Abstract
In most people language is processed predominantly by the left hemisphere, but we don’t know how or why. A popular view is that developmental language disorders result from a poorly lateralized brain, but until recently evidence has been weak and indirect. Modern neuroimaging methods have made it possible to study normal and abnormal development of lateralized function in the developing brain and have confirmed links with language and literacy impairments. However, there is little evidence that weak cerebral lateralization has common genetic origins with language/literacy impairments. Our understanding of the association between atypical language lateralization and developmental disorders may benefit if we reconceptualise the nature of cerebral asymmetry to recognize its multidimensional nature and to take into account variation in lateralization over developmental time. Contrary to popular belief, cerebral lateralization may not be a highly heritable, stable characteristic of individuals; rather, weak lateralization may be a consequence of impaired language learning.
Why are some children poor at language learning?
Human language is distinct from other animal communication systems in its complexity yet it is acquired effortlessly by children. We have known since the nineteenth century that language depends on specialized brain systems in the left cerebral hemisphere (1), and we are starting to understand the neurological basis of language networks at macroscopic, microscopic and molecular levels (2, 3).
The claim that children acquire language effortlessly needs qualification, however. For some, mastery of their native tongue is a struggle: they may be slow to produce their first words and subsequently never achieve the grammatical complexity and fluent understanding shown by their peers. Although such difficulties can occur as the consequence of a genetic syndrome, neurological disease, or hearing loss, usually they have no obvious cause (4). This kind of unexplained, selective problem with language learning is known as specific language impairment (SLI).
There are close links between SLI and developmental dyslexia. Most children with oral language impairments have difficulties learning to read, and it is not uncommon for children with SLI to be identified as dyslexic as they move into school age (5). It would be misleading to suggest that SLI and dyslexia are the same thing, but there is considerable overlap between the two conditions, and very often the specific diagnostic label that is given is more a function of the person making the diagnosis than the actual profile of impairment. For this reason, the term ‘language/literacy impairment’ will be used here to refer generically to both conditions.
The neurobiological basis of both dyslexia and SLI remains unclear. For understandable reasons, brain-imaging studies with affected children are rare. Such studies as have been done seldom find any gross evidence of structural brain abnormality, though differences from controls have been reported for proportions of gray matter in specific regions, and for gyral morphology (6-8). However, these associations are probabilistic: there is substantial heterogeneity among affected individuals, and abnormalities that are found are seldom specific to language/literacy impairment.
One recurring theme in research on neurobiology of language/literacy impairments is the idea that disruption of the normal pattern of left-hemisphere language lateralization may be implicated. This makes intuitive sense: a lateralized brain appears to have evolved in humans under strong selection pressure, yet left-hemisphere language is not universal in all people. It follows that, if a lateralized brain facilitates language learning, then a failure of lateralization could be a cause of poor language development.
Are language/literacy impairments associated with atypical cerebral lateralization?
As early as 1925 Orton (9) had suggested developmental reading problems might be the result of a poorly lateralized brain. This link was developed more fully in the 1980s, when Annett formulated a detailed genetic theory of lateralization that linked developmental dyslexia and language disorders to specific genotypes of a postulated ‘right shift’ factor that was thought to influence both speech laterality and handedness (10). The field was constrained, however, by difficulties in direct assessment of cerebral lateralization for speech. Prior to the development of modern neuroimaging, the only reliable way of assessing cerebral lateralization in an individual was the Wada technique, an invasive pre-surgical procedure in which anaesthetic is injected into one carotid artery to produce a transient inactivation of the corresponding hemisphere (11). This was clearly inappropriate for research use with children, and for many years, most studies of laterality in children with developmental disorders focused on handedness as a proxy measure. However, this is far from ideal, as it is an indirect and imprecise indicator of lateralization for speech and language, which is not clearly associated with language and literacy problems (Box 1).
Box 1. Handedness, cerebral lateralization and language/literacy impairment: myth and reality.
It is commonly asserted as fact that left-handedness is associated with conditions such as dyslexia, and that handedness is highly heritable, and yet the evidence for both claims is lacking. A comprehensive review of the literature found no association between handedness and language/literacy impairments (88). Twin studies confirm that there is some genetic influence on handedness, but around 75% of the variance is non-genetic and specific to the individual, with only 25% explained by genes (33). Molecular genetic studies have found significant associations with handedness, but the effects are complex and small in magnitude, suggesting a complex polygenic etiology, rather than a single gene that determines an individual’s handedness (89). Furthermore, the relationship between handedness and language laterality, though significant, is imperfect: around 96% of right-handers have left hemisphere speech, compared with around 70% of left-handers (90). For these reasons, research using handedness as a proxy for cerebral lateralization will not be included in this review.
With the advent of neuroimaging, it became possible to look more directly at the brain in children. Most studies, though, considered only structural brain asymmetries. There was initial excitement at claims for reduced asymmetry of the planum temporale – a region important for receptive language – in dyslexia, but later studies gave a more confusing picture, and results appeared to depend on precisely how asymmetry is measured (12), and also on the type of specific learning disability (13). Furthermore, planum temporale asymmetry is not correlated with the lateralization of the brain response to a language activation task, though some other asymmetries of brain structure are (14, 15).
It became easier to study cerebral lateralization when functional transcranial Doppler ultrasound (fTCD) was adapted for imaging blood flow in the left and right middle cerebral arteries simultaneously while people performed language tasks (16). A word generation task (e.g. “think of as many words as possible beginning with the letter B”) typically induces enhanced blood flow to the left hemisphere for a period of a few seconds after initiating the task. The method is adequately reliable and results correspond well with those from the Wada technique (17).
The word generation task has been given to adults with SLI (18) and university students with developmental dyslexia (19), revealing reduced language laterality in both groups. In neither study was weak lateralization explained by poor task performance. Subsequently, a handful of studies used functional magnetic resonance imaging (fMRI) to measure language lateralization in children or adults with language/literacy impairments, and these mostly found that cerebral lateralization was weaker than in typically-developing individuals (20-22).
Are there genes that influence cerebral lateralization?
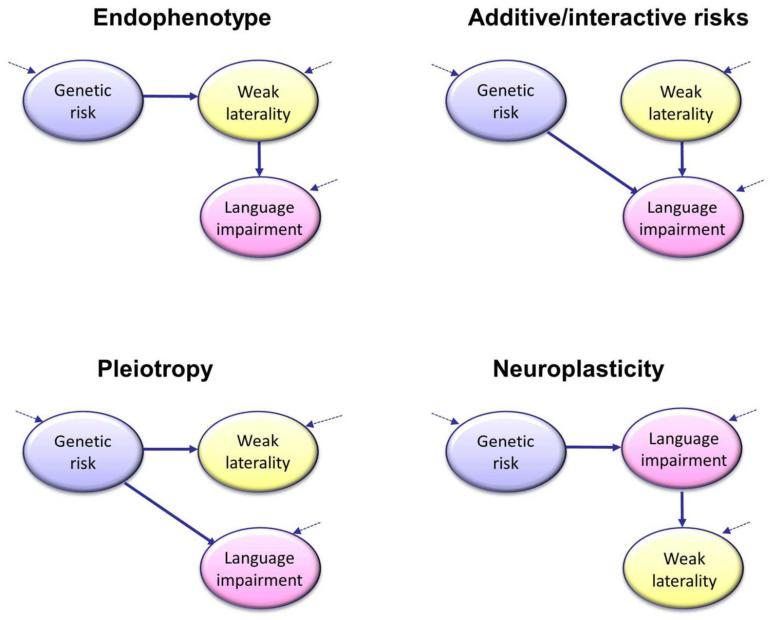
Developmental language and literacy disorders are heritable, and the pattern of inheritance suggests they are complex multifactorial disorders, caused by the combined action of many genes and environmental risk factors, each of small effect (23). Findings vary according to how the phenotype is defined; for children about whom there is some clinical concern, twin studies estimate heritability of language impairment as around 70%. Developmental dyslexia gives similar estimates (24) and there is some overlap in genetic variants that confer risk for language and literacy problems (25). This raises the possibility that the genetic risk for dyslexia and SLI could be mediated via an effect on cerebral lateralization, as shown in the Endophenotype model of Figure 1. An alternative genetic explanation is the Pleiotropy model (Figure 1), which postulates that the same genes that act as risk factors for language/literacy problems also affect cerebral lateralization, but it does not entail that cerebral lateralization mediates the relationship between genes and language/literacy problems. One difference between the two theories concerns the strength of predicted relationship between genes and cerebral asymmetry. If cerebral asymmetry is a mediating factor, then we would expect to see stronger links between genes and cerebral asymmetry than between genes and language/literacy problems (26). This is not the case for the Pleiotropy account.
Figure 1.
Four path diagrams of the association between weak cerebral lateralization for language and language/literacy problems. These causal models are simplified abstractions and not mutually exclusive, but they illustrate the differing predictions about the pattern of associations that might be found between genotypes, language/literacy impairment and cerebral lateralization. The Endophenotype model depicts the situation where genes that influence risk for language impairment do so by affecting cerebral lateralization. This model predicts that cerebral lateralization should be at least as heritable as language impairment, with the same genes affecting both traits. The Pleiotropy model also assumes that the same genes that lead to risk for language impairment also affect cerebral lateralization, but there is no direct causal link: weak laterality and language impairment co-occur because they have common origins, not because one causes the other. In the Additive/interactive risks model, the genetic risk factors for language impairment do not affect lateralization. However, weak laterality, which could have genetic and/or non-genetic origins, exerts an independent causal influence on language impairment, which may add or interact with other genetic risk factors. In the Neuroplasticity model, cerebral lateralization has no causal effect on language; rather, language impairment influences how the brain develops and is associated with weaker cerebral lateralization. The dotted arrows indicate that for each construct there will be sources of variation in addition to those depicted in the model.
Specific genes are known to affect asymmetric development in various animal species, notably worms, fish and songbirds (27); however, our interest here is not so much in overall population biases as in individual differences in cerebral lateralization. There could be genes that lead to lateral biases of the body form in humans, but this need not entail that there is allelic variation in those genes: observed individual differences could be determined by environmental factors or chance. So the key question is whether humans have genes that are polymorphic, (i.e., taking different forms in different people), where the specific version of the gene affects cerebral asymmetry. One way of quantifying the role of genes in accounting for individual variation in brain asymmetry is to compare phenotypic resemblance in people with different degrees of genetic similarity. Twins provide a useful natural experiment, because we can compare monozygotic (MZ) twins, who are genetically identical, with dizygotic (DZ) twins, who share on average 50 per cent of alleles from polymorphic genes. Insofar as genes are important in determining a phenotype, we should see greater phenotypic similarity between MZ twin pairs than DZ twin pairs. In one study, frontal, temporal, parietal and occipital brain volumes were measured in 72 MZ and 67 DZ twin pairs (28). Estimates of heritability were high for raw brain volumes but no figures were reported for heritability of brain asymmetries. Other studies of MZ twins have looked at laterality indices for brain regions that show reliable structural asymmetries, and found only weak correlations between genetically identical individuals (29, 30).
The largest brain imaging twin study to date measured structural integrity of white matter fibers in the left and right sides of the brain using diffusion tensor imaging (DTI) (31). Heritability was computed using maximum likelihood methods (32) for an asymmetry index based on brain images from 11 regions taken from 60 MZ twin pairs and 45 same-sex DZ pairs. Only two regions gave heritability estimates greater than 0.25, and for only one of these, the anterior thalamic radiation, was the fit superior for a model that included a genetic term, with heritability estimate of 0.38. Interpretation of the study is complicated by low power to detect small genetic effects, and by the fact that estimates of heritability may have been inflated because only right-handers were included. We know that brain asymmetry measured by DTI is discordant in around 29% of MZ twin pairs with opposite handedness, who constitute around one in five of all MZ pairs (33); in contrast, discordant brain asymmetry is seen in only 7% of those concordant for right-handedness (29). Note, however, that this study considered only structural brain asymmetry, and it is unclear how well this reflects functional language laterality – the few studies to look at associations between structural and functional asymmetry had small sample sizes (26 or less) and inconsistent findings (34-36).
Functional brain asymmetry was considered in a study of MZ twins who were given three different tasks – a word generation task, lateralized to the left, and two nonverbal tasks that showed rightward asymmetry (37). The analysis focused on how concordance for language and visuospatial tasks varied in relation to concordance for handedness, but if one ignores handedness and collapses across groups, statistical analysis shows that these pairs of genetically identical twins were no more similar than expected by chance. Thus evidence for heritability of functional brain asymmetry seems no stronger than that for structural asymmetries.
An alternative approach is to look directly for association between cerebral lateralization and specific genetic variants associated with language/literacy impairments. To evaluate such candidates, I searched the literature for neuroimaging studies that investigated structural or functional correlates of seven genes with common variants that had been associated with specific language impairment (SLI) and/or dyslexia (25). In addition the FOXP2 gene was included. Rare mutations in FOXP2 are well-established as a cause of severe speech and language disorder. More recently, common polymorphisms have been tentatively linked to language impairment (38).
When conducting an analysis of this kind, it is easy to neglect negative studies, because associations with asymmetry get mentioned only when they are significant. To minimize such bias, I identified studies of brain correlates of polymorphisms in these candidate genes in healthy volunteers by a search of Web of Science, plus a Google search, regardless of whether or not asymmetry was explicitly mentioned in the title or abstract. This produced nine studies focusing on the genes CNTNAP2 (39, 40), (41), (42), (43), (44), DCDC2 (45), DYX1C1 (45), FOXP2 (46), KIAA0319 (45), (46), and MRPL19/C2ORF3 (47). No studies were found for ATP2C2 or CMIP.
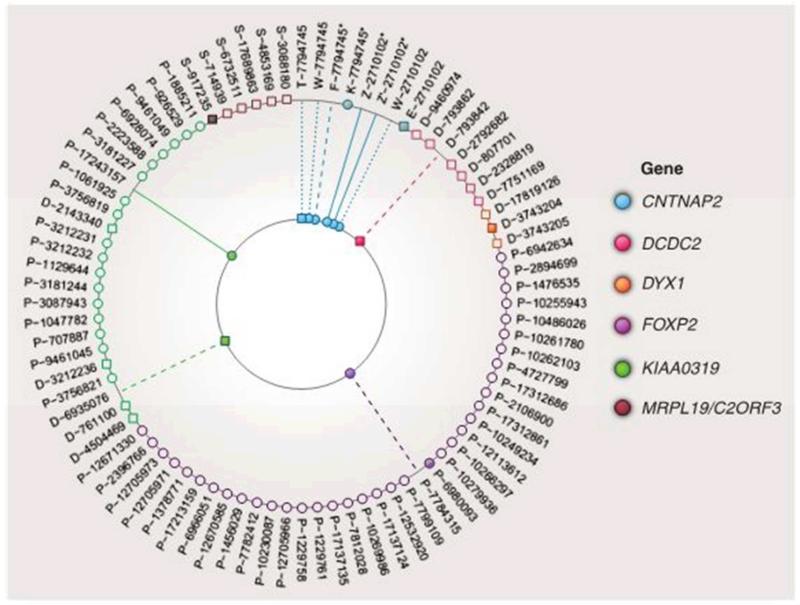
There was wide variation in the specific genotypes and phenotypes studied. Because a single gene can have many functional polymorphisms, it is generally recommended to study haplotypes, i.e. patterns of alleles (26), but this was seldom done. Some studies focused on just one or two single nucleotide polymorphisms (SNPs) in a gene, whereas others evaluated several SNPs at different positions along the gene. Figure 2 summarises the main findings. For FOXP2, 35 of the 37 SNPs tested by Pinel et al. (46) were not associated with brain activation. Of the other two, one (rs6980093) was associated with activation in the left frontal region, but those with different genotypes did not differ on an asymmetry measure. In the same study, 18 SNPs in a region on chromosome 6p22 spanning KIAA0319, THEM2 and TTRAP, were studied in relation to brain activation during a reading task and a language comprehension task. One SNP in KIAA0319 was significantly associated with reduction of left-hemisphere asymmetry during reading. In a different study, significant genetic variation in white matter volume in the left temporo-parietal region was found for another SNP of KIAA0319, but the researchers did not explicitly test for brain asymmetry (45).
Figure 2.
Summary of effects of language/reading associated SNPs on asymmetry of brain structure or function. Unfilled shapes on the rim of the figure correspond to SNPs with no effect on brain structure (squares) or function (circles). Filled shapes on the rim correspond to a bilateral effect. For the remaining shapes, a solid line indicates reduced asymmetry for the risk genotype; a dashed line indicates reduction of left-hemisphere size or function; a dotted line indicates other lateralized brain difference. Genes correspond to color-coding of the dots. SNP reference IDs are shown on the rim of the figure with the study identified by letter. An asterisk denotes a non-standard definition of risk genotype (see text). The study codes are (T) Tan et al (40); (Z) Scott– van Zeeland et al (39) discovery sample; (Z’) Scott-van Zeeland et al, replication sample; (F) Folia et al (43); (W) Whalley et al (41); (E) Dennis et al (42); (D) Darki et al (45); (K) Kos et al (44); (P) Pinel et al (46); (S) Scerri et al (same sample as (D)) (47). See text for more details.
CNTNAP2 stands out in Figure 2 as showing some evidence of lateralized effects in six of the eight samples that were tested for association with SNPs in this gene. Nevertheless, inconsistencies between studies cast doubt on the robustness of the results. Three studies considered SNP rs2710102, which has previously been associated with early language acquisition in autistic (48) and non-autistic samples (49), and also with a measure of nonword repetition in children with language impairments (50). In one study16 adults participants with the CC genotype showed reduced activation of the homolog of Broca’s area in the right hemisphere when performing a sentence-completion task in the scanner (41). Association with risk and non-risk genotypes were studied in 32 children who performed a nonverbal implicit learning task. A left-frontal network was activated in the ‘non-risk’ group but a more bilateral network in the ‘risk’ group (39). In a ‘replication’ sample 39 children were scanned doing a different language-learning task, and two regions were identified as showing similar genetic effects. However, in contrast to other studies, this study grouped both CC and CT together as risk genotypes, with TT as non-risk. A subsequent study failed to replicate these results, using the more usual definition of CC as risk genotype and CT/TT as non-risk (42). Using a complex graph theory analysis of network connectivity, they found significant differences between risk and non-risk genotypes, but, contrary to expectation, the CC genotype appeared to be associated with more efficient networks in both hemispheres.
The other neuroimaging studies of CNTNAP2 focused on another SNP, rs7794745; an initial report had associated this SNP with autism but in a complex manner, with parent-of-origin effects (51). However, the association with autism failed to replicate (52), and this SNP has not been linked to language delay (49, 52) or SLI (50). A structural imaging study of a sample of 314 adults by Tan et al obtained a complex pattern of results that included reduced grey and white matter on the right side in those with the TT vs AT/AA genotypes (40). In contrast, Whalley et al (41) reported increased activation in the right middle temporal gyrus in those with the TT risk genotype (N = 8) compared with 57 people with AT/AA. Two other studies grouped TT and AT genotypes together into a ‘risk’ group, and compared them with AA genotype, but one was too small to be meaningful (43) and the other found reduced electrophysiological responses to grammatical incongruency in those with TT/AT (N = 23) genotypes compared to AA (N = 26), but no evidence of lateralized differences.
Thus, while lateralized differences have been reported by different researchers linked with CNTNAP2 variants, the categorization of genotypes is inconsistent from study to study, as are the results. Furthermore, given that the aim is to identify an endophenotype that might help explain why a genetic variant is associated with neurodevelopmental disorders, it is surprising that only two studies (41, 45) stated how genotype related to cognitive abilities of their participants. One of these studies (45) found that white matter volume in left-hemisphere regions was significantly correlated both with genotype and with reading ability; the differences in brain volume in relation to genotype were substantial (effect size = 0.8), suggesting that a laterality index based on these regions might be a candidate for further study as a potential endophenotype.
Overall, the evidence for genes affecting individual differences in cerebral lateralization is not strong – both in terms of twin study data, and genetic variants associated with phenotypic variation. We cannot, however, dismiss the idea that genetic variants are implicated in cerebral lateralization. There are several problems to grapple with. First, most studies have focused on structural brain asymmetry, rather than functional asymmetry. Second, where functional asymmetry is assessed, there is little consistency in how it is quantified. Third, established genetic associations between genes and language/literacy impairments are typically small in magnitude, with effect sizes for the difference between risk and non-risk genotypes less than 0.2 (Cohen’s d). Very large sample sizes (over 1000) are needed to detect effects of this magnitude; all of the neuroimaging studies reviewed here used much smaller samples and had power to detect only large effects.
Given all these limitations, it would be premature to conclude that there is no genetic influence on cerebral asymmetry, but the evidence to date is not promising for the Endophenotype account (Figure 1): The Pleiotropy account could be tested using family data, insofar as the cross-trait correlation between laterality and language/literacy impairment between individuals should depend on the strength of genetic relationship between them (53). However, for either model to be plausible, we would need better evidence that genes that affect language/literacy impairment also have reliable effects on cerebral asymmetry. Current evidence suggests that, just as with handedness (54), genetic variants may play a measurable, but relatively small, role in accounting for individual variation in lateralization.
Is atypical cerebral lateralization associated with cognitive deficits?
Perhaps the greatest challenge for any causal account comes from studies that look at the association between atypical cerebral lateralization and impairment in a different way: starting with individuals selected for atypical cerebral lateralization, and assessing their language and literacy skills. In one such study, skills in a range of domains, including mastery of foreign languages, academic achievement, and verbal fluency, were not correlated with language lateralization in 31 people with right hemisphere language, 31 with bilateral language and 264 with left hemisphere language (55).
Two smaller studies with children from the general population did, however, find some evidence of benefit of left-sided language lateralization. In a Canadian study, structural brain asymmetry – laterality of the arcuate fasciculus measured on diffusion tensor imaging – was modestly correlated (r = .32) with receptive vocabulary in 68 children, with highest scores for those with strong left lateralization (56). Our group found similar results in a study using fTCD to assess functional brain lateralization in 55 children, with a small but significant correlation of .34 between laterality index and vocabulary score (57). Nevertheless, in both studies there were several right-lateralised children with mean vocabulary scores well above average. And a study of adults found no relationship between left-lateralisation of the arcuate fasciculus and vocabulary – on the contrary, in this study only one of 15 neuropsychological measures – a verbal memory task – showed any association with asymmetry of the arcuate fasciculus, and the effect was contrary to prediction, with worse performance associated with strong lateralization (58).
It would seem that atypical lateralization is compatible with normal, or even above-average, cognitive function, but in studies that over-sample those with developmental difficulties, an association with language/literacy skills becomes apparent. There are two main lines of explanation that might account for this puzzling pattern of findings. First, atypical lateralization may add or interact with genetic risk for language/literacy impairments; second, the asymmetry phenotype could differ for those who have language/literacy impairments and the remainder of the population,
Is atypical cerebral asymmetry a contributory factor for language/literacy impairments?
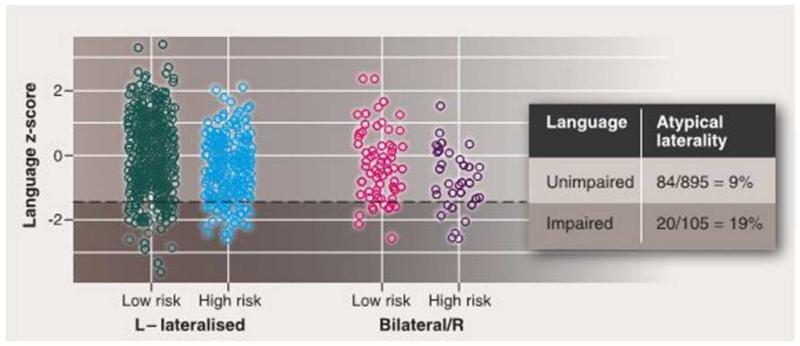
Figure 1 also illustrates the Additive/Interactive Risks model; this treats atypical lateralization as a risk factor for language/literacy impairment, while postulating that the origins of cerebral asymmetry are distinct from the genetic risk for language impairment. The idea here is that atypical cerebral asymmetry adds to or interacts with the genetic risk factors for language impairment. Figure 3 illustrates the logic. Let us consider a hypothetical genotype found in 30% of the population that, on average depress verbal skills so that for those with this risk genotype, the mean scaled score on a language measure is 94, rather than 100 (effect size, d = 0.4). In addition, lack of left-sided cerebral lateralization (bilateral or right-hemisphere speech) is estimated to affect 10% of people and have a similar impact. These effects are assumed to be independent and additive, so that the mean score for someone with both risks would be 88. Figure 3 shows data simulated using these assumptions. Overall, the rate of atypical cerebral asymmetry is roughly doubled among those with a language z-score below −1.5 compared to the rest of the population – even though there is no significant correlation between laterality and language ability in the whole population. The impact of atypical cerebral asymmetry on language would be hard to detect in the general population because of the rarity of this phenotype. A power calculation shows that if there is a 9:1 ratio between two groups, a sample of around 600 cases would be needed to detect an effect size of .4 with 80% probability.
Figure 3.
Simulated data for a common genetic variant present in 30% of the population that depresses language z-score by 0.4. In addition, language scores are depressed by 0.4 z in those with bilateral speech. The genetic variant and bilateral speech have independent origins, but their effects are additive. The rate of bilateral speech is 9% in those with unimpaired language (z-score higher than −1.5) but 19% in those with language impairment (z-score less than or equal to −1.5). If the simulation is modified to give an interaction between genetic risk and bilateral speech, then the association between bilateral speech and language impairment becomes even stronger.
We can go further and suppose that the combination of atypical cerebral asymmetry and risk genotype might be not additive but interactive, such that the impact of the two factors together is more severe than would be predicted from each effect independently. This would further strengthen the association between atypical asymmetry and language impairment. However, the language deficit in those with atypical asymmetry would remain hard to detect in samples from the general population, because those affected by the interactive effect would be in such a minority.
The important point here is that when an association is detected between traits in a clinical sample, it need not indicate shared origins. Genetic causes of language impairment and causes of cerebral lateralization could be quite independent: co-occurrence of these phenotypes may be a consequence of how the sample was selected, if those with two risk factors are more likely to end up in the clinical group. According to this causal account, risk factors for atypical cerebral asymmetry, which could be non-genetic and/or genetic, are separate for genetic risks for language impairment.
Phenotypic variation: Is cerebral lateralization a unitary variable?
Most people have visuospatial functions mediated by the right hemisphere, and language by the left. This fits with the idea that cerebral lateralization allows for division of labor between the hemispheres, with the left specializing in language, while the right specializes in visuospatial functions. In practice, however, some patients with focal brain lesions depart from this pattern, with both language and visuospatial functions controlled by the same hemisphere (59). Furthermore, extent of left-hemisphere lateralization for verbal tasks is not strongly correlated with extent of right-hemisphere lateralization for spatial processing, either in fMRI (60), (61) or fTCD (62), (63), (64), (57). It has been suggested that departures from complementarity simply reflect non-optimal methods for computing a laterality index and consequent unreliability of laterality assessment (65): If measurement is noisy, then correlations between different indices may be swamped by random error. However, some degree of dissociation between different indices of laterality appears to be the rule rather than the exception, even where asymmetry is measured with very little error. For instance, the measurement of handedness is highly reliable, yet handedness and structural brain asymmetries are often poorly correlated (66).
Indeed, studies that use multiple measures of cerebral lateralization suggest that, even within the domain of language, there can be dissociation between functions. Early studies with the Wada test found that some people showed opposite patterns of laterality for two language tasks: naming objects, and saying a well-known series, such as days of the week (67). These cases were rare, and Wada testing is only done with patients prior to epilepsy surgery, so one might dismiss this evidence as reflecting pathology rather than normal variation. Dissociation between different language functions can, however, be seen in the fTCD paradigm, where intercorrelations between laterality indices from different tasks are lower than the split-half reliability of each individual task (68). The evidence is not watertight: the key question is whether the correlation between the laterality index obtained with the same task given on different occasions (i.e., test-retest reliability) is higher than correlations that are observed between different tasks.
If we accept that cerebral lateralization is not a unitary function, this provides a possible resolution of the apparently contradictory findings, whereby individuals with language/literacy impairments have weak cerebral lateralization, but those with weak cerebral lateralization usually have normal cognitive skills. It may be that atypical lateralization can take different forms, only some of which are associated with poor functioning. For instance, could there be a cognitive disadvantage to having language and visuospatial functions mediated by the same hemisphere? To date, this notion has not received support, either from studies of neuropsychological patients (59) or from fTCD data from 6- to 12-year-old children (57). It would, however, be interesting to test a related idea: that within the domain of language, there could be a disadvantage of having different linguistic functions distributed between the two hemispheres. Because it is typically assumed that language laterality is a unitary function, this idea has not been explored.
Does cerebral lateralization change with age and language development?
The final model in Figure 1 is the Neuroplasticity model, in which the causal path between cerebral lateralization and language/literacy development is reversed: i.e., language ability influences cerebral lateralization. This may seem implausible, because structural brain asymmetries are evident in utero, long before the child learns language (69-73). However, this does not rule out a role of maturation or experience on functional laterality, given evidence from infants and toddlers that asymmetric processing of language increases with age (74). A key question is whether this change simply reflects a maturational process under genetic control, or whether it is experience-dependent. Minagawa-Kanai et al. put forward a complex model that included a role for early perceptual asymmetries based on acoustic properties of auditory input, onto which was superimposed an increasing left-sided bias that developed as language engaged lateralized learning systems (74). Processing of phonemes in one’s native language becomes increasingly left-lateralized, whereas detection of prosody becomes more right-lateralized with age. Linguistic status of sounds is critical in determining laterality: the same sound may be processed asymmetrically or symmetrically, depending on whether it is in the phonemic repertoire of the listener’s native language. Pitch provides a particularly striking example: it is usually preferentially processed in the right hemisphere, but for learners of tone languages, where pitch is used phonemically to contrast meanings, a left hemisphere processing bias emerges. Thus, developing cerebral asymmetry could reflect the growing engagement of lateralized systems for language learning and analysis as the child matures (74).
An earlier theory by Locke went further in linking a failure of cerebral lateralization to developmental language difficulties. He argued that if a child’s language learning is disrupted or delayed for any reason, then left-hemisphere syntactic systems will fail to be engaged during a critical period of neurobiological development, and cerebral asymmetry will never be fully established (75)
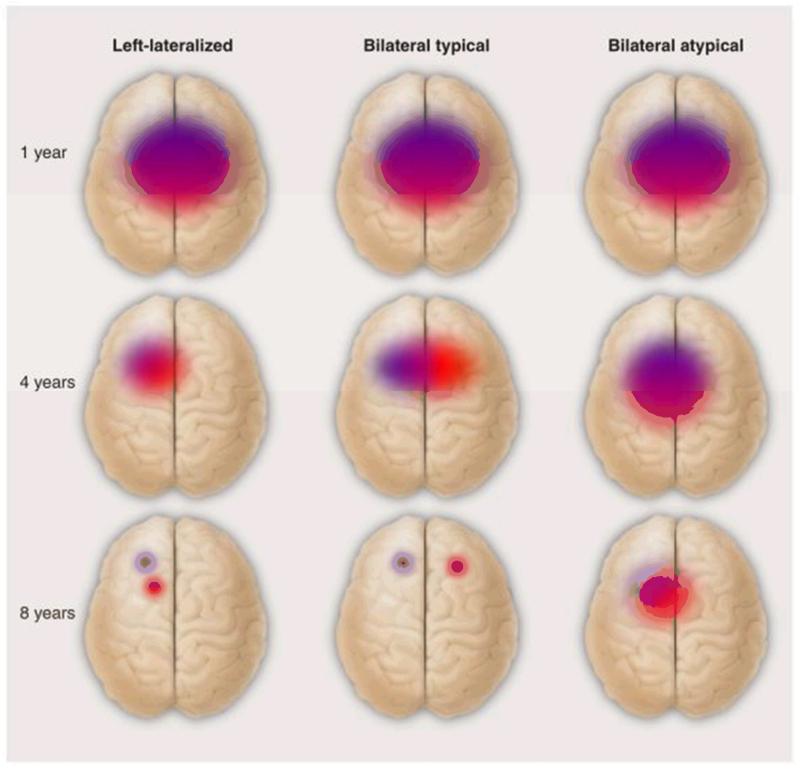
Development of lateralization with age may involve topographic as well as directional changes (Figure 4). Learning of perceptual and motor skills is sometimes associated with a more focal representation in the brain (76). Consistent with this, language-impaired children with poor phonological skills have more diffuse and bilateral processing of speech sounds than typically-developing children (77). If focal representation develops as language competence improves, then we should see different patterns of bilateral cerebral representation, depending on language skill: In those with SLI, the bilaterality would reflect persistence of an immature pattern of diffuse activity, whereas in children with age-appropriate language skills, we should see activation of separate focal regions in left and right hemispheres (Figure 4). To test this idea, we would need to move from treating cerebral lateralization as a single dimension to distinguish different types of atypical lateralization, taking into account the spatial extent of activation associated with language functions in the two hemispheres.
Figure 4.
Illustration of hypothetical distinction between different kinds of typical and atypical language lateralization. Red and purple regions correspond to different language functions (e.g. naming and series repetition as tested in the Wada test). The typical developmental progression is from more diffuse language representation in infancy that steadily becomes more focal as skill develops and synaptic pruning occurs. Usually both language functions will be represented in the left hemisphere, but normal language function is compatible with separation into left and right hemispheres. For children with language impairments, the developmental progression to focal representation is much slower if it occurs at all.
Overview: Alternatives to the Endophenotype account
There is growing support for the idea that atypical functional cerebral lateralization is associated with language/literacy impairments, but the work reviewed here challenges the conventional explanation for this association. At first glance, it is tempting to think of cerebral lateralization as an endophenotype that mediates the relationship between genetic risks and language/literacy impairments. However, there are difficulties for this account: First, most, people with weak cerebral lateralization have no indications of cognitive deficits. Second, an endophenotype account would predict that risk genes should exert a stronger effect on cerebral asymmetry than on the language/literacy phenotype, yet the converse appears to be the case. The literature on genetics of cerebral lateralization is in its infancy and we should not take lack of evidence for association as evidence of lack of association, especially as much of the evidence is from structural rather than functional brain asymmetry. Nevertheless, the data from twin studies suggest that, as with the related trait of handedness (54), individual differences in cerebral lateralization may turn out to be substantially influenced by non-genetic factors – possibly largely determined by random events occurring in early neurodevelopment (78). Genes almost certainly play a part in determining cerebral asymmetry, but their role is likely to be smaller and more probabilistic than generally assumed.
Alternatives to the endophenotype account are seldom considered, yet, as shown in Figure 1, there are several other ways to account for the association between language/literacy impairments and cerebral asymmetry. Pleiotropic genes that exert separate influences on cerebral asymmetry and language development are one possibility, but here again, if heritability of cerebral asymmetry turns out to be much lower than that seen for language/literacy problems, it would be hard to fit existing data into such a model. An alternative is that cerebral asymmetry does not share genetic origins with language/literacy problems, but acts as a separate risk factor, that adds to or interacts with other risks. A final possibility is that atypical asymmetry in those with dyslexia or SLI is more a consequence than a cause of the language/literacy impairments. These possibilities are not mutually exclusive.
Future directions
Measurement issues
Even when structural brain asymmetries are the focus of research, there has been disagreement about how they should be measured, and how far they relate to functional asymmetries in handedness or language processing. Progress has been made, but systematic asymmetries can still be difficult to localize and to distinguish from random fluctuations (66). This problem is magnified for functional measures of language lateralization. Furthermore, there is no agreement as to whether functional brain asymmetry is unidimensional or multifactorial, because when two laterality indices disagree, we typically are unable to tell if the difference is meaningful, a consequence of poor reliability of measurement (65), or indicative of genuine plasticity in functional lateralisation. Now that methods such as fMRI and fTCD make it possible to study brain activity in healthy volunteers, we are in a position to do larger-scale studies that assess laterality for different tasks on repeated occasions, which will help us determine whether it is reasonable to derive a single scale from different measures, or whether a spectrum of laterality measures is more appropriate.
The phenomenon of bilateral language processing is under-researched, yet is of considerable interest because of claims of links to various neurodevelopmental disorders. If, as argued above, lateralization of different language functions can fractionate, then there may be different cognitive consequences for those who have opposite biases for different components of language, compared to those whose language processing is predominantly in one hemisphere. In addition, it may be important to distinguish between diffuse vs. focal activation associated with language processing.
Genetic and non-genetic influences on laterality
Once we have methods of adequate validity and reliability, we will be able to forge ahead with studies designed to identify genes that bias the human brain to left-sided language processing. The methods noted above, including twin and family studies, as well as molecular genetics, have potential to inform our understanding of the etiology of individual differences in lateralization. Genes known to be involved in determining the left-right axis of the body may be more promising candidates for this purpose than those implicated in language/literacyimpairments (79).
It is worth pausing, however, to consider whether in the search for genes involved in cerebral lateralization we may have downplayed a role for non-genetic factors. These could potentially be of two kinds: systematic or stochastic. One systematic influence is the position of the fetus in utero, where most commonly the right ear faces outward (80). Potentially, a range of non-genetic prenatal influences could lead to small perceptual or motor asymmetries, which in turn could influence asymmetry of higher cognitive functions. The second kind of factor to consider is stochastic influences, i.e. random fluctuations in the expression of individual genes (78). Even if we find genes that bias the human brain to left-sided language, we should not assume that variability in human asymmetry must be heritable: there could be a fixed gene (i.e. with no genetic variation) that has a probabilistic effect. Genetically identical individuals could show different degrees of asymmetry because of purely stochastic influences.
Studies of MZ twins could illuminate our understanding of cerebral asymmetry in two important ways. First, they can help identify environmental factors associated with phenotypic differences – for instance, we could test whether prenatal positioning can account for postnatal differences in asymmetry. Second, they could help distinguish between the causal models outlined here. For instance, can we find MZ twins who are concordant for language/literacy impairments, yet discordant for cerebral asymmetry? A consideration of the associations between traits both within and between related individuals can be helpful in distinguishing causal models (53).
Although language is specific to humans, lateralization of vocal behavior is not; song-birds, notably the zebra finch, provide an intriguing model organism for studying biological bases of structural and functional brain asymmetry (81). This work has potential for our understanding of the impact of genes on cerebral lateralization; the genome of the zebra finch has been mapped, and much is known about genetic influences on song learning (82), as well as the role of experience (81). It has even been suggested that there may be overlap in the mechanisms involved in song-learning in birds and language-learning in humans (83). It would be a natural next step to test how gene expression relates to lateralization in the zebra finch brain.
Neuroplasticity of cerebral lateralization
Work with songbirds is useful for not only for genetics – it has also emphasized that cerebral asymmetry is not fixed at birth, but can change substantially throughout development, and be subject to experience. We still understand relatively little about such processes in humans, and we are limited by ethical considerations that preclude the kinds of experiential manipulations that are used with other species. Nevertheless, we can learn a great deal from the study of natural experiments. There is already fascinating research with deaf children learning sign language, indicating that processing of a visual language shows remarkably similar lateralization to processing of spoken language (84). But what of children who have milder hearing impairments, who learn oral language but suffer from language delays (85) – or those who have cochlear implants, where language outcomes are highly variable (86)? Are the language delays typically associated with hearing impairment accompanied by a delay in establishing language laterality? Another group of interest is children who can hear normally, but cannot speak because of physical or motor impairment affecting the articulators. We know that, where there is adequate general intelligence, it is possible for a child with cerebral palsy to develop good grammatical and phonological skills, even in the case of total anarthria (inability to speak) (87). It would be fascinating to study cerebral lateralization in such children while they do a phonological judgement task, to establish whether their good receptive language skills are underpinned by an asymmetric language system. And finally, with development of child-friendly methods such as fTCD we start to be able to look at development of lateralization in late-talkers – children who have few spoken words around 2 years of age, many of whom catch up with their peer group by the age of 4 or 5 years – to consider how cerebral asymmetry changes as language skills are acquired.
Acknowledgments
The author is supported by a Principal Research Fellowship and Programme Grant 082498/Z/07/Z from the Wellcome Trust. I would like to thank Kate Watkins, Silvia Paracchini, Dianne Newbury, Will Brandler and Elsje van Bergen for valuable comments on a draft of this paper, and Tim Brock for his generous help with design of Figure 2.
References
- 1.Berker EA, Berker AH, Smith A. Translation of Broca’s 1865 report: localization of speech in the third left frontal convolution. Archs Neurol. 1986;43:1065. doi: 10.1001/archneur.1986.00520100069017. [DOI] [PubMed] [Google Scholar]
- 2.Amunts K. In: The Two Halves of the Brain: Information Processing in the Cerebral Hemispheres. Hugdahl K, Westerhausen R, editors. MIT Press; Cambridge, Massachusetts: 2010. pp. 145–176. [Google Scholar]
- 3.Wada JA. Is functional hemispheric lateralization guided by structural cerebral asymmetry? Canad J Neurol Sci. 2009;36:S25. [PubMed] [Google Scholar]
- 4.Bishop DVM. What causes specific language impairment in children? Curr Dir Psychol Sci. 2006;15:217. doi: 10.1111/j.1467-8721.2006.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop DVM, Snowling MJ. Developmental dyslexia and Specific Language Impairment: Same or different? Psychol Bull. 2004;130:858. doi: 10.1037/0033-2909.130.6.858. [DOI] [PubMed] [Google Scholar]
- 6.Webster RI, Shevell MI. Neurobiology of specific language impairment. J Child Neurol. 2004;19:471. doi: 10.1177/08830738040190070101. [DOI] [PubMed] [Google Scholar]
- 7.Pernet C, Poline J, Demonet J, Rousselet G. Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neurosci. 2009;10:67. doi: 10.1186/1471-2202-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckert M. Neuroanatomical markers for dyslexia: A review of dyslexia structural imaging studies. The Neuroscientist. 2004;10:362. doi: 10.1177/1073858404263596. [DOI] [PubMed] [Google Scholar]
- 9.Orton ST. “Word-blindness” in school children. Archs Neurol Psychiat. 1925;14:581. [Google Scholar]
- 10.Annett M. Left, right, hand and brain: the Right Shift Theory. Erlbaum, Hillsdale, NJ: 1985. [Google Scholar]
- 11.Abou-Khalil B. Methods for determination of language dominance: the Wada test and proposed noninvasive alternatives. Curr Neurol Neurosci Rep. 2007;7:483. doi: 10.1007/s11910-007-0075-6. [DOI] [PubMed] [Google Scholar]
- 12.Eckert MA, Leonard CM. Structural imaging in dyslexia: the planum temporale. Ment Res Dev Dis Res Rev. 2000;6:198. doi: 10.1002/1098-2779(2000)6:3<198::AID-MRDD7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Leonard CM, et al. Anatomical risk factors that distinguish dyslexia from SLI predict reading skill in normal children. J Commun Disord. 2002;35:501. doi: 10.1016/s0021-9924(02)00120-x. [DOI] [PubMed] [Google Scholar]
- 14.Keller SS, et al. Can the language-dominant hemisphere be predicted by brain anatomy? J Cogn Neurosci. 2011;23:2013. doi: 10.1162/jocn.2010.21563. [DOI] [PubMed] [Google Scholar]
- 15.Dorsaint-Pierre R, et al. Asymmetries of the planum temporale and Heschl’s gyrus: relationship to language lateralization. Brain. 2006;129:1164. doi: 10.1093/brain/awl055. [DOI] [PubMed] [Google Scholar]
- 16.Knecht S, et al. Reproducibility of functional transcranial Doppler sonography in determining hemispheric language lateralization. Stroke. 1998;29:1155. doi: 10.1161/01.str.29.6.1155. [DOI] [PubMed] [Google Scholar]
- 17.Knecht S, et al. Noninvasive determination of language lateralization by functional transcranial Doppler sonography: A comparison with the Wada test. Stroke. 1998;29:82. doi: 10.1161/01.str.29.1.82. [DOI] [PubMed] [Google Scholar]
- 18.Whitehouse AJO, Bishop DVM. Cerebral dominance for language function in adults with specific language impairment or autism. Brain. 2008;131:3193. doi: 10.1093/brain/awn266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Illingworth S, Bishop DVM. Atypical cerebral lateralisation in adults with compensated developmental dyslexia demonstrated using functional transcranial Doppler ultrasound. Brain Lang. 2009;111:61. doi: 10.1016/j.bandl.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badcock N, Bishop D, Hardiman M, Barry JG, Watkins K. Co-localisation of abnormal brain structure and function in Specific Language Impairment. Brain Lang. 2011;120:310. doi: 10.1016/j.bandl.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Guibert C, et al. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia) Brain. 2011;134:3044. doi: 10.1093/brain/awr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun YF, Lee JS, Kirby R. Brain imaging findings in dyslexia. Pediatr. Neonatol. 2010 Apr;51:89. doi: 10.1016/S1875-9572(10)60017-4. [DOI] [PubMed] [Google Scholar]
- 23.Bishop DVM. Genes, cognition and communication: insights from neurodevelopmental disorders. The Year in Cognitive Neuroscience: Ann NY Acad Sci. 2009;1156:1. doi: 10.1111/j.1749-6632.2009.04419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pennington BF, Bishop DVM. Relations among speech, language, and reading disorders. Ann Rev Psychol. 2009;60:283. doi: 10.1146/annurev.psych.60.110707.163548. [DOI] [PubMed] [Google Scholar]
- 25.Newbury DF, et al. Investigation of dyslexia and SLI risk variants in reading- and language-impaired subjects. Behav Genet. 2011;41:90. doi: 10.1007/s10519-010-9424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green AE, et al. Using genetic data in cognitive neuroscience: from growing pains to genuine insights. Nat Rev Neurosci. 2008;9:710. doi: 10.1038/nrn2461. [DOI] [PubMed] [Google Scholar]
- 27.Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci. 2006;7:655. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- 28.Geschwind DH, Bruce LM, Charles D, Dorit C. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc Nat Acad Sci. 2002;99:3176. doi: 10.1073/pnas.052494999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Häberling IS, Badzakova-Trajkov G, Corballis MC. Asymmetries of the arcuate fasciculus in monozygotic twins: Genetic and nongenetic influences. PLOS One. 2013;8:e52315. doi: 10.1371/journal.pone.0052315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinmetz H, Herzog A, Schlaug G, Huang YX, Jancke L. Brain (a)symmetry in monozygotic twins. Cerebral Cortex. 1995;5:296. doi: 10.1093/cercor/5.4.296. [DOI] [PubMed] [Google Scholar]
- 31.Jahanshad N, et al. Genetic influences on brain asymmetry: A DTI study of 374 twins and siblings. Neuroimage. 2010;52:455. doi: 10.1016/j.neuroimage.2010.04.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3:119. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- 33.Medland SE, Duffy DL, Wright MJ, Geffen GM, Martin NG. Handedness in twins: Joint analysis of data from 35 samples. Twin Res Hum Genet. 2006;9:46. doi: 10.1375/183242706776402885. [DOI] [PubMed] [Google Scholar]
- 34.Powell HW, et al. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32:388. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Vernooij MW, et al. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: A combined fMRI and DTI study. Neuroimage. 2007;35:1064. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 36.Propper RE, et al. A combined fMRI and DTI examination of functional language lateralization and arcuate fasciculus structure: Effects of degree versus direction of hand preference. Brain Cogn. 2010 Jul;73:85. doi: 10.1016/j.bandc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Badzakova-Trajkov G, Häberling IS, Corballis MC. Cerebral asymmetries in monozygotic twins: An fMRI study. Neuropsychologia. 2010;48:3086. doi: 10.1016/j.neuropsychologia.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Rice ML, Smith SD, Gayán J. Convergent genetic linkage and associations to language, speech and reading measures in families of probands with Specific Language Impairment. J Neurodev Dis. 2009;1:264. doi: 10.1007/s11689-009-9031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott-Van Zeeland AA, et al. Altered functional connectivity in frontal lobe circuits Is associated with variation in the autism risk gene CNTNAP2. Sci Trans Med. 2010;2:56ra80. doi: 10.1126/scitranslmed.3001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan GC, Doke TF, Ashburner J, Wood NW, Frackowiak RS. Normal variation in fronto-occipital circuitry and cerebellar structure with an autism-associated polymorphism of CNTNAP2. Neuroimage. 2010;53:1030. doi: 10.1016/j.neuroimage.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whalley HC, et al. Genetic variation in CNTNAP2 alters brain function during linguistic processing in healthy individuals. Am J Med Genet B. 2011;156B:941. doi: 10.1002/ajmg.b.31241. [DOI] [PubMed] [Google Scholar]
- 42.Dennis EL, et al. Altered structural brain connectivity in healthy carriers of the autism risk gene, CNTNAP2. Brain Conn. 2011;1:447. doi: 10.1089/brain.2011.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Folia V, Forkstam C, Ingvar M, Hagoort P, Petersson KM. Implicit artificial syntax processing: Genes, preference, and bounded recursion. Biolinguistics. 2011:5. [Google Scholar]
- 44.Kos M, et al. CNTNAP2 and language processing in healthy individuals as measured with ERPs. PLOS One. 2012:7. doi: 10.1371/journal.pone.0046995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darki F, Peyrard-Janvid M, Matsson H, Kere J, Klingberg T. Three dyslexia susceptibility genes, DYX1C1, DCDC2, and KIAA0319, affect temporo-parietal white matter structure. Biol Psychiat. 2012;72:671. doi: 10.1016/j.biopsych.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Pinel P, et al. Genetic variants of FOXP2 and KIAA0319/TTRAP/THEM2 locus are associated with altered brain activation in distinct language-related regions. J Neurosci. 2012;32:817. doi: 10.1523/JNEUROSCI.5996-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scerri TS, et al. The dyslexia candidate locus on 2p12 Is associated with general cognitive ability and white matter structure. PLOS One. 2012;7:e50321. doi: 10.1371/journal.pone.0050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alarcón M, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitehouse AJO, Bishop DVM, Ang QW, Pennell CE, Fisher SE. CNTNAP2 variants affect early language development in the general population. Genes Brain Behav. 2011;10:451. doi: 10.1111/j.1601-183X.2011.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vernes SC, et al. A functional genetic link between distinct developmental language disorders. New Eng J Med. 2008;359:2337. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arking DE, et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anney R, et al. Individual common variants exert weak effects on the risk for autism spectrum disorders. Hum Molec Genet. 2012;21:4781. doi: 10.1093/hmg/dds301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ligthart L, Boomsma DI. Causes of comorbidity: pleiotropy or causality? Shared genetic and environmental influences on migraine and neuroticism. Twin Res Hum Genet. 2012;15:158. doi: 10.1375/twin.15.2.158. [DOI] [PubMed] [Google Scholar]
- 54.Medland SE, et al. Genetic influences on handedness: Data from 25,732 Australian and Dutch twin families. Neuropsychologia. 2009;47:330. doi: 10.1016/j.neuropsychologia.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knecht S, et al. Behavioural relevance of atypical language lateralization in healthy subjects. Brain. 2001;124:1657. doi: 10.1093/brain/124.8.1657. [DOI] [PubMed] [Google Scholar]
- 56.Lebel C, Beaulieu C. Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp. 2009;30:3563. doi: 10.1002/hbm.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Groen MA, Whitehouse AJ, Badcock NA, Bishop DV. Does cerebral lateralisation develop? A study using functional transcranial Doppler ultrasound assessing lateralisation for language production and visuospatial memory. Brain Beh. 2012;2:256. doi: 10.1002/brb3.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Catani M, et al. Symmetries in human brain language pathways correlate with verbal recall. Proc Nat Acad Sci. 2007;104:17163. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bryden MP, Hecaen M, Deagostini M. Patterns of cerebral organization. Brain Lang. 1983;20:249. doi: 10.1016/0093-934x(83)90044-5. [DOI] [PubMed] [Google Scholar]
- 60.Pinel P, Dehaene S. Beyond hemispheric dominance: brain regions underlying the joint lateralization of language and arithmetic to the left hemisphere. J Cog Neuro. 2010;22:48. doi: 10.1162/jocn.2009.21184. [DOI] [PubMed] [Google Scholar]
- 61.Badzakova-Trajkov G, Haberling IS, Roberts RP, Corballis MC. Cerebral asymmetries: Complementary and independent processes. PLOS One. 2010;5:9. doi: 10.1371/journal.pone.0009682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flöel A, Buyx A, Breitenstein C, Lohmann H, Knecht S. Hemispheric lateralisation of spatial attention in right- and left-hemispheric language dominance. Brain Beh Res. 2005;158:269. doi: 10.1016/j.bbr.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 63.Whitehouse AJO, Bishop DVM. Hemispheric division of function is the result of independent probabilistic biases. Neuropsychologia. 2009;47:1938. doi: 10.1016/j.neuropsychologia.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosch RE, Badcock NA, Bishop DVM. Lateralised visual attention is unrelated to language lateralisation, and not influenced by task difficulty - a functional transcranial Doppler study. Neuropsychologia. 2012;50:810. doi: 10.1016/j.neuropsychologia.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cai Q, Van der Haegen L, Brysbaert M. Complementary hemispheric specialization for language production and visuospatial attention. Proc Nat Acad Sci. 2013;110(4):E322–330. doi: 10.1073/pnas.1212956110. doi: 10.1073/pnas.1212956110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 67.Milner B, Branch C, Rasmussen T. In: Disorders of Language (Ciba Foundation Symposium) De Reuck AVS, O’Connor M, editors. Churchill; London: 1964. [Google Scholar]
- 68.Bishop DVM, Watt H, Papadatou-Pastou M. An efficient and reliable method for measuring cerebral lateralization during speech with functional transcranial Doppler ultrasound. Neuropsychologia. 2009;47:587. doi: 10.1016/j.neuropsychologia.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dubois J, et al. Structural asymmetries in the infant language and sensori-motor networks. Cerebral Cortex. 2009;19:414. doi: 10.1093/cercor/bhn097. [DOI] [PubMed] [Google Scholar]
- 70.Kasprian G, et al. The prenatal origin of hemispheric asymmetry: An in utero neuroimaging study. Cerebral Cortex. 2011;21:1076. doi: 10.1093/cercor/bhq179. [DOI] [PubMed] [Google Scholar]
- 71.Habas PA, et al. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cerebral Cortex. 2012;22:13. doi: 10.1093/cercor/bhr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kivilevitch Z, Achiron R, Zalel Y. Fetal brain asymmetry: in utero sonographic study of normal fetuses. Am J Obstet Gynecol. 2010:202. doi: 10.1016/j.ajog.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 73.Zhang ZH, et al. Development of fetal brain of 20 weeks gestational age: Assessment with post-mortem Magnetic Resonance Imaging. Eur J Radiol. 2011;80:E432. doi: 10.1016/j.ejrad.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 74.Minagawa-Kawai Y, Cristià A, Dupoux E. Cerebral lateralization and early speech acquisition: A developmental scenario. Dev Cog Neurosci. 2011;1:217. doi: 10.1016/j.dcn.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Locke JL. A theory of neurolinguistic development. Brain Lang. 1997;58:265. doi: 10.1006/brln.1997.1791. [DOI] [PubMed] [Google Scholar]
- 76.Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;31:681. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- 77.Bishop DVM, Hardiman MJ, Barry JG. Auditory deficit as a consequence rather than endophenotype of Specific Language Impairment: Electrophysiological evidence. PLOS One. 2012;7:e35851. doi: 10.1371/journal.pone.0035851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raj A, van Oudenaarden A. Nature, nurture, or chance: Stochastic gene expression and its consequences. Cell. 2008;135:216. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scerri TS, et al. PCSK6 is associated with handedness in individuals with dyslexia. Hum Mol Genet. 2011;20:608. doi: 10.1093/hmg/ddq475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Previc FH. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychol Rev. 1991;98:299. doi: 10.1037/0033-295x.98.3.299. [DOI] [PubMed] [Google Scholar]
- 81.George I. In: The Two Halves of the Brain: Information Processing in the Cerebral Hemispheres. Hugdahl K, Westerhausen R, editors. MIT Press; Cambridge, Massachusetts: 2010. pp. 92–120. [Google Scholar]
- 82.Warren WC, et al. The genome of a songbird. Nature. 2010;464:757. doi: 10.1038/nature08819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bolhuis J, Okanoya K, Scharff C. Twitter evolution: converging mechanisms in birdsong and human speech. Nat Rev Neurosci. 2010 Nov;11:747. doi: 10.1038/nrn2931. [DOI] [PubMed] [Google Scholar]
- 84.MacSweeney M, Capek CM, Campbell R, Woll B. The signing brain: the neurobiology of sign language. Trends Cogn Sci. 2008;12:432. doi: 10.1016/j.tics.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 85.Briscoe J, Bishop DVM, Norbury CF. Phonological processing, language, and literacy: A comparison of children with mild-to-moderate sensorineural hearing loss and those with specific language impairment. J Child Psychol Psychiatry. 2001;42:329. [PubMed] [Google Scholar]
- 86.Markman TM, et al. Language development after cochlear implantation: an epigenetic model. J Neurodev Dis. 2011;3:388. doi: 10.1007/s11689-011-9098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bishop DVM, Byers-Brown B, Robson J. The relationship between phoneme discrimination, speech production and language comprehension in cerebral-palsied individuals. J Speech Hear Res. 1990;33:210. doi: 10.1044/jshr.3302.210. [DOI] [PubMed] [Google Scholar]
- 88.Bishop DVM. Handedness and developmental disorder. Blackwell Scientific and Philadelphia: J.B. Lippincott; Oxford: 1990. ( Clinics in Developmental Medicine, 110). [Google Scholar]
- 89.Francks C. Understanding the genetics of behavioural and psychiatric traits will only be achieved through a realistic assessment of their complexity. Laterality. 2009;14:11. doi: 10.1080/13576500802536439. [DOI] [PubMed] [Google Scholar]
- 90.Rasmussen T, Milner B. In: Cerebral Localization. Zülch K, Creutzfeldt O, Galbraith G, editors. Springer Verlag; New York: 1975. pp. 238–257. [Google Scholar]