Abstract
The current analysis reexamines the relationship between supplemental vitamin E and all-cause mortality. All randomized, controlled trials testing the treatment effect of vitamin E supplementation in adults for at least one year were sought. MEDLINE, the Cochrane Library, and Biological Abstracts databases were searched using the terms “vitamin E,” “alpha-tocopherol,” “antioxidants,” “clinical trial,” and “controlled trial” for studies published through April 2010; results were limited to English, German, or Spanish language articles. Studies were also obtained through reference mining. All randomized controlled trials using vitamin E, with a supplementation period of at least one year, to prevent or treat disease in adults were identified and abstracted independently by two raters. Mortality data from trials with a supplementation period of at least one year were pooled. The selected trials (n = 57) were published between 1988 and 2009. Sample sizes ranged from 28 to 39,876 (median = 423), yielding 246,371 subjects and 29,295 all-cause deaths. Duration of supplementation for the 57 trials ranged from one to 10.1 years (median = 2.6 years). A random effects meta-analysis produced an overall risk ratio of 1.00 (95% confidence interval: 0.98, 1.02); additional analyses suggest no relationship between dose and risk of mortality. Based on the present meta-analysis, supplementation with vitamin E appears to have no effect on all-cause mortality at doses up to 5,500 IU/d.
Keywords: all-cause mortality, α-tocopherol, clinical trials, meta-analysis, oral supplements, Vitamin E
Introduction
Many chronic diseases associated with aging, such as cancer, cardiovascular disease, cataracts and dementia, have been linked to cellular damage from oxidative stress [1]. In theory, antioxidants should be able to treat or prevent conditions associated with oxidative stress. Though some epidemiological studies [2–4] and clinical trials [5–8] of vitamin E have shown positive results, these are in the minority. In 2008, the Data Safety Monitoring Board of The Selenium and Vitamin E Cancer Prevention Trial (SELECT), a large prostate cancer prevention trial, determined during a planned interim analysis that there was no difference in the primary endpoint (prostate cancer) of the trial and recommended that supplementation be stopped due to futility [9]. Though vitamin E supplementation for prevention or treatment of disease has not been supported by the accumulated evidence, it is generally considered safe [1].
When evidence from individual trials is inconclusive or contradictory, a meta-analysis can enhance understanding of an issue by combining evidence from multiple trials. Yet, meta-analyses may also be contradictory or inconclusive since any given meta-analysis on a topic need not contain the same individual studies or employ the same statistical methodology as any other on that same subject. A 2004 meta-analysis [10], for example, found that the risk of all-cause mortality increased slightly but significantly for those subjects taking at least 400 IU/d vitamin E (risk difference (RD): 39/10,000 persons; 95% confidence interval (CI): 3 to 74 per 10,000 persons). The included trials were generally small and involved subjects with chronic diseases, and studies reporting fewer than 10 deaths were excluded. A 2009 meta-analysis reanalyzed these same studies using a Bayesian rather than a frequentist approach and found no increased risk of mortality associated with vitamin E at any dose [11]. A 2007 meta-analysis that included additional trials, however, reported that when trials using selenium in combination with vitamin E and trials deemed to have a high risk of bias (due to inadequate descriptions of blinding, follow-up, allocation generation of the allocation sequence, or allocation concealment) were excluded from the analysis, vitamin E supplementation was associated with a 4% increased risk of all-cause mortality (95% CI: 1.01, 1.07) [12].
Estimates based on the 1999–2000 National Health and Nutrition Examination Survey suggest that at least 20% of Americans age 60 and older take 400 IU or more vitamin E on a daily basis [13]. The purpose of the current analysis is to re-examine the safety of supplemental vitamin E, particularly for older people, in light of additional evidence provided by several large trials.
Methods
Data Sources and Searches
All randomized, controlled trials testing the treatment effect of vitamin E supplementation were sought for this analysis. MEDLINE, the Cochrane Library, and Biological Abstracts/RRM databases were searched using the terms “vitamin E,” “alpha-tocopherol,” “antioxidant,” “clinical trial,” and “controlled trial” for articles published through April 2010. Results were restricted to English, German, or Spanish language articles. Studies were also obtained by reference mining. Unpublished original research was not sought.
Study Selection
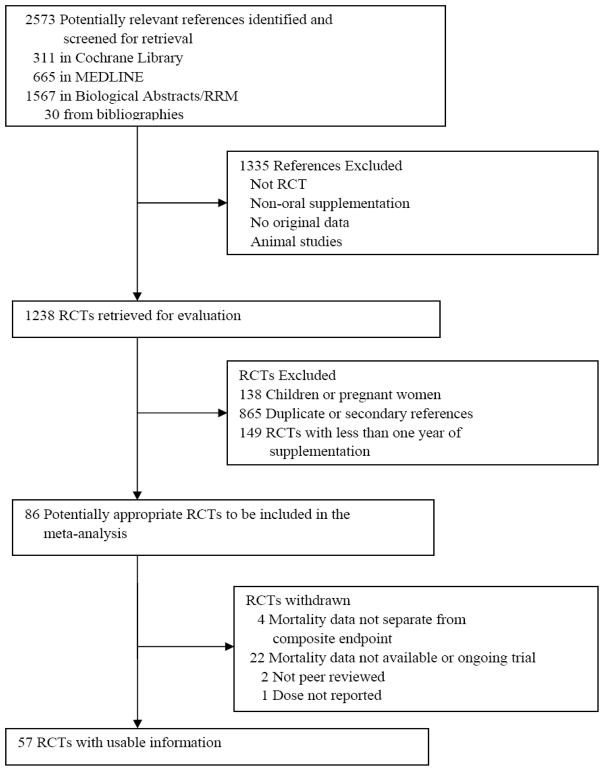
Inclusion criteria were (1) peer review (indicated by publication in a peer-reviewed journal), (2) randomized treatment conditions, (3) comparator arms, (4) adult participants (excluding pregnant women), (5) parallel or factorial designs, and (6) the assignment of participants to supplemental vitamin E, taken orally, alone or in combination with other drugs and supplements for at least one year (Figure 1). Studies were assessed for inclusion by two of the authors (ELA and RJK); one reviewer (ELA) screened all articles identified by the search, and one reviewer (RJK) assisted in making inclusion determinations. Zero event trials were included since their exclusion could produce biased pooled effect estimates and widened confidence intervals [14–16]. Twenty-five studies met the inclusion criteria but did not have available or sufficiently detailed mortality data [17–37], two were not peer reviewed [38, 39], and one did not report dose [40]. These 25 studies were therefore excluded. Fifty-seven studies were identified that satisfied all the inclusion criteria and are described in Tables 1 and 2 [5–9, 41–93].
Fig. 1.
Flow diagram for identification of selected trials
Table 1a.
Stratified analyses.
| Studies | Participants | RR (95% CI) | Studies | Participants | RR (95% CI) | |
|---|---|---|---|---|---|---|
| Quality Measure | Adequate | Inadequate | ||||
| Description of randomization | 38 | 202,150 | 1.00 (0.98,1.03) | 18 | 44,193 | 0.96 (0.90,1.02) |
| Description of blinding method | 34 | 170,610 | 1.01 (0.99,1.03) | 22 | 75,733 | 0.98 (0.94,1.02) |
| Baseline Mortality Risk | Above | Below | ||||
| Control arm death rate vs. median | 29 | 139,598 | 1.00 (0.97,1.03) | 28 | 106,773 | 0.99 (0.93,1.06) |
| Dose (IU/d) | Less than | Greater than or equal to | ||||
| 100 | 10 | 77,931 | 0.97 (0.93,1.02) | 47 | 168,440 | 1.01 (0.98,1.04) |
| 400 | 20 | 147,215 | 0.99 (0.97,1.02) | 37 | 99,156 | 1.02 (0.97,1.06) |
| 500 | 32 | 215,601 | 1.00 (0.98,1.02) | 25 | 30,770 | 1.03 (0.95, .11) |
| 800 | 45 | 242,971 | 1.00 (0.98,1.02) | 12 | 3,400 | 1.04 (0.91,1.20) |
| Age | Included | Excluded | ||||
| Subjects under age 55 | 35 | 221,662 | 1.00 (0.98,1.02) | 16 | 23,583 | 1.01 (0.92,1.10) |
| Subjects under age 60 | 50 | 243,243 | 1.00 (0.98,1.02) | 7 | 3,128 | 0.94 (0.78,1.13) |
| Vitamin E Type | Natural Source | Synthetic | ||||
| 16 | 64,648 | 1.02 (0.96,1.08) | 16 | 174,859 | 1.01 (0.92,1.10) | |
Data Abstraction and Quality Assessment
Two of the authors (ELA and JLM) independently abstracted summary data from each of the studies; disagreements were resolved by additional review of the publications. For each study, the design, number of subjects randomized to each treatment, age, gender, health status of the subjects, length of follow up, duration of supplementation, the dose and form of vitamin E used in the trial, other medications, if any, used in the trial, and the number of deaths in each treatment group were recorded. Compliance assessment methods and aspects of study quality including randomization method, blinding method, and description of follow-up were also recorded.
Data Synthesis and Analysis
Total deaths in the vitamin E (14,636 deaths out of 123,149 participants) and no vitamin E (14,659 deaths out of 123,222 participants) groups were compared using risk ratios and 95% confidence intervals, where the exposure was defined as vitamin E supplementation and the outcome was all-cause mortality. A continuity correction factor, delta = 0.5, was added to each cell in the analysis to allow for the calculation of risk estimates in studies where no deaths occurred. Sensitivity analyses were conducted using 0.5, 0.25, 0.1, and the inverse of the sample size in the opposite treatment arm as delta values [94]. The 57 studies were combined using the intent-to-treat principle, with the exception of one trial that did not include the intent-to-treat randomization scheme in the published data [51]. For studies using a factorial design, risk ratios were calculated based on a vitamin E main effect, where all subjects receiving vitamin E were compared to all subjects not receiving vitamin E. Pooled risk ratios (RRs) were obtained using a random effects model with studies weighted by the inverse of the sample variance of the log RR [95]. Stratified analyses were conducted to investigate the effect of dose on mortality. An initial cut-point of 400 IU/d was chosen based on the findings of Miller et al. (2004) [10], and a sensitivity analysis was conducted with cut-points of 100 IU/d, 500 IU/d, and 800 IU/d.
Stratified analyses were also conducted to investigate the effects of the subjects’ baseline mortality risk and study quality. Coding of mortality risk depended on the death rate in the control arm. If the control death rate was above the median for all studies (M = 4.8%), then subjects were judged to have a greater than average baseline mortality risk. If the control death rate was below the median, subjects were judged to have a lower than average baseline mortality risk. Coding of study quality was done by two raters (ELA and JLM). Three factors were considered: (1) adequate description of the randomization method (e.g., computer generated random numbers), (2) adequate description of the blinding method (e.g., placebos matching in appearance, taste, and smell), and (3) adequate description of reasons for drop-outs and withdrawals. A determination of adequacy meant that the methods described were sufficiently detailed and appropriate. Additional stratified analyses were performed to assess the effect of age using cut points of age 55 and age 60, as well as treatment type (vitamin E studied alone versus other combined with other treatments), and type of vitamin E (synthetic versus natural source).
Statistical analyses were performed using NCSS (Number Cruncher Statistical System) and SAS/STAT® 9.1.3 software [95,96].
Results
The pooled trials were published between 1988 and 2009. Sample sizes ranged from 28 to 39,876 (M = 423), yielding 246,371 subjects and 29,295 all-cause deaths (Appendices A and B). Duration of supplementation for the 58 trials ranges from one to 10.1 years (M = 2.6 years).
Appendix A. Selected trial characteristics.
| Study | N | Design | Treatment Type† |
Compliance assessment |
Population characteristics | Risk§ |
|---|---|---|---|---|---|---|
| Avenell et al., 2005[41] | 910 | Parallel | 1 | Not specified | Community dwelling elderly | L |
| Girodon et al., 1997[42] | 81 | Factorial | 1 | Directly observed therapy, pill counts, biomarkers | Institutionalized elderly | H |
| Girodon et al., 1999[43] | 725 | Factorial | 1 | Directly observed therapy, pill counts, biomarkers | Institutionalized elderly | H |
| CTNSARC, 2009[44]* | 1020 | Parallel | 1 | Pill counts | Community dwelling elderly | H |
| Blot et al., 1993[45] | 29584 | Factorial | 1 | Pill counts | High risk for gastric cancer | H |
| Hercberg et al., 2004[46] | 13017 | Parallel | 1 | CRFs | Healthy | L |
| Chandra et al., 1992[47] | 96 | Parallel | 1 | Interview, pill counts | Independent living elderly | L |
| Pike and Chandra, 1995[48] | 47 | Parallel | 3 | Interview, pill counts | Healthy | L |
| Wright et al., 2006[49] | 29133 | Factorial | 1 | Pill counts | Smoking | L |
| Li et al., 1993[50] | 3318 | Parallel | 3 | Pill counts, biomarkers | High risk for gastric cancer | L |
| Takamatsu et al., 1995[51] | 161 | Parallel | 2 | Pill counts, biomarkers | Healthy | L |
| Meydani et al., 2004[52] | 617 | Parallel | 1 | Pill counts, biomarkers | Institutionalized elderly | H |
| ARMDS, 1996[53]* | 71 | Parallel | 1 | Run-in period | Age-related macular degeneration | H |
| You et al., 2001[54] | 3411 | Factorial | 3 | Pill counts | High risk for gastric cancer | L |
| Graat et al., 2002[55] | 652 | Factorial | 1 | Pill counts, biomarkers | Independent living elderly | L |
| Salonen et al., 2003[56] | 520 | Factorial | 1 | Pill counts | Hypercholesterolemia | L |
| Cook et al., 2007[57] | 8171 | Factorial | 1 | Self-report | Healthy | H |
| Lee et al., 2005[8] | 39876 | Factorial | 3 | CRFs | Healthy | L |
| GISSI, 1999[58]* | 11324 | Factorial | 4 | Pill refills | Heart attack | H |
| PPP, 2001[59]* | 4495 | Factorial | 4 | Pill refills | Elevated cardiovascular risk | L |
| Bukin et al., 1997[60] | 36 | Parallel | 2 | Not addressed | Chronic atrophic gastritis with small intestinal metaplasis | L |
| Mooney et al., 2005[61] | 284 | Parallel | 1 | Pill counts, biomarkers | Smoking | L |
| Milman et al., 2008[62] | 1434 | Parallel | 1 | Self-report | Type 2 diabetes | L |
| de Waart et al., 2001[63] | 218 | Parallel | 2 | Not addressed | Smoking | L |
| McKeown-Eyssen et al., 1988[64] | 200 | Parallel | 3 | Biomarkers | Colon polyps | L |
| Bairati et al., 2006[65] | 540 | Parallel | 1 | Not specified | Head and neck cancer | H |
| Hodis et al., 2002[66] | 353 | Parallel | 2 | Pill counts, biomarkers | No heart disease | L |
| Lonn et al., 2005[67] | 9541 | Factorial | 4 | Not specified | Vascular disease or diabetes | H |
| Lippman et al., 2008[9] | 35533 | Factorial | 1 | Pill counts | Healthy | L |
| AREDS, 2001[68]* | 4757 | Parallel | 1 | Pill refills, pill counts | Age-related macular degeneration | H |
| Sesso et al., 2008[69] | 14,641 | Factorial | 1 | Self-report | Healthy | H |
| Greenberg et al., 1994[70] | 864 | Factorial | 1 | Interview, biomarkers | Adenoma(s) removed | H |
| Antoniadi et al., 2008[71] | 58 | Parallel | 2 | Pill counts | Hemodialysis patients | H |
| de la Maza et al., 1995[72] | 74 | Parallel | 2 | Pill counts | Alcoholic cirrhosis | H |
| Richer et al., 2004[73] | 90 | Parallel | 1 | Interview | Age-related macular degeneration | H |
| Wluka et al., 2002[74] | 136 | Parallel | 2 | Pill counts | Osteoarthritis | L |
| Magliano et al., 2006[75] | 409 | Parallel | 2 | Pill counts | Smoking | H |
| McNeil et al., 2004[76] | 1204 | Parallel | 4 | Pill counts, biomarkers | Healthy | L |
| Pathak et al., 2005[78] | 136 | Parallel | 3 | Interview, pill counts | Lung cancer | H |
| Stephens et al., 1996[79] | 2002 | Parallel | 2 | Pill refills, biomarkers | Atherosclerosis | H |
| Plummer et al., 2007[80] | 1980 | Parallel | 1 | Pill counts | High risk for gastric cancer | L |
| Takagi et al., 2003[81] | 93 | Parallel | 2 | Not addressed | Liver cirrhosis | H |
| CLIPS, 2006[82]* | 366 | Factorial | 3 | Pill counts, interview | Peripheral artery disease | L |
| Chylack et al., 2002[7] | 297 | Parallel | 1 | Pill refills, biomarkers | Age-related cataracts | L |
| MRC/BHF HPS, 2002[83]* | 20536 | Factorial | 3 | Pill counts | Coronary disease, occlusive arterial disease, or diabetes | H |
| Bugianesi et al., 2005[84] | 110 | Parallel | 2 | Pill counts | Nonalcoholic fatty liver disease | L |
| Fang et al., 2002[85] | 40 | Parallel | 1 | Interview, biomarkers | Heart transplant | L |
| Boaz et al., 2000[5] | 196 | Parallel* | 3 | Not addressed | Chronic hemodialysis | H |
| Brown et al., 2001[86] | 160 | Factorial | 3 | Pill counts | Coronary disease | L |
| Waters et al., 2002[87] | 423 | Factorial | 4 | Pill counts | Coronary stenosis | L |
| Desnuelle et al., 2001[88] | 288 | Parallel | 4 | Not addressed | Amyotrophic lateral sclerosis | H |
| Stevic et al., 2001[89] | 28 | Parallel | 1 | Not available | Amyotrophic lateral sclerosis | H |
| Singh et al., 2007[90] | 127 | Parallel | 1 | Biomarkers | Coronary artery disease | L |
| Sano et al., 1997[91] | 341 | Factorial | 4 | Biomarkers | Alzheimer’s disease | H |
| Marras et al., 2005[92] | 800 | Factorial | 4 | Not specified | Parkinson’s disease | H |
| Petersen et al., 2005[93] | 790 | Factorial | 4 | Not specified | Mild cognitive impairment | L |
| Graf et al., 2005[94] | 160 | Parallel | 4 | Not addressed | Amyotrophic lateral sclerosis | H |
AREDS = Age-Related Eye Disease Study. ARMDS = Age-Related Macular Degeneration Study. CLIPS = Critical Leg Ischaemia Prevention Study. CTNSARC = Clinical Trial of Nutritional Supplements and Age-Related Cataract. GISSI = Gruppos Italiano per lo Studio della Sopravvivenza nell’Infarto miocardio. MRC/BHF HPS = Medical Research Council/British Heart Foundation Heart Protection Study. PPP = Primary Prevention Project.
Treatment type: 1 = Vitamin E plus other antioxidant(s); 2 = Vitamin E alone; 3 = Vitamin E plus other antioxidant(s) and medication(s); 4 = Vitamin E plus other medication(s).
Risk: H = Elevated mortality risk (control arm death rate higher than median); L = Low mortality risk (control arm death rate lower than median).
Appendix B. Additional trial characteristics.
| Study | Supplement Duration (Years) |
Baseline Age |
Sex (%M) |
Form** | Appropriate Randomization Scheme |
Appropriate Blinding Method |
Sufficient Follow-up |
|---|---|---|---|---|---|---|---|
| Avenell et al., 2005[41] | 1 | 72 | 96 | 0 | Y | Y | Y |
| Girodon et al., 1997[42] | 2 | 84 | 25 | 9 | Y | N | Y |
| Girodon et al., 1999[43] | 2 | 83 | 25 | 9 | Y | Y | Y |
| CTNSARC, 2009[44] | 9 | 68 | 55 | 9 | Y | Y | Y |
| Blot et al., 1993[45] | 5.25 | 55 | 50 | 0 | N | N | Y |
| Hercberg et al., 2004[46] | 7.54 | 49 | 39 | 0 | Y | Y | Y |
| Chandra et al., 1992[47] | 1 | 74 | 73 | 9 | Y | Y | Y |
| Pike and Chandra, 1995[48] | 1 | 69 | 28 | 0 | Y | Y | Y |
| Wright et al., 2006[49] | 6.1 | 58 | 100 | 0 | Y | Y | Y |
| Li et al., 1993[50] | 6 | 54 | 44 | 0 | Y | N | Y |
| Takamatsu et al., 1995[51] | 6 | 47 | 37 | 1 | N | Y | Y |
| Meydani et al., 2004[52] | 1 | 85 | 34 | 0 | Y | Y | Y |
| ARMDS, 1996[53]* | 1.5 | 72 | 93 | 9 | N | Y | Y |
| You et al., 2001[54] | 3.25 | 47 | 51 | 9 | N | Y | Y |
| Graat et al., 2002[55] | 1 | 73 | 50 | 1 | Y | Y | Y |
| Salonen et al., 2003[56] | 6 | 60 | 49 | 1 | N | Y | Y |
| Cook et al., 2007[57] | 9.4 | 61 | 0 | 1 | N | Y | Y |
| Lee et al., 2005[8] | 10.1 | 55 | 0 | 1 | Y | Y | Y |
| GISSI, 1999[58]* | 3.5 | 59 | 85 | 0 | Y | N | Y |
| PPP, 2001[59]* | 3.6 | 64 | 58 | 0 | Y | N | Y |
| Bukin et al., 1997[60] | 1 | 55 | 39 | 1 | N | N | Y |
| Mooney et al., 2005[61] | 1.25 | 37 | 55 | 0 | N | N | Y |
| Milman et al., 2008[62] | 1.5 | 69 | 48 | 1 | Y | Y | Y |
| de Waart et al., 2001[63] | 1.8 | 60 | 100 | 0 | N | N | Y |
| McKeown-Eyssen et al., 1988[64] | 2 | 58 | 66 | 0 | N | Y | Y |
| Bairati et al., 2006[65] | 3 | 63 | 79 | 0 | Y | N | Y |
| Hodis et al., 2002[66] | 3 | 56 | 45 | 0 | Y | N | Y |
| Lonn et al., 2005[67] | 4.5 | 66 | 74 | 1 | Y | Y | Y |
| Lippman et al., 2008[9] | 5.46 | 62 | 100 | 0 | Y | Y | Y |
| AREDS, 2001[68]* | 6.3 | 68 | 45 | 0 | Y | Y | Y |
| Sesso et al., 2008[69] | 8 | 64 | 100 | 0 | Y | Y | Y |
| Greenberg et al., 1994[70] | 4 | 61 | 79 | 0 | Y | Y | Y |
| Antoniadi et al., 2008[71] | 1 | 60 | 40 | 9 | Y | N | N |
| de la Maza et al., 1995[72] | 1 | 49 | 85 | 9 | N | Y | Y |
| Richer et al., 2004[73] | 1 | 75 | 97 | 1 | Y | Y | Y |
| Wluka et al., 2002[74] | 2 | 64 | 42 | 1 | N | N | Y |
| Magliano et al., 2006[75] | 4 | 64 | 45 | 1 | Y | Y | Y |
| McNeil et al., 2004[76] | 4 | 66 | 44 | 1 | Y | Y | Y |
| Pathak et al., 2005[78] | 2 | 58 | 83 | 0 | Y | N | Y |
| Stephens et al., 1996[79] | 1.4 | 62 | 84 | 1 | Y | Y | Y |
| Plummer et al., 2007[80] | 3 | NA | 33 | 0 | Y | Y | Y |
| Takagi et al., 2003[81] | 5 | 63 | 40 | 9 | N | N | Y |
| CLIPS, 2006[82]* | 2 | 66 | 77 | 0 | Y | Y | Y |
| Chylack et al., 2002[7] | 3 | 68 | 41 | 0 | Y | Y | Y |
| MRC/BHF HPS, 2002[83]* | 5 | 40–80¶ | 70 | 0 | Y | Y | Y |
| Bugianesi et al., 2005[84] | 1 | 42 | 83 | 9 | Y | N | Y |
| Fang et al., 2002[85] | 1 | 51 | 88 | 9 | Y | Y | Y |
| Boaz et al., 2000[5] | 1.42 | 64 | 69 | 1 | Y | Y | N |
| Brown et al., 2001[86] | 3 | 53 | 87 | 1 | N | Y | Y |
| Waters et al., 2002[87] | 3 | 65 | 0 | 0 | Y | Y | Y |
| Desnuelle et al., 2001[77] | 1 | 64 | 55 | 9 | Y | Y | Y |
| Stevic et al., 2001[88] | 1 | 57 | 75 | 9 | -- | -- | -- |
| Singh et al., 2007[89] | 2 | 59 | 74 | 1 | N | N | Y |
| Sano et al., 1997[90] | 2 | 73 | 35 | 0 | Y | N | Y |
| Marras et al., 2005[91] | 2.6 | 61 | 66 | 0 | N | N | Y |
| Petersen et al., 2005[92] | 3 | 73 | 54 | 9 | Y | N | Y |
| Graf et al., 2005[93] | 1.5 | 58 | 65 | 0 | N | N | Y |
Dose is average of all doses assigned to parallel arms.
Range; mean not reported. [16].
Form: 1 = Natural; 0 = Synthetic; 9 = Unknown
It is worth noting that 86% of the subjects and 91% of the deaths are contributed by 10 very large trials (N > 5,000): GISSI-Prevenzione [58], SELECT [9], The Women’s Antioxidant Cardiovascular Study (WACS) [57], The Women’s Health Study (WHS) [8], The Heart Outcomes Prevention Evaluation Study (HOPE) [67], The Alpha-tocopherol and Beta-carotene Cancer Prevention Study (ATBC) [49], The Linxian Nutrition Intervention Trial [45], The Supplementation with Vitamins, Minerals, and Antioxidants Study (SU.VI.MAX) [46], The Medical Research Council/British Heart Foundation Heart Protection Study (MRC/BHF HPS) [83], and The Physician’s Health Study II (PHS II) [69]. Duration of supplementation in these trials ranges from 3.5 to over 10 years. Three of the trials [45,46,49], however, use very low doses of vitamin E and are less likely to contribute much information about potential harms of vitamin E if risk depends on dose. WHS and WACS participants received supplements every other day instead of daily. These two trials, in addition to HOPE, use natural source vitamin E, while the remaining trials use synthetic.
Vitamin E was given in combination with other supplements (including other antioxidants) or medications in all but 11 of the trials. Of the remaining 46 trials, 10 trials used vitamin E in combination with medications only; 10 used vitamin E in combination with other antioxidants, medications or non-antioxidant supplements; and 26 used vitamin E only in combination with other antioxidants (see Appendix A). The dose of vitamin E was converted to international units per day (IU/d) relative to all rac-α-tocopheryl acetate [1]. Where different doses of vitamin E were assigned to parallel groups, the study dose was taken to be the daily average of all administered doses (Table 2). WHS and WACS assigned participants to 600 IU of vitamin E every other day [8,57]; these subjects were recorded as having been exposed to 300 IU of vitamin E per day, which may bias the results if there is a true dose-response effect. Thirty-five (61%) studies are parallel group designs. All but 10 studies are double-blind and placebo controlled: of these, one is double-blind but does not use a placebo control; one is single-blind and placebo-controlled; four are placebo controlled, but blinding is not fully described; and five are open label studies. Compliance assessment was not specified or not addressed in 12 studies (see Appendix A).
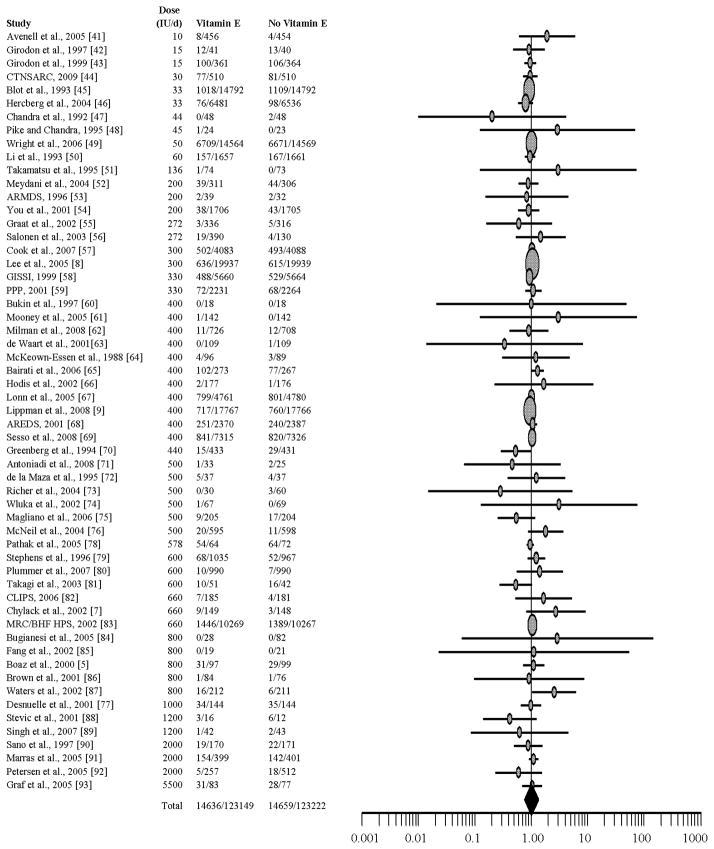
The effect of supplemental vitamin E on mortality varied from a low RR of 0.20 to a high RR of 3.09 (M = 1.00). Results from 4 of 57 studies show significant treatment effects (two protective and two harmful) associated with vitamin E supplementation and all-cause mortality [45,65,70,87].
An inverse-variance weighted random effects meta-analysis of the 57 studies yields a pooled RR of 1.00 (95% CI: 0.98, 1.02), which indicates no treatment effect associated with supplemental vitamin E (Figure 2). Cochran’s Q test for heterogeneity of these 57 studies is not significant (df = 56; p = 0.52), and Higgins’ I2 = 0, which indicates that only a very low percentage of the variation across the studies is due to heterogeneity rather than chance [97]. Inspection of a radial plot suggested that four studies may be outliers [45,65,70,87]. Excluding these four trials does not change the conclusion of no treatment effect: RR = 1.00 (95% CI: 0.98, 1.02).
Fig. 2.
Forest plot of risk ratio. Horizontal lines represent 95% confidence intervals. Symbols proportional to sample size.
Stratified analyses were conducted to evaluate the influence of study quality, baseline mortality risk, dose, age, form of vitamin E given (i.e., natural or synthetic), and treatment type (Tables 1a and 1b). There is no significant treatment effect associated with vitamin E in any of the strata. No significant treatment effects are associated with supplemental vitamin E, though it is perhaps worth noting that the risk ratio estimate increases as the dose cut point increases. To further explore the impact of study quality, a separate analysis was conducted on the subset of 47 double-blind, placebo-controlled studies (total participants = 197,078). Again, there was no significant treatment effect associated with vitamin E supplementation: RR = 1.01 (95% CI: 0.99, 1.03).
Table 1b.
Stratified analysis of study treatment.
| Study Treatment | Studies (n) | Participants (n) | RR (95% CI) |
|---|---|---|---|
| Vitamin E alone | 11 | 3,636 | 0.95 (0.73, 1.25) |
| Vitamin E plus antioxidants | 26 | 145,170 | 0.99 (0.95, 1.03) |
| Vitamin E plus medications | 10 | 68,231 | 1.02 (0.97, 1.07) |
| Vitamin E plus antioxidants and medications | 10 | 29,334 | 1.01 (0.92, 1.09) |
Discussion
This random-effects meta-analysis of vitamin E and all-cause mortality based on 57 studies, which is the largest and most inclusive to date, shows that vitamin E supplementation is associated with neither a reduction nor an increase in the risk of all-cause mortality. This finding differs from two recently published meta-analyses of vitamin E and all-cause mortality [10,12], both of which found small but significantly increased risks of mortality for those taking supplemental vitamin E. One possible explanation for the different findings is the number of included trials. Miller and colleagues (2004) [10] reported on only 19 trials and appeared prior to the release of mortality data from several large, well-conducted trials including WHS [8], WACS [57], PHS II [69], and SELECT [9]. Bjelakovic and colleagues (2007) [12] reported on 51 trials but only found a significantly increased risk of mortality when a subset of 26 studies was considered. Both analyses were completed prior to the publication of WACS [57], PHS II [69], and SELECT [9] mortality data, which contribute 98,221 subjects and 5,495 deaths.
If vitamin E does have an adverse effect on mortality, no biological mechanism has yet been identified although vitamin E supplementation has been reported to increase blood pressure [98]. Unfortunately, cause-specific analysis of mortality is not possible because cause of death is not typically reported in the trials that were reviewed. Meta-analyses of vitamin E and liver diseases [99], gastrointestinal cancers [100], and cardiovascular disease [101] have found no relationship between vitamin E supplementation and overall mortality or cause-specific deaths.
Miller and colleagues (2004) [10] posited a quadratic-linear spline model that assumes a quadratic trend for doses below 400 IU and a linear trend for doses above 400 IU; they found a small but significantly increased risk of mortality for doses above 400 IU. Using a Bayesian analysis of the same data, Berry et al. (2009) [11] found no association between dose and risk of mortality. Of note, Bjelakovic et al. [12] found no relationship between vitamin E dose and risk of mortality in any of their analyses. No increased risk of mortality was observed in the current study. This suggests that inclusion of more trials may attenuate the result observed in the Miller et al. paper. (2004) [10].
In 2005, following a series of neutral effect vitamin E trials including HOPE [67], GISSI [58], ATBC [49], and MRC/BHF HPS [83], Brown and Crowley posed the question, “Is there any hope for vitamin E?” [102]. The recent negative findings, both in terms of treatment effects and mortality, of the large vitamin E trials WHS [8], WACS [57], PHS II [69], and SELECT [9] appear to answer that question. These four trials have effectively established that vitamin E holds no promise as a cancer or cardiovascular disease prophylactic or therapy, so it is unlikely that future large trials will be conducted. Thus, it is also unlikely that the publication of future trials will change the results of this meta-analysis.
Limitations
Meta-analysis can provide increased statistical power to detect small effect sizes. It is also fraught with potential sources of bias. Selection bias is typically foremost among these. Even if one could be confident that all published articles have been retrieved, there are many more unpublished articles that often cannot be accessed. It is possible that the inclusion of these inaccessible data in the meta-analysis would change the results. Moreover, bias present in the individual studies due to study design may influence the results and conclusions of the meta-analysis.
Additionally, combining data from different populations may not be appropriate. In the current analysis, the gender and average age of participants are fairly consistent across studies (Appendix A). Both chronological and biological age have been shown to independently predict survival [103], and it is possible that if there was an interaction between age and supplementation, aggregate measures of chronological age only would not be sufficient to produce the result. Also, the characteristics and health conditions of patients vary (Appendix A), and the studies differ by time on treatment, length of follow-up period, dose, and other important factors. While Cochran’s Q and Higgins’ I2 do not indicate significant heterogeneity, some unobserved heterogeneity may be present. However, the data strongly indicate that given the differences among trials in study populations, treatments given, doses used, and length of supplement duration, there is very little heterogeneity in the observed mortality between those who receive supplemental vitamin E and those who do not.
The inability to assess the adherence of study patients is another significant limitation. Results from an intent-to-treat analysis are conservative effect estimates because it assumes that all patients adhered to their assigned conditions, and that all patients completed the trial, which is very rarely true. Published compliance data from the CHAOS trial, for example, indicates that of the 38 deaths due to ischemic heart disease in the vitamin E group, only 6 were known to be compliant, 21 were known to be non-compliant, and 11 had unknown compliance [104]. Although many of the studies reported that adherence was assessed, often through biomarkers, the exact numbers of compliant versus non-compliant subjects is rarely given. Therefore, this and prior analyses of mortality due to vitamin E supplementation could yield different results if compliance data (drop-in and drop-out rates) were more detailed and entered as factors in the meta-analyses.
Perhaps more importantly, although several large studies have followed supplemented patients for up to 10 years, most follow patients for much shorter periods of time. The median for the 57 studies included here is 2.6 years. If it is the case that vitamin E supplementation effects mortality but only after very long periods of time, then many of the included studies would likely not demonstrate that effect due to insufficient length of follow-up.
A final limitation of meta-analysis is that the treatment of interest may not be studied in isolation. The studies in this meta-analysis comprised multiple interventions, including vitamin E studied alone, in combination with other antioxidants, in combination with other medications, and in combination with both additional antioxidants and medications. While the biological mechanisms in combined therapies could result in additional risk or risk reduction, stratified analysis based on these treatment combinations did not reveal any significant differences.
Conclusion
For the populations reviewed here, supplementation with vitamin E appears neither beneficial nor harmful in terms of mortality. Based on the information available, supplementation with vitamin E as alpha-tocopherol cannot be endorsed as a means of reducing mortality for the specified populations. In the absence of evidence to the contrary, vitamin E supplementation should not be recommended as a means of improving longevity.
Footnotes
An earlier version was presented as a poster at the 2006 American Public Health Association Annual Meeting in Boston, MA on November 7, 2006.
References
- 1.Institute of Medicine. A report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food Nutrition Board. Washington, DC: National Academies Pr; 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. [Google Scholar]
- 2.Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz G, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. NEJM. 1993;328:1450–6. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 3.Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC. Vitamin E consumption and the risk of coronary disease in women. NEJM. 1993;328:1444–9. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 4.Hayden KM, Welsh-Bohmer KA, Wengreen HJ, Zandi PP, Lyketsos CG, Breitner JCS. Risk of mortality with vitamin E supplements: The Cache County Study. Am J Med. 2007;120:180–4. doi: 10.1016/j.amjmed.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 5.Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 2000;356:1213–18. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 6.Boshtam M, Rafiei M, Sadeghi K, Sarraf-Zadegan N. Vitamin E can reduce blood pressure in mild hypertensives. Int J Vitam Nutr Res. 2002;72(5):309–14. doi: 10.1024/0300-9831.72.5.309. [DOI] [PubMed] [Google Scholar]
- 7.Chylack LT, Brown NP, Bron A, Hurst M, Kopcke W, Thien U, Schalch W. The Roche European American Cataract Trial (REACT): A randomized clinical trial to investigate the efficacy of an oral antioxidant micronutrient mixture to slow progression of age-related cataract. Opthal Epi. 2002;9:49–80. doi: 10.1076/opep.9.1.49.1717. [DOI] [PubMed] [Google Scholar]
- 8.Lee I, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer. The Women’s Health Study: A Randomized Controlled Trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 9.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2008;301(1) doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: High dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2004;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 11.Berry D, Wathen JK, Newell M. Bayesian model averaging in meta-analysis: vitamin E supplementation and mortality. Clinical Trials. 2009;6:28–41. doi: 10.1177/1740774508101279. [DOI] [PubMed] [Google Scholar]
- 12.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Sytematic review and meta-analysis. JAMA. 2007;297:842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 13.Ford ES, Ajani UA, Mokdad AH. Brief communication: The prevalence of high intake of vitamin E from the use of supplements among U.S. adults. Ann Intern Med. 2005;143:116–20. doi: 10.7326/0003-4819-143-2-200507190-00010. [DOI] [PubMed] [Google Scholar]
- 14.Bradburn MJ, Deeks JJ, Berlin JA, Localio AR. Much ado about nothing: A comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26:57–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- 15.Diamond GABL, Kaul S. Uncertain effects of rosiglitazone on the risk of myocardial infarction and cardiovascular death. Ann Intern Med. 2007;147:578–81. doi: 10.7326/0003-4819-147-8-200710160-00182. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich JO, Adhikari NKJ, Beyene J. Inclusion of zero event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodology. 2007;7:5. doi: 10.1186/1471-2288-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E. The St. Francis Heart Study Randomized Clinical Trial. JACC. 2005;46:166–72. doi: 10.1016/j.jacc.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 18.Berson EL, Rosner B, Sandberg MA, Hayes KC, Nicholson BW, Weigel-DiFranco C, Willett W. A randomized trial of vitamin A and vitamin E for retinitis pigmentosa. Arch Opthalmol. 1993;111:761–72. doi: 10.1001/archopht.1993.01090060049022. [DOI] [PubMed] [Google Scholar]
- 19.Chesney CM, Elam MB, Herd JA, Davis KB, Garg R, Hunninghake D, et al. Effects of niacin, warfarin, and antioxidant therapy on coagulation parameters in patients with peripheral arterial disease in the Arterial Disease Multiple Intervention Trial (ADMIT) Am Heart J. 2000;140:631–6. doi: 10.1067/mhj.2000.109648. [DOI] [PubMed] [Google Scholar]
- 20.DeCosse JJ, Miller HH, Lesser ML. Effect of wheat fiber and vitamins C and E on rectal polyps in patients with familial adenomatous polyposis. JNCI. 1989;81:1290–7. doi: 10.1093/jnci/81.17.1290. [DOI] [PubMed] [Google Scholar]
- 21.Gritz DC, Srinivasan M, Smith SD, Kim U, Lietman TM, Wilkins JH, et al. The antioxidants in prevention of cataracts study: Effects of antioxidant supplements on cataract progression in South India. Br J Ophthalmol. 2006;90:847–51. doi: 10.1136/bjo.2005.088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofstad B, Almendingen K, Vatn M, Andersen SN, Owen RW, Larsen S, Osnes M. Growth and recurrence of colorectal polyps: A double-blind 3-year intervention with calcium and antioxidants. Digestion. 1998;59:148–56. doi: 10.1159/000007480. [DOI] [PubMed] [Google Scholar]
- 23.Lamm DL, Riggs DR, Shriver JS, vanGilder PF, Rach JF, DeHaven JI. Megadose vitamins in bladder cancer: A double-blind clinical trial. J Urol. 1994;151:21–6. doi: 10.1016/s0022-5347(17)34863-2. [DOI] [PubMed] [Google Scholar]
- 24.Ronucci L, Di Donato P, Carati L, Ferrari A, Perini M, Bertoni G, et al. Antioxidant vitamins or lactulose for the prevention of the recurrence of colorectal adenomas. Dis Colon Rectum. 1993;36:227–34. doi: 10.1007/BF02053502. [DOI] [PubMed] [Google Scholar]
- 25.Steiner M, Glantz M, Lekos A. Vitamin E plus aspirin compared with aspirin alone in patients with transient ischemic attacks. Am J Clin Nutr. 1995;62:1381S–4S. doi: 10.1093/ajcn/62.6.1381S. [DOI] [PubMed] [Google Scholar]
- 26.Stone PH, Lloyd-Jones DM, Kinlay S, Frei B, Carlson W, Rubenstein J, et al. Effect of intensive lipid lowering, with or without antioxidant vitamins, compared with moderate lipid lowering on myocardial ischemia in patients with stable coronary artery disease: The Vascular Basis for the Treatment of Myocardial Ischemia Study. Circulation. 2005;111:1747–55. doi: 10.1161/01.CIR.0000160866.90148.76. [DOI] [PubMed] [Google Scholar]
- 27.Sumitra K, Pragasam V, Sakthivel R, Kalaiselvi P, Varalakshmi P. Beneficial effect of vitamin E supplementation on the biochemical and kinetic properties of Tamm-Horsfall glycoprotein in hypertensive and hyperoxaluric patients. Nephrol Dial Transplant. 2005;20:1407–15. doi: 10.1093/ndt/gfh794. [DOI] [PubMed] [Google Scholar]
- 28.Zaridze D, Evstifeeva T, Boyle P. Chemoprevention of oral leukoplakia and chronic esophagia in an area of high incidence of oral and esophageal cancer. Ann Epidemiol. 1993;3:225–34. doi: 10.1016/1047-2797(93)90023-w. [DOI] [PubMed] [Google Scholar]
- 29.Lu L, Erhard P, Salomon RG, Weiss MF. Serum vitamin E and oxidative protein modification in hemodialysis: A randomized clinical trial. Am J Kidney Dis. 2007;50:305–13. doi: 10.1053/j.ajkd.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Bonelli L, Camoriano A, Ravelli P, Missale G, Bruzzi P, Aste H. Reduction of the incidence of metachronous adenomas of the large bowel by means of antioxidants 1998. Brussels, Belgium: Se-Te Press; 1998. pp. 91–4. [Google Scholar]
- 31.Garelnabi M, White-Welkey J, Abramson J, Veledar E, Weintraub W, Parthasarathay S. Impact of diet, antioxidant, and exercise-induced oxidative stress on lipids: A randomized, double-blind trial. Circulation. 2006;113:E363. [Google Scholar]
- 32.Stewart S, Prince M, Bassendine M, Hudson M, James O, Jones D, et al. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol. 2007;47:277–83. doi: 10.1016/j.jhep.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 33.Nanayakkara PWB, von Guldener C, ter Wee PM, Scheffer PG, van Ittersum FJ, Twisk JW, et al. Effect of a treatment strategy consisting of pravastatin, vitamin E, and homocysteine lowering on carotid intima-media thickness, endothelial function, and renal function in patients with mild to moderate chronic kidney disease: Results from the Anti-Oxidant Therapy in Chronic Renal Insufficiency (ATIC) Study. Arch Intern Med. 2007;167:1262–70. doi: 10.1001/archinte.167.12.1262. [DOI] [PubMed] [Google Scholar]
- 34.Orndahl G, Grimby G, Grimby A, Johansson G, Wilhelmsen L. Functional deterioration and selenium-vitamin E treatment in myotonic dystrophy. A placebo-controlled study. J Int Med. 1994;235:205–10. doi: 10.1111/j.1365-2796.1994.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 35.Olmedilla B, Granado F, Blanco I, Vaquero M. Lutein, but not a-tocopherol, supplementation improves visual function in patients with age-related cataracts: A 2-y double-blind, placebo-controlled pilot study. Nutrition. 2003;19:21–4. doi: 10.1016/s0899-9007(02)00861-4. [DOI] [PubMed] [Google Scholar]
- 36.Kelly K, Kittelson J, Franklin WA, Kennedy TC, Klein CE, Keith RL, et al. A randomized phase II chemoprevention trial of 13-CIS retinoic acid with or without α tocopherol or observation in subjects at high risk for lung cancer. Cancer Prevention Research. 2009;2:440–9. doi: 10.1158/1940-6207.CAPR-08-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peyser CE, Folstein M, Chase GA, Starkstein S, Brandt J, Cockrell JR, et al. Trial of d-a-tocopherol in Huntington’s disease. Am J Psychiatry. 1995;152:1771–5. doi: 10.1176/ajp.152.12.1771. [DOI] [PubMed] [Google Scholar]
- 38.Tomeo AC, Geller M, Watkins TR, Gapor A, Bierenbaum ML. Antioxidant effects of tocotrienols in patients with hyperlipidemia and carotid stenosis. Lipids. 1995;30:1179–83. doi: 10.1007/BF02536621. [DOI] [PubMed] [Google Scholar]
- 39.Hernaandez J, Syed S, Weiss G, Fernandes G, von Merveldt D, Troyer DA, et al. The modulation of prostate cancer risk with alpha-tocopherol: A pilot randomized, controlled clinical trial. J Urol. 2005;174:519–22. doi: 10.1097/01.ju.0000165151.08560.6a. [DOI] [PubMed] [Google Scholar]
- 40.Dufour J, Oneta CM, Gonvers J, Bihl F, Cerny A, Cereda J, et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin E in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–43. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 41.Avenell A, Campbell MK, Cook JA, Hannaford PC, Kilonzo MM, McNeill G, et al. Effect of multivitamin and multimineral supplements on morbidity from infections in older people (MAVIS trial): pragmatic, randomized, double blind, placebo controlled, trial. Br Med J. 2005;331:324–9. doi: 10.1136/bmj.331.7512.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girodon F, Lombard M, Galan P, Brunet-Lecomte P, Monget A, Arnaud J, et al. Effect of micronutrient supplementation on infection in institutionalized elderly subjects: A controlled trial. Ann Nutr Metab. 1997;41:98–107. doi: 10.1159/000177984. [DOI] [PubMed] [Google Scholar]
- 43.Girodon F, Galan P, Monget A, Boutron-Ruault M, Brunet-Lecomte P, Preziosi P, et al. Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients. Arch Intern Med. 1999;159:748–54. doi: 10.1001/archinte.159.7.748. [DOI] [PubMed] [Google Scholar]
- 44.Clinical Trial of Nutritional Supplements and Age-Related Cataract Study Group. A randomized, double-masked, placebo-controlled trial of multivitamin supplementation for age-related lens opacities. Ophthalmology. 2008;115:599–607. doi: 10.1016/j.ophtha.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Blot WJ, Li J, Taylor PR, Guo W, Dawsey S, Wang G, et al. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. JNCI. 1993;85:1483–92. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 46.Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, et al. The SU.VI.MAX Study. A randomized, placebo-controlled trial of the health-effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335–42. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 47.Chandra RK. Effect of vitamin and trace-element supplementation on immune responses and infection in elderly subjects. Lancet. 1992;340:1124–7. doi: 10.1016/0140-6736(92)93151-c. [DOI] [PubMed] [Google Scholar]
- 48.Pike J, Chandra RK. Effect of vitamin and trace element supplementation on immune indices in healthy elderly. Int J Vitam Nutr Res. 1995;65:117–120. [PubMed] [Google Scholar]
- 49.Wright ME, Lawson KA, Weinstein SJ, Pietinen P, Taylor PR, Virtamo J, Albanes D. Higher baseline serum concentrations of vitamin E are associated with lower total and cause-specific mortality in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 2006;84:1200–7. doi: 10.1093/ajcn/84.5.1200. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Taylor PR, Li B, Dawsey S, Wang G, Ershow AG, et al. Nutrition intervention trials in Linxian, China: Multiple vitamin/mineral supplementation, cancer incidence, and disease-specific mortality among adults with esophageal dysplasia. JNCI. 1993;85:1492–98. doi: 10.1093/jnci/85.18.1492. [DOI] [PubMed] [Google Scholar]
- 51.Takamatsu S, Takamatsu M, Satoh K, Imaizumi T, Yoshida H, Hiramoto M, et al. Effects on health of dietary supplementation with 100 mg d-a-tocopheryl acetate, daily for 6 years. J Int Med Res. 1995;23:342–57. doi: 10.1177/030006059502300504. [DOI] [PubMed] [Google Scholar]
- 52.Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF, Hamer DH. Vitamin E and respiratory tract infections in elderly nursing home residents. A randomized controlled trial. JAMA. 2004;292:828–36. doi: 10.1001/jama.292.7.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Multicenter ophthalmic and nutritional age-related macular degeneration study—part 2: antioxidant intervention and conclusions. Age Related Macular Degeneration Study Group. J Am Optom Assoc. 1996;67:30–49. [PubMed] [Google Scholar]
- 54.You WC, Chang YS, Heinrich J, Ma JL, Liu WD, Zhang L, et al. An intervention trial to inhibit the progression of precancerous gastric lesions: compliance, serum micronutrients and S-allyl cysteine levels, and toxicity. Eur J Cancer Prev. 2001;10:257–63. doi: 10.1097/00008469-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Graat JM, Schouten EG, Kok FJ. Effect of multivitamin-mineral supplementation on acute respiratory tract infections in elderly persons. A randomized controlled trial. JAMA. 2002;288:715–21. doi: 10.1001/jama.288.6.715. [DOI] [PubMed] [Google Scholar]
- 56.Salonen RM, Nyyssonen K, Kaikkonnen J, Porkkala-Sarataho E, Voutilained S, Rissanen TH, et al. Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression. The Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation. 2003;107:947–53. doi: 10.1161/01.cir.0000050626.25057.51. [DOI] [PubMed] [Google Scholar]
- 57.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFayden J, Danielson E, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: Results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167(15):1610–18. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppos Italiano per lo Studio della Sopravvivenza nell’Infarto miocardio. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 59.Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 60.Bukin YV, Draudlin-Krylenko VA, Kuvshinov YP, Poddubniy BK, Shabanov MA. Decrease of ornithine decarboxylase activity in premalignant gastric muscosa and regression of small intestinal metaplasia in patients supplemented with high doses of vitamin E. Cancer Epidemiol Biomarkers Prev. 1997;6:543–6. [PubMed] [Google Scholar]
- 61.Mooney LA, Madsen AM, Tang D, Orjuela MA, Tsai W, Garduno ER, Perera FP. Antioxidant vitamin supplementation reduces benzo(a)pyrene-DNA adducts and potential cancer risk in female smokers. Cancer Epidemiol Biomarkers Prev. 2005;14:237–42. [PubMed] [Google Scholar]
- 62.Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: A prospective double-blinded clinical trial. Arterio Thromb Vasc Biol. 2008;28(2):341–7. doi: 10.1161/ATVBAHA.107.153965. [DOI] [PubMed] [Google Scholar]
- 63.de Waart FG, Kok FJ, Smilde TJ, Hijmans A, Wollershein H, Stalenhoef AF. Effect of glutathione S-transferase M1 genotype on progression of atherosclerosis in lifelong male smokers. Atherosclerosis. 2001;158:227–31. doi: 10.1016/s0021-9150(01)00420-8. [DOI] [PubMed] [Google Scholar]
- 64.McKeown-Eyssen G, Holloway C, Jazmaji V, Bright-See E, Dion P, Bruce WR. A randomized trial of vitamins C and E in the prevention of recurrence of colorectal polyps. Cancer Res. 1988;48:4701–5. [PubMed] [Google Scholar]
- 65.Bairati I, Meyer F, Jobin E, Gelinas M, Fortin A, Nabid A, et al. Antioxdant vitamins supplementation and mortality: A randomized trial in head and neck cancer patients. Int J Cancer. 2006;119:2221–4. doi: 10.1002/ijc.22042. [DOI] [PubMed] [Google Scholar]
- 66.Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevanian A, Liu C, et al. Alpha-Tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not artherosclerosis. The Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106:1453–9. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- 67.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JMO, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer. A randomized controlled trial. The HOPE and HOPE-TOO Investigators. JAMA. 2005;293:1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 68.AREDS Research Group. Associations of mortality with ocular disorders and an intervention of high-dose antioxidants and zinc in the Age-Related Eye Disease Study. AREDS report no 13. Arch Ophthalmol. 2004;122:716–26. doi: 10.1001/archopht.122.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFayden J, et al. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians’ Health Study II Randomized Controlled Trial. JAMA. 2008;300(18):2123–33. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greenberg ER, Baron JA, Tosteson TD, Freeman DH, Jr, Beck GJ, Bond JH, et al. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp Prevention Study Group. NEJM. 1994;331:141–7. doi: 10.1056/NEJM199407213310301. [DOI] [PubMed] [Google Scholar]
- 71.Antoniadi G, Eleftheriadis T, Liakopoulos V, Kakasi E, Kartsios C, Passadakis P, Vargemezis V. Effect of one-year oral α-tocopherol administration on hemodialysis patients. Therapeutic Apherisis and Dialysis. 2008;12:237–42. doi: 10.1111/j.1744-9987.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 72.de la Maza MP, Petermeann M, Bunout D, Hirsch S. Effects of long-term vitamin E supplementation in alcoholic cirrhotics. J Am Coll Nutr. 1995;14:192–6. doi: 10.1080/07315724.1995.10718493. [DOI] [PubMed] [Google Scholar]
- 73.Richer S, Stiles W, Statkuke L, Pulido J, Frankowski J, Rudy D, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial) Optometry. 2004;75:216–30. doi: 10.1016/s1529-1839(04)70049-4. [DOI] [PubMed] [Google Scholar]
- 74.Wluka AE, Stuckey S, Brand C, Cicuttini F. Supplementary vitamin E does not affect the loss of cartilage volume in knee osteoarthritis: a 2-year double blind randomized placebo controlled study. J Rheumatol. 2002;29:2585–91. [PubMed] [Google Scholar]
- 75.Magliano D, McNeil J, Branley P, Shiel L, Demos L, Wolfe R, et al. The Melbourne Atherosclerosis Vitamin E Trial (MAVET): a study of high dose vitamin E in smokers. Eur J Cardiovasc Prev Rehabil. 2006;113:241–7. doi: 10.1097/01.hjr.0000219108.10167.46. [DOI] [PubMed] [Google Scholar]
- 76.McNeil JJ, Robman L, Tikellis G, Sinclair MI, McCarty CA, Taylor HR. Vitamin E supplementation and cataract: randomized controlled trial. Opthalmology. 2004;111:75–84. doi: 10.1016/j.ophtha.2003.04.009. [DOI] [PubMed] [Google Scholar]
- 77.Desnuelle C, Dib M, Garrel C, Favier A and the ALS riluzole-tocopherol Study Group. A double-blind, placebo-controlled randomized clinical trial of a-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis. ALS Motor Neur Dis. 2001;2:9–18. doi: 10.1080/146608201300079364. [DOI] [PubMed] [Google Scholar]
- 78.Pathak AK, Bhutani M, Guleria R, Bal S, Mohan A, Mohanti BK, et al. Chemotherapy alone vs. chemotherapy plus high dose multiple antioxidants in patients with advanced non small cell lung cancer. J Am Coll Nutr. 2005;24:16–21. doi: 10.1080/07315724.2005.10719438. [DOI] [PubMed] [Google Scholar]
- 79.Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchison MJ. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–9. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 80.Plummer M, Vivas J, Lopez G, Bravo JC, Peraza S, Carillo E, et al. Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: A randomized trial in a high-risk population. JNCI. 2007;99:137–46. doi: 10.1093/jnci/djk017. [DOI] [PubMed] [Google Scholar]
- 81.Takagi H, Kakizaki S, Sohara N, Sato K, Tsukioka G, Tago Y, et al. Pilot clinical trial of the use of alpha-tocopherol for the prevention of hepatocellular carcinoma in patients with liver cirrhosis. Int J Vitam Nutr Res. 2003;73:411–5. doi: 10.1024/0300-9831.73.6.411. [DOI] [PubMed] [Google Scholar]
- 82.Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. Critical Leg Ischaemia Prevention (CLIPS) Group. J Int Med. 2006;261:276–84. doi: 10.1111/j.1365-2796.2006.01763.x. [DOI] [PubMed] [Google Scholar]
- 83.MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 84.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–90. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 85.Fang JC, Kinlay S, Beltrame J, Hikiti H, Wainstein M, Behrendt D, et al. Effect of vitamins C and E on progression of transplant-associated ateriosclerosis: A randomized trial. Lancet. 2002;359:1108–13. doi: 10.1016/S0140-6736(02)08154-0. [DOI] [PubMed] [Google Scholar]
- 86.Brown BG, Zhao X, Chait A, Fisher LD, Cheung MC, Morse JS, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. NEJM. 2001;345:1583–92. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 87.Waters DD, Alderman EL, Hsia J, Howard BV, Cobb FR, Rogers WJ, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomised controlled trial. JAMA. 2002;288:2432–40. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 88.Stević Z, Nikolić A, Blagojević DP, Saicić ZS, Kocev NI, Apostolski SA, Spasić MB. A controlled trial of combination of methionine and antioxidants in ALS patients. Jugoslav Med Biohem. 2001;20:223–8. [Google Scholar]
- 89.Singh U, Otvos J, Dasgupta A, de Lemos JA, Devaraj S, Jialal I. High-dose a-tocopherol therapy does not affect HDL subfractions in patients with coronary artery disease on statin therapy. Clin Chem. 2007;53:525–8. doi: 10.1373/clinchem.2006.078865. [DOI] [PubMed] [Google Scholar]
- 90.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. NEJM. 1997;336:1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 91.Marras C, Oakes D, Tanner CM, Fahn S. Vitamin E supplementation was not associated with mortality in the DATATOP cohort. Ann Intern Med. 2005;143:152–3. doi: 10.7326/0003-4819-143-2-200507190-00022. [DOI] [PubMed] [Google Scholar]
- 92.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. NEJM. 2005;352:1–10. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 93.Graf M, Ecker D, Horowski B, Kramer B, Riederer P, Gerlach M, et al. High dose vitamin E therapy in amyotrophic lateral sclerosis as add-on therapy to riluzole: Results of a placebo-controlled double-blind study. J Neur Trans. 2005;112:649–60. doi: 10.1007/s00702-004-0220-1. [DOI] [PubMed] [Google Scholar]
- 94.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–75. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 95.Hintze J. Number Cruncher Statistical Systems [Computer software] 2000. Kaysville, UT: NCSS; 2000. [Google Scholar]
- 96.SAS Institute Inc. SAS 9.2. [Computer software] 9.2. Cary, NC: SAS Institute Inc; 2000–2004. [Google Scholar]
- 97.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clarke MW, Burnett JR, Croft KD. Vitamin E in human health and disease. Crit Rev Clin Lab Sci. 2008;45:417–50. doi: 10.1080/10408360802118625. [DOI] [PubMed] [Google Scholar]
- 99.Bjelakovic G, Gluud LL, Nikolova D, Bjelakovic M, Nagorni A, Gluud C. Meta-analysis: antioxidant supplements for liver diseases - the Cochrane Hepato-Biliary Group. Aliment Pharmacol Ther. 2010;32(3):356–67. doi: 10.1111/j.1365-2036.2010.04371.x. [DOI] [PubMed] [Google Scholar]
- 100.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: A systematic review and meta-analysis. Lancet. 2004;364:1219–28. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 101.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomized trials. Lancet. 2003;361:2017–33. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 102.Brown BG, Crowley J. Is there any hope for vitamin E? JAMA. 2005;293(11):1387–90. doi: 10.1001/jama.293.11.1387. [DOI] [PubMed] [Google Scholar]
- 103.Bulpitt CJ, Antikainen RL, Markowe HL, Shipley MJ. Mortality according to a prior assessment of biological age. Curr Aging Sci. 2009;2(3):193–9. doi: 10.2174/1874609810902030193. [DOI] [PubMed] [Google Scholar]
- 104.Mitchinson MJ, Stephens NG, Parsons A, Bligh E, Schofield PM, Brown MJ. Mortality in the CHAOS trial. Lancet. 1999;353:381–2. doi: 10.1016/S0140-6736(05)74955-2. [DOI] [PubMed] [Google Scholar]