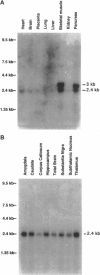
Abstract
The inheritance of much early-onset Alzheimer disease (AD) has been linked to a dominant-acting locus on chromosome 14. Recently, the gene likely responsible for this genetic linkage has been identified and termed AD3. Five mutations have been found in AD3 that segregate with the disease phenotype in seven AD families and are not present in unaffected individuals. Here we report the existence of a gene encoding a seven transmembrane domain protein very similar to that encoded by AD3 in structure and sequence. This gene is located on chromosome 1, is expressed in a variety of tissues, including brain, and is predicted to harbor mutations causing nonchromosome 14 familial AD. The presence of several S/TPXX DNA binding motifs in both the AD3 protein and the AD3-like protein /AD4 protein suggests a possible role in intracellular signaling and gene expression or in linking chromatin to the nuclear membrane. Ways in which mutations in either gene could lead to AD are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Abraham C. R., Selkoe D. J., Potter H., Price D. L., Cork L. C. Alpha 1-antichymotrypsin is present together with the beta-protein in monkey brain amyloid deposits. Neuroscience. 1989;32(3):715–720. doi: 10.1016/0306-4522(89)90292-3. [DOI] [PubMed] [Google Scholar]
- Abraham C. R., Shirahama T., Potter H. Alpha 1-antichymotrypsin is associated solely with amyloid deposits containing the beta-protein. Amyloid and cell localization of alpha 1-antichymotrypsin. Neurobiol Aging. 1990 Mar-Apr;11(2):123–129. doi: 10.1016/0197-4580(90)90045-2. [DOI] [PubMed] [Google Scholar]
- Ashall F., Goate A. M. Role of the beta-amyloid precursor protein in Alzheimer's disease. Trends Biochem Sci. 1994 Jan;19(1):42–46. doi: 10.1016/0968-0004(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Cai X. D., Golde T. E., Younkin S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Evans K. C., Berger E. P., Cho C. G., Weisgraber K. H., Lansbury P. T., Jr Apolipoprotein E is a kinetic but not a thermodynamic inhibitor of amyloid formation: implications for the pathogenesis and treatment of Alzheimer disease. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):763–767. doi: 10.1073/pnas.92.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R., Gerace L. Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell. 1993 Jul 2;73(7):1267–1279. doi: 10.1016/0092-8674(93)90355-t. [DOI] [PubMed] [Google Scholar]
- Games D., Adams D., Alessandrini R., Barbour R., Berthelette P., Blackwell C., Carr T., Clemens J., Donaldson T., Gillespie F. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995 Feb 9;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Kamboh M. I., Sanghera D. K., Ferrell R. E., DeKosky S. T. APOE*4-associated Alzheimer's disease risk is modified by alpha 1-antichymotrypsin polymorphism. Nat Genet. 1995 Aug;10(4):486–488. doi: 10.1038/ng0895-486. [DOI] [PubMed] [Google Scholar]
- Katsunuma T., Tsuda M., Kusumi T., Ohkubo T., Mitomi T., Nakasaki H., Tajima T., Yokoyama S., Kamiguchi H., Kobayashi K. Purification of a serum DNA binding protein (64DP) with a molecular weight of 64,000 and its diagnostic significance in malignant diseases. Biochem Biophys Res Commun. 1980 Mar 28;93(2):552–557. doi: 10.1016/0006-291x(80)91112-2. [DOI] [PubMed] [Google Scholar]
- L'Hernault S. W., Arduengo P. M. Mutation of a putative sperm membrane protein in Caenorhabditis elegans prevents sperm differentiation but not its associated meiotic divisions. J Cell Biol. 1992 Oct;119(1):55–68. doi: 10.1083/jcb.119.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan D., Greenwald I. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature. 1995 Sep 28;377(6547):351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E., Wasco W., Poorkaj P., Romano D. M., Oshima J., Pettingell W. H., Yu C. E., Jondro P. D., Schmidt S. D., Wang K. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995 Aug 18;269(5226):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E., Wijsman E. M., Nemens E., Anderson L., Goddard K. A., Weber J. L., Bird T. D., Schellenberg G. D. A familial Alzheimer's disease locus on chromosome 1. Science. 1995 Aug 18;269(5226):970–973. doi: 10.1126/science.7638621. [DOI] [PubMed] [Google Scholar]
- Ma J., Yee A., Brewer H. B., Jr, Das S., Potter H. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994 Nov 3;372(6501):92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- Mullan M., Houlden H., Windelspecht M., Fidani L., Lombardi C., Diaz P., Rossor M., Crook R., Hardy J., Duff K. A locus for familial early-onset Alzheimer's disease on the long arm of chromosome 14, proximal to the alpha 1-antichymotrypsin gene. Nat Genet. 1992 Dec;2(4):340–342. doi: 10.1038/ng1292-340. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B., Beyreuther K. Molecular biology of Alzheimer's disease. Annu Rev Biochem. 1989;58:287–307. doi: 10.1146/annurev.bi.58.070189.001443. [DOI] [PubMed] [Google Scholar]
- Naidoo N., Cooperman B. S., Wang Z. M., Liu X. Z., Rubin H. Identification of lysines within alpha 1-antichymotrypsin important for DNA binding. An unusual combination of DNA-binding elements. J Biol Chem. 1995 Jun 16;270(24):14548–14555. doi: 10.1074/jbc.270.24.14548. [DOI] [PubMed] [Google Scholar]
- Namba Y., Tomonaga M., Kawasaki H., Otomo E., Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991 Feb 8;541(1):163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Finch E. A., Dawes L. R. Expression of the Alzheimer amyloid precursor gene transcripts in the human brain. Neuron. 1988 Oct;1(8):669–677. doi: 10.1016/0896-6273(88)90166-3. [DOI] [PubMed] [Google Scholar]
- Pericak-Vance M. A., Bebout J. L., Gaskell P. C., Jr, Yamaoka L. H., Hung W. Y., Alberts M. J., Walker A. P., Bartlett R. J., Haynes C. A., Welsh K. A. Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am J Hum Genet. 1991 Jun;48(6):1034–1050. [PMC free article] [PubMed] [Google Scholar]
- Potter H., Nelson R. B., Das S., Siman R., Kayyali U. S., Dressler D. The involvement of proteases, protease inhibitors, and an acute phase response in Alzheimer's disease. Ann N Y Acad Sci. 1992 Dec 31;674:161–173. doi: 10.1111/j.1749-6632.1992.tb27485.x. [DOI] [PubMed] [Google Scholar]
- Potter H. Review and hypothesis: Alzheimer disease and Down syndrome--chromosome 21 nondisjunction may underlie both disorders. Am J Hum Genet. 1991 Jun;48(6):1192–1200. [PMC free article] [PubMed] [Google Scholar]
- Quon D., Wang Y., Catalano R., Scardina J. M., Murakami K., Cordell B. Formation of beta-amyloid protein deposits in brains of transgenic mice. Nature. 1991 Jul 18;352(6332):239–241. doi: 10.1038/352239a0. [DOI] [PubMed] [Google Scholar]
- Rogaev E. I., Sherrington R., Rogaeva E. A., Levesque G., Ikeda M., Liang Y., Chi H., Lin C., Holman K., Tsuda T. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature. 1995 Aug 31;376(6543):775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- Rozemuller J. M., Abbink J. J., Kamp A. M., Stam F. C., Hack C. E., Eikelenboom P. Distribution pattern and functional state of alpha 1-antichymotrypsin in plaques and vascular amyloid in Alzheimer's disease. A immunohistochemical study with monoclonal antibodies against native and inactivated alpha 1-antichymotrypsin. Acta Neuropathol. 1991;82(3):200–207. doi: 10.1007/BF00294446. [DOI] [PubMed] [Google Scholar]
- Rozemuller J. M., Stam F. C., Eikelenboom P. Acute phase proteins are present in amorphous plaques in the cerebral but not in the cerebellar cortex of patients with Alzheimer's disease. Neurosci Lett. 1990 Oct 30;119(1):75–78. doi: 10.1016/0304-3940(90)90759-3. [DOI] [PubMed] [Google Scholar]
- Sanan D. A., Weisgraber K. H., Russell S. J., Mahley R. W., Huang D., Saunders A., Schmechel D., Wisniewski T., Frangione B., Roses A. D. Apolipoprotein E associates with beta amyloid peptide of Alzheimer's disease to form novel monofibrils. Isoform apoE4 associates more efficiently than apoE3. J Clin Invest. 1994 Aug;94(2):860–869. doi: 10.1172/JCI117407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg G. D., Bird T. D., Wijsman E. M., Orr H. T., Anderson L., Nemens E., White J. A., Bonnycastle L., Weber J. L., Alonso M. E. Genetic linkage evidence for a familial Alzheimer's disease locus on chromosome 14. Science. 1992 Oct 23;258(5082):668–671. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Pericak-Vance M. A., Wijsman E. M., Moore D. K., Gaskell P. C., Jr, Yamaoka L. A., Bebout J. L., Anderson L., Welsh K. A., Clark C. M. Linkage analysis of familial Alzheimer disease, using chromosome 21 markers. Am J Hum Genet. 1991 Mar;48(3):563–583. [PMC free article] [PubMed] [Google Scholar]
- Schupf N., Kapell D., Lee J. H., Ottman R., Mayeux R. Increased risk of Alzheimer's disease in mothers of adults with Down's syndrome. Lancet. 1994 Aug 6;344(8919):353–356. doi: 10.1016/s0140-6736(94)91398-6. [DOI] [PubMed] [Google Scholar]
- Scinto L. F., Daffner K. R., Dressler D., Ransil B. I., Rentz D., Weintraub S., Mesulam M., Potter H. A potential noninvasive neurobiological test for Alzheimer's disease. Science. 1994 Nov 11;266(5187):1051–1054. doi: 10.1126/science.7973660. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Cell biology of the amyloid beta-protein precursor and the mechanism of Alzheimer's disease. Annu Rev Cell Biol. 1994;10:373–403. doi: 10.1146/annurev.cb.10.110194.002105. [DOI] [PubMed] [Google Scholar]
- Sherrington R., Rogaev E. I., Liang Y., Rogaeva E. A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995 Jun 29;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop P., Haines J., Rogaev E., Mortilla M., Vaula G., Pericak-Vance M., Foncin J. F., Montesi M., Bruni A., Sorbi S. Genetic evidence for a novel familial Alzheimer's disease locus on chromosome 14. Nat Genet. 1992 Dec;2(4):330–334. doi: 10.1038/ng1292-330. [DOI] [PubMed] [Google Scholar]
- Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M. SPXX, a frequent sequence motif in gene regulatory proteins. J Mol Biol. 1989 May 5;207(1):61–84. doi: 10.1016/0022-2836(89)90441-5. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Cheung T. T., Cai X. D., Odaka A., Otvos L., Jr, Eckman C., Golde T. E., Younkin S. G. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994 May 27;264(5163):1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Tahara E., Ito H., Taniyama K., Yokozaki H., Hata J. Alpha 1-antitrypsin, alpha 1-antichymotrypsin, and alpha 2-macroglobulin in human gastric carcinomas: a retrospective immunohistochemical study. Hum Pathol. 1984 Oct;15(10):957–964. doi: 10.1016/s0046-8177(84)80125-2. [DOI] [PubMed] [Google Scholar]
- Takada S., Tsuda M., Fujinami S., Yamamura M., Mitomi T., Katsunuma T. Incorporation of alpha-1-antichymotrypsin into carcinoma cell nuclei of human stomach adenocarcinoma transplanted into nude mice. Cancer Res. 1986 Jul;46(7):3688–3691. [PubMed] [Google Scholar]
- Tanzi R. E., Vaula G., Romano D. M., Mortilla M., Huang T. L., Tupler R. G., Wasco W., Hyman B. T., Haines J. L., Jenkins B. J. Assessment of amyloid beta-protein precursor gene mutations in a large set of familial and sporadic Alzheimer disease cases. Am J Hum Genet. 1992 Aug;51(2):273–282. [PMC free article] [PubMed] [Google Scholar]
- Terry R. D., Katzman R. Senile dementia of the Alzheimer type. Ann Neurol. 1983 Nov;14(5):497–506. doi: 10.1002/ana.410140502. [DOI] [PubMed] [Google Scholar]
- Van Broeckhoven C., Backhovens H., Cruts M., De Winter G., Bruyland M., Cras P., Martin J. J. Mapping of a gene predisposing to early-onset Alzheimer's disease to chromosome 14q24.3. Nat Genet. 1992 Dec;2(4):335–339. doi: 10.1038/ng1292-335. [DOI] [PubMed] [Google Scholar]
- Winey M., Hoyt M. A., Chan C., Goetsch L., Botstein D., Byers B. NDC1: a nuclear periphery component required for yeast spindle pole body duplication. J Cell Biol. 1993 Aug;122(4):743–751. doi: 10.1083/jcb.122.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T., Castaño E. M., Golabek A., Vogel T., Frangione B. Acceleration of Alzheimer's fibril formation by apolipoprotein E in vitro. Am J Pathol. 1994 Nov;145(5):1030–1035. [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T., Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992 Feb 3;135(2):235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Ghiso J., Frangione B. Peptides homologous to the amyloid protein of Alzheimer's disease containing a glutamine for glutamic acid substitution have accelerated amyloid fibril formation. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1247–1254. doi: 10.1016/0006-291x(91)91706-i. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Golabek A., Matsubara E., Ghiso J., Frangione B. Apolipoprotein E: binding to soluble Alzheimer's beta-amyloid. Biochem Biophys Res Commun. 1993 Apr 30;192(2):359–365. doi: 10.1006/bbrc.1993.1423. [DOI] [PubMed] [Google Scholar]
- Worman H. J., Evans C. D., Blobel G. The lamin B receptor of the nuclear envelope inner membrane: a polytopic protein with eight potential transmembrane domains. J Cell Biol. 1990 Oct;111(4):1535–1542. doi: 10.1083/jcb.111.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Worman H. J. Primary structure analysis and lamin B and DNA binding of human LBR, an integral protein of the nuclear envelope inner membrane. J Biol Chem. 1994 Apr 15;269(15):11306–11311. [PubMed] [Google Scholar]