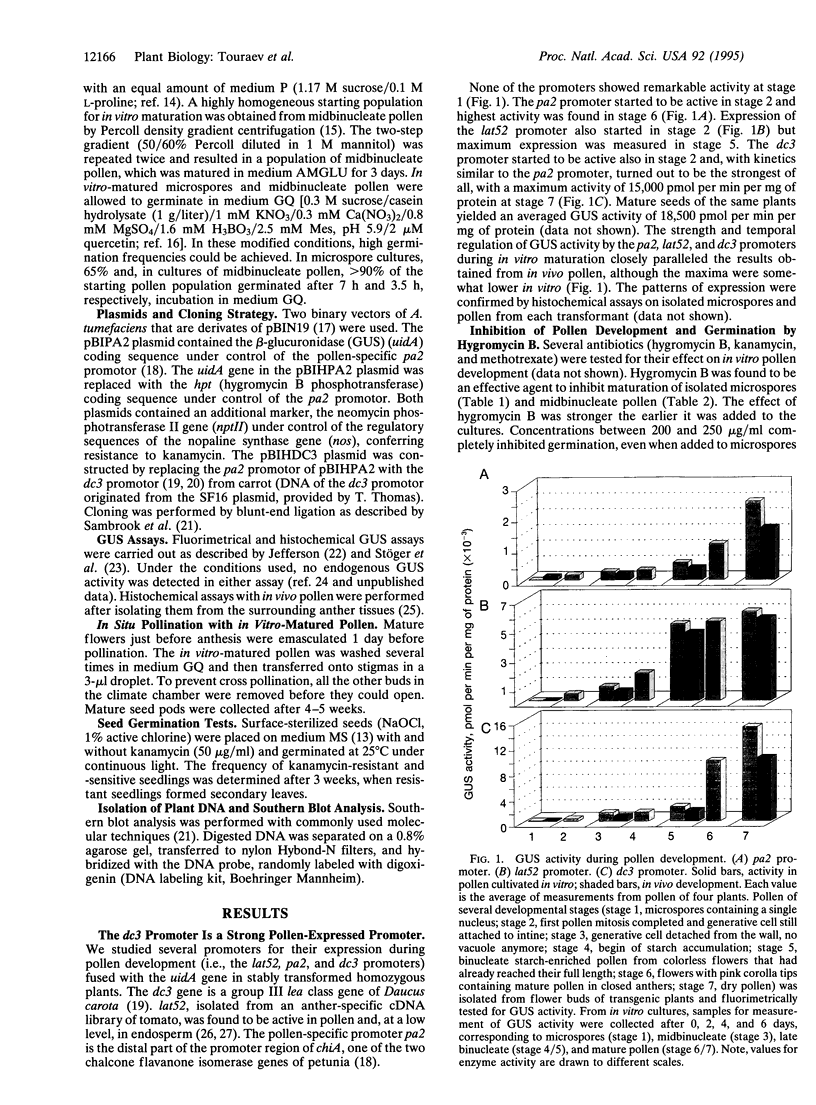
Abstract
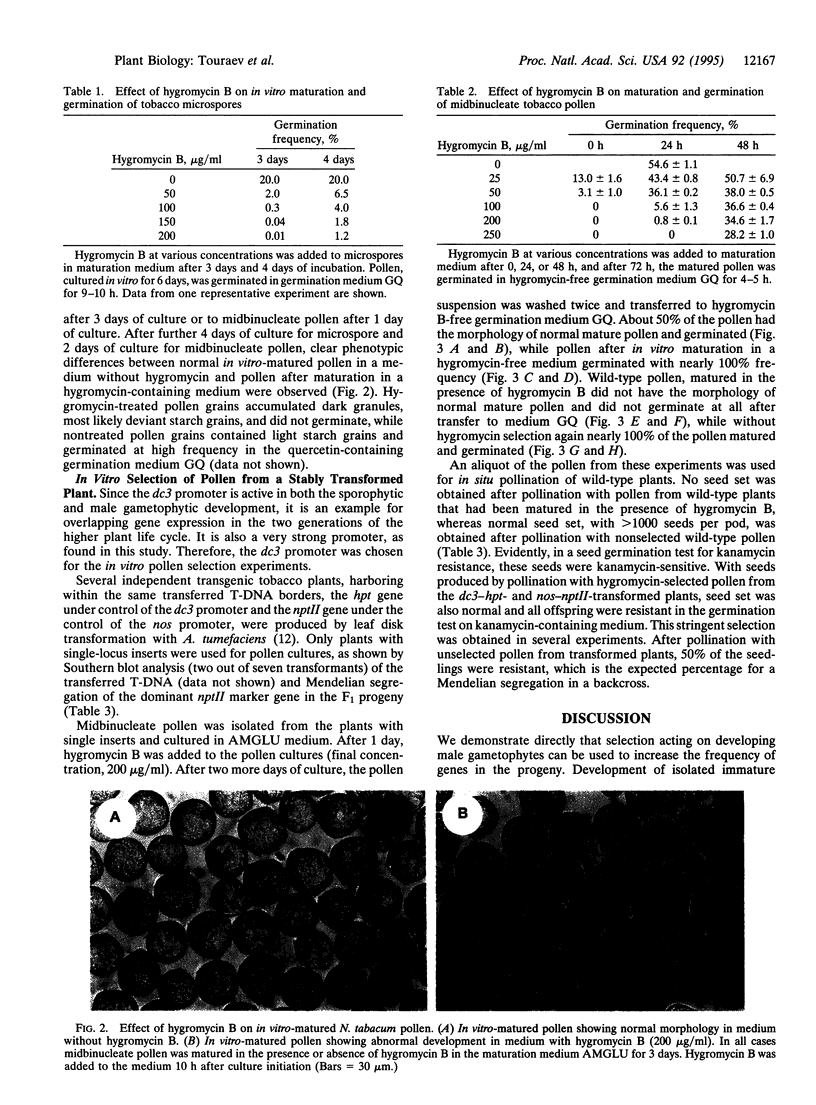
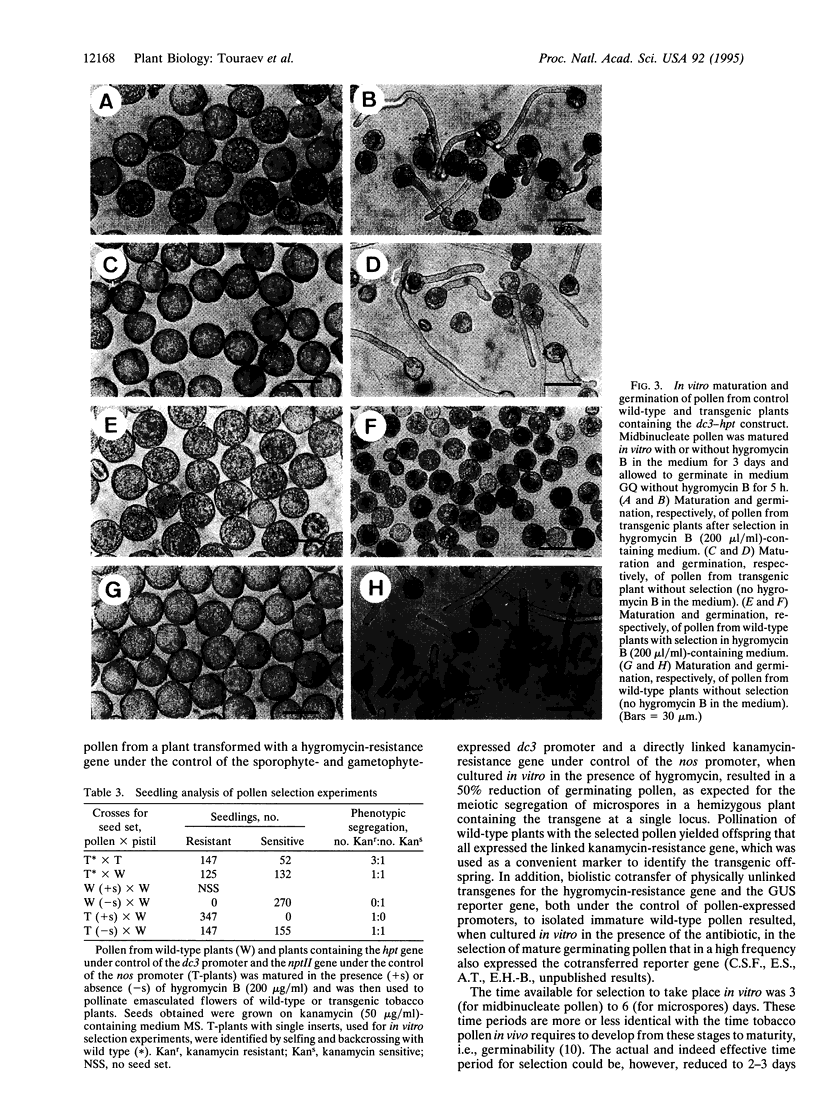
A transgenic reconstruction experiment has been performed to determine the feasibility of male gametophytic selection to enhance transmission of genes to the next sporophytic generation. For tobacco pollen from a transgenic plant containing a single hygromycin-resistance (hygromycin phosphotransferase, hpt-) gene under control of the dc3 promoter, which is active in both sporophytic and gametophytic tissues, 3 days of in vitro maturation in hygromycin-containing medium was sufficient to result in a 50% reduction of germinating pollen, as expected for meiotic segregation of a single locus insert. Pollination of wild-type plants with the selected pollen yielded 100% transgenic offspring, as determined by the activity of the linked kanamycin-resistance gene--present within the same transferred T-DNA borders--under control of the nos promoter. This is direct proof that selection acting on male gametophytes can be a means to alter the frequency of genes in the progeny.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Alwen A., Benito Moreno R. M., Vicente O., Heberle-Bors E. Plant endogenous beta-glucuronidase activity: how to avoid interference with the use of the E. coli beta-glucuronidase as a reporter gene in transgenic plants. Transgenic Res. 1992 Mar;1(2):63–70. doi: 10.1007/BF02513023. [DOI] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas J. P. Pollen gene expression: molecular evidence. Int Rev Cytol. 1992;140:3–18. doi: 10.1016/s0074-7696(08)61091-8. [DOI] [PubMed] [Google Scholar]
- Seffens W. S., Almoguera C., Wilde H. D., Vonder Haar R. A., Thomas T. L. Molecular analysis of a phylogenetically conserved carrot gene: developmental and environmental regulation. Dev Genet. 1990;11(1):65–76. doi: 10.1002/dvg.1020110108. [DOI] [PubMed] [Google Scholar]
- Twell D., Wing R., Yamaguchi J., McCormick S. Isolation and expression of an anther-specific gene from tomato. Mol Gen Genet. 1989 Jun;217(2-3):240–245. doi: 10.1007/BF02464887. [DOI] [PubMed] [Google Scholar]
- Twell D., Yamaguchi J., McCormick S. Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development. 1990 Jul;109(3):705–713. doi: 10.1242/dev.109.3.705. [DOI] [PubMed] [Google Scholar]
- Ylstra B., Touraev A., Moreno R. M., Stöger E., van Tunen A. J., Vicente O., Mol J. N., Heberle-Bors E. Flavonols stimulate development, germination, and tube growth of tobacco pollen. Plant Physiol. 1992 Oct;100(2):902–907. doi: 10.1104/pp.100.2.902. [DOI] [PMC free article] [PubMed] [Google Scholar]