovascularization with EPCs have led to widen applications for these cells. These areas include brain tissue regeneration, for which EPCs have been successfully applied. Human cord blood-derived CD34+ cells were systemically transplanted in immunocompromised mice within 48 h of sustaining a stroke, inducing neovascularization at the site of the ischemic zone and providing a favorable environment for neuronal regeneration.21 PB CD34+ cell transplantation has been also reported to promote tissue healing via revascularization include full-thickness skin wounds of diabetic mice, peripheral nerve injuries, and spinal cord injuries in rat22–24 (Table 1). Our group also reported on the therapeutic effectiveness of circulating CD34+ cell transplantation for ligament healing.25 Applications of CD34+ cells for therapeutic use were summarized in Table 1.
Almost all fractures are usually healed with appropriate condition and treatment but sometimes fail due to many systemic and local factors. Among them, adequate blood supply is quite important and its breakdown often result in either delayed unions or established nonunions. Traditionally, complex vascular procedures or soft tissue transfers with adequate blood supply was tried to restore the local blood flow and heal the fracture in certain types of injuries.26,27 Taking regenerative medicine and tissue engineering into consideration, neovascularization has been accordingly recognized to be an attractive strategy for bone healing and regeneration that appears to benefit quite well from an osteogenic reciprocity that exists between endothelial cells and osteoblasts.27 Therefore, we conducted several studies to prove the efficacy of circulating CD34-positive cells, which have potential for osteogenesis28–30 and angiogenesis/vasculogenesis, for bone fracture healing.31–38 In this review, the current concepts and strategies in circulating CD34+ cell-based therapy and its potential applications for bone repair will be highlighted.
Circulating Endothelial/Osteoprogenitor Cells for Bone Healing
All long bone fractures has a high (5–10%) incidence mainly due to an inadequate local blood supply around the injury zone.39,40 To this end, human PB CD34+ cells/EPCs are to be investigated to address the problem of delayed and atrophic nonunions in fracture healing. Because an adequate blood supply to the fracture site is essential for recovery from fracture,26,41 EPCs are one of the ideal candidates for promoting neoangiogenesis. EPCs are focused on overcoming this task in large part because angiogenesis and the development of native bone have closely related to each other and the association led to the discovery of a developmental reciprocity between endothelial cells and osteoblasts.27 EPCs are also promising targets because vascular bone grafting, which requires painstaking microvascular surgical procedures,39 is considered to be the only traditional effective approach for enhancing local angiogenesis at the fracture site.
Systemic transplantation of PB CD34+ cells for bone healing
As the preclinical study using animal model, we have reported several successful outcomes showing the efficacy of PB CD34+ cells/EPCs for fracture healing.31–34 First, when human PB CD34+ cells were systemically transplanted into the immunodeficient rat with nonhealing femoral fracture model, these cells were recruited to the fracture site and provided a favorable environment for fracture healing by enhancing angiogenesis/vauculogenesis and osteogenesis, and finally contributed to functional fracture healing.32 In brief, human PB CD34+ cells, mononuclear cells (MNCs), or phosphate-buffered saline (PBS) were systemically transplanted into a nonhealing femoral fracture model in immunodeficient rats. Fracture healing in CD34+ cell-transplanted group was significantly enhanced radiologically, histologically, and biomechanically when compared with the MNC and PBS groups. Two mechanisms were considered to explain this healing potential of the transplanted CD34+ cells: (1) differentiation potential of CD34+ cells into osteoblasts and endothelial cells, (2) the paracrine effect of CD34+ cells by secreting vascular endothelial growth factor (VEGF). Osteogenic and endotheliogenic differentiation by human cells was confirmed by the recruitment of human cells at the fracture site by labeling the transplanted cells, and was then further confirmed by reverse transcriptase–polymerase chain reaction (RT-PCR) and immunohistochemical staining at the fracture site, which showed molecular and histological expression of human-specific endothelial and osteoblast markers 2 weeks after transplantation. Of note, the overlapping origin of the endothelial and osteogenic markers was also confirmed by single cell RT-PCR, and we found that approximately osteocalcin mRNA expression was confirmed in 20% of human PB CD34+ cells. Previous reports showing that CD34+ and/or CD133+ cells could differentiate into osteoblasts and hematopoietic and endothelial cells in vitro28–30 support our findings. The second mechanism was confirmed by enhanced intrinsic angiogenesis and osteogenesis using rat antibody for osteoblasts and endothelial cells at the fracture site in the CD34+ cell-transplanted group. In addition, as the loss of function test, human-specific VEGF expression was confirmed and the blockade with sFlt1 (a VEGF antagonist) at the fracture site in the CD34+ cell-transplanted group resulted in reducing angiogenesis, osteogenesis, and fracture healing. The first series of studies conclude that transplantation of PB CD34+ cells provided favorable environment for leading to appropriate fracture healing by indirect paracrine effect and direct differentiation into osteoblasts and endothelial cells. Further, the osteogenic properties of PB CD34+ cells was also confirmed through in vitro experiments.33 These combined results reveal the therapeutic potential of PB CD34+ cells for fracture healing (Fig. 1).
FIG. 1.
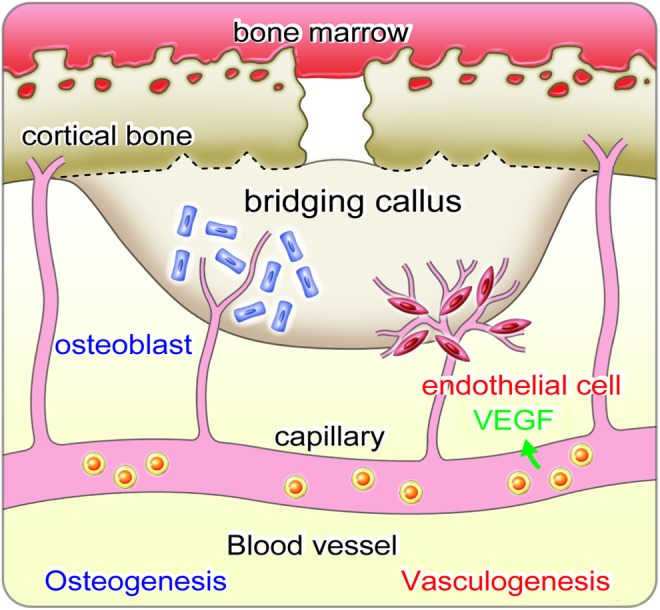
Kinetics of CD34+ cells in bone fracture. When bone fracture occurred, CD34+ cells (endothelial progenitor cell-rich population) are mobilized from bone marrow into peripheral blood, and they are recruited to the fracture site through circulation. Then, recruited CD34+ cells at the injured site develop a favorable environment for fracture healing by releasing vascular endothelial growth factor (VEGF) differentiating osteoblasts and endothelial cells. These combined mechanisms finally enhance vasculogenesis/angiogenesis and osteogenesis, leading to accelerate bridging callus formation and functional recovery from fracture. Color images available online at www.liebertpub.com/teb
Physiological role of EPCs in bone healing
Although EPC transplantation was found to be effective in fracture healing, the kinetic role of EPCs on fracture healing was still unclear. So, mobilization of EPCs from BM and incorporation of EPCs into fracture site were conformed.31 During the early phase of endochondral ossification in mouse fracture model, neovascularization showed its peaks at 7-day postfracture, which was confirmed by serial laser Doppler perfusion imaging and quantitative assessment of staining endothelial cells. At the fracture site, BM cKit+Sca1+Lineage- (Lin-) and PB Sca1+ Lin-cells, known as EPC population, were significantly increased. Double immunohistochemistry for CD31 and Sca1 indicated vasculogenesis by Sca1+ EPCs. Further, EPCs was found to enhance neovascularization by transplanting BM from transgenic donors that expresses LacZ into a fracture in wild-type models; these cells were regulated by the endothelial cell-specific Tie-2 promoter. In this BM transplantation model, these EPCs were mobilized from BM and incorporated into a fracture site prior to healing following systemic administration of PB Sca+Lin-Green Fluorescent Protein (GFP)+ cells into an animal. These findings conclude that fracture onset induces the mobilization of EPCs from BM and incorporation into the fracture sites through circulation for augment neovascularization and fracture healing. Similar to our report, other groups have also reported the mobilization of EPCs for bone healing in mouse and rat fracture and destruction of osteogenesis models42–46 (Table 2). In addition, recent studies demonstrated that patients with fractures showed significant larger amount of CD34+/AC133+ cells/EPCs in circulation47,48 (Table 2).
Table 2.
Biological Studies on the Use of EPCs for Bone Healing
| Author | Species | Model | Target materials | Research purpose |
|---|---|---|---|---|
| Cetrulo et al.46 | nude rat | Systemic Transplantation to tibial distraction osteogenesis model | Human cultured EPCs | Mobilization and incorporation of EPCs |
| Laing et al.47 | Mouse | Femoral fracture model | EPCs (Sca1+cKit+MNCs) | Mobilization of EPCs |
| Laing et al.47 | Human | Tibial fracture | CD34+/CD133+ cells | Mobilization of CD34+/CD133+ cells |
| Matsumoto et al.31 | Mouse | Femoral fracture model | EPCs (KSL cells, Sca1+Lin- MNCs) | Mobilization and incorporation of EPCs |
| Lee et al.43 | Rat | Tibial distraction osteogenesis model | EPCs (DiI-Ac-LDL/FITC-lectin double-stained MNCs) | Mobilization of EPCs |
| Ma et al.48 | Human | Long bone traumatic fracture (Femur, Tibia, Fumerus) | CD34+/CD133+ cells | Mobilization of CD34+/CD133+ cells |
MNC, mononuclear cell.
Local transplantation of granulocyte colony stimulating factor-mobilized CD34+ cells for bone healing
The clinical applications for PB CD34+ cells largely depend on the availability of these cells in sufficient quantities. While we demonstrated that intravenous transplantation of PB CD34+ cells could effectively heal bone fracture, these cells were found to migrate to other tissues, including the lungs, liver, thymus, and brain, which may raise concerns of possible unforeseen side effects in these organs. In an attempt to find alternative approaches to systemic infusion of these cells to promote bone healing, we performed local transplantation of granulocyte colony stimulating factor (G-CSF)-mobilized CD34+ cells to the femoral nonunion sites of immunodeficient rats, and proved successful fracture union, as evidenced by radiological and histological assessments.33 In addition, the effectiveness of the treatment was proved in a dose-dependent manner; fracture healing was significantly enhanced by transplantation of high (105) and moderate (104) doses of CD34+ cells compared with low (103) dose of CD34+ cells or PBS. Further, the dose-dependent contribution was also confirmed using molecular and histological techniques (RT-PCR and immunohistochemical staining, respectively) using tissue around the fracture site, which was most notable via detection of endothelial and osteoblast markers at 2-weeks post-transplantation.
Comparison between MNC and CD34+ cell transplantation for bone healing
In the field of revascularization, the therapeutic efficacy in enhancing neovascularization at ischemic sites has been demonstrated for transplantation of both PB CD34+ cells and PB total MNCs in hindlimb ischemia or myocardial ischemia. In the clinical setting, the use of PB MNCs, which can be easily collected in a short time and at a low cost, is more attractive than the use of PB CD34+ cells. However, some reports have shown higher therapeutic potency in ischemic neovascularization for CD34+ cells than for total MNCs. Based on this controversy, we performed experiments to support the hypothesis that transplantation of PB G-CSF-mobilized MNCs may also contribute to fracture healing via vasculogenesis/angiogenesis and osteogenesis. In this series of studies, FACS analysis showed that approximately 1% of PB MNCs were CD34+ cells, which led us to compare fracture healing with 1×107 MNCs and 1×105 CD34+ cells. Local transplantation of PB MNCs also contributed to bone healing via angiogenesis/vasculogenesis and osteogenesis, as confirmed by immunohistochemistry and RT-PCR analysis using a human-specific marker. In addition, using a rat-specific marker, the paracrine effect was also confirmed. This enhancement of angiogenesis/vasculogenesis and osteogenesis led to much greater radiological and histological fracture healing in the cell-treated groups than in the PBS group. However, the therapeutic potential of PB MNCs for fracture healing was inferior to that of purified CD34+ cells even if the PB MNCs administered contained the same number of CD34+ cells.34 The reason why the therapeutic effect of MNC transplantation is inferior to CD34+ cell transplantation in fracture healing is still being investigated. Total MNCs are a mixed cell population, and lymphocytes and monocytes/macrophages are the most numerous MNCs. These inflammatory cells could exert a negative influence on osteogenesis. We speculate that inflammation after transplantation of total MNCs could interfere with the long-term survival and differentiation of transplanted cells.
Clinical Trial with Circulating CD34+ Cells for Bone Healing
Recently, Fadini et al. reviewed the close relationship between “circulating calcifying cells” and circulating CD34+ cells/EPCs, indicating the osteogenic potential of circulating CD34+ cells in appropriate local environments.49 Prior to our published reports, CD34+ and CD133+ cells were reported to have a potential differentiation into osteoblasts,28–30 and osteoblasts expressing CD34 marker were also shown to line the cartilage cavities around the site of a tibial osteotomy in a rabbit model.50 Intra-capsular injection of human PB EPCs were reported to migrate to the ischemic zones and promote angiogenesis in immunodeficient rat distraction osteogenesis model.46 Further, in vitro expansion of autologous EPCs was reported to enhance the healing of a bone defect model in rat51,52 and critical-sized bone defects in sheep53 (Table 3). These findings indicate that EPCs/CD34+ cells have a therapeutic potential for bone healing including large bone defects and nonunions or delayed unions.
Table 3.
Therapeutic Studies on the Use of EPCs for Bone Healing
| Author | Animal | Model | Target materials | Research purpose |
|---|---|---|---|---|
| Matsumoto et al.32 | Nude rat | Systemic transplantation to nonhealing femoral fracture model | Human circulating CD34+ cells | Mobilization and incorporation of CD34+ cells Enhanced angiogenesis/vasculogenesis and osteogenesis Radiological, histological, and biomechanical bone healing |
| Mifune et al.33 | Nude rat | Local transplantation to nonhealing femoral fracture model | Human circulating CD34+ cells | Enhanced angiogenesis/vasculogenesis and osteogenesis Radiological, histological, and biomechanical bone healing |
| Rozen et al.53 | Sheep | Local transplantation to tibial critical size defect model | Cultured EPCs | Radiological and histological bone healing |
| Atesok et al.52 | Rat | Local transplantation to femoral segmental bone defect model | Cultured EPCs | Radiological and histological bone healing |
| Li et al.51 | Rat | Local transplantation to femoral segmental bone defect model | Cultured EPCs | Radiological and biomechanical bone healing |
| Fukui et al.34 | Nude rat | Local transplantation to nonhealing femoral fracture model | Human circulating CD34+ cells and MNCs | Enhanced angiogenesis/vasculogenesis and osteogenesis Radiological, histological, and biomechanical bone healing |
In the clinical setting, PB cells are attractive because they can be isolated using relatively minimally invasive, safe, and efficacious methods. This gives PB cells an advantage over BM mesenchymal stem cells (MSCs), which have been used for bone healing,54–56 as BM MSCs can only be isolated via BM aspiration under anesthesia, which is considered a form of surgical intervention. Recently, autologous injection of cultured osteoblasts, which were isolated from BM and cultured for 4 weeks in osteogenic conditioned medium, for long-bone fractures was reported by Kim et al.57 They showed statistically significant acceleration of fracture healing in the experimental groups treated with these cells compared with the nontreated group.57 In contrast, PB cell aspiration does not require anesthesia. Although we recognize that PB cells do have an advantage in their harvesting method, further investigation is needed to compare their efficacy in bone healing.
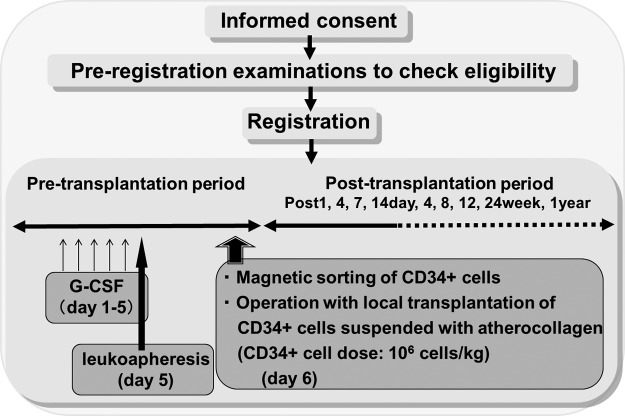
Based on the scientific evidence, we performed and reported the first clinical case of tibial nonunion treated with autologous, G-CSF-mobilized CD34+ cells with an atelocollagen scaffold immediately after autologous bone grafting from the iliac crest.58 Following this report, we began a phase I/IIa clinical trial: Autologous local transplantation of G-CSF-mobilized PB CD34-positive cells for patients with tibial or femoral nonunion.59 The inclusion criteria are1 a tibial or femoral fracture,2 a nonreactive and noninfectious nonunion,3 20–70 years-old, and4 informed written consent. Patients were selected and registered after informed consent was obtained and preregistration examinations were completed to confirm eligibility. Schema is shown in Figure 2. After leukapheresis following 5 days of G-CSF injection, each patient's cells were magnet sorted to separate the CD34+ cells. Treatment was conventional surgery for the nonunion combined with autologous transplantation of G-CSF-mobilized PB CD34+ cells (106 cells/kg) suspended in atherocollagen gel. Radiological fracture healing at week 12 was achieved in five of seven patients (71.4%), which was greater than the threshold (18.1%) predefined by the historical outcome of standard care (Table 4). The interval between cell transplantation and union, the secondary endpoint, was 12.6±5.4 (range, 8–24) weeks for clinical healing and 16.1±10.2 (range, 8–36) weeks for radiological healing. Neither death nor life-threatening adverse events were observed during the 1-year follow-up after the treatment. The promising outcomes in this phase I/IIa clinical trial encourage the application of CD34+ cells for nonunion/delayed union as a novel therapeutic modality. To elucidate the safety and efficacy of CD34+ cell transplantation or a combination of cell transplantation and bone grafting for bone fracture healing, randomized clinical trials with an appropriate control group are needed.
FIG. 2.
Schema of clinical trial. Based on inclusion and exclusion criteria, patients are selected and registered after informed consent and preregistration examinations to check eligibility. In the pretransplantation period, after leukoapheresis following 5 days granulocyte colony stimulating factor (G-CSF) injection, patient receives magnet sorting of CD34+ cells at day 6. Patient receives conventional surgery for the nonunion combined with autologous transplantation of G-CSF-mobilized peripheral blood CD34+ cells (106 cells/kg) suspended in atherocollagen gel at day 6. In the post-transplantation period, the efficacy of the treatment is assessed by clinical and radiological healing at each point assessed toward 1 year after the treatment.
Table 4.
Radiological Union of the CD34+ Cell-Treated Nonunions Compared to the Historical Controls
| CD34+ cell therapy (n=7) | Historical control (n=11) | p-Value | |
|---|---|---|---|
| Age (range, years) | 33.9±7.8 (20–45) | 37.1±14.9 (21–56) | N.S. |
| Tibia/Femur | 5/2 | 7/4 | N.S. |
| Union rate at week 12 (%) | 5/7 (71.4%) | 2/11 (18.1%) | <0.01 |
N.S., not significant.
Alternative to or Supportive Therapy for Circulating CD34+ Cell Transplantation
Although above-mentioned new cell therapy is indicated to be effective in the clinical setting, two main issues remain to be improved in the future. One is obtaining enough cell number without the use of G-CSF, the other is upregulating potential of cells transplanted.
Ex vivo-expanded BM CD34+ cells for bone healing
In the clinical setting of trauma surgery, the use of BM CD34+ cells that can be easily collected at the fracture site in a primary operation is a more convenient cell source than PB CD34+ cells. However, since EPCs represent less than 1% of all BM cells and less than 0.01% of all PB MNCs, the number of CD34+ cells obtained from either BM or PB is not sufficient for routine clinical applicaton. Indeed, results from preclinical studies have suggested that more than 10 L of autologous PB would be needed to provide a sufficient number of EPCs to induce angiogenesis in one patient. In addition, aging and cardiovascular risk factors will also make it difficult to obtain enough EPCs for cell therapy. The functionality and the number of EPCs are likely to be critical factors for effective cell therapy. Therefore, ex vivo expansion of EPCs may be critical in the future. Based on the background, we reported the functions of culture-expanded BM CD34+ cells, and supported the hypothesis that local transplantation of culture-expanded BM EPCs had a similar or more potent therapeutic effect on fracture healing than freshly isolated nonexpanded BM CD34+ cells.35 In this report, a 7-day culture expansion technique (Stem Span media without serum, supplemented with five growth factors, VEGF, interleukin-6, SCF, thrombopoietin, Flt-3 ligand, and antibiotics) allowed us to obtain 23 times more BM CD34+ cells at 60% purity (by CD34 positivity). Moreover, the culture-expanded BM CD34+ cells exhibited striking therapeutic efficacy for unhealed fractures, promoting neovascularization and osteogenesis at the sites of fracture even compared with the same number of freshly isolated BM CD34+ cells. These results suggest that this novel cell therapy requires only a small amount of BM, thus avoiding complicated procedures such as multiple administrations of G-CSF and long-time apheresis of PB in a clinical setting. Autologous culture-expanded BM CD34+ cell transplantation would be simple but powerful therapeutic strategy for nonunion fractures.
Growth factors and cytokines that enhance bone healing
Successful G-CSF-mobilized cell applications in the hematologic and cardiovascular fields have involved the mobilization of EPCs to obtain sufficient, therapeutic quantities of PB CD34+ cells.18 Our group reported the effective application of G-CSF for bone healing in a canine bone defect model,60 in which the local use of G-CSF combined with hydrogel enhanced angiogenesis and osteogenesis at the fracture site compared with hydrogel only. G-CSF has been used in thousands of clinical cases; however, severe complications such as spleen rupture, interstitial pneumonitis, and acute coronary syndrome have been reported in a small number of cases. These rare but potential risks of using G-CSF and the high cost of CD34+ cell isolation need to be overcome before they can be considered a viable source. The potential risks of using G-CSF might be alleviated by the use of alternative cytokines and growth factors. Many cytokines also augment the mobilization and/or recruitment of BM-derived EPCs, and angiogenic growth factors such as VEGF and stromal cell-derived factor (SDF)-1; estrogen; and pharmaceutical drugs such as statins. Our group reported the effective application of statin for bone healing via recruitment of BM-derived EPCs in a rat model.34 In this study, approximately 70% of animals in the simvastatin-conjugated gelatin hydrogel group achieved fracture union radiographically and histologically, whereas only 7% of animals achieved fracture healing in the control group with only hydrogel. Functional bone healing was also significantly greater in animals with increased angiogenesis- and osteogenesis-related growth factor expression in periosteal granulation tissue in the simvastatin-conjugated gelatin hydrogel group than in the control group. However, these factors act not only on immature stem/progenitor cells but also on hematopoietic cells and mature endothelial cells. Therefore, the identification of a novel molecule that specifically regulates immature populations involved in EPC kinetics in BM is warranted.
One possible candidate molecule, Lnk, shares a pleckstin homology domain, an Src homology 2 domain, and potential tyrosine phosphorylation sites with APS and SH-2B. It belongs to a family of adaptor proteins implicated in the integration and regulation of multiple signaling events,61,62 and has also been suggested to act as a negative regulator in the stem cell factor (SCF)-cKit signaling pathway.63,64 Recently, Takaki et al. reported that Lnk is expressed in hematopoietic cell lineages, and that the BM cells of Lnk-deficient mice are competitively superior in hematopoietic population to those of wild-type mice.63 They also demonstrated that not only were the numbers of HSCs/HPCs [cKit-positive, Sca1-positive, lineage marker-negative (KSL) cell] increased but the self-renewal capacity of some HSCs/HPCs were also markedly increased in Lnk-deficient mice.65 In addition, they identified the functional domains of Lnk and developed a dominant-negative Lnk mutant that inhibits the functions of endogenously-expressed Lnk in HSCs/HPCs, which thereby potentiates the HPCs for engraftment.66 Enhancement of the HSC/EPC-mediated osteogenesis/angiogenesis via Lnk, one of the novel factors responsible for stem/progenitor cell mobilization from BM, directed our research interests toward developing a therapeutic strategy utilizing circulating EPCs for skeletal medicine. Therefore, we investigated the hypothesis that the lack of Lnk signaling, dependent on the SCF-cKit signaling pathway, enhances the regenerative response via vasculogenesis and osteogenesis in fracture healing via HSC/EPC mobilization and recruitment to the sites of fracture in Lnk-deficient mice. Molecular, physiological, and morphological approaches showed that vasculogenesis/angiogenesis and osteogenesis were promoted in Lnk-deficient mice through the mobilization and recruitment of HSCs/EPCs via activation of the SCF-cKit signaling pathway in the peri-fracture zone, which established a favorable environment for bone healing and remodeling. In addition, osteoblasts from Lnk-deficient mice had a greater potential for terminal differentiation in response to SCF-cKit signaling in vitro.37 In the second series of studies, we clarified that downregulation of the Lnk system through transfection of a Lnk siRNA in mice contributed to favorable conditions for fracture healing by enhancing vasculogenesis/angiogenesis and osteogenesis.38 In this study, we also demonstrated that Lnk was also expressed in KSL cells and osteoblasts, and that Lnk siRNA-transfected KSL cells and osteoblasts showed increased proliferation activity and high vasculogenic and osteogenic activity. These findings suggest that inhibition of Lnk may have therapeutic potential by promoting an environment conducive to vasculogenesis/angiogenesis and osteogenesis and by facilitating terminal differentiation of osteoblasts leading to enhanced fracture healing.
Summary and Perspective
In the circulation of humans, Zvaifler et al. first discovered blood mesenchymal precursor cells (BMPCs), which adhered to plastic and glass and proliferate logarithmically without growth factors, and thereby suggested that these cells were relatively easy to expand in vitro.67 Besides limited reproducibility of these experiments, recent studies focusing on peripherally circulating skeletal-lineage cells from humans have received attention. Eghbali-Fatourechi et al. proved via flow cytometry and immunostaining that cells expressing osteocalcin and alkaline phosphatase are indeed present in the circulation of humans.68,69 Otsuru et al. reported that BM-derived osteoblast progenitor cells in the circulation could form ectopic bone after transplantation with a BMP-2-containing collagen pellet into the skeletal muscle beds of mice.70 Moreover, they demonstrated that circulating BM-derived osteoblast progenitor cells (MOPCs) were recruited to the bone-forming site via the CXCR4/SDF-1 pathway.71 Ratanavaraporn et al. recently reported that the gelatin hydrogels incorporating combined SDF-1 and BMP-2, which was implanted at a rat ulna critical-sized defect, provided enhanced angiogenic and osteogenic activity and recruitment of osteogenic cells, compared with that of those incorporating either SDF-1 or BMP-2.72 They concluded that the combined release of SDF-1 and BMP-2 enhanced the recruitment of osteogenic cells and angiogenesis, resulting in the synergistic effect on bone regeneration.
Recently, there has been increased interest in circulating stem/progenitor cells due to their ease of isolation and their great potential for bone repair. Our group has investigated the therapeutic potential of human circulating CD34+ cells for bone healing and repair and focused on their clinical feasibility, providing an attractive strategy for cell-based therapy. Although circulating EPCs, circulating osteoblast-lineage cells, and MOPCs need different isolation procedures, they exhibit similar cell characteristics and behaviors in bone healing and repair, indicating that there is some functional and perhaps phenotypic overlap in these populations. The recent advances in circulating stem/progenitor cell research provide new clinical applications for patients suffering from large bone defects, fracture nonunions, and delayed unions. To further expand the clinical application of these cells and their therapeutic effects, we hope that further in-depth investigations will focus on the inter-relationships of these cell types and attempt to provide a greater understanding of the molecular mechanisms underlying their therapeutic effects.
Acknowledgments
The authors express special thanks for Takahiro Niikura and Snag Yang Lee, Kobe University, for collecting data and assisting surgery. We also appreciate the great cooperation of Ms. Maki Shibakawa as a clinical research coordinator.
Disclosure Statement
This study was supported in part by a grant from the Translation Research Support Program (2007–2012) of the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1.Blau H.M., Brazelton T.R., and Weimann J.M.The evolving concept of a stem cell: entity or function? Cell 105,829, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Slack J.M.Stem cells in epithelial tissues. Science 287,1431, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Korbling M., and Estrov Z.Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med 349,570, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Weissman I.L.Stem cells: units of development, units of regeneration, and units in evolution. Cell 100,157, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Asahara T., Murohara T., Sullivan A., Silver M., van der Zee R., Li T., et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275,964, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Asahara T., Masuda H., Takahashi T., Kalka C., Pastore C., Silver M., et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85,221, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Pardanaud L., Yassine F., and Dieterlen-Lievre F.Relationship between vasculogenesis, angiogenesis and haemopoiesis during avian ontogeny. Development 105,473, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Risau W., Sariola H., Zerwes H.G., Sasse J., Ekblom P., Kemler R., et al. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development 102,471, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Takahashi T., Kalka C., Masuda H., Chen D., Silver M., Kearney M., et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5,434, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki H., Kawamoto A., Ishikawa M., Oyamada A., Nakamori S., Nishimura H., et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation 113,1311, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Kalka C., Masuda H., Takahashi T., Kalka-Moll W.M., Silver M., Kearney M., et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A 97,3422, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawamoto A., Gwon H.C., Iwaguro H., Yamaguchi J.I., Uchida S., Masuda H., et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 103,634, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Kawamoto A., Tkebuchava T., Yamaguchi J., Nishimura H., Yoon Y.S., Milliken C., et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 107,461, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Murohara T., Ikeda H., Duan J., Shintani S., Sasaki K., Eguchi H., et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest 105,1527, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemoli R.M., Catani L., Talarico S., Loggi E., Gramenzi A., Baccarani U., et al. Mobilization of bone marrow-derived hematopoietic and endothelial stem cells after orthotopic liver transplantation and liver resection. Stem Cells 24,2817, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kollet O., Shivtiel S., Chen Y.Q., Suriawinata J., Thung S.N., Dabeva M.D., et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest 112,160, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kocher A.A., Schuster M.D., Szabolcs M.J., Takuma S., Burkhoff D., Wang J., et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 7,430, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Losordo D.W., Schatz R.A., White C.J., Udelson J.E., Veereshwarayya V., Durgin M., et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation 115,3165, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Terai S., Ishikawa T., Omori K., Aoyama K., Marumoto Y., Urata Y., et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells 24,2292, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kudo F.A., Nishibe T., Nishibe M., and Yasuda K.Autologous transplantation of peripheral blood endothelial progenitor cells (CD34+) for therapeutic angiogenesis in patients with critical limb ischemia. Int Angiol 22,344, 2003 [PubMed] [Google Scholar]
- 21.Taguchi A., Soma T., Tanaka H., Kanda T., Nishimura H., Yoshikawa H., et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest 114,330, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivan-Loukianova E., Awad O.A., Stepanovic V., Bickenbach J., and Schatteman G.C.CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res 40,368, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Kijima Y., Ishikawa M., Sunagawa T., Nakanishi K., Kamei N., Yamada K., et al. Regeneration of peripheral nerve after transplantation of CD133+ cells derived from human peripheral blood. J Neurosurg 110,758, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Kamei N., Kwon S.M., Alev C., Nakanishi K., Yamada K., Masuda H., et al. , Ex-vivo expanded human blood-derived CD133+ cells promote repair of injured spinal cord. J Neurol Sci 328,41, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Tei K., Matsumoto T., Mifune Y., Ishida K., Sasaki K., Shoji T., et al. Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem Cells 26,819, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Gerstenfeld L.C., Cullinane D.M., Barnes G.L., Graves D.T., and Einhorn T.A.Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem 88,873, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Karsenty G., and Wagner E.F.Reaching a genetic and molecular understanding of skeletal development. Dev Cell 2,389, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Bick T., Rozen N., Dreyfuss E., Soudry M., and Lewinson D.Osteogenic differentiation of circulating endothelial progenitor cells. J Bone Min Res 22,S143, 2007 [Google Scholar]
- 29.Chen J.L., Hunt P., McElvain M., Black T., Kaufman S., and Choi E.S.Osteoblast precursor cells are found in CD34+ cells from human bone marrow. Stem Cells 15,368, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Tondreau T., Meuleman N., Delforge A., Dejeneffe M., Leroy R., Massy M., et al. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells 23,1105, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto T., Mifune Y., Kawamoto A., Kuroda R., Shoji T., Iwasaki H., et al. Fracture induced mobilization and incorporation of bone marrow-derived endothelial progenitor cells for bone healing. J Cell Physiol 215,234, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto T., Kawamoto A., Kuroda R., Ishikawa M., Mifune Y., Iwasaki H., et al. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol 169,1440, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mifune Y., Matsumoto T., Kawamoto A., Kuroda R., Shoji T., Iwasaki H., et al. Local delivery of granulocyte colony stimulating factor-mobilized CD34-positive progenitor cells using bioscaffold for modality of unhealing bone fracture. Stem Cells 26,1395, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Fukui T., Matsumoto T., Mifune Y., Shoji T., Kuroda T., Kawakami Y., et al. Local transplantation of granulocyte colony-stimulating factor-mobilized human peripheral blood mononuclear cells for unhealing bone fractures. Cell Transplant 21,707, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Kawakami Y., Ii M., Alev C., Kawamoto A., Matsumoto T., Kuroda R., et al. Local transplantation of ex vivo expanded bone marrow-derived CD34-positive cells accelerates fracture healing. Cell Transplant 21,2689, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto T., Kuroda R., Mifune Y., Kawamoto A., Shoji T., Miwa M., et al. Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone 43,434, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto T., Ii M., Nishimura H., Shoji T., Mifune Y., Kawamoto A., et al. Lnk-dependent axis of SCF-cKit signal for osteogenesis in bone fracture healing. J Exp Med 207,2207, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawakami Y., Ii M., Matsumoto T., Kawamoto A., Kuroda R., Akimaru H., et al. A small interfering RNA targeting Lnk accelerates bone fracture healing with early neovascularization. Lab Invest 93,1036, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Merchan E.C., and Forriol F.Nonunion: general principles and experimental data. Clin Orthop Relat Res 419,4, 2004 [PubMed] [Google Scholar]
- 40.Marsh D.Concepts of fracture union, delayed union, and nonunion. Clin Orthop Relat Res 355Suppl,S22, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Colnot C.I., and Helms J.A.A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev 100,245, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Laing A.J., Dillon J.P., Condon E.T., Coffey J.C., Street J.T., Wang J.H., et al. A systemic provascular response in bone marrow to musculoskeletal trauma in mice. J Bone Joint Surg Br 89,116, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Lee D.Y., Cho T.J., Kim J.A., Lee H.R., Yoo W.J., Chung C.Y., et al. Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone 42,932, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Atesok K., Li R., and Schemitsch E.Endothelial progenitor cells: a novel cell-based therapy in orthopaedic surgery. J Am Acad Orthop Surg 20,672, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Atesok K., Matsumoto T., Karlsson J., Asahara T., Atala A., Doral M.N., et al. An emerging cell-based strategy in orthopaedics: endothelial progenitor cells. Knee Surg Sports Traumatol Arthrosc 20,1366, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Cetrulo C.L., Jr., Knox K.R., Brown D.J., Ashinoff R.L., Dobryansky M., Ceradini D.J., et al. Stem cells and distraction osteogenesis: endothelial progenitor cells home to the ischemic generate in activation and consolidation. Plast Reconstr Surg 116, 1053.; discussion 65, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Laing A.J., Dillon J.P., Condon E.T., Street J.T., Wang J.H., McGuinness A.J., et al. Mobilization of endothelial precursor cells: systemic vascular response to musculoskeletal trauma. J Orthop Res 25,44, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Ma X.L., Sun X.L., Wan C.Y., Ma J.X., and Tian P.Significance of circulating endothelial progenitor cells in patients with fracture healing process. J Orthop Res 30,1860, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Fadini G.P., Rattazzi M., Matsumoto T., Asahara T., and Khosla S.Emerging role of circulating calcifying cells in the bone-vascular axis. Circulation 125,2772, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ford J.L., Robinson D.E., and Scammell B.E.Endochondral ossification in fracture callus during long bone repair: the localisation of “cavity-lining cells” within the cartilage. J Orthop Res 22,368, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Li R., Atesok K., Nauth A., Wright D., Qamirani E., Whyne C.M., et al. Endothelial progenitor cells for fracture healing: a microcomputed tomography and biomechanical analysis. J Orthop Trauma 25,467, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Atesok K., Li R., Stewart D.J., and Schemitsch E.H.Endothelial progenitor cells promote fracture healing in a segmental bone defect model. J Orthop Res 28,1007, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Rozen N., Bick T., Bajayo A., Shamian B., Schrift-Tzadok M., Gabet Y., et al. Transplanted blood-derived endothelial progenitor cells (EPC) enhance bridging of sheep tibia critical size defects. Bone 45,918, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Quarto R., Mastrogiacomo M., Cancedda R., Kutepov S.M., Mukhachev V., Lavroukov A., et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 344,385, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Horwitz E.M., Prockop D.J., Fitzpatrick L.A., Koo W.W., Gordon P.L., Neel M., et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 5,309, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Petite H., Viateau V., Bensaid W., Meunier A., de Pollak C., Bourguignon M., et al. Tissue-engineered bone regeneration. Nat Biotechnol 18,959, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Kim S.J., Shin Y.W., Yang K.H., Kim S.B., Yoo M.J., Han S.K., et al. A multi-center, randomized, clinical study to compare the effect and safety of autologous cultured osteoblast(Ossron) injection to treat fractures. BMC Musculoskelet Disord 10,20 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuroda R., Matsumoto T., Miwa M., Kawamoto A., Mifune Y., Fukui T., et al. Local transplantation of G-CSF-mobilized CD34+ cells in a patient with tibial nonunion: a case report. Cell Transplant 20,1491, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Kuroda R., Matsumoto T., Niikura T., Kawakami Y., Fukui T., Lee S., et al. Local transplantation of G-CSF-mobilized CD34+ cells for patients with femoral and tibial nonunion: phase 1/2 clinical trial. Stem Cells Transl Med 328,41, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishida K., Matsumoto T., Sasaki K., Mifune Y., Tei K., Kubo S., et al. Bone regeneration properties of granulocyte colony-stimulating factor via neovascularization and osteogenesis. Tissue Eng Part A 16,3271, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Ahmed Z., and Pillay T.S.Adapter protein with a pleckstrin homology (PH) and an Src homology 2 (SH2) domain (APS) and SH2-B enhance insulin-receptor autophosphorylation, extracellular-signal-regulated kinase and phosphoinositide 3-kinase-dependent signalling. Biochem J 371,405, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takaki S., Watts J.D., Forbush K.A., Nguyen N.T., Hayashi J., Alberola-Ila J., et al. Characterization of Lnk. An adaptor protein expressed in lymphocytes. J Biol Chem 272,14562, 1997 [DOI] [PubMed] [Google Scholar]
- 63.Takaki S., Morita H., Tezuka Y., and Takatsu K.Enhanced hematopoiesis by hematopoietic progenitor cells lacking intracellular adaptor protein, Lnk. J Exp Med 195,151, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takaki S., Sauer K., Iritani B.M., Chien S., Ebihara Y., Tsuji K., et al. Control of B cell production by the adaptor protein lnk. Definition of a conserved family of signal-modulating proteins. Immunity 13,599, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ema H., Sudo K., Seita J., Matsubara A., Morita Y., Osawa M., et al. Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice. Dev Cell 8,907, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Takizawa H., Kubo-Akashi C., Nobuhisa I., Kwon S.M., Iseki M., Taga T., et al. Enhanced engraftment of hematopoietic stem/progenitor cells by the transient inhibition of an adaptor protein, Lnk. Blood 107,2968, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Zvaifler N.J., Marinova-Mutafchieva L., Adams G., Edwards C.J., Moss J., Burger J.A., et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res 2,477, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khosla S., and Eghbali-Fatourechi G.Z.Circulating cells with osteogenic potential. Ann N Y Acad Sci 1068,489, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Eghbali-Fatourechi G.Z., Lamsam J., Fraser D., Nagel D., Riggs B.L., and Khosla S.Circulating osteoblast-lineage cells in humans. N Engl J Med 352,1959, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Otsuru S., Tamai K., Yamazaki T., Yoshikawa H., and Kaneda Y.Bone marrow-derived osteoblast progenitor cells in circulating blood contribute to ectopic bone formation in mice. Biochem Biophys Res Commun 354,453, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Otsuru S., Tamai K., Yamazaki T., Yoshikawa H., and Kaneda Y.Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells 26,223, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Ratanavaraporn J., Furuya H., Kohara H., and Tabata Y.Synergistic effects of the dual release of stromal cell-derived factor-1 and bone morphogenetic protein-2 from hydrogels on bone regeneration. Biomaterials 32,2797, 2011 [DOI] [PubMed] [Google Scholar]