Abstract
We investigated the hypotriglyceridemic mechanism of action of linalool, an aromatic monoterpene present in teas and fragrant herbs. Reporter gene and time-resolved fluorescence resonance energy transfer assays demonstrated that linalool is a direct ligand of PPARα. Linalool stimulation reduced cellular lipid accumulation regulating PPARα-responsive genes and significantly induced FA oxidation, and its effects were markedly attenuated by silencing PPARα expression. In mice, the oral administration of linalool for 3 weeks reduced plasma TG concentrations in Western-diet-fed C57BL/6J mice (31%, P < 0.05) and human apo E2 mice (50%, P < 0.05) and regulated hepatic PPARα target genes. However, no such effects were seen in PPARα-deficient mice. Transcriptome profiling revealed that linalool stimulation rewired global gene expression in lipid-loaded hepatocytes and that the effects of 1 mM linalool were comparable to those of 0.1 mM fenofibrate. Metabolomic analysis of the mouse plasma revealed that the global metabolite profiles were significantly distinguishable between linalool-fed mice and controls. Notably, the concentrations of saturated FAs were significantly reduced in linalool-fed mice. These findings suggest that the appropriate intake of a natural aromatic compound could exert beneficial metabolic effects by regulating a cellular nutrient sensor.
Keywords: peroxisome proliferator-activated receptor-α, triglyceride, linalool, agonist
Hypertriglyceridemia is an independent risk factor in the development of CVD and a key component of metabolic syndrome (1, 2). A meta-analysis showed that a 1 mM increase in the fasting TG level was associated with 14% and 37% higher incidences of CVD in men and women, respectively (3). A TG level reduction of the same magnitude could be achieved by dietary interventions, possibly based on the intake of fruits, vegetables, and teas (4, 5).
The ingestion of fragrant herbal teas has been suggested as a simple and effective way of preventing CVD, with green tea being the most widely used (6). Studies on the hypolipidemic effects of teas have particularly focused on the catechins in green tea as potent active compounds (7, 8). However, catechins alone cannot explain the lipid-lowering effects of teas because data on the effects of catechins in human trials have not been consistently positive (9, 10). The majority of human epidemiological and interventional studies have indicated beneficial effects of catechin on metabolic syndrome (11). However, the number of human studies in this field is still considered to be limited because some published clinical studies have shown that catechin has no effects on metabolic risk factors, including insulin sensitivity and secretion, as well as on glucose tolerance, cholesterol, and TG levels (10). In addition, noncatechin phytochemicals derived from teas have been reported to exert hypolipidemic effects independently or synergistically. In particular, teas are rich in flavor compounds, among which the terpenoids could be potent, active compounds to enhance hepatic cholesterol and FA metabolism (12, 13). However, the metabolic functions of terpenoid aroma compounds in teas have largely been ignored.
Linalool is a major and common terpenoid contained in most herbal essential oils and teas, including both green and black teas, and has been implicated in aroma and flavoring (14, 15). Oral exposure to linalool from formulated food products, including beverages, was estimated at up to 140 μg/kg/day, including the dietary intake of linalool from natural sources such as vegetables and spices (16). Linalool has been traditionally used for medicinal purposes because of its potent antioxidative activities (17, 18). Recent studies have suggested that linalool may have a novel biological activity in TG metabolism, as the oral administration of fragrant herbal essential oils containing linalool, including Plantago asiatica and Melissa officinalis essential oils, was shown to improve dyslipidemia by reducing plasma TG concentrations (13, 19).
The metabolic functions of the nuclear receptor and nutrient sensor peroxisome PPARα in the regulation of plasma TG concentrations have been intensively studied. It is well known that PPARα activation is a favorable drug target for liver and plasma TG level reductions (20, 21). Similar to other nuclear receptors, PPARα is activated by ligand binding. It then heterodimerizes with retinoid X receptor and binds to PPAR response elements in the promoter regions of target genes responsible for FA uptake, FA oxidation, and TG-rich VLDL secretion (20–23). Conformational changes during the ligand-dependent activation of PPARα facilitate the coordinated dissociation of corepressors and the recruitment of coactivator proteins to enable transcriptional activation (24, 25).
Previous luciferase-based activity screening with ∼900 natural compounds and extracts revealed that linalool is a potential PPARα agonist. Subsequently, we performed two preliminary animal feeding experiments with linalool, and the results consistently included significant reductions in TG concentrations. Therefore, in this study, we investigated the biological mechanism of action of linalool as a novel PPARα agonist that is responsible for the hypotriglyceridemic effects of teas and aromatic herbs. To this end, we examined the agonistic activity of linalool for PPARα and performed three independent animal studies to confirm hypotriglyceridemic activities. In addition, we performed transcriptome and metabolome analyses and adopted selected conventional biomarker approaches.
MATERIALS AND METHODS
Animals
Male C57BL/6J (6 weeks old; Samtako Animal Breeding Center, Osan, Korea), human apoE2 (R158C) transgenic mice (17–20 weeks old; Taconic Farms, Germantown, NY), and PPARα-deficient mice (Taconic, Hudson, NY) were used. Mice were given a Western-type diet [21% (w/w) fat and 0.15% (w/w) cholesterol (Feedlab, Seoul, Korea)] for 4 weeks with oral administration of the vehicle (control group) and 100 mg/kg body weight/day linalool (Sigma-Aldrich, St. Louis, MO) or fenofibrate (Sigma-Aldrich) for 3 weeks (n = 3–7 per group). Linalool was dissolved in polyethylene glycol 200 (Fisher Chemicals, Loughborough, UK), and fenofibrate was prepared in an aqueous solution containing 10% DMSO and 0.5% methylcellulose. Controls were fed the vehicle (polyethylene glycol 200). Blood samples were obtained by retro-orbital sinus bleeding and cardiac puncture under general anesthesia with 2.5% tribromoethanol (20 ml/kg, ip). Food intake was measured twice a week during the feeding period. After the feeding period, the mice were euthanized after overnight fasting, and their livers were removed, snap-frozen in liquid nitrogen, and stored at −80°C prior to analysis. All experimental procedures involving mice were approved by the Institutional Animal Care and Use Committee of Korea University (animal protocol number: KUIACUC-20090421-2).
Cell culture and treatment
CHO-K1 cells were maintained in DMEM/Ham’s F-12 medium (Hyclone, Logan, UT) containing 10% heat-inactivated FBS (Hyclone) and 1% penicillin/streptomycin (Welgene Inc., Seoul, Korea). HEK293T and HepG2 cells were cultured in DMEM (Hyclone) supplemented with 10% heat-inactivated FBS and 1% penicillin/streptomycin. For lipid loading to mimic hyperlipidemic conditions, HepG2 cells were incubated with palmitate (400 μM) and oleate (400 μM), which were conjugated to 0.5% (w/v) FA-free BSA, for 24 h. Caco-2 cells were grown in DMEM supplemented with 20% heat-inactivated FBS, 1% nonessential amino acids (Welgene Inc.), 25 mM HEPES, 1% penicillin/streptomycin, and 0.1% gentamycin (Welgene Inc.). All cells were maintained at 37°C in an atmosphere containing 5% CO2. Linalool was dissolved in DMSO and was then added at various concentrations to cells for 24 h. The final DMSO concentration was 0.1%. Control cells were incubated with vehicle (0.1% DMSO).
Transcriptional reporter assay
CHO-K1 cells were seeded in a 24-well plate (1 × 105 cells per well) and incubated overnight before being transfected using Metafectene (Biontex, Munich, Germany) with 0.3 μg each of PPARα expression vector and DR-1 (PPAR recognition site) luciferase reporter plasmids and 20 ng of a β-galactosidase reporter plasmid. Transfected cells were incubated for 24 h and then treated with linalool or fenofibrate for 24 h. The cells were lysed, and luciferase activity was measured with the Firefly Luciferase Assay Kit (Biotium, Hayward, CA) using a luminometer (Victor3, PerkinElmer, Waltham, MA). Relative promoter activity was computed by normalization to β-galactosidase activity, as determined with the β-Galactosidase Enzyme Assay System (Promega Corp., Madison, WI).
PPARα knockdown by a lentivirus carrying PPARα-selective shRNA
HEK293T cells were cotransfected for 4 h with a lentiviral pGIPZ plasmid carrying PPARα-selective shRNA (mature antisense sequence 5′-TGAGTCGAATCGTTCGCCG-3′ Open Biosystems, Huntsville, AL), a psPAX2 packaging plasmid, and a pMD2G envelop plasmid using HilyMax reagent (Dojindo, Kumamoto, Japan). The medium containing the transfection reagent was removed and replaced with fresh complete DMEM supplemented with 10% FBS and penicillin/streptomycin. Twelve hours later, the culture medium containing lentiviral particles was harvested from HEK293T cells, filtered through a 0.45 μm filter, and transferred to a polypropylene storage tube. This procedure was repeated three times. The virus was stored in aliquots at −80°C until use. HepG2 cells were then infected with appropriate amounts of lentiviral particles in medium supplemented with polybrene for 8 h. Fresh complete medium was added, and the infection was repeated twice. Infected HepG2 cells were selected with puromycin, and immunoblotting was performed to examine the efficiency of the protein knockdown. As a control, scrambled shRNA was used following the same procedure.
Nuclear protein preparation and PPARα promoter binding assay
Nuclear proteins were prepared from HepG2 cells treated with linalool for 24 h using the Nuclear Extraction Kit (Cayman, Ann Arbor, MI), according to the manufacturer’s instructions. Sample protein contents were determined by the Bradford method. PPARα promoter binding activity in the nuclear proteins was measured using an ELISA-based PPARα transcription factor assay kit (Cayman) to detect PPARα bound to PPAR response element (PPRE)-containing double-stranded DNA sequences, according to the manufacturer’s instructions.
PPARα coactivator recruitment assay
PPARα ligand binding activity was determined by LanthaScreen™ TR-FRET PPARα Coactivator Assay (catalog no. PV4684, Invitrogen, Carlsbad, CA), as described previously (26). Recombinant human PPARα ligand binding domain (LBD; amino acids 192–468; NP_005027) was synthesized and tagged to glutathione S-transferase, terbium-labeled anti-PPARα antibodies were provided (catalog no. PV4691; included in the PV4684 kit), and fluorescein-labeled coactivator [PPARγ coactivator 1, (PGC1α)] peptides (EAEEPSLLKKLLLAPANTQ; catalog no. PV4421) were provided in the PV4684 kit. Recruitment of fluorescein-PGC1α peptides in response to linalool or fenofibrate treatment was measured by monitoring the fluorescence resonance energy transfer (FRET) from the terbium anti-glutathione S-transferase antibody to the fluorescein on the peptide. The FRET signal was determined by excitation at 340 nm and emission at 520 nm for terbium, as well as 490 nm for fluorescein, using a SpectraMax spectrophotometer (Molecular Devices, Sunnyvale, CA). Data were analyzed using GraphPad Prism software (La Jolla, CA).
Absorption of linalool in Caco-2 cells
The assay was performed as previously described (27), and its reproducibility was confirmed with a reference compound, propranolol. Caco-2 cells were seeded at a density of 1.6 × 105 cells/cm2 on collagen-coated Transwell filter inserts (Corning Costar Corp., Cambridge, MA). Cells were grown and differentiated into confluent monolayers for 21 days. The integrity of the monolayers was evaluated by measuring transport of Lucifer yellow, as described previously (28). HBSS containing linalool (0.25 μM in 0.5 ml) was added to the apical side, while the basolateral side received 1.5 ml of HBSS. After incubation, apical and basolateral HBSS buffers containing linalool were collected and analyzed using a GC-mass selective detector (GC-MSD; Agilent 7890A GC/5975C MSD, Agilent Technologies, Avondale, PA) equipped with a nonpolar fused silica capillary DB-5MS column (30 m × 25 mm, 0.25 μm film thickness; J and W Scientific, Folsom, CA). Data were acquired and processed using ChemStation software (Agilent Technologies). The apparent permeability coefficient (Papp) was used to predict the absorption potential of linalool (29).
Cellular and plasma lipids, lipoprotein profiling, and DiI and Oil Red O staining
Lipid-loaded HepG2 cells were treated with linalool for 24 h. Cellular TG and cholesterol concentrations were quantified as described previously, and cellular lipids were extracted with 2 ml of a 2:1 (v/v) mixture of hexane and isopropanol at room temperature (30). Plasma TG concentrations were determined enzymatically using the Cobas C111 analyzer. Lipoproteins were analyzed using a fast protein LC (FPLC) system (AKTA Purifier 10; GE Healthcare, Piscataway, NJ) equipped with two Superose 6 10/300 GL columns (GE Healthcare) connected in series. Pooled mouse plasma (150 and 200 μl for C57BL/6J and apoE2 transgenic mice, respectively) was injected onto the column and separated with elution buffer containing 154 mM NaCl, 1 mM EDTA, and 0.02% NaN3 (pH 8.2) at a flow rate of 0.35 ml/min. The effluent was collected, and TG levels were determined enzymatically. DiI (1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate) and Oil Red O statin were performed in lipid-loaded HepG2 cells treated with linalool. Formalin (10%, v/v) fixed cells were washed with water and isopropanol (60%, v/v) and stained with DiI (2 μM; Molecular Probes, Eugene, OR) or Oil Red O (0.35%, v/v) for 1 h. After washing with water, images were acquired using an inverted microscope (Eclipse Ti-s, Nikon).
FA oxidation
HepG2 cells were seeded at a density of 1 × 106 cells/well and grown for 24 h in 6-well plates. For the FA oxidation assay, the growth medium was removed, and the cell monolayers were washed three times with PBS. Next, HepG2 cells were lipid-loaded with DMEM containing 1.75 mM [1-14C]palmitate (57 mCi/mM; Perkin Elmer) conjugated to 1 mg/ml FA-free BSA for 18 h. The medium was removed and centrifuged to remove cellular debris. It was then placed in a sealed container with a reservoir containing 0.5 ml of 1 M NaOH. Then, 0.5 ml of concentrated HCl was added to the medium, and the amount of l4CO2-liberated l4CO2 and l4C-labeled acid-soluble metabolite was measured to give the total palmitate oxidation rate. [1-14C]palmitate-containing medium without cells was run in parallel and used for background detection. Cells were lysed in NaOH, and the protein concentrations of the resulting lysates were determined for normalization (31).
Quantitative real-time PCR analysis
Total RNA was extracted from the livers and HepG2 cells using RNAiso Plus (Takara, Shiga, Japan). cDNA was synthesized from 2 μg of total RNA using M-MLV Reverse Transcriptase (Mbiotech, Seoul, Korea) and oligo(dT) primers. The levels of gene expression were measured using the iQ5 Real-Time PCR Detection System (Bio-Rad, Hercules, CA) and RealMasterMix SYBR ROX reagent (5 Prime, Hamburg, Germany). Relative levels of gene expression were calculated using iQ5 Optical System Software version 2 (Bio-Rad), with the expression of each target gene being normalized to that of β-actin or GAPDH.
Immunoblotting
Total protein was prepared, as described previously (13). Proteins (40 µg) were subjected to 10% SDS-PAGE and then transferred onto nitrocellulose membranes. After blocking, the membranes were probed with a primary antibody [anti-PPARα, anti-acyl-CoA synthetase 1 (ACS1), anti-FA transporter protein 4 (FATP4), anti-acyl-CoA oxidase 1 (ACOX 1), anti-uncoupling protein 2 (UCP2), anti-APOC3, anti-LPL, and anti-α-tubulin] and then incubated with secondary antibody (anti-rabbit IgG-HRP, anti-goat IgG-HRP, or anti-mouse IgG-HRP). All antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Immunoreactive bands were imaged with the ChemiDoc XRS imaging system (Bio-Rad) using PowerOpti-ECL Western blotting detection reagent (Anigen, Seoul, Korea). The relative band intensities were determined using Gel-Pro Analyzer 4.0 software (Media Cybernetics, Silver Spring, MD). For each sample, target protein levels were normalized to those of the internal reference α-tubulin.
Oligonucleotide microarray analysis
Two-color oligonucleotide microarray experiments were performed using untreated control and lipid-accumulated cells or control and lipid-accumulated cells stimulated with either linalool (1 mM) or fenofibrate (0.1 mM). Total RNA was prepared from HepG2 cells using RNAiso Plus, RNase-free DNase I Set (Qiagen, Valencia, CA), and the RNeasy MinElute Cleanup Kit (Qiagen). During reverse transcription, cDNA was labeled with the Cy3-dUTP and Cy5-dUTP (GeneChem Inc., Seoul, Korea) and purified with QIAquick PCR Purification Kit (Qiagen) before being hybridized to the 32 K Human OneArray™ (Phalanx Biotech Group, Hsinchu, Taiwan), which contains 30,968 human genome probes. Hybridized arrays were scanned with a GenePix 4000B scanner (Axon Instruments, Palo Alto, CA) and analyzed with GenePix 5.1 software (Axon Instruments). Probe-level gene expression values were computed, background corrected, and normalized by the Loess method using GenePix 5.1 and Acuity 4.0 software (Axon Instruments). Genes showing significant changes in expression in response to lipid accumulation, linalool, or fenofibrate were determined by Student’s t-test (P < 0.05), and commonly detected genes were used to compare the effects of the three treatments on transcriptional profiles, assessed with the HeatMap Viewer tool in GenePattern software (http://genepattern.broadinstitute.org/pages/index.jsf).
Metabolite analysis
The extraction, derivatization, and analysis of metabolites from plasma were conducted as previously described (32, 33). A total of 30 µL of each plasma sample was extracted through incubation at 20°C for 5 min with 1 ml of an extraction solvent (3:3:2, v/v/v, isopropanol-acetonitrile-water). After centrifugation (16,000 rpm, 4°C for 5 min), 0.5 ml of supernatant was removed into a 1.5 ml tube and then dried in a centrifugal vacuum concentrator at 25°C for 6 h. The completely dried pellet was subsequently derivatized prior to GC/MS analysis. The carbonyl functional groups were protected by methoximation using 10 μl of a 40 mg/ml methoxyamine hydrochloride in pyridine (Sigma-Aldrich) at 30°C for 90 min. To increase the volatility of the metabolites, 90 μl of N-methyl-N-(trimethylsilyl) trifluoroacetamide (Fluka, St. Louis, MO) was added to the methoxymized sample at 37°C for 30 min. GC/TOF-MS analysis was conducted using an Agilent 7890A GC system (Hewlett-Packard, Atlanta, GA) coupled to a Pegasus III TOF mass spectrometer (LECO, St. Joseph, MI) equipped with a Rtx-5Sil MS column (30 m × 0.25 mm, 0.25 μm film thickness; Restek, Bellefonte, PA). For metabolite analysis, 1 μl of the derivatized sample was injected in splitless mode, and the sample was subjected to the following conditions: 50°C for 1 min, followed by an increase to 330°C at 20°C per min, and incubation at 330°C for 5 min. Mass spectra were acquired in a scan range of m/z 85–500, with the electron impact and temperature of the ion source set to 70 eV and 250°C, respectively. The mass spectra were preprocessed by automated peak detection and mass spectral deconvolution using LECO ChromaTOF software (version 2.32) and then matched against the retention index and mass spectrum information of customized reference mass spectrum libraries from the BinBase database, which were acquired using authentic standard compounds with identical data acquisition parameters. The identified metabolites were used for the unsupervised principal components analysis (PCA) of multivariate statistics using Statica software (version 7.1; StatSoft, Tulsa, OK, USA).
Statistical analysis
Data are presented as the means ± SE. The values of the treatment groups were compared with those of controls by t-test. Differences with P values < 0.05 were considered statistically significant.
RESULTS
Linalool directly binds to the PPARα LBD, induces transactivation, and is bioavailable
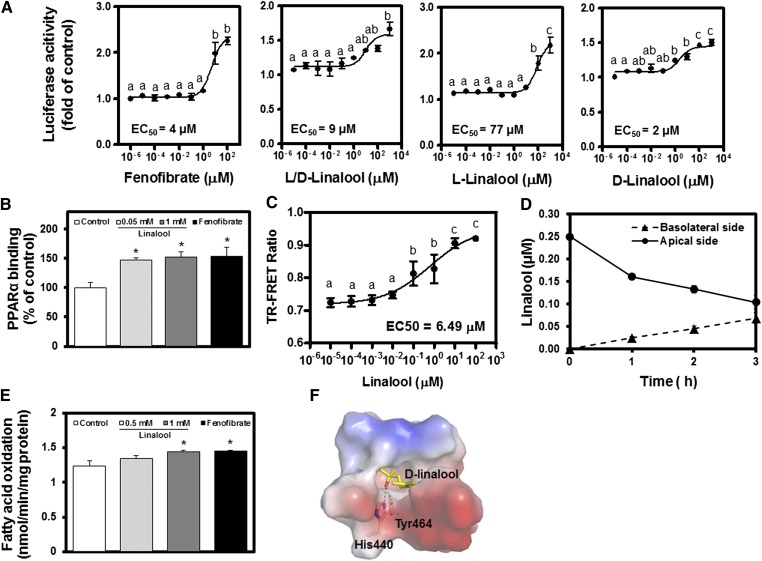
We first investigated PPARα activity regulated with linalool. Linalool (l/d-linalool) induced PPARα transactivation in a dose-dependent manner (Fig. 1A). The results confirmed that each isomeric form, l- and d-, could contribute to the effects; however, d-linalool was more potent than the l-isoform. In this study, we investigated the effects of a mixture of l- and d-linalool (linalool) on the PPARα-dependent lipid metabolism because linalool occurs naturally as two isomeric forms that were both significantly effective. The binding of activated PPARα to the PPRE sequence was confirmed by the binding assay (Fig. 1B). PPARα binding to PPRE was induced significantly in HepG2 cells stimulated with linalool. Ligand binding to PPARα caused conformational changes and induced coactivator recruitment, which can be assessed by the time-resolved (TR)-FRET assay. In the TR-FRET assay, linalool displayed a dose-dependent induction of the recruitment of a PGC1α coactivator peptide to PPARα LBD (EC50 = 5.45 μM, Fig. 1C), suggesting that linalool could act as a PPARα ligand to induce target gene transcription. In the Caco-2 cell-based in vitro bioavailability assay, 0.5 mM linalool showed a time-dependent transport from the apical to the basolateral side of Caco-2 monolayers (Fig. 1D). After 3 h of incubation, the amounts of linalool in the apical and basolateral compartments were 0.1 and 0.07 μM, respectively. The apparent permeability coefficient (Papp) of linalool was calculated as 11.3 ± 2.1 × 10−6 cm/s at the end of 3 h, which is considered indicative of a moderate permeability (34). FA oxidation, which can be induced by PPARα activation, was significantly increased in cells stimulated with linalool (Fig. 1E). In silico molecular docking analysis suggested that d-linalool interacts directly in the active site pocket of PPARα LBD via hydrogen bonding with His440 and Tyr464, thus acting as a PPARα ligand to induce target gene transcription (Fig. 1F). These results demonstrate that linalool is bioavailable, interacts with PPARα protein directly, and thus induces PPARα transactivation.
Fig. 1.
Linalool induces PPARα transactivation via direct binding, thus inducing FA oxidation. A: PPARα-dependent transactivation of linalool in CHO-K1 cells was examined by luciferase assay (treatment for 24 h; n = 3 per group). Fenofibrate (0.1 mM), a positive control; d- and l-isoforms and a d/l mixture (linalool) were examined. B: PPARα DNA binding assay using nuclear extracts from HepG2 cells treated with linalool for 24 h (n = 3 per group). C: TR-FRET assay using PPARα LBD (n = 3–6 per group). D: Bioavailability of linalool (500 µM) quantified by human Caco-2 permeability assay (n = 3 per group). E: FA oxidation was assessed in lipid-loaded (palmitate and oleate) HepG2 cells stimulated with linalool for 24 h and then as described in Materials and Methods. F: In silico molecular docking analysis. d-Linalool binds to the ligand-binding domain of PPARα. Data represent the means ± SE. * P < 0.05 versus the nontreated controls.
Linalool significantly reduces cellular TG concentrations and activates PPARα target genes in HepG2 cells
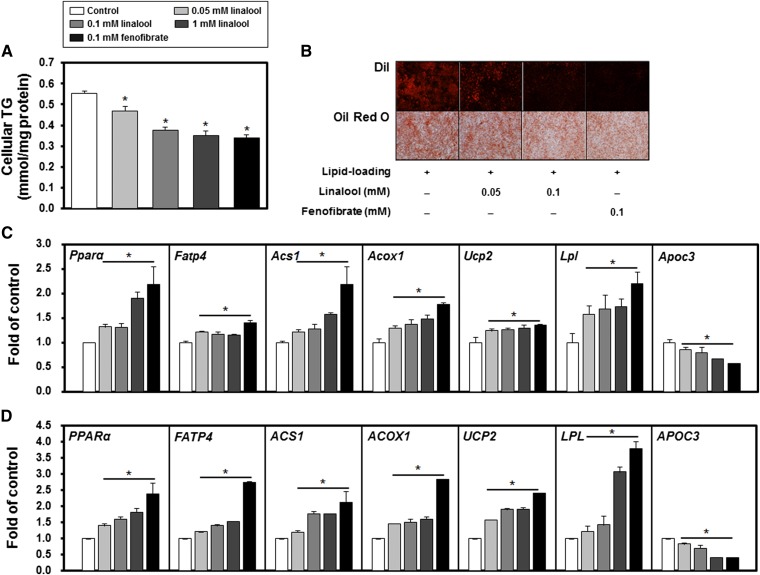
The effects of linalool on TG metabolism and PPARα target gene expression were examined in cultured hepatocytes. Linalool reduced cellular TG concentrations in a dose-dependent manner compared with controls (e.g., 37% at 1 mM, P < 0.05; Fig. 2A). Oil Red O and DiI staining also showed a significant reduction in intracellular TG accumulation by linalool (Fig. 2B). The quantitative real-time PCR (qPCR) and immunoblotting analysis revealed that the gene (Fig. 2C) and protein (Fig. 2D; supplementary Fig. I) expressions of PPARα and the target genes responsible for the hypotriglyceridemic effects were significantly altered by linalool stimulation (e.g., FATP4 and ACS1 for FA uptake; ACOX 1 and UCP2 for FA oxidation; and LPL and APOC3 for TG hydrolysis). Linalool dose-dependently increased the mRNA and protein expression of PPARα (1.9- and 1.8-fold for mRNA and protein expression, respectively, at 1 mM, P < 0.05). The increase in PPARα expression induced by linalool stimulation contributed to the regulation of the expression of its target genes, including upregulation of FATP4, ACS1, ACOX 1, UPC2, and LPL expression and downregulation of APOC3 expression (P < 0.05). In addition, the PPARα-dependent mechanism of action of linalool was further confirmed by specific PPARα knockdown in HepG2 cells using a lentivirus carrying a PPARα-selective shRNA. PPARα shRNA reduced endogenous PPARα protein expression by 80%, and under these conditions, linalool (0.05 and 0.1 mM) did not alter the protein expression of PPARα or the expression of its responsive genes (supplementary Fig. II). Combined, these results demonstrate that linalool is a PPARα ligand and regulates cellular lipid metabolism by altering target gene transcription in vitro.
Fig. 2.
Linalool reduces intracellular TG concentrations in HepG2 cells by a PPARα-dependent mechanism. A: Intracellular TG concentrations. Cellular lipids were extracted and measured, as described in Materials and Methods (n = 3 per group). B: DiI and Oil Red O staining in lipid-loaded (palmitate and oleate) cells with linalool treatment for 24 h. For lipid staining, HepG2 cells were lipid-loaded after incubating with palmitate (400 μM) and oleate (400 μM), which were conjugated to 0.5% (w/v) FA-free BSA, for 24 h. C: PPARα and its responsive gene expressions. D: PPARα and its target protein expressions. For C and D, HepG2 cells were cultured with vehicle, linalool, or fenofibrate for 24 h, and then the total RNA and protein were isolated. No lipid loading was performed prior to the treatment. The mRNA and protein expression levels of PPARα and its targets were evaluated by real-time PCR and immunoblotting and were normalized to the levels of GAPDH mRNA and α-tubulin protein, respectively. HepG2 cells were incubated with linalool (1 mM) or fenofibrate (0.1 mM) for 24 h. Data represent means ± SE. * P < 0.05 versus the nontreated control.
Linalool reduces plasma TG concentrations by activating PPARα in Western-diet-fed C57BL/6J and human apoE2 transgenic mice but has no effect in PPARα-deficient mice
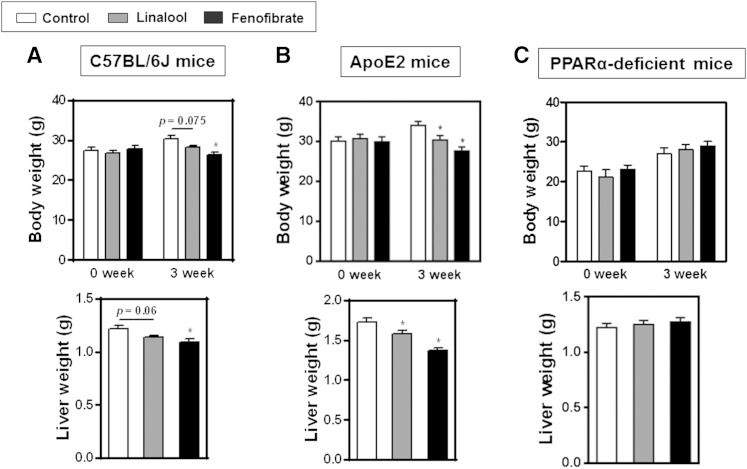
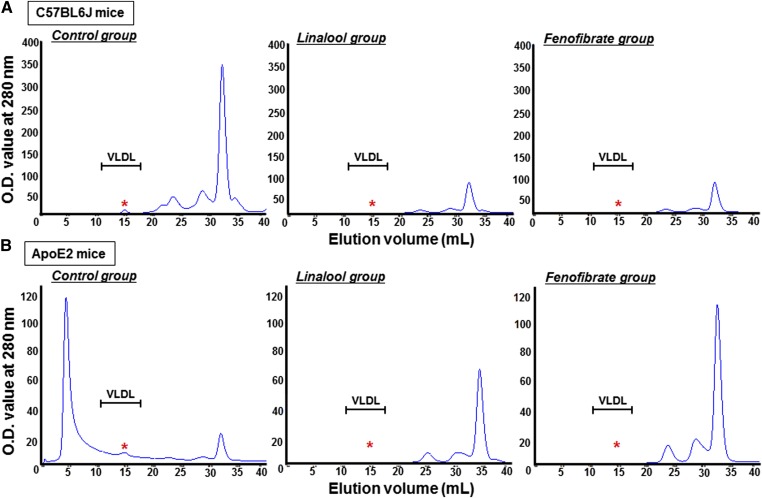
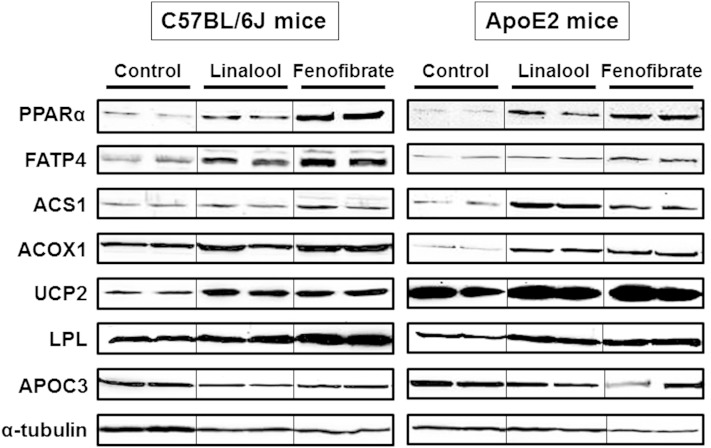
In vivo experiments were performed with C57BL/6J-, apoE2-, and PPARα-deficient mice. Western-diet-fed animals were orally administered with linalool (100 mg/kg body weight/day) for 3 weeks. Cumulated food intake in both C57BL/6J and apoE2 mice was decreased by linalool or fenofibrate supplementation compared with controls as previously reported (data not shown) (35). Body weight of C57BL/6J and apoE2 mice was lower than that of controls after linalool or fenofibrate feeding for 3 weeks with more marked changes in apoE2 mice than C57BL/6J mice (supplementary Fig. III). Linalool administration reduced plasma TG levels by 31% and 50% in C57BL/6J (Fig. 3A) and apoE2 mice (Fig. 4A), respectively, compared with those in control groups (P < 0.05). When the same amount of fenofibrate (100 mg/kg body weight/day) was orally administered to mice, 76% and 89% of the reductions in plasma TG levels were observed in C57BL/6J and apoE2 mice, respectively, which were more profound than those of the linalool group. Lipoprotein profiling analysis by FPLC showed that the TG concentrations in VLDL were dramatically reduced in the linalool group relative to controls (Figs. 3B, 4B; supplementary Fig. IV). Prominent effects of linalool in plasma cholesterol concentrations were also found in our previous study, with −12% and −45% in the total and LDL cholesterol levels of linalool-supplemented group (0.57 mg linalool/mouse/day for 3 weeks), respectively, versus the control group (P < 0.05) (12). In the qPCR and immunoblotting assays of selected biomarkers, hepatic PPARα expression was significantly increased in C57BL/6J mice (+2.1-fold, Fig. 3C) and apoE2 mice (+1.4-fold, Fig. 4C). Liver mRNA expression of PPARα target genes, including FATP4, ACS1, ACOX 1, UCP2, and LPL, was upregulated, whereas the APOC3 mRNA expression was suppressed. Protein expression, which was assessed by immunoblotting, showed trends similar to those for mRNA expression (Figs. 3D, 4D; supplementary Fig. V). However, hyportriglyceridemic activities and PPARα regulation were not observed in PPARα-deficient mice (Fig. 5). Plasma TG concentration and the expression of PPARα and of its responsive genes and proteins were not altered in PPARα-deficient mice fed linalool (Fig. 5A–C). These data demonstrate that linalool ameliorates hypertriglyceridemia by PPARα-dependent mechanisms.
Fig. 3.
Linalool ameliorates hypertriglyceridemia in Western-diet-fed C57BL6 mice by hepatic PPARα activation. Western-diet-fed hypertriglyceridemic C57BL6J mice (n = 3–5 per group) were orally administered with vehicle, linalool (100 mg/kg body weight/day), or fenofibrate (100 mg/kg body weight/day) for 3 weeks, as described in Materials and Methods. A: Fasting plasma TG levels. B: Plasma TG profiling with FPLC analysis. FPLC fractions obtained from overnight fasting plasma were determined as described in Materials and Methods. C: PPARα and its responsive gene expressions. D: PPARα and its target protein expressions. Total RNA and protein were extracted from liver tissues, and the expression levels of PPARα and targets were analyzed by real-time PCR and immunoblotting, respectively (n = 3 per group). mRNA and protein expression levels were normalized to β-actin mRNA and α-tubulin protein, respectively. Data represent means ± SE. * P < 0.05 versus the control group. In C and D, means without common letters differed, P < 0.05.
Fig. 4.
Linalool activates hepatic PPARα and attenuates hypertriglyceridemia in Western-diet-fed human apoE2 knock-in transgenic mice. Hypertriglyceridemic apoE2 mice (n = 3–7 per group) were fed a Western diet supplemented or not (control group) with 100 mg/kg body weight/day linalool or fenofibrate by oral gavage for 3 weeks. A: Analysis of fasting plasma TG levels. B: TG levels in lipoprotein fractions isolated by FPLC analysis from overnight fasting plasma, as described in Materials and Methods. C: PPARα and its responsive gene expressions. D: PPARα and its target protein expressions. Total RNA and protein were isolated from the livers, and the expression levels of PPARα and its targets were quantified by real-time PCR and immunoblotting, respectively (n = 3 per group). β-actin and α-tubulin were used for the normalization of mRNA and protein expression, respectively. Data represent means ± SE. * P < 0.05 versus the control group. In C and D, means without common letters differed, P < 0.05.
Fig. 5.
Hypotriglyceridemic effects of linalool were not observed in PPARα-deficient mice. Western-diet-fed PPARα-deficient mice (n = 6–7 per group) were orally administered with vehicle, linalool (100 mg/kg body weight/day), or fenofibrate (100 mg/kg body weight/day) for 3 weeks. A: Analysis of fasting plasma TG levels. B: PPARα and its target gene expressions assessed with qPCR. β-actin was used as a reference. C: Immunoblotting analysis. α-tubulin was a reference for normalization. Data represent means ± SE. * P < 0.05 versus the control group.
Linalool stimulation of lipid-loaded HepG2 cells rewired the hepatic transcriptome profile, with the effects of linalool being comparable to those of fenofibrate
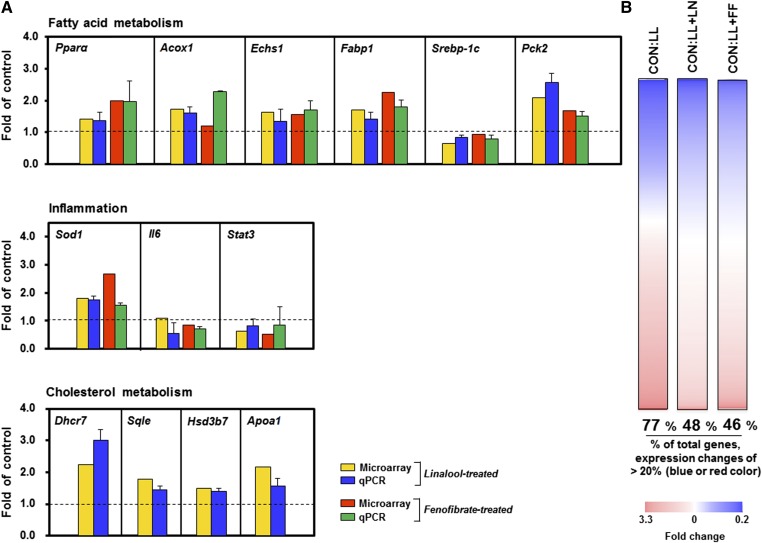
Next, transcriptome profiling was assessed with microarray analysis in lipid-loaded HepG2 cells stimulated with linalool. A total of 8,988 genes were commonly significantly expressed in all experimental groups, including control (CON), lipid-loaded (LL), and lipid-loaded hepatocytes stimulated with either linalool (1 mM; LL+LN) or the hypotriglyceridemic drug fenofibrate (0.1 mM; LL+FF). These genes were analyzed to compare the transcriptome profiles of cells treated with linalool and those treated with fenofibrate. The 13 genes associated with lipid metabolism from the selected genes were measured by qPCR analysis to verify the results of the microarray. The expression patterns of the selected genes in qPCR analyses were similar to those in the microarray analysis (Fig. 6A). Nonbiased genome profiling analysis revealed that lipid loading in hepatocytes notably changed the hepatic transcriptome profile relative to controls. The total of 6,922 genes (77% of the selected genes) showed >20% changes in expression [fold change >1.2 (blue color) or fold change <0.8 (red color)], suggesting that hepatic lipid accumulation could induce dramatic alternations in the global gene expressions compared with normal conditions. However, linalool-stimulated lipid-loaded (LL+LN) cells showed expression changes of >20% in 48% (4,314 genes) of the selected genes relative to controls. Thus, 29% of the selected genes (2,608 genes) resulted in <20% changes in expression after linalool stimulation, compared with the lipid-loading conditions. Fenofibrate-stimulated cells (LL+FF) showed expression changes of >20% in 46% of the selected genes (4,134 genes). Thus, 31% of the selected genes (2,788 genes) fell into the <20% change category compared with lipid-loading conditions after fenofibrate stimulation (Fig. 6B). These results indicate that linalool and fenofibrate shifted aberrant gene expression patterns under lipid-loading conditions toward those of controls without lipid loading. In addition, the gene expression patterns of a high concentration of linalool-treated cells were comparable to those of fenofibrate-stimulated cells.
Fig. 6.
Linalool rewires the hepatic transcriptome and ameliorates lipid metabolism in lipid-loaded HepG2 cells. The alterations of the gene expression patterns in lipid-loading (LL) and lipid-loaded stimulated linalool (LL+LN) or fenofibrate (LL+FF) cells were compared with those of vehicle-treated controls (CON). A: Lipid-loaded (palmitate and oleate) hepatocytes were treated with linalool or fenofibrate. Microarray gene expression heat maps compare hepatic transcriptional profiles (8,988 genes; n = 3 per group; P < 0.05) in TG-accumulated HepG2 cells treated with linalool or fenofibrate. For each gene (row), the heat map colors depict the gene expression levels in the each group (column). Significantly upregulated (blue) and downregulated (red) expression compared with nontreated controls. The percentage of changed selected genes (presented as fold change 1 ± 0.2) in lipid-loaded cells and linalool- or fenofibrate-treated cells was calculated. The middle part of the white portion indicates the unchanged selected genes relative to controls. B: Confirmation of gene expression with qPCR. Real-time PCR validation of the 13 transcripts involved in FA, cholesterol, and inflammatory metabolism (n = 3 per group). Fold changes are LL versus LL+LN or LL+FF.
An excessive accumulation of TG in hepatocytes is mainly linked to defects in the energy metabolism (36, 37). Pathway analysis indicated that linalool caused a significant alteration in energy metabolism, including FA oxidation and synthesis, glycolysis, and TCA cycle. Key genes involved in the metabolic pathways were detectable (supplementary Table I). Decreases in the transcription factor sterol regulatory element binding protein (SREBP)-1C and lipogenic target gene Acc1 were found in the linalool-treated group. As we showed in the PPARα-targeted mechanism of linalool, the expression of the genes involved in FA oxidation, including Echs1, Acaa, Acox1, and Acadm, was induced by linalool. Microarray data also showed that the impaired glycolysis under hepatic lipid accumulation conditions could be improved by linalool by significant reductions in glucokinase (Gck), pyruvate kinase (Pk), and phosphofructokinase (Pkfl). The expression of TCA-cycle-related genes, which link glycolysis and fat oxidation to respiration, was increased under linalool-treated conditions (i.e., Sdhb, Mdh, and Suclg1). Linalool also positively affected hepatic lipid metabolism through the upregulation of Hsd17b4, Cyp27a1, Hsd3b7, and Akr1c3 expression for the conversion of excessive cholesterols to bile acids. Therefore, the linalool-mediated regulation of these key genes might play a prominent role in the improvement of the aberrant hepatic transcriptome by lipid accumulation and eventual protection against hypertriglyceridemia.
Linalool altered the plasma FA metabolite
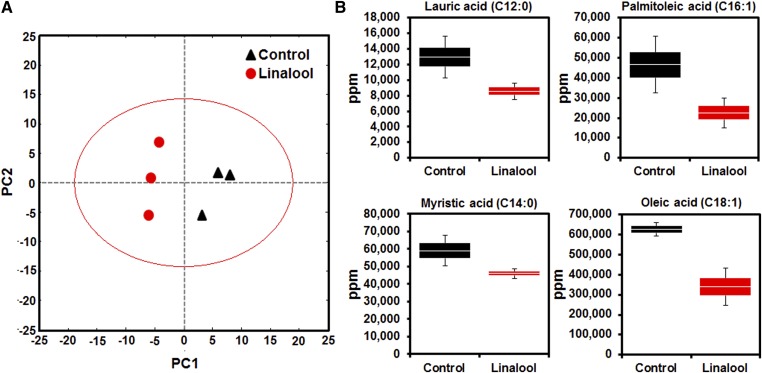
Plasma metabolomic analysis was performed to examine the metabolic effects of linalool in C57BL/6J mice. A total of 103 metabolites were detected in both control and linalool-fed mice. PCA of the 103 metabolites showed a differential distribution of control and linalool-treated mice along the principal component (PC) 1 and PC2 dimensions, describing 38% and 23% of the total variance, respectively (Fig. 7A). PC1 primarily contributed to the separation of control and linalool-treated groups. Interestingly, the results showed that the top loading metabolites for PC1 included long-chain FAs, such as oleic and lauric acids, which were major contributors to the differentiation of PCA values between control and linalool-fed groups and were thus associated with the hypotriglyceridemic effects of linalool (supplementary Table II).
Fig. 7.
Linalool alters plasma FA metabolite composition reflecting hypotriglyceridemic properties in nontargeted metabolomic analysis. Metabolites were extracted from plasma from vehicle- or linalool-treated C57BL/6J mice with diet-induced hypertriglyceridemia. The metabolites were then derivatized and analyzed, as described in Materials and Methods (n = 3 per group). A: PCA was performed with 103 metabolites detected in both the control and linalool treatment groups. B: Plasma levels of selected metabolites (lauric acid, palmitoleic acid, myristic acid, and oleic acid). Data are shown as box-and-whisker plots.
Because an association between plasma TG and FA concentrations in metabolic diseases has been reported in human studies, the effects of linalool on plasma FA composition were assessed with metabolomic analysis. We selected 13 long-chain FA metabolites that had been altered by linalool treatment (supplementary Table III), out of which four FA metabolites, lauric acid, palmitoleic acid, myristic acid, and oleic acid, showed greatly reduced levels, as described in the previous studies that reported alterations in the plasma FA metabolite composition in the plasma under TG-lowered conditions in vivo (38). However, the stearic acid concentrations were not changed. Box-whisker plots show that the intensities of the metabolic signals for FAs (n = 3) were additive in these regions and were lower in the linalool-treated group than in controls (Fig. 7B). These data demonstrate that linalool significantly altered the plasma metabolite profile and reduced the concentrations of most of the long-chain FA concentrations as a result of PPARα activation.
DISCUSSION
Aromatic compounds generally have potent radical scavenging activities (17, 18) and possess the ability to directly interact with proteins (e.g., binding and interaction with olfactory receptors), thereby playing important biological roles (39, 40). Studies have shown that the oral administration of aromatic compounds has notable biological effects in animals and humans, suggesting their potential as active therapeutic and preventive agents (12, 19, 41). Linalool, a major aromatic terpenoid of teas and herbal essential oils, has been traditionally used for medicinal purposes because of its potent antioxidative activities (17, 18). Recent reports have shown that the oral administration of fragrant herbal essential oils containing linalool, including Plantago asiatica and Melissa officinalis essential oils, improved dyslipidemia by reducing plasma TG concentrations (13, 19). Here, we demonstrate that the hypotriglyceridemic effects of linalool can be achieved by PPARα agonistic properties.
PPARα is a ligand-activated nuclear receptor regulating the plasma TG concentration. Ligand binding alters the conformation of PPARα, enabling it to recruit the coactivators required for the transcriptional activation (42, 43). We confirmed that linalool induced the transactivation of PPARα in a reporter gene assay and that it stimulated the recruitment of PGC1α coactivator peptide due to conformational changes in PPARα via ligand binding in a TR-FRET assay. These findings demonstrate that linalool directly interacts with the PPARα LBD and functions as an agonistic ligand. In this case, d-linalool bound more tightly to PPARα than l-linalool. The activities of the PPARβ/δ and γ subtypes were not altered by linalool (data not shown). In cultured cell studies, a radiolabeled assay confirmed that linalool promoted FA oxidation significantly and regulated PPARα and its responsive gene and protein expressions, thus reducing intracellular TG accumulations, although not in cells with PPARα gene knockdowns.
The role of linalool in TG and FA metabolism was investigated in vivo in three independent Western-diet-fed animal studies in C57BL/6J, human apoE2 transgenic, and PPARα-deficient mice. The oral administration of linalool for 3 weeks regulated hepatic PPARα and its responsive genes, finally reducing plasma TG levels in both human apoE2 and wild-type (C57BL/6J) mice. Consistently, FPLC analysis showed a significant suppression of TG concentrations in VLDL in the linalool-supplemented group, compared with controls. Notably, the effects of linalool in PPARα-dependent mechanisms and TG reduction were not shown in PPARα-deficient mice. The results from both cultured cell and animal experiments indicate that the hypotriglyceridemic properties of linalool are achieved via PPARα activation.
Nonbiased genomic analysis suggested that both linalool and fenofibrate partially normalized the hyperlipidemic hepatic transcriptome. The data verified again that PPARα is a target molecule of linalool. Furthermore, global gene expression patterns showed that linalool rewired the aberrant hyperlipidemic hepatic transcriptome, shifting it toward that of control conditions. Pathway analysis indicated that linalool caused a significant alteration in energy metabolism, inducing FA oxidation, while suppressing the FA synthesis pathways in line with the documented biological functions of PPARα activators and the results from our experiments. These results demonstrated that linalool ameliorated the hepatic TG metabolism by rewiring the transcriptome profile from hyperlipidemic patterns toward those found in control hepatocytes. The results of the hepatic transcriptome profiling revealed that linalool administration was associated with the regulation of FA and carbohydrate (glucose) metabolism gene expression. Linalool activated the expression of Acadvl, Echs1, Acox1, Acaa1, and Acadm, Hadh, and Fabp1, which are key genes in FA utilization. In addition, linalool suppressed the expression of several key lipogenic genes, including Srebp-1c, Acc1, and Elovl3. Collectively, these activities decreased the number of acyl-CoA substrates for TG synthesis. In glucose metabolism, the inhibition of Pk, Gck, and Pfkl expression in the liver is known to reduce the rates of glycolysis, acetyl-CoA formation, and lipogenesis under conditions of a high energy charge. Transcriptome profiling indicated that reductions in the expressions of Pk as well as in other Gck and Pkfl were reduced in the linalool-stimulated group, suggesting the excessive glycolysis-dependent lipogenesis and subsequent hepatic TG accumulation were ameliorated by linalool. The induction of Suclg2, a key regulatory enzyme of the TCA cycle, also suggested an enhanced oxidation of acetyl-CoA. Taken together, the data from the hepatic transcriptome profile supported the hypotriglyceridemic effects of linalool.
Plasma metabolome analysis also suggested the hypotriglyceridemic effects of linalool, showing changes in the plasma FA metabolite levels of linalool-treated mice compared with controls. Notably, among the 13 FFAs analyzed, the concentrations of 11 were lower in the linalool group than in controls, corresponding to a significant TG reduction in the plasma of linalool-supplemented mice. The association between serum TG levels and FA composition under metabolic disease conditions was previously reported in several human studies. Schwab et al. (2008) reported that a plasma TG reduction affected plasma FA metabolite levels in obese subjects following a dietary intervention. These authors showed that short-chain and saturated FAs were significantly downregulated, which was associated with an improvement in insulin sensitivity (38). We also found that the plasma levels of saturated FA metabolites, such as lauric acid, palmitic acid, and myristic acid, but not stearic acid, were significantly reduced in linalool-treated mice. Furthermore, interestingly, our data showed very similar changes in FA metabolite compositions (especially, C16 and C18) as those in previously reported studies investigating the hypolipidemic effects of phytochemical supplementation in animals (44, 45). It has been reported that the decreased circulation of saturated FAs may be considered a beneficial effect because an increased influx of saturated FAs into peripheral tissues can lead to the accumulation of lipids in the tissues (46). In combination with transcriptional changes, it is thought that the PPARα-targeted mechanism and regulation of gene expression involved in FA production and oxidation, as suggested in hepatic transcriptome profiling, could very strongly support the reduction of long-chain TGs in metabolite analysis. Therefore, metabolite profiling demonstrated a significant association between the enhancement of FA metabolite composition and the attenuation of hypotriglyceridemia by linalool treatment.
The results of the in vitro permeability of human intestinal Caco-2 cells implied that linalool is efficiently absorbed in the human intestine (Papp = 11.3 ± 2.1 × 10−6 cm/s). For example, the hypertension drug propranolol, which shows a similar permeability to that of linalool, has been reported to display a 90% absorption in humans (34). Consistently, linalool also showed a high availability in vivo, based on the Screening Information Dataset published by United Nations Environment Programme. That study reported that linalool is rapidly and completely absorbed from intestinal tract following oral uptake in rats using 14C-labeled substances (16). Previously, it has been demonstrated that inhalation is able to deliver aromatic compounds to the blood via the absorption of the compound through the lungs. For example, inhalation experiments with several herbal essential oils showed a significant delivery of aromatic compounds in the plasma, acting in a dose-dependent manner (47, 48). Thus, the aromatic compounds in teas could possibly be delivered to the blood by both inhalation and intestinal uptake to circulate in the blood and to exert metabolic effects on the target tissues, including the liver.
The antioxidant activity of linalool has been well-established in previous studies (17, 18). We reported that linalool could protect against hypercholesterolemia induced by high-fat diet through the downregulation of hepatic SREBP2-mediated cholesterol synthesis. Together with these known bioactivities of linalool, the hypotriglyceridemic effects, which we proved in this study, are able to provide strength in the availability of linalool for the prevention and treatment of CVD. Linalool might contribute to the improvement of CVD through a significant reduction of TG and cholesterol production and accumulation as a PPARα ligand and through the inhibition of lipid peroxidation and inflammation via antioxidative activities, thereby leading to the attenuation of atherosclerosis.
In conclusion, we showed that linalool, a major and common aromatic compound contained in most herbal essential oils and teas, reduced plasma TG concentrations, had PPARα agonistic effects, and rewired the hepatic transcriptome (mimicking the effects of fenofibrate) and plasma metabolome. Our results suggest that the intake of herbs and teas containing linalool may contribute to the prevention and improvement of hypertriglyceridemia.
Supplementary Material
Acknowledgments
The authors thank Haewon Kim for technical assistance and Prof. Hyun-Gyu Song for the advice on FRET analysis.
Footnotes
Abbreviations:
- ACOX 1
- acyl-CoA oxidase 1
- ACS1
- acyl-CoA synthetase 1
- FATP
- FA transporter protein
- FPLC
- fast protein LC
- FRET
- fluorescence resonance energy transfer
- Gck
- glucokinase
- LBD
- ligand binding domain
- PC
- principal component
- PCA
- principal components analysis
- PGC1α
- PPARγ coactivator 1
- Pk
- pyruvate kinase
- Pkfl
- phosphofructokinase
- PPRE
- PPAR response element
- qPCR
- quantitative real-time PCR
- TR-FRET
- time-resolved fluorescence resonance energy transfer
- UCP2
- uncoupling protein 2
This work was supported by the Basic Science Research Program of MEST/NRF, Republic of Korea (2013R1A2A2A01016176), and the BK21-PLUS program.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five figures and three tables.
REFERENCES
- 1.Holmes D. R., Jr, Elveback L. R., Frye R. L., Kottke B. A., Ellefson R. D. 1981. Association of risk factor variables and coronary artery disease documented with angiography. Circulation. 63: 293–299. [DOI] [PubMed] [Google Scholar]
- 2.Miller M., Stone N. J., Ballantyne C., Bittner V., Criqui M. H., Ginsberg H. N., Goldberg A. C., Howard W. J., Jacobson M. S., Kris-Etherton P. M., et al. 2011. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 123: 2292–2333. [DOI] [PubMed] [Google Scholar]
- 3.Austin M. A., Hokanson J. E., Edwards K. L. 1998. Hypertriglyceridemia as a cardiovascular risk factor. Am. J. Cardiol. 81: 7B–12B. [DOI] [PubMed] [Google Scholar]
- 4.Aprikian O., Duclos V., Guyot S., Besson C., Manach C., Bernalier A., Morand C., Remesy C., Demigne C. 2003. Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. J. Nutr. 133: 1860–1865. [DOI] [PubMed] [Google Scholar]
- 5.Skulas-Ray A. C., Kris-Etherton P. M., Teeter D. L., Chen C. Y., Vanden Heuvel J. P., West S. G. 2011. A high antioxidant spice blend attenuates postprandial insulin and triglyceride responses and increases some plasma measures of antioxidant activity in healthy, overweight men. J. Nutr. 141: 1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuriyama S., Shimazu T., Ohmori K., Kikuchi N., Nakaya N., Nishino Y., Tsubono Y., Tsuji I. 2006. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 296: 1255–1265. [DOI] [PubMed] [Google Scholar]
- 7.Basu A., Lucas E. A. 2007. Mechanisms and effects of green tea on cardiovascular health. Nutr. Rev. 65: 361–375. [DOI] [PubMed] [Google Scholar]
- 8.Unno T., Tago M., Suzuki Y., Nozawa A., Sagesaka Y. M., Kakuda T., Egawa K., Kondo K. 2005. Effect of tea catechins on postprandial plasma lipid responses in human subjects. Br. J. Nutr. 93: 543–547. [DOI] [PubMed] [Google Scholar]
- 9.Iwai N., Ohshiro H., Kurozawa Y., Hosoda T., Morita H., Funakawa K., Okamoto M., Nose T. 2002. Relationship between coffee and green tea consumption and all-cause mortality in a cohort of a rural Japanese population. J. Epidemiol. 12: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown A. L., Lane J., Coverly J., Stocks J., Jackson S., Stephen A., Bluck L., Coward A., Hendrickx H. 2009. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br. J. Nutr. 101: 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thielecke F., Boschmann M. 2009. The potential role of green tea catechins in the prevention of the metabolic syndrome - a review. Phytochemistry. 70: 11–24. [DOI] [PubMed] [Google Scholar]
- 12.Cho S. Y., Jun H. J., Lee J. H., Jia Y., Kim K. H., Lee S. J. 2011. Linalool reduces the expression of 3-hydroxy-3-methylglutaryl CoA reductase via sterol regulatory element binding protein-2- and ubiquitin-dependent mechanisms. FEBS Lett. 585: 3289–3296. [DOI] [PubMed] [Google Scholar]
- 13.Jun H. J., Lee J. H., Jia Y., Hoang M. H., Byun H., Kim K. H., Lee S. J. 2012. Melissa officinalis essential oil reduces plasma triglycerides in human apolipoprotein E2 transgenic mice by inhibiting sterol regulatory element-binding protein-1c-dependent fatty acid synthesis. J. Nutr. 142: 432–440. [DOI] [PubMed] [Google Scholar]
- 14.Lee S., Park M. K., Kim K. H., Kim Y. S. 2007. Effect of supercritical carbon dioxide decaffeination on volatile components of green teas. J. Food Sci. 72: S497–S502. [DOI] [PubMed] [Google Scholar]
- 15.Pripdeevech P., Machan T. 2011. Fingerprint of volatile flavour constituents and antioxidant activities of teas from Thailand. Food Chem. 125: 797–802. [Google Scholar]
- 16.Organisation for Economic Co-operation and Development (OECD) Screening Information Dataset (SIDS). 2002. Linalool, CAS N°: 78-70-6: SIDS Initial Assessment Report for SIAM 14. United Nations Environment Programme (UNEP) Publications, Paris, France. [Google Scholar]
- 17.Peana A. T., D’Aquila P. S., Panin F., Serra G., Pippia P., Moretti M. D. 2002. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine. 9: 721–726. [DOI] [PubMed] [Google Scholar]
- 18.Naderi G. A., Asgary S., Ani M., Sarraf-Zadegan N., Safari M. R. 2004. Effect of some volatile oils on the affinity of intact and oxidized low-density lipoproteins for adrenal cell surface receptors. Mol. Cell. Biochem. 267: 59–66. [DOI] [PubMed] [Google Scholar]
- 19.Chung M. J., Park K. W., Kim K. H., Kim C. T., Baek J. P., Bang K. H., Choi Y. M., Lee S. J. 2008. Asian plantain (Plantago asiatica) essential oils suppress 3-hydroxy-3-methyl-glutaryl-co-enzyme A reductase expression in vitro and in vivo and show hypocholesterolaemic properties in mice. Br. J. Nutr. 99: 67–75. [DOI] [PubMed] [Google Scholar]
- 20.Gervois P., Fruchart J. C., Staels B. 2007. Drug Insight: mechanisms of action and therapeutic applications for agonists of peroxisome proliferator-activated receptors. Nat. Clin. Pract. Endocrinol. Metab. 3: 145–156. [DOI] [PubMed] [Google Scholar]
- 21.Staels B., Dallongeville J., Auwerx J., Schoonjans K., Leitersdorf E., Fruchart J. C. 1998. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 98: 2088–2093. [DOI] [PubMed] [Google Scholar]
- 22.Lefebvre P., Chinetti G., Fruchart J. C., Staels B. 2006. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J. Clin. Invest. 116: 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan Y. J., Cai Y., Lungo W., Fu P., Locker J., French S., Sucov H. M. 2000. Peroxisome proliferator-activated receptor alpha-mediated pathways are altered in hepatocyte-specific retinoid X receptor alpha-deficient mice. J. Biol. Chem. 275: 28285–28290. [DOI] [PubMed] [Google Scholar]
- 24.Xu H. E., Stanley T. B., Montana V. G., Lambert M. H., Shearer B. G., Cobb J. E., McKee D. D., Galardi C. M., Plunket K. D., Nolte R. T., et al. 2002. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPARalpha. Nature. 415: 813–817. [DOI] [PubMed] [Google Scholar]
- 25.Jia Y., Qi C., Kashireddi P., Surapureddi S., Zhu Y. J., Rao M. S., Le Roith D., Chambon P., Gonzalez F. J., Reddy J. K. 2004. Transcription coactivator PBP, the peroxisome proliferator-activated receptor (PPAR)-binding protein, is required for PPARalpha-regulated gene expression in liver. J. Biol. Chem. 279: 24427–24434. [DOI] [PubMed] [Google Scholar]
- 26.Jia Y., Kim J. Y., Jun H. J., Kim S. J., Lee J. H., Hoang M. H., Kim H. S., Chang H. I., Hwang K. Y., Um S. J., et al. 2013. Cyanidin is an agonistic ligand for peroxisome proliferator-activated receptor-alpha reducing hepatic lipid. Biochim. Biophys. Acta. 1831: 698–708. [DOI] [PubMed] [Google Scholar]
- 27.Brand W., van der Wel P. A., Rein M. J., Barron D., Williamson G., van Bladeren P. J., Rietjens I. M. 2008. Metabolism and transport of the citrus flavonoid hesperetin in Caco-2 cell monolayers. Drug Metab. Dispos. 36: 1794–1802. [DOI] [PubMed] [Google Scholar]
- 28.Hidalgo I. J., Raub T. J., Borchardt R. T. 1989. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 96: 736–749. [PubMed] [Google Scholar]
- 29.Artursson P. 1990. Epithelial transport of drugs in cell culture. I: A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J. Pharm. Sci. 79: 476–482. [DOI] [PubMed] [Google Scholar]
- 30.Jia Y., Kim J. Y., Jun H. J., Kim S. J., Lee J. H., Hoang M. H., Hwang K. Y., Um S. J., Chang H. I., Lee S. J. 2012. The natural carotenoid astaxanthin, a PPAR-alpha agonist and PPAR-gamma antagonist, reduces hepatic lipid accumulation by rewiring the transcriptome in lipid-loaded hepatocytes. Mol. Nutr. Food Res. 56: 878–888. [DOI] [PubMed] [Google Scholar]
- 31.Furth E. E., Sprecher H., Fisher E. A., Fleishman H. D., Laposata M. 1992. An in vitro model for essential fatty acid deficiency: HepG2 cells permanently maintained in lipid-free medium. J. Lipid Res. 33: 1719–1726. [PubMed] [Google Scholar]
- 32.A J., Trygg J., Gullberg J., Johansson A. I., Jonsson P., Antti H., Marklund S. L., Moritz T. 2005. Extraction and GC/MS analysis of the human blood plasma metabolome, Anal Chem. 77:8086–8094. [DOI] [PubMed] [Google Scholar]
- 33.Lee D. Y., Fiehn O. 2008. High quality metabolomic data for Chlamydomonas reinhardtii. Plant Methods. 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu M., Ling J., Lin H., Chen J. 2004. Use of Caco-2 cell monolayers to study drug absorption and metabolism. In Methods in Pharmacology and Toxicology. Optimization in Drug Discovery: In Vitro Methods. Z. Yan and G. W. Caldwell, editors. Humana Press, Totowa, NJ. 19–35. [Google Scholar]
- 35.Larsen P. J., Jensen P. B., Sorensen R. V., Larsen L. K., Vrang N., Wulff E. M., Wassermann K. 2003. Differential influences of peroxisome proliferator-activated receptors gamma and -alpha on food intake and energy homeostasis. Diabetes. 52: 2249–2259. [DOI] [PubMed] [Google Scholar]
- 36.Browning J. D., Horton J. D. 2004. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Postic C., Girard J. 2008. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest. 118: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwab U., Seppanen-Laakso T., Yetukuri L., Agren J., Kolehmainen M., Laaksonen D. E., Ruskeepaa A. L., Gylling H., Uusitupa M., Oresic M. 2008. Triacylglycerol fatty acid composition in diet-induced weight loss in subjects with abnormal glucose metabolism–the GENOBIN study. PLoS ONE. 3: e2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oka Y., Omura M., Kataoka H., Touhara K. 2004. Olfactory receptor antagonism between odorants. EMBO J. 23: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Touhara K., Sengoku S., Inaki K., Tsuboi A., Hirono J., Sato T., Sakano H., Haga T. 1999. Functional identification and reconstitution of an odorant receptor in single olfactory neurons. Proc. Natl. Acad. Sci. USA. 96: 4040–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung M. J., Cho S. Y., Bhuiyan M. J., Kim K. H., Lee S. J. 2010. Anti-diabetic effects of lemon balm (Melissa officinalis) essential oil on glucose- and lipid-regulating enzymes in type 2 diabetic mice. Br. J. Nutr. 104: 180–188. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y. X. 2010. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res. 20: 124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chawla A., Repa J. J., Evans R. M., Mangelsdorf D. J. 2001. Nuclear receptors and lipid physiology: opening the X-files. Science. 294: 1866–1870. [DOI] [PubMed] [Google Scholar]
- 44.Graf D., Seifert S., Jaudszus A., Bub A., Watzl B. 2013. Anthocyanin-rich juice lowers serum cholesterol, leptin, and resistin and improves plasma fatty acid composition in Fischer rats. PLoS ONE. 8: e66690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ayerza R., Coates W. 2005. Ground chia seed and chia oil effects on plasma lipids and fatty acids in the rat. Nutr. Res. 25: 995–1003. [Google Scholar]
- 46.Nielsen H. B., Febbraio M. A., Ott P., Krustrup P., Secher N. H. 2007. Hepatic lactate uptake versus leg lactate output during exercise in humans. J. Appl. Physiol. 103: 1227–1233. [DOI] [PubMed] [Google Scholar]
- 47.Muchtaridi A., Subarnas A., Apriyantono A., Mustarichie R. 2010. Identification of compounds in the essential oil of nutmeg seeds (Myristica fragrans Houtt.) that inhibit locomotor activity in mice. Int. J. Mol. Sci. 11: 4771–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muchtaridi, Diantini A., Subarnas A. 2011. Analysis of Indonesian spice essential oil compounds that inhibit locomotor activity in mice. Pharmaceuticals. 4: 590–602. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.