Abstract
While the risk of lung cancer associated separately with smoking and radiation exposure has been widely reported, it is not clear how smoking and radiation together contribute to the risk of specific lung cancer histological types. With individual smoking histories and radiation dose estimates, we characterized the joint effects of radiation and smoking on type-specific lung cancer rates among the Life Span Study cohort of Japanese atomic bomb survivors. Among 105,404 cohort subjects followed between 1958 and 1999, 1,803 first primary lung cancer incident cases were diagnosed and classified by histological type. Poisson regression methods were used to estimate excess relative risks under several interaction models.
Adenocarcinoma (636 cases), squamous-cell carcinoma (330) and small-cell carcinoma (194) made up 90% of the cases with known histology. Both smoking and radiation exposure significantly increased the risk of each major lung cancer histological type. Smoking-associated excess relative risks were significantly larger for small-cell and squamous-cell carcinomas than for adenocarcinoma. The gender-averaged excess relative risks per 1 Gy of radiation (for never-smokers at age 70 after radiation exposure at age 30) were estimated as 1.49 (95% confidence interval 0.1–4.6) for small-cell carcinoma, 0.75 (0.3–1.3) for adenocarcinoma, and 0.27 (0–1.5) for squamous-cell carcinoma. Under a model allowing radiation effects to vary with levels of smoking, the nature of the joint effect of smoking and radiation showed a similar pattern for different histological types in which the radiation-associated excess relative risk tended to be larger for moderate smokers than for heavy smokers. However, in contrast to analyses of all lung cancers as a group, such complicated interactions did not describe the data significantly better than either simple additive or multiplicative interaction models for any of the type-specific analyses.
Introduction
Lung cancers arise in various cell types. While the mixture of different histological types varies considerably between countries and populations and has changed over time, squamous cell carcinoma (SqCC), small cell carcinoma (SmCC) and adenocarcinoma (AC) account for the majority of microscopically diagnosed cases (1). Cigarette smoking is associated with increased rates for most types of lung cancer with the largest (relative) increases for squamous cell carcinoma and small cell carcinoma (2, 3). Adenocarcinoma predominates in never-smokers for most countries, but recent increases in adenocarcinoma rates also seem to be attributed to smoking (4, 5). Ionizing radiation exposure has been shown to be associated with significant increases in lung cancer rates (6, 7). There has also been considerable interest in the nature of the joint effect of radiation and smoking among uranium miners (8), populations exposed to residential radon (9–11), radiation-treated Hodgkin's disease patients (12, 13) and Japanese atomic bomb survivors (14, 15).
In the Life Span Study (LSS) cohort of atomic bomb survivors, a rather complex radiation-smoking interaction was found for the risk of all lung cancers as a group. This interaction was best described as super-multiplicative for low-intensity smokers and additive or subadditive for high-intensity smokers (15). In addition, smoking-associated relative risks of lung cancer in this Japanese cohort were somewhat smaller than those reported from Western populations (16–18). These results pertained to the risk of lung cancer as a group, but little is known about the nature of the joint effects of radiation and smoking on different histological types of lung cancer. In this paper, we describe the distribution of lung cancer by histological type in the LSS and characterize, to the extent possible, the radiation-smoking interaction for the three major histological types of lung cancer.
Materials and Methods
Study Cohort and Case Ascertainment
The LSS cohort consists of 120,321 subjects who were residents of Hiroshima and Nagasaki in October 1950, including 93,741 atomic bomb survivors who were within 10 kilometers of the hypocenters at the time of bombing and an age, gender, and city frequency-matched group of 26,580 people who were temporarily away from the cities at that time, as described elsewhere (6, 19). After excluding subjects who did not have dose estimates (7,070 people), could not be traced (71 people), or were known to have died or had cancer prior to the inception of the Hiroshima and Nagasaki Tumor registries on January 1, 1958 (7,776 people), 105,404 cohort members were eligible for analyses in this study.
As in the previous report, lung cancer diagnoses and histological types were derived from a pathology review carried out to identify LSS lung cancer cases through the end of 1999. At the first stage of the case review, 5,711 members with potential lung tumors were identified using information from the Hiroshima and Nagasaki Tumor and Tissue Registries, an autopsy program of the Radiation Effects Research Foundation, and mortality data collected as part of the LSS mortality follow-up. Additional information, including registry records, tissue slides, and relevant clinical and pathology records were sought for those cases. In reviewing the available data, the study pathologists identified and classified 2,368 lung cancers based on the latest WHO diagnostic criteria (20). Lung cancers were considered ineligible if they were not the first primary tumor (216 cases), if the individual was not a resident in Hiroshima or Nagasaki Prefecture at the time of diagnosis (171 cases diagnosed primarily from death certificates) or was diagnosed prior to 1958 (47 cases), or if a dose estimate was unavailable (131 cases), resulting in 1,803 eligible cases for use in analysis.
Radiation and Smoking Data
The radiation dose-response analyses were based on individual weighted DS02 lung doses (19) calculated as the γ-ray dose plus 10 times the neutron dose in Gy with adjustment to reduce bias in risk estimates associated with the uncertainty in individual dose estimates (21).
Smoking histories were obtained for 61% of the cohort members from a series of mail surveys conducted in the LSS between 1965 and 1991 and from interviews conducted in a clinical subcohort of the LSS. For the present analyses, individual smoking history was summarized by a time-dependent indicator of smoking status (never/past/current/unknown), the year (if any) in which smoking data were first obtained, and, for ever-smokers, ages started or stopped smoking and the average number of cigarettes smoked per day. For smokers, smoking duration was defined as the difference between the reported age at start of smoking and the minimum of the current age and the age at which one reported having stopped smoking. Time since quitting was defined as the difference between the current age and the age at smoking cessation for reported past smokers and as 0 otherwise. Cohort members for whom data on smoking habits had never been obtained were treated as having unknown smoking status throughout their time at risk. In addition, for the period prior to the date at which information on an individual's smoking habits was first obtained, his/her smoking status was treated as unknown. This was done to avoid biasing risk estimates by overcounting person years in known smoking-status categories. Additional details on the smoking variables are available elsewhere (15).
Statistical Analysis
Primary analyses were based on a highly stratified person-year table containing about 120,000 cells with non-zero person-years at risk. Each cell contained the number of person-years, cases (by histological type) and person-year weighted means of attained age, age at exposure, calendar year, radiation dose, and smoking summary variables. Details of the categorization are given in the previous report (15).
Analyses were carried out using Poisson regression models for cancer rate functions of the form:
where λ0 is a function of background factors B that describes the incidence rate for unexposed never-smokers (baseline rate), and RR is a relative risk function that can depend on smoking- (S) and radiation-dose- (D) related variables as well as variables that modify the effects. We considered several forms for the joint effects of smoking and radiation:
where φ and ρ describe the excess risks associated with smoking- and radiation-related variables, respectively. Under the additive model, ρ is the excess relative risk (ERR) of exposed subjects relative to unexposed never-smokers, while under the multiplicative model it is the ERR relative to unexposed subjects with the same smoking history. Similar interpretation applies to the smoking excess risk. The generalized models allow for departures from either additive or multiplicative interactions by allowing the radiation ERR to vary as a function ω of smoking variables.
An analysis model is specified by the interaction type (AM, MM, GAM or GMM) and a set of model-component forms (λ0, φ, ρ and ω). For all type-specific analyses in this study, we used the same set of component forms considered in analysis for all lung cancers combined (15), which are described as follows:
The baseline rate model λ0(B) assumed the log rate to be proportional to gender-specific quadratic functions of log attained age with additional effects of city and birth year. In addition, cohort members who were not in the cities at the times of bombing were distinguished from the other members with an indicator. Thus, radiation effects were quantified relative to rates for survivors who were in the cities at the times of bombing but received little radiation from the bombs.
The smoking excess risk φ(S) was described as a function of the cumulative amount smoked (packs of cigarettes smoked per day times years smoked), or pack-years (p), times an effect-modification function that depended on gender (g), birth-cohort (b), smoking duration (y) and time since quitting (q): (x003D5)(S) = θgpexp{ξb + η1log(y) + η2log(y)2 + vlog(q + 1)}. For the follow-up during which smoking status was unknown, the “smoking” effect was allowed to vary with gender and birth-cohort strata.
The radiation excess risk ρ(D) was described by the product of a gender-specific function of dose (d) and an effect-modification function that depended on age at exposure (e) and attained age (a). With the linear dose-response, the excess risk was described as ρ(D) = δgdαβexp{γe}. Departure from the linear dose-response was tested by using various nonlinear functions.
The generalized radiation-smoking interaction was expressed as a function of smoking intensity (s), ω(S) = exp{ω1log(s) + ω2log(s)2} with ω(S) ≡ 1 for lifelong never-smokers.
Model parameters were estimated by maximum likelihood using Epicure software (22). Likelihood ratio tests were used to compute P values for hypothesis tests (two sided) and to determine confidence intervals (CIs).
Although we made some efforts to develop more parsimonious models for specific histological types, goodness of fit measures such as the Akaike information criterion (AIC) (23) suggested that improvements gained by using more parsimonious models were limited. Therefore, the results presented herein were limited to those based on the component forms described above to facilitate comparisons across types. The rule for these comparisons was that AIC differences (for a given outcome) of less than about 6 should not be regarded as significant (24).
In the main text we focus on the nature of radiation and of smoking effects on rates for the most common histological types (AC, SqCC, SmCC) of lung cancer. Results for the relatively large proportion of cases for which histological type could not be determined and those for the combined cases of the three common histological types are presented as supplementary materials.
Results
Table 1 provides information on the number of cases and crude rates by histology and sex in the study cohort. Adenocarcinoma accounted for 35% of all lung cancer cases and almost half of those with identified types. Squamous cell carcinoma accounted for 18% of all cases and 25% of the type-identified cases, and small cell carcinoma for 11% and 15%, respectively. The histological type could not be determined for 28% of all cases (labeled “not otherwise specified”), about half of which were reported as lung cancers solely on the basis of death certificates. Crude rates were generally higher for men than for women with the smallest relative differences for adenocarcinoma and not otherwise specified.
Table 1. LSS Lung Cancer Cases and Crude Rate (Cases per 10,000 Person-Years) bv Histological Type and Sex.
| Male | Female | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Cases | (%) | Rate | Cases | (%) | Rate | Cases | (%) | Rate | |
| Epithelial cancers | |||||||||
| Adenocarcinoma | 315 | (29.4) | 2.99 | 321 | (43.9) | 1.84 | 636 | (35.3) | 2.27 |
| Squamous-cell carcinoma | 254 | (23.7) | 2.41 | 76 | (10.4) | 0.43 | 330 | (18.3) | 1.18 |
| Small-cell carcinoma | 129 | (12.0) | 1.22 | 65 | (8.9) | 0.37 | 194 | (10.8) | 0.69 |
| Large-cell carcinoma | 33 | (3.1) | 0.31 | 13 | (1.8) | 0.07 | 46 | (2.6) | 0.16 |
| Other epithelial cancers | 45 | (4.2) | 0.43 | 29 | (4.0) | 0.17 | 74 | (4.1) | 0.26 |
| Non epithelial cancers | |||||||||
| Mesothelioma | 13 | (1.2) | 0.12 | 4 | (0.5) | 0.02 | 17 | (0.9) | 0.06 |
| Lymphoma | 2 | (0.2) | 0.02 | 2 | (0.3) | 0.01 | 4 | (0.2) | 0.01 |
| Unclassified lung cancers | |||||||||
| Not otherwise specified (NOS) | 281 | (26.2) | 2.67 | 221 | (30.2) | 1.26 | 502 | (27.8) | 1.79 |
| Total | 1072 | (100) | 10.17 | 731 | (100) | 4.18 | 1803 | (100) | 6.43 |
| Person-years | 1,053,713 | 1,749,254 | 2,802,967 | ||||||
| Subjects | 42.889 | 62,515 | 105.404 | ||||||
Table 2 provides summary information on case counts and crude rates for the three major types. Crude rates were similar for Hiroshima and Nagasaki and increased over the follow-up period in part due to the aging of the cohort, but trend for adenocarcinoma was remarkable.
Table 2. Subjects, Cases and Crude Rate (Cases per 10,000 Person-Years) by Histological Subtype of Lung Cancer in the Study Cohort (1958–1999).
| Adenocarcinoma | Squamous cell | Small cell | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Subjects | Cases | Rate | Cases | Rate | Cases | Rate | |
| City | |||||||
| Hiroshima | 73,414 | 439 | 2.20 | 246 | 1.23 | 141 | 0.71 |
| Nagasaki | 31,990 | 197 | 2.44 | 84 | 1.04 | 53 | 0.66 |
| Attained age (years) | |||||||
| 0–39 | 4 | 0.06 | 0 | 0 | 0 | 0 | |
| 40–49 | 19 | 0.38 | 2 | 0.04 | 5 | 0.10 | |
| 50–59 | 113 | 1.90 | 33 | 0.56 | 22 | 0.37 | |
| 60–69 | 211 | 3.96 | 103 | 1.93 | 63 | 1.18 | |
| 70–79 | 183 | 5.28 | 131 | 3.78 | 76 | 2.19 | |
| 80+ | 106 | 6.82 | 61 | 3.92 | 28 | 1.80 | |
| Age at exposure (years) | |||||||
| 0–9 | 22,681 | 53 | 0.76 | 8 | 0.12 | 4 | 0.06 |
| 10–19 | 23,057 | 124 | 1.72 | 46 | 0.64 | 23 | 0.32 |
| 20–39 | 30,073 | 282 | 3.07 | 119 | 1.29 | 92 | 1.00 |
| 40+ | 29,593 | 177 | 3.78 | 157 | 3.35 | 75 | 1.60 |
| Calendar year | |||||||
| 1958–1969 | 113 | 1.00 | 83 | 0.75 | 55 | 0.49 | |
| 1970–1979 | 124 | 1.75 | 86 | 1.20 | 42 | 0.59 | |
| 1980–1989 | 195 | 3.40 | 90 | 1.57 | 51 | 0.89 | |
| 1990–1999 | 204 | 5.08 | 71 | 1.76 | 46 | 1.14 | |
Table 3 provides type-specific case counts and crude rates by radiation dose, smoking status at diagnosis and gender. Crude rates for each type appeared to be strongly associated with smoking status. Crude rates were consistently higher for men than for women, although sex-specific rates were similar in men and women for small cell carcinoma among the current-smokers and for adenocarcinoma among never-smokers. While crude rates were higher for adenocarcinoma than for squamous cell carcinoma or small cell carcinoma, the crude risks for smokers relative to never-smokers were several-fold larger for squamous cell carcinoma or small cell carcinoma than for adenocarcinoma. Information on smoking status was available for 59% of men and 62% of women, among whom 86% of men but only 18% of women reported having smoked, while among these ever-smokers, 30% of men and 34% of women reported having quit as of their last survey responses (not shown). While interpretations of risk patterns with radiation dose by smoking status in Table 3 are hampered by the small number of cases, the “total” row for each type suggests a radiation-related increase in the risks. Tables S2 and S3 in the supplementary material (http://dx.doi.org/10.1667/RR2819.1.S1) present information similar to Tables 2 and 3, respectively, for all lung cancers, the not otherwise specified group, and the three major types as a group.
Table 3. Cases and Crude Rate (Cases per 10,000 Person-Years) bv Radiation Dose, Smoking Status at Diagnosis, and Sex.
| Radiation dose (Gy) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| < 0.005 | 0.005–0.2 | 0.2–1 | 1 + | Total | |||||||
|
|
|
|
|
|
|||||||
| Histologic type | Smoking status | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate |
| Men | |||||||||||
| Adenocarcinoma | Never | 1 1 | 4.0 | 4 | 2.0 | 0 | 0.0 | 0 | 0.0 | 15 | 2.7 |
| Past | 20 | 5.0 | 16 | 4.9 | 3 | 3.5 | 2 | 7.4 | 41 | 4.9 | |
| Current | 100 | 7.7 | 50 | 6.4 | 15 | 6.4 | 6 | 7.1 | 171 | 7.1 | |
| Unknown | 54 | 1.3 | 25 | 1.2 | 4 | 0.8 | 5 | 3.3 | 88 | 1.5 | |
| Total | 185 | 3.1 | 95 | 2.9 | 22 | 2.5 | 15 | 4.6 | 315 | 3.0 | |
| Squamous cell | Never | 2 | 0.7 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 3 | 0.5 |
| Past | 11 | 2.8 | 9 | 2.7 | 1 | 1.2 | 2 | 7.4 | 23 | 2.7 | |
| Current | 83 | 6.4 | 36 | 4.6 | 16 | 6.8 | 6 | 7.1 | 141 | 5.9 | |
| Unknown | 56 | 1.4 | 18 | 0.9 | 10 | 2.0 | 3 | 2.0 | 87 | 1.5 | |
| Total | 152 | 2.5 | 64 | 1.9 | 27 | 3.1 | 11 | 3.9 | 254 | 2.4 | |
| Small cell | Never | 1 | 0.4 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 2 | 0.4 |
| Past | 5 | 1.3 | 3 | 0.9 | 1 | 1.2 | 0 | 0.0 | 9 | 1.1 | |
| Current | 35 | 2.7 | 21 | 2.7 | 6 | 2.6 | 5 | 6.0 | 67 | 2.8 | |
| Unknown | 23 | 0.6 | 17 | 0.8 | 6 | 1.2 | 5 | 3.3 | 51 | 0.8 | |
| Total | 64 | 1.1 | 42 | 1.3 | 13 | 1.5 | 10 | 5.6 | 129 | 1.2 | |
| Subjects | 24,644 | 13,436 | 3,609 | 1,200 | 42,889 | ||||||
| Women | |||||||||||
| Adenocarcinoma | Never | 72 | 2.3 | 37 | 1.9 | 31 | 5.8 | 12 | 9.5 | 152 | 2.7 |
| Past | 5 | 3.3 | 3 | 2.1 | 1 | 2.6 | 0 | 0.0 | 9 | 2.6 | |
| Current | 16 | 3.8 | 11 | 4.0 | 7 | 6.8 | 5 | 17.7 | 39 | 4.7 | |
| Unknown | 68 | 1.1 | 42 | 1.3 | 9 | 1.0 | 2 | 0.9 | 121 | 1.1 | |
| Total | 161 | 1.6 | 93 | 1.7 | 48 | 3.0 | 19 | 4.9 | 321 | 1.8 | |
| Squamous cell | Never | 8 | 0.3 | 3 | 0.2 | 2 | 0.4 | 0 | 0.0 | 13 | 0.2 |
| Past | 4 | 2.6 | 1 | 0.7 | 1 | 2.6 | 0 | 0.0 | 6 | 1.8 | |
| Current | 8 | 1.9 | 6 | 2.2 | 8 | 7.7 | 1 | 5.5 | 23 | 2.8 | |
| Unknown | 19 | 0.3 | 7 | 0.2 | 3 | 0.5 | 5 | 2.5 | 34 | 0.3 | |
| Total | 39 | 0.4 | 17 | 0.3 | 14 | 0.9 | 6 | 1.6 | 76 | 0.4 | |
| Small cell | Never | 3 | 0.1 | 3 | 0.2 | 0 | 0.0 | 2 | 1.5 | 8 | 0.1 |
| Past | 0 | 0.0 | 1 | 0.7 | 1 | 2.6 | 0 | 0.0 | 2 | 0.6 | |
| Current | 9 | 2.2 | 7 | 2.5 | 4 | 3.9 | 2 | 7.1 | 22 | 2.7 | |
| Unknown | 23 | 0.4 | 7 | 0.2 | 3 | 0.3 | 0 | 0.0 | 33 | 0.3 | |
| Total | 35 | 0.4 | 18 | 0.3 | 8 | 0.5 | 4 | 1.0 | 65 | 0.4 | |
| Subjects | 35,417 | 19,864 | 5772 | 1442 | 62,515 | ||||||
For each of the three histological subtypes, Table 4 presents estimates and 95% CIs for selected parameters in the radiation and smoking excess risk functions under the generalized multiplicative model together with AIC values for the four interaction models, as described above, and models with only radiation or smoking effects. While the generalized multiplicative model performed better than the other interaction models for all lung cancers as a group (15), there was no strong evidence to prefer or reject any of the four interaction models in any type-specific analysis. The simple interaction models (additive or multiplicative) had the smallest AIC values but the differences were not large. Table S4 in the supplementary material (http://dx.doi.org/10.1667/RR2819.1.S1) shows that the generalized multiplicative model was significant over the simpler model for the not otherwise specified group (P = 0.003) or when the three major types were combined (P = 0.03).
Table 4. Parameter Estimates (959% CI) for Smoking Effects and Radiation Effects from a Generalized Multiplicative Model and AIC Values for the Alternative Joint Effect Models.
| Adenocarcinoma | Squamous cell | Small cell | |
|---|---|---|---|
| Smoking parameters | |||
| ERRa (male) | 2.42 (1.4, 3.8) | 12.71 (4.8,51.2) | 17.51 (4.6, 112.4) |
| ERRa (female) | 3.41 (0.9, 7.3) | 21.12 (9.7, 44.7) | 41.39 (16.8, 107.9) |
| Birth year (percentage change per decade decrease) | 6.02 (−25.1, 47) | 45.32 (3.7, 104.2) | 40.24 (−3.8, 103.9) |
| Log (duration/50) | 1.58 (−1.5, 5.5) | −0.72 (−2.5, 1) | −0.91 (−2.9, 0.7) |
| Log (duration/50)2 | −18.43 (−41.3, −4.9) | −2.27 (−6.6, 0.4) | −0.23 (−4.4, 0.5) |
| Log (years since quitting +1) | −0.39 (−12.9, 0.2) | −0.37 (−0.7, −0.1) | −0.59 (−1.1, −0.3) |
| Radiation parameters | |||
| ERR/Gyb (gender-averaged) | 0.75 (0.3. 1.3) | 0.27 (0.0. 1.5) | 1.49 (0.1,4.6) |
| ERR/Gyb (male) | 0.17 (0.0, 0.8) | 0.07 (0.0, 0.7) | 2.21 (0.2, 7.6) |
| ERR/Gyb (female) | 1.34 (0.6, 2.3) | 0.48 (0.0, 2.6) | 0.78 (0.0, 3.3) |
| Female/male ratio | 7.94 (1.8, Inf) | 6.89(1.6, Inf) | 0.35 (0.0, 2.3) |
| Age at exposure (percentage change per decade increase) | 8.74 (−33, 72) | 212.4 (26, 3476) | 4.36 (−48,221) |
| Attained age (power) | −2.34 (−5.3,0.8) | −8.91 (−25.9, −0.7) | −2.63(−9.7, 4.6) |
| P for generalized interaction | 0.26 | 0.54 | 0.18 |
| AIC | |||
| Radiation only | 4714 | 2762 | 1941 |
| Smoking only | 4691 | 2630 | 1854 |
| Additive | 4666 | 2618 | 1848 |
| Multiplicative | 4667 | 2617 | 1850 |
| Generalized additive | 4668 | 2622 | 1852 |
| Generalized multiplicative | 4668 | 2619 | 1850 |
Risk at age 70 for an unexposed person who was born in 1915 and smoked a pack of cigarettes per day for 50 years since age 20.
Risk for a never-smoker at age 70 after an exposure at 1 Gy at age 30.
Baseline Rate
Fitted baseline incidence rates were higher for adenocarcinoma than for the other two histological types at all ages. For each histological type, baseline rates were greater for men than for women, with the female:male ratio at age 70 estimated as 0.62 for adenocarcinoma, 0.49 for squamous cell carcinoma and 0.57 for small cell carcinoma. Age-specific rates tended to be lower for younger cohorts, decreasing by 23% (P < 0.001) per decade increase in birth year for adenocarcinoma, 14% (P = 0.28) for squamous cell carcinoma, and 16% (P = 0.28) for small cell carcinoma. Baseline rate estimates were significantly higher in Nagasaki than in Hiroshima for all lung cancers combined (P = 0.002) and adenocarcinoma (P = 0.03) but the city difference was not significant for either squamous cell carcinoma (P > 0.5) or small cell carcinoma (P = 0.39). Overall, there was little variation in fitted baseline rates among the interaction models for each type (not shown).
Smoking Effects
The effect of cumulative smoking amount was statistically significant for each type (P < 0.001), with consistently higher risks for women than for men with the same smoking history (Table 4, upper section). The smoking effect, expressed as the ERR at 50 pack-years for people with no exposure (unexposed), was significantly higher for small cell carcinoma (17.5/41.4 for men/women) and squamous cell carcinoma (12.7/21.1) than for adenocarcinoma (2.4/3.4). The difference in smoking effects among the models was relatively large for small cell carcinoma (the gender-averaged ERRs per 50 pack-years were estimated as 27, 29, 35 and 35 for MM, GMM, AM and GAM, respectively). Smoking ERR estimates were consistently larger under the additive model than under the multiplicative model, reflecting the fact that the ERR in the additive model is relative to unexposed never-smokers while, in the multiplicative model, it is relative to never-smokers with the same radiation exposure.
Smoking cessation led to a significant reduction in risk compared to the risk that would have been incurred if the individual had continued smoking for small cell carcinoma (P < 0.001) and squamous cell carcinoma (P = 0.004). The reduction in risk for adenocarcinoma was not statistically significant (P = 0.44), but similar in magnitude to that for the other types. There were some indications of variation in smoking effects among birth-cohort groups for squamous cell carcinoma (P = 0.03) and small cell carcinoma (P = 0.08); the ERR tended to be increasing for earlier-birth cohorts for both of these types, while there was no indication of a significant birth-cohort effect for adenocarcinoma (P > 0.5).
Figure 1 plots the fitted gender- and age-specific incidence rates (upper panel) and smoking ERR (lower panel) for lifetime never-smokers, smokers who have continuously smoked a pack (20 cigarettes) per day since age 20, and smokers who started smoking a pack per day at age 20 and quit at age 50 (among unexposed people born in 1915). Adenocarcinoma had the smallest smoking ERR, but its incidence was still the highest of the three types among smokers of the same smoking history. While the smoking ERRs were consistently higher for females than for males, the absolute rates were higher among males than among females for adenocarcinoma and squamous cell carcinoma, but not for small cell carcinoma.
Fig. 1.
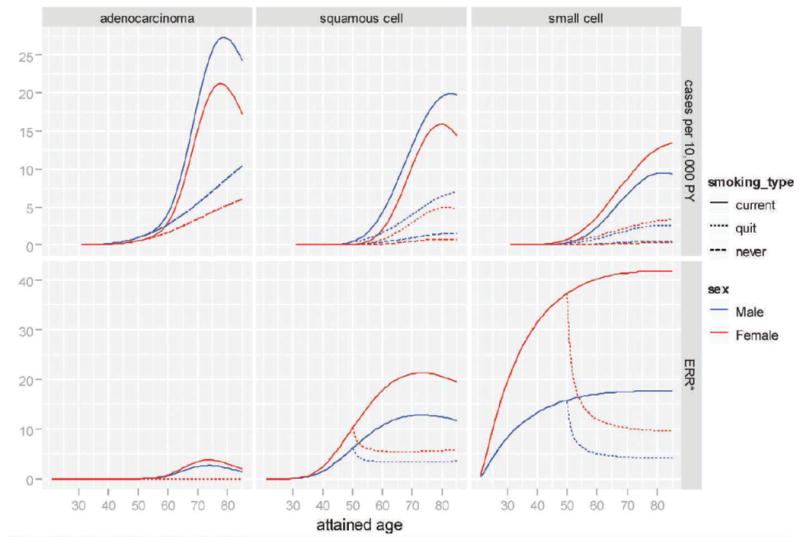
Age- and gender-specific absolute rate (upper panel) and excess relative risk of smoking (lower panel). Solid curves are for smokers who started at age 20 and never stopped. [The dotted curves are for past smokers who started at age 20 and stopped at age 50. The dashed curves are for lifetime never-smokers. The curves correspond to risk for an unexposed person born in 1915.
Radiation Effects
Radiation exposure significantly increased the risk for each histological type. As shown in Table 4, the radiation effect, expressed as the gender-averaged ERR per Gy (at age 70 for never-smokers exposed at age 30), was highest for small cell carcinoma (1.49, 95% CI: 0.1–4.6), followed by those for adenocarcinoma (0.75, 0.3–1.3) and for squamous cell carcinoma (0.27, 0.0–1.5). The large radiation ERR for small cell carcinoma, however, had a wide confidence interval due to the small number of cases among never-smokers. There was no evidence of a statistically significant departure from the simple linear dose response in any of the type-specific analyses.
The ERR/Gy tended to be higher among females than among males for both adenocarcinoma (female:male ratio = 7.9, P = 0.01) and for squamous cell carcinoma (6.9, P = 0.01). For small cell carcinoma, however, radiation ERRs were slightly, but not significantly, lower for women than for men (0.35, P = 0.27). Given the smoking history, the radiation effect tended to decrease with increasing attained age: rapidly and significantly for squamous cell carcinoma (P = 0.03), but somewhat less rapidly and not significantly for either adenocarcinoma (P = 0.13) or for small cell carcinoma (P = 0.43). Age-specific radiation risks for squamous cell carcinoma showed a rapid increase with increasing age at exposure (P = 0.01). However, there was little evidence of age at exposure effects for either adenocarcinoma (P > 0.5) or small cell carcinoma (P > 0.5). Alternatively, we fitted a model with the radiation ERR replacing both attained age and age at exposure with time since exposure only. For each type, as well as all lung cancers combined, these models showed a slight improvement in AIC, with risk estimates little affected, indicating that the time dependent variation of the radiation risk might be more parsimoniously explained by a risk decrease with time since exposure only (not shown).
Smoking-Radiation Joint Effects
Figure 2 presents two summaries of estimated radiation ERRs/Gy as a function of smoking intensity. The upper panel presents gender-averaged ERRs/Gy relative to those for unexposed people with the same smoking history under the four interaction models and the lower panel presents gender-averaged and gender-specific ERRs/Gy relative to those for unexposed never-smokers under the generalized multiplicative model. These were all computed as the risks at age 70 for those who were exposed at age 30 and smoked a fixed number of cigarettes per day since age 20.
Fig. 2.
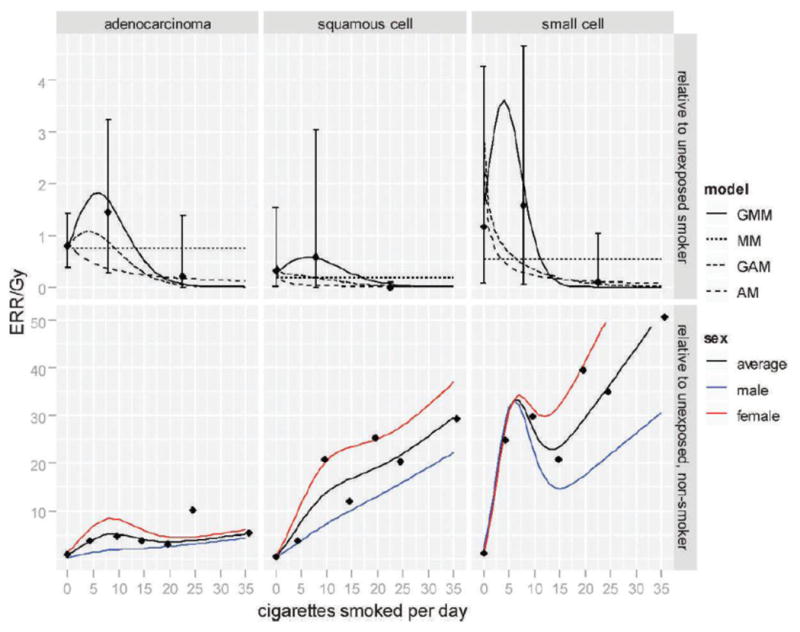
Excess relative risk at 1 Gy as a function of smoking intensity, relative to unexposed smokers with the same smoking history (upper panel) and to unexposed non-smokers (lower panel). The curves correspond to risk at age 70, for one exposed at age 30 who smoked a pack/day for 50 years since age 20. In the upper panel, gender-averaged risks were plotted for each fitted interaction model. In the lower panel, the gender-averaged and gender-specific risks were plotted for the generalized multiplicative model. All points are categorical estimates of the ERRs (with 95% confidence intervals in the upper panel) based on a generalized multiplicative model in which smoking intensity categories replaced the linear-quadratic function of log intensity.
Although inclusion of the generalized interaction term ω(S) did not significantly improve the fit over the simple interactions in any of the type-specific analyses, the fitted curves in Fig. 2 suggest that, given the duration of smoking, the nature of the variation in the ERR/Gy with smoking intensity might be similar across the three histological groups. As the smoking intensity increased, the ERR/Gy tended to increase relatively rapidly (up to about 10 cigarettes per day) and then decrease toward no radiation-associated excess risk (at 20 or more cigarettes per day). This trend was most evidently demonstrated in analysis for small cell carcinoma. Similar patterns were observed for the not otherwise specified group and the three major types as a group (see Supplementary Material: http://dx.doi.org/10.1667/RR2819.1.S1, Fig. S2) and for both of these groups, the generalized models described the data markedly better than simple additive or multiplicative models (see Supplementary Material: http://dx.doi.org/10.1667/RR2819.1.S1,Table S4).
Table 5 summarizes the distribution of observed and fitted cases over dose categories for the generalized multiplicative model. The fitted cases are broken down into baseline cases, excess cases associated with radiation exposure, smoking, or radiation-smoking interaction, and among those with no smoking data over predictions for unexposed never-smokers in the same dose group. About 26% (111 of 427 cases) of the cases among subjects with smoking history data appeared to be associated with smoking for adenocarcinoma, while this proportion was much higher for squamous cell carcinoma (81%; 170 of 209) and for small cell carcinoma (84%; 92 of 110). About 7% of the adenocarcinoma cases, 3% of the squamous cell carcinoma cases, and 4% of the small cell carcinoma cases appeared to be associated with radiation exposure; this proportion increased to 56%, 26% and 22%, respectively, among the highest dose category (>1 Gy). Excess cases associated with the radiation-smoking interaction accounted for 1–3% of the cases with known smoking history for each type and about 10% of the radiation-associated cases for adenocarcinoma, 53% for squamous cell carcinoma and 44% for small cell carcinoma. Regardless of the type of interaction, the total estimated numbers of smoking-associated cases were generally similar for each type but the model with no smoking adjustment provided the largest estimate of radiation-related excess cases.
Table 5. Observed and Fitted Cases by Dose Category for the Generalized Multiplicative Model with Totals for Alternative Models.
| Excess cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Model | Dose (Gy) | Cases | Baseline (no smoking) | Radiation only | Radiation and smoking | Smoking only | Unknown smoking | Total | Total radiation excess | Total smoking excess |
| Adenocarcinoma | ||||||||||
| Generalized multiplicative model | <0.005 | 346 | 271.3 | 0.2 | 0.0 | 60.6 | 6.6 | 67.4 | 0.2 | 60.6 |
| 0.005–0.2 | 188 | 146.4 | 7.0 | 0.7 | 31.6 | 3.9 | 43.2 | 7.7 | 32.3 | |
| 0.2–1 | 70 | 41.6 | 18.6 | 2.2 | 10.7 | 1.3 | 32.8 | 20.8 | 12.9 | |
| 1 + | 32 | 11.2 | 16.5 | 1.6 | 3.5 | 0.4 | 22.0 | 18.1 | 5.1 | |
| Total | 636 | 470.5 | 42.3 | 4.5 | 106.3 | 12.3 | 165.5 | 46.8 | 110.8 | |
| Radiation only model | 586.0 | 50.0 | 50.0 | |||||||
| Additive model | 465.5 | 48.0 | 0.0 | 107.2 | 15.3 | 170.5 | 48.0 | 107.2 | ||
| Multiplicative model | 474.1 | 43.2 | 3.8 | 103.8 | 11.0 | 161.9 | 47.0 | 107.6 | ||
| Generalized additive model | 465.1 | 43.5 | 4.4 | 106.7 | 16.3 | 170.9 | 47.9 | 111.0 | ||
| Squamous cell carcinoma | ||||||||||
| Generalized multiplicative model | <0.005 | 191 | 34.6 | 0.0 | 0.0 | 92.1 | 51.9 | 144.0 | 0.0 | 92.1 |
| 0.005–0.2 | 81 | 19.6 | 0.9 | 0.7 | 50.3 | 26.8 | 78.7 | 1.6 | 51.0 | |
| 0.2-1 | 41 | 5.6 | 2.2 | 2.3 | 17.0 | 10.2 | 31.7 | 4.5 | 19.3 | |
| 1 + | 17 | 1.5 | 1.7 | 2.7 | 5.1 | 4.7 | 14.2 | 4.4 | 7.8 | |
| Total | 330 | 61.3 | 1.9 | 5.7 | 164.6 | 93.5 | 268.7 | 10.6 | 170.3 | |
| Radiation only model | 310.0 | 20.0 | 20.0 | |||||||
| Additive model | 63.2 | 14.0 | 0.0 | 168.3 | 84.6 | 266.8 | 14.0 | 168.3 | ||
| Multiplicative model | 63.0 | 4.8 | 4.3 | 165.2 | 92.8 | 267.0 | 9.1 | 169.5 | ||
| Generalized additive model | 61.8 | 13.8 | 2.6 | 166.4 | 85.5 | 268.2 | 16.3 | 168.9 | ||
| Small cell carcinoma | ||||||||||
| Generalized multiplicative model | <0.005 | 99 | 14.2 | 0.0 | 0.0 | 48.6 | 39.2 | 87.8 | 0.0 | 48.6 |
| 0.005–0.2 | 60 | 7.9 | 0.6 | 0.4 | 27.4 | 19.9 | 48.3 | 1.0 | 27.8 | |
| 0.2-1 | 21 | 2.3 | 1.6 | 1.1 | 9.6 | 8.8 | 21.4 | 3.0 | 11.0 | |
| 1 + | 1 1 | 0.6 | 1.7 | 1.1 | 3.0 | 5.4 | 11.5 | 3.1 | 4.4 | |
| Total | 194 | 24.9 | 3.9 | 3.1 | 88.6 | 73.5 | 169.1 | 7.0 | 91.7 | |
| Radiation only model | 174.9 | 19.1 | 19.1 | |||||||
| Additive model | 22.6 | 14.5 | 0.0 | 88.6 | 68.3 | 171.4 | 14.5 | 88.6 | ||
| Multiplicative model | 28.2 | 2.2 | 7.8 | 83.2 | 72.6 | 165.8 | 10.0 | 91.0 | ||
| Generalized additive model | 22 2 | 15.6 | −0.7 | 88.8 | 68.2 | 171.8 | 14.9 | 88.1 | ||
Discussion
It has been recognized that the association between smoking and lung cancer incidence varies by histological type (25–28). Our analyses demonstrated that smoking ERRs were markedly higher for small cell carcinoma (18/41 per 50 pack-years for men/women born in 1915) and squamous cell carcinoma (13/21) than for adenocarcinoma (2.4/3.4). These results were comparable to the risk estimates reported in another large Japanese cohort study (29). For each of the three histological types, and all lung cancers as a group, the estimated smoking risks appeared to be smaller than those reported for Western populations (1, 18). As suggested by the “smoking-only” excess case estimates (Table 5), histology-type differences in excess cases were not as marked as those in the ERRs with the largest increases for squamous cell carcinoma and the smallest increases for small cell carcinoma.
Previous studies of radiation effects by lung cancer histological type have provided rather inconsistent results. Prior studies among atomic bomb survivors (30–32) were based on limited numbers of cases diagnosed histopathologically by means of autopsies, surgical resections, or biopsies. Land et al. noted that radiation-induced cancers appeared more likely to be small cell carcinoma and less likely to be adenocarcinoma both in atomic bomb survivors (exposed predominately to penetrating γ rays) and another cohort of American uranium miners (exposed to low-energy α particles with low entrance) (30). In studies of radiation-treated patients, the largest ERRs/Gy were seen for adenocarcinoma and large-cell carcinoma among Hodgkin's disease patients (13), while squamous cell carcinoma was the most closely related to radiotherapy among breast cancer patients with a significant effect only among smokers (33). In a Swedish case-control study of residential-radon exposure and lung cancer, stronger associations with increasing levels of residential-radon exposure were suggested for small cell carcinoma and adenocarcinoma than for squamous cell carcinoma (9), while a larger excess odds ratio was observed for small cell carcinoma than for adenocarcinoma and squamous cell carcinoma in a combined analysis of seven case-control studies in North America (11). In our study, conducting formal tests comparing ERRs across types was difficult in the presence of relatively strong modification of radiation effects by smoking levels. However, visual inspection of Fig. 2, as well as ERR/Gy coefficient estimates in Table 4, suggests that the relative effect of radiation might be larger for small cell carcinoma and adenocarcinoma than for squamous cell carcinoma in the LSS.
It has been previously noted that the age-specific radiation-associated excess risk for lung cancers tends to increase with increasing age at exposure in the LSS (6, 15). Our type-specific analyses suggest that this increase could be largely due to that for squamous cell carcinoma. The possibility of non-decreasing patterns of radiation-associated cancer risks with increasing age-at-exposure has been a matter of recent interest (34–36). Using a biologically-based model that allows radiation to act as both an initiator and a promoter of lung cancer, Shuryak et al. suggested that variations in the age at exposure pattern for different cancer types might be explained by differences in the relative importance of initiation and promotion effects of the exposure (36). In this context, our findings suggest that promotion (or late stage) effects of radiation might be more important for lung cancer, particularly squamous cell carcinoma, than they are for many other types of solid cancers.
To our knowledge, this is the first epidemiological study to quantify the joint interaction effects of radiation and smoking on lung cancers of different histological types. For all lung cancers as a group, many previous reports concluded that the interaction could be more multiplicative than additive (8, 9, 12, 13). Our type-specific analyses did not conclusively identify the type of interaction, in contrast to our previous analysis pooling all lung cancers (15) in which the generalized multiplicative model fit statistically better than the multiplicative model or additive model. However, despite the lack of statistical significance, our analyses suggest that the type-specific radiation effects might share a complex dependence on smoking intensity like that seen in the pooled analysis. Specifically, the effect of a given radiation dose tends to be larger for moderate smokers than for heavier smokers given the duration of smoking. The slight variations in this pattern observed among different histological types seem to reflect the differences in the strength of association with smoking or radiation. The lung cancer not otherwise specified group may be mostly a collection of the three major types, with some unknown numbers of metastatic cancer misclassified as lung cancer. It is of interest to note that the interaction pattern seen for this group is similar to that for all three types of lung cancer combined. The reductions in radiation-associated risk with increasing smoking intensity might be related to the inverse exposure rate effect in the smoking-associated lung cancer risks that is seen in the LSS and other populations (15, 37); namely, smoking at a lower intensity for a longer duration tends to be more deleterious than smoking at a higher intensity for a shorter duration. Potential explanations for this leveling-off include intensity-dependent molecular mechanisms and nicotine-related smoking behavioral factors (37). Our findings suggest that radiation might contribute most markedly to lung cancer risks in smokers of intensities at which the intensity-dependent modification of the smoking effects is the largest.
With largely complete cancer ascertainment, long-term follow up of a large cohort of men and women of all ages, and of detailed smoking information, the LSS cohort is an excellent resource for the study of the joint effect of radiation and smoking on lung cancer risks. However, the LSS data have some limitations, particularly with regard to the completeness of the smoking data. In the analyses we assumed that people's smoking habits had not changed since the last time they provided information on smoking, which was no later than 1992. In view of the age of the cohort members it is unlikely that many have begun to smoke in recent years, but it is likely that many have quit smoking. This will possibly lead to some downward bias in estimates of the effect of smoking cessation, but how this impacts estimates of radiation effects is unclear. In addition, despite the size of the cohort and the relatively large number of cases, the amount of information relevant to inference about the nature of the radiation-smoking interaction was still limited due to the highly skewed dose distribution and the unavailability of histology information for about 30% of the cases. Furthermore, the smaller relative risks associated with smoking in Japan make it challenging to apply the results to non-Japanese populations.
In summary, our analyses of the LSS demonstrated that both smoking and radiation significantly increased the risks for the three major types of lung cancer (AC, SqCC and SmCC). Despite some variation in magnitude of the main effects of smoking and radiation among the types, all of these lung cancer types shared a relatively consistent pattern for the smoking-radiation interaction; radiation effects tended to be largest for those who smoked modest amounts and much lower for heavy smokers. This pattern might be related to the inverse exposure-rate pattern generally observable for smoking-related cancer risks. Our findings provide information on smoking effects for various types of lung cancer in Japan and contribute to the sparse literature on the radiation-smoking interaction effects on lung cancer histological type.
Supplementary Material
Acknowledgments
This work was supported by the Radiation Effects Research Foundation (RP1-94), Hiroshima and Nagasaki, Japan, and the U.S. National Cancer Institute intramural research program (NO2CP-2009-00005). The Radiation Effects Research Foundation is a private nonprofit foundation funded by the Japanese Ministry of Health, Labour and Welfare and the U.S. Department of Energy, the latter in part through the National Academy of Sciences.
References
- 1.Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW., Jr Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst. 1997;89:1580–86. doi: 10.1093/jnci/89.21.1580. [DOI] [PubMed] [Google Scholar]
- 2.Sobue T, Ajiki W, Tsukuma H, Oshima A, Hanai A, Fujimoto I. Trends of lung cancer incidence by histologic type: a population-based study in Osaka, Japan. Jpn J Cancer Res. 1999;90:6–15. doi: 10.1111/j.1349-7006.1999.tb00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssen-Heijnen ML, Coebergh JW. Trends in incidence and prognosis of the histological subtypes of lung cancer in North America, Australia, New Zealand and Europe. Lung Cancer. 2001;31:123–37. doi: 10.1016/s0169-5002(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 4.Burns DM, Anderson CM, Gray N. Do changes in cigarette design influence the rise in adenocarcinoma of the lung? Cancer Caus Contr. 2011;22:13–22. doi: 10.1007/s10552-010-9660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito H, Matsuo K, Tanaka H, Koestler DC, Ombao H, Fulton J, et al. Nonfilter and filter cigarette consumption and the incidence of lung cancer by histological type in Japan and the United States: analysis of 30-year data from population-based cancer registries. Int J Cancer. 2011;128:1918–28. doi: 10.1002/ijc.25531. [DOI] [PubMed] [Google Scholar]
- 6.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 7.Sokolnikov ME, Gilbert ES, Preston DL, Ron E, Shilnikova NS, Khokhryakov VV, et al. Lung, liver and bone cancer mortality in Mayak workers. Int J Cancer. 2008;123:905–11. doi: 10.1002/ijc.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Research Council Committee on the Biological Effects of Radiation. The Health Effects on Exposure to Indoor Radon (BEIR VI) National Academy Press; Washington, DC: 1998. [Google Scholar]
- 9.Pershagen G, Akerblom G, Axelson O, Clavensjo B, Damber L, Desai G, et al. Residential radon exposure and lung cancer in Sweden. N Engl J Med. 1994;330:159–64. doi: 10.1056/NEJM199401203300302. [DOI] [PubMed] [Google Scholar]
- 10.Darby S, Hill D, Auvinen A, Barros-Dios JM, Baysson H, Bochicchio F, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 2005;330:223. doi: 10.1136/bmj.38308.477650.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krewski D, Lubin JH, Zielinski JM, Alavanja M, Catalan VS, Field RW, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology. 2005;16:137–45. doi: 10.1097/01.ede.0000152522.80261.e3. [DOI] [PubMed] [Google Scholar]
- 12.Travis LB, Gospodarowicz M, Curtis RE, Clarke EA, Andersson M, Glimelius B, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J Natl Cancer Inst. 2002;94:182–92. doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert ES, Stovall M, Gospodarowicz M, Van Leeuwen FE, Andersson M, Glimelius B, et al. Lung cancer after treatment for Hodgkin's disease: focus on radiation effects. Radiat Res. 2003;159:161–73. doi: 10.1667/0033-7587(2003)159[0161:lcatfh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Pierce DA, Sharp GB, Mabuchi K. Joint effects of radiation and smoking on lung cancer risk among atomic bomb survivors. Radiat Res. 2003;159:511–20. doi: 10.1667/0033-7587(2003)159[0511:jeoras]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa K, Preston DL, Lonn S, Funamoto S, Yonehara S, Matsuo T, et al. Radiation and smoking effects on lung cancer incidence among atomic bomb survivors. Radiat Res. 2010;174:72–82. doi: 10.1667/RR2083.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stellman SD, Takezaki T, Wang L, Chen Y, Citron ML, Djordjevic MV, et al. Smoking and lung cancer risk in American and Japanese men: an international case-control study. Cancer Epidemiol Biomarkers Prev. 2001;10:1193–9. [PubMed] [Google Scholar]
- 17.Crispo A, Brennan P, Jockel KH, Schaffrath-Rosario A, Wichmann HE, Nyberg F, et al. The cumulative risk of lung cancer among current, ex- and never-smokers in European men. Br J Cancer. 2004;91:1280–6. doi: 10.1038/sj.bjc.6602078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354:333–42. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 19.Cullings HM, Fujita S, Funamoto S, Grant EJ, Kerr GD, Preston DL. Dose estimation for atomic bomb survivor studies: its evolution and present status. Radiat Res. 2006;166:219–54. doi: 10.1667/RR3546.1. [DOI] [PubMed] [Google Scholar]
- 20.Travis WD, Sobin LH. Histological typing of lung and pleural tumours. Springer-Verlag, Berlin; New York: 1999. [Google Scholar]
- 21.Pierce DA, Stram DO, Vaeth M. Allowing for random errors in radiation dose estimates for the atomic bomb survivor data. Radiat Res. 1990;123:275–84. [PubMed] [Google Scholar]
- 22.Preston DL, Lubin JH, Pierce DA, McConney ME. Epicure Users Guide. Hirosoft International Corporation. Seattle, WA: 1993. [Google Scholar]
- 23.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–23. [Google Scholar]
- 24.Walsh L. A short review of model selection techniques for radiation epidemiology. Radiat Environ Biophys. 2007;46:205–13. doi: 10.1007/s00411-007-0109-0. [DOI] [PubMed] [Google Scholar]
- 25.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–9. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 26.Radzikowska E, Glaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival Population-based study of 20 561 cases. Ann Oncol. 2002;13:1087–93. doi: 10.1093/annonc/mdf187. [DOI] [PubMed] [Google Scholar]
- 27.Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15:5626–45. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yun YH, Lim MK, Jung KW, Bae JM, Park SM, Shin SA, et al. Relative and absolute risks of cigarette smoking on major histologic types of lung cancer in Korean men. Cancer Epidemiol Biomarkers Prev. 2005;14:2125–30. doi: 10.1158/1055-9965.EPI-05-0236. [DOI] [PubMed] [Google Scholar]
- 29.Sobue T, Yamamoto S, Hara M, Sasazuki S, Sasaki S, Tsugane S. Cigarette smoking and subsequent risk of lung cancer by histologic type in middle-aged Japanese men and women: the JPHC study. Int J Cancer. 2002;99:245–51. doi: 10.1002/ijc.10308. [DOI] [PubMed] [Google Scholar]
- 30.Land CE, Shimosato Y, Saccomanno G, Tokuoka S, Auerbach O, Tateishi R, et al. Radiation-associated lung cancer: a comparison of the histology of lung cancers in uranium miners and survivors of the atomic bombings of Hiroshima and Nagasaki. Radiat Res. 1993;134:234–43. [PubMed] [Google Scholar]
- 31.Thompson DE, Mabuchi K, Ron E, Soda M, Tokunaga M, Ochikubo S, et al. Cancer incidence in atomic bomb survivors Part II: Solid tumors, 1958–1987. Radiat Res. 1994;137:S17–67. [PubMed] [Google Scholar]
- 32.Yamamoto T, Kopecky KJ, Fujikura T, Tokuoka S, Monzen T, Nishimori I, et al. Lung cancer incidence among Japanese A-bomb survivors, 1950–80. J Radiat Res (Tokyo) 1987;28:156–71. doi: 10.1269/jrr.28.156. [DOI] [PubMed] [Google Scholar]
- 33.Prochazka M, Hall P, Gagliardi G, Granath F, Nilsson BN, Shields PG, et al. Ionizing radiation and tobacco use increases the risk of a subsequent lung carcinoma in women with breast cancer: case-only design. J Clin Oncol. 2005;23:7467–74. doi: 10.1200/JCO.2005.01.7335. [DOI] [PubMed] [Google Scholar]
- 34.Little MP. Heterogeneity of variation of relative risk by age at exposure in the Japanese atomic bomb survivors. Radiat Environ Biophys. 2009;48:253–62. doi: 10.1007/s00411-009-0228-x. [DOI] [PubMed] [Google Scholar]
- 35.Shuryak I, Hahnfeldt P, Hlatky L, Sachs RK, Brenner DJ. A new view of radiation-induced cancer: integrating short- and long-term processes Part I: approach. Radiat Environ Biophys. 2009;48:263–74. doi: 10.1007/s00411-009-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shuryak I, Sachs RK, Brenner DJ. Cancer risks after radiation exposure in middle age. J Natl Cancer Inst. 2010;102:1628–36. doi: 10.1093/jnci/djq346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lubin JH, Virtamo J, Weinstein SJ, Albanes D. Cigarette smoking and cancer: intensity patterns in the alpha-tocopherol, beta-carotene cancer prevention study in Finnish men. Am J Epidemiol. 2008;167:970–5. doi: 10.1093/aje/kwm392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


