Summary
The ketogenic diet (KD) is an effective therapy for pediatric refractory epilepsies, however, whether the KD changes the pathologic network oscillations generated by an epileptic brain remains unknown. We have reported that hippocampal CA3 regions of epileptic Kv1.1α knockout (KO) mice generate pathologic sharp waves (SPWs) and high frequency oscillations (HFOs) that have higher incidence, longer duration and fast ripples compared to wild-type. Synaptic activity of hyperexcitable KO mossy fibers significantly decreased CA3 principal cell spike-timing reliability contributing to this network pathology. Also, we have demonstrated that the KD reduces seizures by 75% in KO mice. Here, we determined whether 10-14 day in vivo KD treatment exerts disease modifying effects that alter the spontaneous SPW-HFO complexes generated by the hippocampal CA3 region of KO mice in vitro using extracellular multielectrode array recordings. We found that KD treatment significantly attenuated the pathologic features of KO SPWs and ripples and reduced the incidence of fast ripples. The KD also improved spike-timing reliability of KO CA3 principal cells, decreased mossy fiber excitability, increased mossy fiber-CA3 paired pulse ratios and reduced EPSP-spike coupling in the CA3 region. Collectively, these data indicate that KD treatment modulates CA3-generated pathologic oscillations by dampening hyperactive mossy fiber synapses.
Keywords: sharp wave, high frequency oscillations, fast ripple, ketogenic diet, epilepsy, Kv1.1
Introduction
The high fat – low carbohydrate/protein ketogenic diet (KD) is a broad-spectrum anti-seizure therapy that is efficacious against many seizure types, severities, etiologies and patient ages.1 However, the strict dietary regimen and unpalatability of the KD limits its clinical use to pediatric patients with refractory epilepsy. Nevertheless, within this difficult-to-treat patient population, the KD reduces ~50% of seizures in approximately half of the patients and ~90% of seizures in one-third of patients.1 Due to recognition of KD efficacy in refractory epilepsy, clinical and basic research interest in the KD has increased dramatically in an effort to determine the mechanisms of the KD’s anti-seizure efficacy.
Many elegant studies indicate that the mechanisms of the KD may involve disease modifying genetic and epigenetic changes that positively modulate neuroprotective and anti-inflammatory pathways.11 However, the effect of the KD on epileptic neuronal networks remains poorly understood. Previously, we demonstrated that the KD reduces seizures by ~75% in epileptic Kv1.1α knockout (KO) mice6, a clinically relevant model to several types of epilepsy including developmental epilepsies, intractable temporal lobe epilepsy with complex partial seizures, human autosomal dominant lateral temporal lobe epilepsy, human limbic encephalitis associated with seizures, and sudden unexplained death in epilepsy (see Simeone et al.13 for references). Recently, we reported that that the CA3 region of acute hippocampal slices from KO mice generates ‘pathologic’ sharp wave-high frequency oscillations (SPW-HFOs) similar to intracranial recordings from epileptic foci in humans.4,13 We demonstrated that increased activity at mossy fiber-CA3 synapses results in decreased spike timing reliability of CA3 principal cells and the emergence of ‘pathologic’ SPW-HFOs. In the present study, we used this paradigm to demonstrate for the first time that KD treatment of epileptic animals attenuates synaptic and neuronal mechanisms that contribute to the emergence of pathologic network oscillations.
Methods
Breeding pairs of heterozygous Kcna1-null mice on a C3HeB/FeJ congenic background were purchased from Jackson Laboratories (Bar Harbor, Maine) and colonies were maintained in the Animal Resource Facilities at Creighton University School of Medicine. Tail clips were taken at P10–P15 and sent to Transnetyx Inc. for genotyping (Cordova, TN, U.S.A.). At P20, mice were weaned onto either a standard diet or a KD (6:1, fat to carbohydrates plus proteins; Bio-Serv F3666, Frenchtown, NJ, U.S.A.). Mice were given food and water ad libitum and kept on a 12-hour light/dark cycle. Reported results were obtained from 11 WT and 11 KO mice fed a standard diet and 7 KD-treated KO mice (P31-35). All procedures involving animals were in accordance with National Institutes of Health guidelines, the EU Directive 2010/63/EU and were approved by the Institutional Animal Care and Use Committee at Creighton University School of Medicine.
All recording procedures and signal analyses were performed as previously described13 and provided in the supplemental methods section.
All data are reported as the mean ± standard error. Statistical significance was determined with an one-way ANOVA followed by Tukey’s multiple comparisons test, unless otherwise specified, using Prism 6 software (Graphpad Software, Inc.). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.).
Results
SPW-HFOs
We have replicated our previous findings and demonstrate that KO SPW-HFOs have spontaneous pathologic features compared to WT, including abnormal inter-event intervals, durations, HFO frequencies and the emergence of fast ripples.13 As depicted in the traces and time-frequency analyses in Fig. 1A, KD treatment diminishes the severity of the pathologic features of SPW-HFOs of KO mice. Specifically, SPWs of KO hippocampi have a 46±4% decrease in inter-SPW intervals reflecting a 2-fold increase of incidence and a 49±3% increase in duration compared to WT (Fig. 1B). KD treatment of KO mice slowed the incidence of SPWs by increasing inter-SPW intervals by 59±17% and decreasing durations by 25±2% (Fig. 1B). Similarly, KO hippocampi generated HFOs in the ripple range (80-200 Hz) with higher incidence (inter-ripple intervals decreased by 72±1%), longer durations (by 64±2%) and lower intra-ripple frequencies (12.2±0.4%) (Fig. 1C). KD treatment of KO mice increased inter-ripple intervals by 183±11%, reduced durations by 33±1% and increased intra-ripple frequencies by 11.9±0.8% compared to standard diet fed KO mice (Fig. 1C).
Figure 1. The ketogenic diet diminishes pathologic features of SPW-HFO generated in KO hippocampal slices.
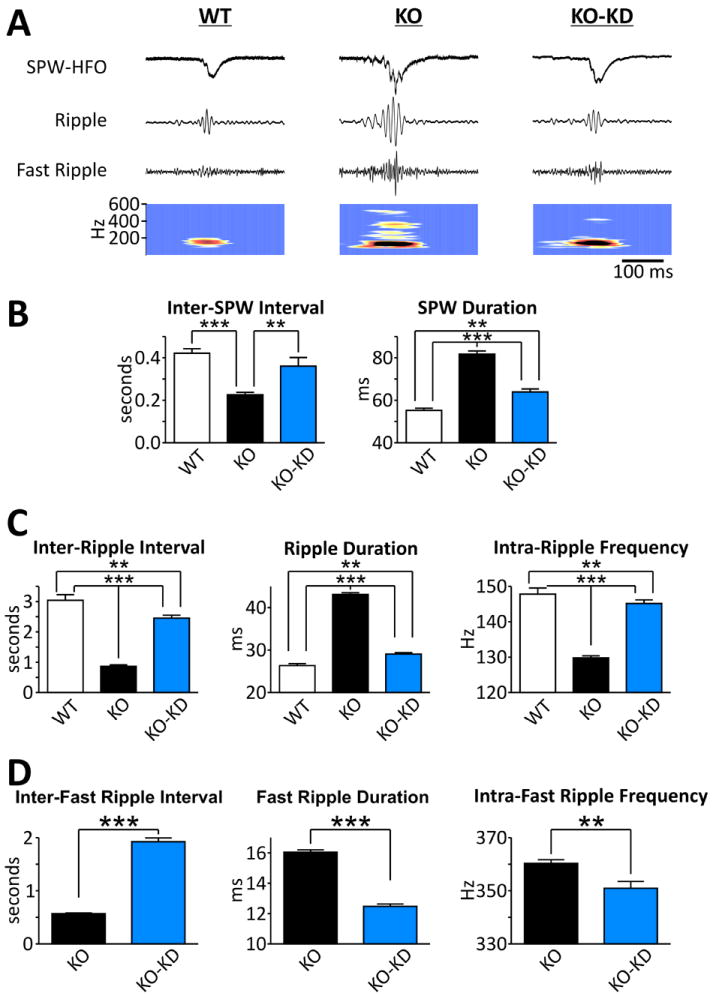
A. Examples of SPW-HFOs recorded in the CA3 stratum radiatum region of hippocampal slices from WT, KO and KD treat KO (KO-KD) mice. Top trace: raw signal. Second trace: raw signal band pass filtered between 100-175 Hz. Third trace: raw signal band pass filtered between 200-600 Hz. Bottom trace: Time-frequency map of raw signal band pass filtered between 100-600 Hz.
B. SPW intervals and durations are increased by KD treatment [n = 6 (WT), 11 (KO), 11 (KO-KD) slices]. Significance determined by an one-way ANOVA followed by Tukey’s multiple comparison test, *p<0.05, **p<0.01, ***p<0.001.
C. KD treatment increases ripple interval and frequency, whereas duration is decreased [n = 584/6 (WT), 3971/11 (KO), 1311/11 (KO-KD) events/slices]. Significance determined by an one-way ANOVA followed by Tukey’s multiple comparison test, *p<0.05, **p<0.01, ***p<0.001.
D. Fast ripple intervals are increased and durations are decreased by KD treatment, but internal fast ripple frequencies are unaffected [n = 6130/11 (KO), 1677/11 (KO-KD) events/slices]. Significance determined by an unpaired Student’s t-test, ***p<0.001.
Unlike WT, KO hippocampi also generate HFOs in the fast ripple range (200-600 Hz) similar to human epileptic foci.4,13 The KD attenuated fast ripples by increasing the inter-fast ripple intervals by 238±12%, decreasing the duration by 22±1% and reducing the intra-fast ripple frequencies by 2.6±0.7% (Fig. 1D).
CA3 Single Units
The emergence of fast ripples in slices from KO mice and other models of epilepsy has been linked to a decrease in the reliability of timing between sequential spikes of CA3 principal cells [i.e., increase in jitter or the mean standard deviation of the inter-spike interval (ISI)].8,13 In the present study, we examined the timing of extracellularly recorded single units occurring as doublets between SPWs and as burst firing during SPWs. Single units of individual principal cells were identified as previously described13 (see Supplemental Methods). We restricted examination of doublet firing frequencies in the range of 130-180Hz resulting in mean ISIs of 6.5 ms (154 Hz; Fig. 2A,B), which approximated intra-ripple frequencies. Within these parameters the jitter of single units of KO CA3 principal cells (n=19) was 17.1±2.6% higher compared to WT (n=20; p<0.05). KD-treatment reduced KO doublet jitter by 13.5±4.3% (n=18; p<0.5) (Fig. 2B).
Figure 2. Ketogenic diet treatment improves spike timing and neurotransmission.
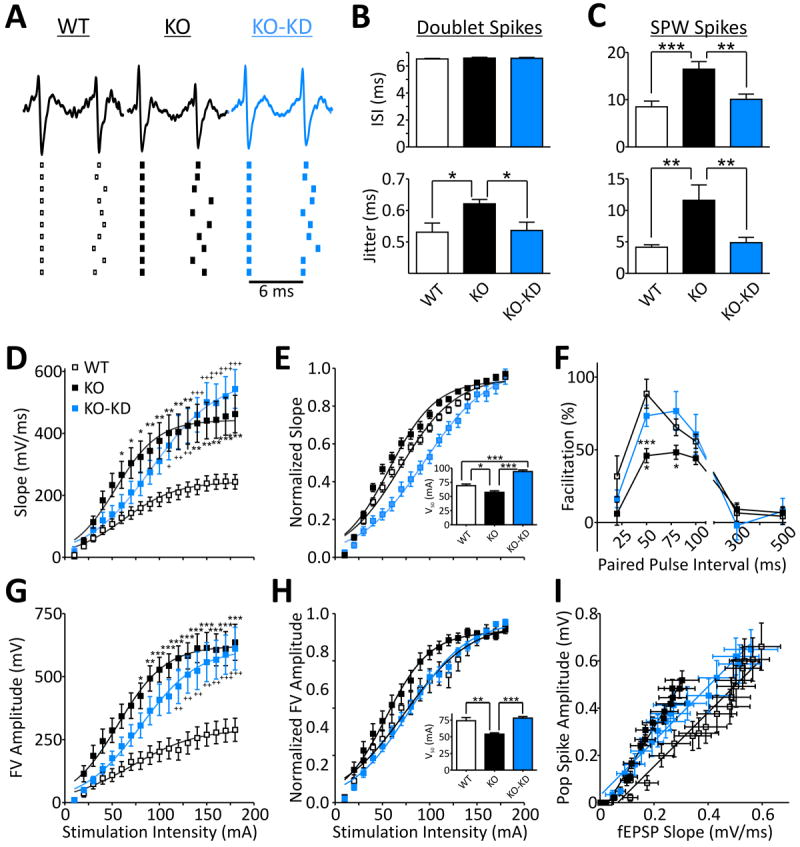
A. Example traces of doublet spikes and illustration of spike timing of ten subsequent doublets from the same neurons.
B. Quantification of doublet CA3 principal cell inter-spike intervals (ISI) and spike jitter indicating KO cells have increased jitter when similar spikes are compared between groups. [n = 20 (WT), 19 KO, 18 (KO-KD) cells]. Significance determined by an one-way ANOVA followed by Tukey’s multiple comparison test, *p<0.05.
C. ISIs and jitter of single units firing during SPWs are increased in KO and rescued with KD-treatment. [n = 20 (WT), 17 KO, 16 (KO-KD) cells]. Significance determined by an one-way ANOVA followed by Tukey’s multiple comparison test, **p<0.01, ***p<0.001.D. KD-treatment has no effect on KO field potential slopes of dendritic CA3 responses to mossy fiber stimulation. Fit with a Boltzmann equation. Significance determined with a two-way ANOVA for genotype/treatment and stimulation intensity (n = 6 slices). Genotype/treatment [F(2,270)=76.74, p<0.001) and stimulation intensity [F(17,270)=31.07, p<0.001] had significant effects and interaction [F(34,270)=1.904, p<0.01]. The two-way ANOVA was followed by Tukey’s multiple comparison test, *p<0.05, **p<0.01, ***p<0.001 (WT vs. KO); +p<0.05, ++p<0.01,+++p<0.001 (WT vs. KO-KD).
E. KD-treatment significantly increases the half maximal stimulation intensities (V50) of the normalized I/O curves of field potential slopes. Fit with a Boltzmann equation. Significance for V50’s determined with a one-way ANOVA followed by Tukey’s multiple comparison test, *p<0.05, ***p<0.001 (n = 6 slices).
F. KD restores mossy fiber-CA3 paired pulse facilitation. Significance determined by a two-way ANOVA for genotype/treatment and stimulation interval (n = 6 slices). Genotype/treatment [F(2,54)=5.823, p<0.05) and stimulation interval [F(5,54)=38.83, p<0.001] had significant effects. The two-way ANOVA was followed by Tukey’s multiple comparison test, *p<0.05 (KO vs. KO-KD), ***p<0.001 (WT vs. KO).
G. KD-treatment has no effect on KO fiber volley amplitudes of mossy fibers. Fit with a Boltzmann equation. Significance determined with a two-way ANOVA for genotype/treatment and stimulation intensity (n = 6 slices). Genotype/treatment [F(2,306)=110.6, p<0.001) and stimulation intensity [F(17,306)=27.84, p<0.001] had significant effects and interaction [F(34,306)=1.62, p<0.05]. The two-way ANOVA was followed by Tukey’s multiple comparison test: *p<0.05, **p<0.01, ***p<0.001 (WT vs. KO); ++p<0.01,+++p<0.001 (WT vs. KO-KD).
H. KD-treatment significantly increases the half maximal stimulation intensities (V50) of the normalized I/O curves of fiber volley amplitudes. Fit with a Boltzmann equation. Significance for V50’s determined with a one-way ANOVA followed by Tukey’s multiple comparison test, *p<0.05, ***p<0.001 (n = 6 slices).
I. KD-treatment has an intermediate effect on CA3 field EPSP slopes and population spike slopes compared to KO and WT. The net effect produces a shift in the extracellular field EPSP-population spike relationship to the right with a slope similar to WT indicating a decrease in the excitability of CA3 neurons (n = 6 slices).
When assessing single units bursting during SPWs we did not restrict firing patterns. SPW single unit ISIs and jitter were much higher than during doublets because of the diverse firing patterns of individual principal cells. KO ISIs and jitter were 94±18% and 179±59% larger than WT, respectively (n=17, 20; Fig. 2C). KD-treatment reduced KO ISIs and jitter by 39±7% and 70±5%, respectively (n=16; Fig. 2C). Collectively, these results indicate that KD-treatment improves the spike timing reliability of KO CA3 principal cells.
Mossy Fiber-CA3 Synapse
Previously we found that hyperexcitability of dentate granule cell mossy fibers and axon terminals increases synaptic activity (i.e., synaptic strength and presumably synaptic noise) in the CA3 region contributing to the impairment of spike timing and the emergence of pathologic SPW-HFOs in slices from KO mice.13 Therefore, we determined the influence of the KD on evoked fiber volleys and field potentials recorded in CA3 stratum lucidum after stimulation of mossy fibers in the hilar region. The KO mossy fiber-CA3 dendritic field potentials had 97±5% larger field potential slopes (Fig. 2D) that were hyperexcitable with 17±4% lower half maximal stimulation intensities (V50) compared to WT (Fig. 2E). Also, KO paired pulse facilitation (50 ms ISI) was 47±6% lower suggesting increased neurotransmitter release probabilities from KO mossy fibers (Fig. 2F). The KO hyperexcitability was most likely presynaptic as mossy fibers exhibited 140±10% larger fiber volleys (Fig. 2G) and 27±5% lower V50’s compared to WT (Fig. 2H). KD-treatment of KO mice reduced mossy fiber hyperexcitability by increasing field potential and fiber volley V50’s by 64±16% and 45±5%, respectively (Fig. 2E,H). KD-treatment also increased paired pulse facilitation (50 ms ISI) by 64±18% suggesting significant decreases in release probabilities compared to KO (Fig. 2F). However, KD-treatment had no effect on fiber volley amplitudes and field potential slopes of KO mice (p>0.05) (Fig. 2D,G).
CA3 Excitability
Spike timing is sensitive to neuronal excitability.5 Synaptic activity, as well as loss of Kv1.1 expression, can alter neuronal excitability.13 KD attenuation of KO mossy fiber hyperexcitability and improvement of KO spike timing suggested that CA3 neuronal excitability would also be reduced by KD-treament. We assessed the coupling of excitatory inputs and firing efficiency by recording extracellular potentials in the CA3 pyramidal cell layer upon mossy fiber stimulation (10-180μA) and determining mossy fiber-CA3 fEPSP-population spike (E-S) ratios.13 Similar to our previous report, population spikes of similar amplitude were elicited with smaller fEPSP slopes in KO slices compared to WT (Fig. 2I). Overall responses from KD-treated KO mice were intermediate, but had more resemblance to WT in the maximum fEPSPs (WT, 597±71; KO, 304±63; KO-KD, 559±60μV; one way ANOVA, F(2,14)=6.192, p<0.05; KO = p<0.05 vs. WT and KO-KD) and population spike amplitudes (WT, 660±100; KO, 518±40; KO-KD, 648±84μV; one way ANOVA, F(2,14)=1.053, p=0.37; p>0.05). This resulted in KD E-S coupling ratios obtained from the slope of the linear regression of the E-S plots to be identical to WT (WT, 1.14±0.06; KO, 1.87±0.08; KO-KD, 1.15±0.05; one way ANOVA, F(2,14)=42.68, p<0.001; KO = p<0.001 vs. WT and KO-KD) (n = 6). These results suggest that KD-treatment dampens the excitability of KO CA3 neurons.
Discussion
Here, we provide evidence that in vivo KD-treatment decreases the hyperexcitability of in vitro epileptic networks by reducing aberrant mossy fiber synaptic release. Specifically, KD-treatment rescued presynaptic fiber volley excitability, mossy fiber-CA3 synaptic excitability and paired pulse facilitation. The decrease of synaptic activity reduced CA3 neuronal excitability, improved the spike timing reliability of CA3 principal cells and reduced the incidence and duration of network spontaneous SPWs. In vitro, in-phase firing of a sub-network of principal cells during SPWs underlies ripples, whereas out-of-phase firing is promoted by increased synaptic strength or noise and results in the emergence of fast ripples.8,13 Thus, KD improvement of spike timing may directly lead to significant reduction of fast ripple emergence. Further supporting the hypothesis that modulation of synaptic activity mediates KD effects on network hyperexcitability, we recently demonstrated similar results upon isolating the KO CA3 region from afferent inputs.13 Even though KD-treatment rescued many facets of excitability, fast ripples were not completely abolished. This failure may be due to the lack of KD effect on the large KO fiber volleys and field potentials which reflect a persistent increase in synaptic strength despite decreased mossy fiber excitability and release probabilities. Further investigation is needed to determine the source and role of these increased KO fiber volleys and field potentials. Overall, our findings support and expand previous studies which demonstrated that KD treatment reduced evoked hyperexcitability in hippocampal slices from rats with kainate-induced spontaneous recurrent seizures14 and dampened baseline and challenged hippocampal excitability recorded in vivo and in vitro from wild-type rats.2,3
How does the KD decrease synaptic activity? KD-induced increase of ketone bodies (KBs) and polyunsaturated fatty acids (PUFAs) have relevant physiological consequences via direct, acute effects on ion channels, neurotransmission and ATP/adenosine production.11 However, in our study, the ≥ 2-hour incubation of slices prior to recording in normal aCSF suggests that the effect persists in the absence of circulating KBs and PUFAs and likely reflects KD-induced disease-modifying effects. We recently demonstrated that KO hippocampal mitochondrial respiratory complex I (MRCI) and uncoupling are chronically dysfunctional similar to that found in human epilepsy10 and that pharmacological inhibition of MRCI in WT slices results in reduced paired pulse facilitation and exacerbates seizure like events in vitro.12 Furthermore, we demonstrated that treatments improving KO MRCI and uncoupling function reduce seizures, increase paired pulse facilitation and V50’s, but do not change the large KO field potential slopes12, results similar to the KD effects presented here (Fig. 2C,D). We and others have found that the KD improves hippocampal MRCI, uncoupling, calcium sequestration, ATP production and reduces ROS generation in WT and KO mice and promotes mitochondrial biogenesis with upregulation of mitochondrial complex genes (unpublished data).2,15 Collectively, these data indicate that chronic dysfunction of mitochondria will have significant effects on neurotransmission and network excitability in addition to the well established cell death pathways.11 Therefore, KD improvement of mitochondria function may directly impact network excitability.2,11
In conclusion, our findings may be relevant to understanding the mechanisms by which the KD prevents general network hyperexcitability from progressing to seizure because the mechanisms of fast ripple emergence may directly participate in the generation of seizures.4,9 As SPWs and ripples are thought to actively participate in memory consolidation, positive modulation of pathologic SPW-HFOs may also be involved in the mechanism by which the KD exerts beneficial effects on cognitive dysfunction in epilepsy and other neurological disorders.7
Supplementary Material
Acknowledgments
This work was supported by the Health Future Foundation (TAS), Epilepsy Foundation (TAS), Citizens United for Research in Epilepsy Foundation (TAS) and NIH NS072179-02 (KAS). The project described was also supported by the National Center for Research Resources grant G20RR024001. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Please note, the last author has published under the names K Dorenbos, KA Fenoglio, KA Fenoglio-Simeone and KA Simeone.
Biography

Footnotes
Disclosure of Conflicts of Interest
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Acharya MM, Hattiangady B, Shetty AK. Progress in neuroprotective strategies for preventing epilepsy. Prog Neurobiol. 2008;84:363–404. doi: 10.1016/j.pneurobio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bough KJ, Wetherington J, Hassel B, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60(2):223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 3.Bough KJ, Schwartzkroin PA, Rho JM. Calorie restriction and ketogenic diet diminish neuronal excitability in rat dentate gyrus in vivo. Epilepsia. 2003;44(6):752–760. doi: 10.1046/j.1528-1157.2003.55502.x. [DOI] [PubMed] [Google Scholar]
- 4.Engel J, Jr, Bragin A, Staba R, et al. High-frequency oscillations: What is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 5.Epsztein J, Sola E, Represa A, et al. A selective interplay between aberrant EPSPKA and INaP reduces spike timing precision in dentate granule cells of epileptic rats. Cerebral Cortex. 2010;20(4):898–911. doi: 10.1093/cercor/bhp156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenoglio-Simeone KA, Wilke JC, Milligan HL, et al. Ketogenic diet treatment abolishes seizure periodicity and improves diurnal rhythmicity in epileptic Kcna1-null mice. Epilepsia. 2009;50:2027–2034. doi: 10.1111/j.1528-1167.2009.02163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallböök T, Ji S, Maudsley S, Martin B. The effects of the ketogenic diet on behavior and cognition. Epilepsy Res. 2012;100(3):304–309. doi: 10.1016/j.eplepsyres.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibarz JM, Foffani G, Cid E, et al. Emergent dynamics of fast ripples in the epileptic hippocampus. J Neurosci. 2010;30:16249–16261. doi: 10.1523/JNEUROSCI.3357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jefferys JG, de la Prida LM, Wendling F, et al. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012;98(3):250–264. doi: 10.1016/j.pneurobio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudin AP, Zsurka G, Elger CE, et al. Mitochondrial involvement in temporal lobe epilepsy. Exp Neurol. 2009;218(2):326–32. doi: 10.1016/j.expneurol.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Masino SA, Rho JM. Mechanisms of Ketogenic Diet Action. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet] 4. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- 12.Simeone KA, Matthews SA, Samson KK, et al. Targeting deficiencies in mitochondrial respiratory complex I and functional uncoupling exerts anti-seizure effects in a genetic model of temporal lobe epilepsy and in a model of acute temporal lobe seizures. Exp Neurol. 2013 doi: 10.1016/j.expneurol.2013.11.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simeone TA, Simeone KA, Samson KK, et al. Loss of the Kv1.1 potassium channel promotes pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices. Neurobiol Dis. 2013;54:68–81. doi: 10.1016/j.nbd.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stafstrom CE, Wang C, Jensen FE. Electrophysiological observations in hippocampal slices from rats treated with the ketogenic diet. Dev Neurosci. 1999;21(3-5):393–399. doi: 10.1159/000017389. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan PG, Rippy NA, Dorenbos K, et al. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55(4):576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


