Individuals with corpus callosum malformations are phenotypically diverse, and often present with broad neurodevelopmental disorders. Edwards et al. review the clinical features of these patients and provide a comprehensive classification of syndromes associated with callosal agenesis, based on a neural developmental framework that will guide future advances in the field.
Keywords: corpus callosum, axon guidance, neuronal specification, neurogenesis, midline patterning
Abstract
The corpus callosum is the largest fibre tract in the brain, connecting the two cerebral hemispheres, and thereby facilitating the integration of motor and sensory information from the two sides of the body as well as influencing higher cognition associated with executive function, social interaction and language. Agenesis of the corpus callosum is a common brain malformation that can occur either in isolation or in association with congenital syndromes. Understanding the causes of this condition will help improve our knowledge of the critical brain developmental mechanisms required for wiring the brain and provide potential avenues for therapies for callosal agenesis or related neurodevelopmental disorders. Improved genetic studies combined with mouse models and neuroimaging have rapidly expanded the diverse collection of copy number variations and single gene mutations associated with callosal agenesis. At the same time, advances in our understanding of the developmental mechanisms involved in corpus callosum formation have provided insights into the possible causes of these disorders. This review provides the first comprehensive classification of the clinical and genetic features of syndromes associated with callosal agenesis, and provides a genetic and developmental framework for the interpretation of future research that will guide the next advances in the field.
Introduction
The corpus callosum is the largest of the interhemispheric white matter tracts in the brain. It comprises >190 million topographically organized axons, each forming homotopic or heterotopic connections, often between distant regions of cerebral cortex (Wahl et al., 2007, 2009). These connections participate in an array of cognitive functions including language, abstract reasoning, and the integration of complex sensory information between the hemispheres (Brown et al., 1999; Paul et al., 2003). The corpus callosum is classically divided into four distinct segments based on early histological studies (Witelson, 1989; see Fig. 1). Recent advances in diffusion tensor imaging and tractography have provided remarkable insight into the diversity of interhemispheric callosal connections within each segment, and has helped to clarify what happens to these connections when embryonic or foetal development is disturbed (Wahl et al., 2007, 2009).
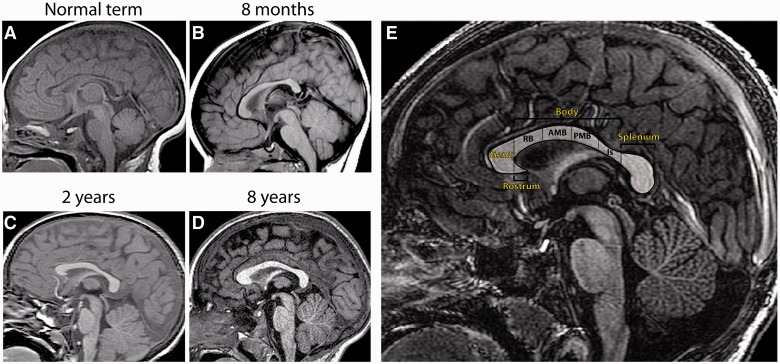
Figure 1.
T1-weighted sagittal MRI scans showing the structure of the normal human corpus callosum in the full-term infant (A), 8-month-old (B), 2-year-old (C), 8-year-old (D) and adult (E). (A) At birth, the corpus callosum has assumed its general shape but is thinner throughout. The thickness of the corpus callosum (vertical dimension) increases generally throughout childhood and adolescence. Growth in the anterior sections is most pronounced within the first 10 years of life (compare C with D), and posterior growth predominates during adolescence (compare D with E). There is also marked interindividual variation in corpus callosum size and shape. (E) Normal adult corpus callosum, showing subdivisions established by Witelson (1989). The corpus callosum is initially divided into genu, rostrum, body and splenium. The body can be further subdivided into the isthmus, and the anterior, middle and posterior segments. RB = rostral body; AMB = anterior midbody; PMB = posterior midbody; Is = isthmus.
Agenesis of the corpus callosum (ACC) is an exceedingly heterogeneous condition that can result from disruption of numerous developmental steps from early midline telencephalic patterning to neuronal specification and guidance of commissural axons. It can occur as an isolated finding on MRI, but is more commonly associated with a broader disorder of brain development (Schell-Apacik et al., 2008; Tang et al., 2009). Accordingly, the cognitive and neurological consequences in patients with ACC vary considerably from mild behavioural problems to severe neurological deficits. Deficits in problem solving and social skills are common, and these often fall within the autistic spectrum (Lau et al., 2013; Siffredi et al., 2013). Interestingly, isolated ACC predominantly carries a favourable prognosis (Moutard et al., 2003; Sotiriadis et al., 2012) and these individuals exhibit a different cognitive outcome from the disconnection syndrome characterized in commissurotomy patients (Paul et al., 2007). Individuals with ACC therefore provide a unique opportunity to study not only the mechanisms of callosal development, but also the broader principles that determine how the brain responds to disruptions in neurodevelopment.
The increased use and resolution of comparative genomic hybridization have implicated many more genes and genomic loci in corpus callosum development (O'Driscoll et al., 2010), and have revealed a great diversity of genetic causes for ACC syndromes. At present, however, the cause of 55–70% of cases with ACC cannot be identified by clinical evaluation (Bedeschi et al., 2006; Schell-Apacik et al., 2008). The apparently sporadic nature of ACC makes genetic studies difficult (Sherr et al., 2005; Schell-Apacik et al., 2008), and it is possible that the cause of ACC in a proportion of these patients is non-genetic, such as foetal exposure to alcohol. Indeed, it is often the associated brain abnormalities found on imaging that point to the underlying developmental process that is disturbed.
Syndromes incorporating ACC can be broadly classified by the stage in development that is primarily affected using an approach similar to previous classifications of cortical malformations (Barkovich et al., 2012). ACC can occur in association with disorders of neuronal and/or glial proliferation, neuronal migration and/or specification, midline patterning, axonal growth and/or guidance, and post-guidance development. Much of what is known about normal corpus callosum formation has emerged from studies using mouse models of callosal agenesis. Indeed, our understanding of the processes underpinning callosal development in mice has served as a foundation for much of what is currently known about human patients with ACC. The purpose of this review is to systematically outline the clinical features of all human syndromes associated with ACC, and relate these to the genetic causes and developmental processes likely to be disturbed.
Imaging and classifying agenesis of the corpus callosum
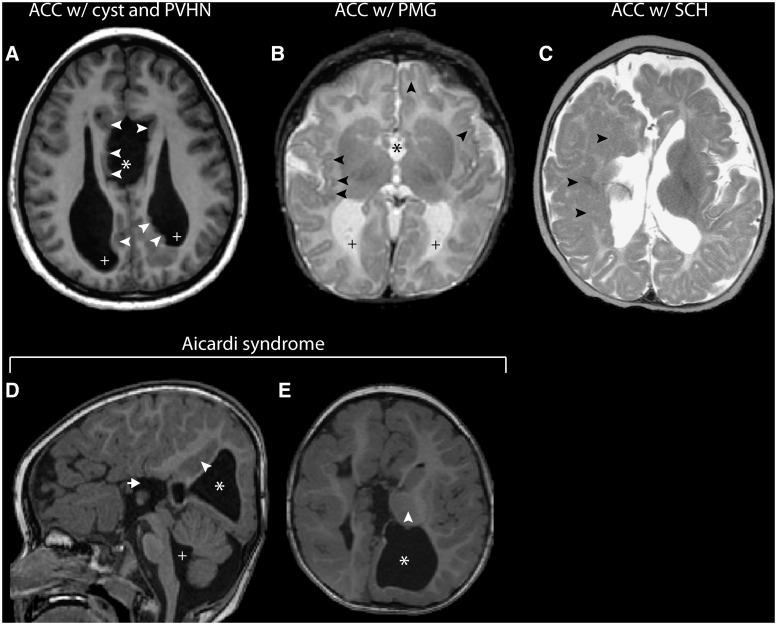
ACC encompasses either total absence (complete ACC) or absence from birth of at least one, but not all, of the anatomically defined regions of the corpus callosum (partial ACC), which results in a shorter anterior-posterior length (Fig. 2). Hypoplasia denotes a corpus callosum that is thinner than usual, but has a normal anterior–posterior extent (Fig. 2). Routine sonography remains the primary tool for identifying ACC from mid-trimester onwards, when widening of the interhemispheric fissure, absence of the cavum septum pellucidum and colpocephaly can be identified (Santo et al., 2012). Sonography, however, often fails to detect more subtle cases of partial ACC or callosal hypoplasia (Ghi et al., 2010; Paladini et al., 2013), as well as associated white matter dysgeneses. For this reason, prenatal MRI remains the preferred imaging modality for direct visualization of the corpus callosum in cases with suspected ACC, and associated abnormalities not detected by sonography. This is particularly important for offering early counselling to parents, as additional cerebral abnormalities identified by MRI might suggest broader disorders of neurodevelopment that are linked with more severe neurological impairment (Tang et al., 2009).
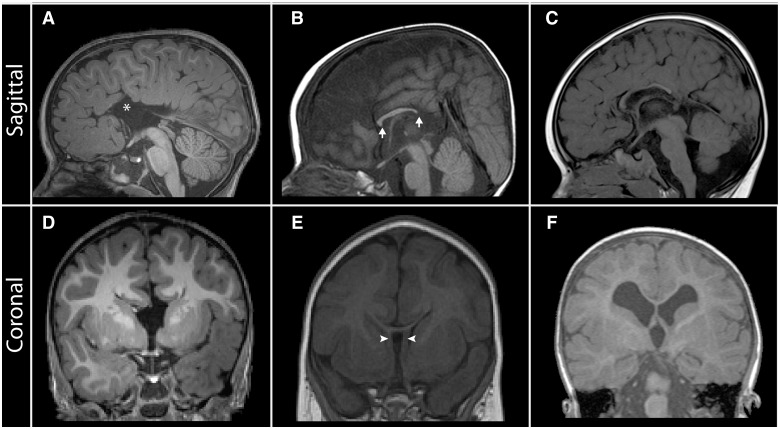
Figure 2.
Neuroanatomical features revealed by T1-weighted midsagittal and coronal MRI in patients with corpus callosum abnormalities. (A and D) Patient with complete ACC associated with dorsal expansion of the third ventricle (asterisk), absence of the cingulate gyrus and sulcus, and absence of the septum pellucidum. (B and E) Patient with partial ACC; the splenium is absent and the rostrum is not fully formed (arrows). In addition, the leaves of the septum pellucidum are unfused (E; arrowheads). (C and F) Patient with hypoplasia of the corpus callosum. All segments are present but are diffusely thinned; there is also markedly reduced cerebral white matter volume (F).
Advances in tractography based on diffusion tensor imaging have significantly improved our understanding of how the corpus callosum connects with the cortex in normal individuals, and how these connections are disturbed and re-routed in patients with ACC. Of particular interest are the so-called ‘sigmoid bundles’, which asymmetrically connect the frontal lobe with the contralateral occipitoparietal cortex. Sigmoid bundles have been reported in patients with partial ACC (Fig. 3), and may represent a pathologic plasticity that has so far not been associated with the better characterized longitudinal bundles of Probst, which exhibit conserved topographical organization, albeit confined to the ipsilateral cortex (Tovar-Moll et al., 2007; Wahl et al., 2009). The mechanisms that account for this apparent plasticity of interhemispheric wiring in patients with partial ACC, and whether these patterns of heterotopic connections are compensatory or detrimental, remain areas of current research.
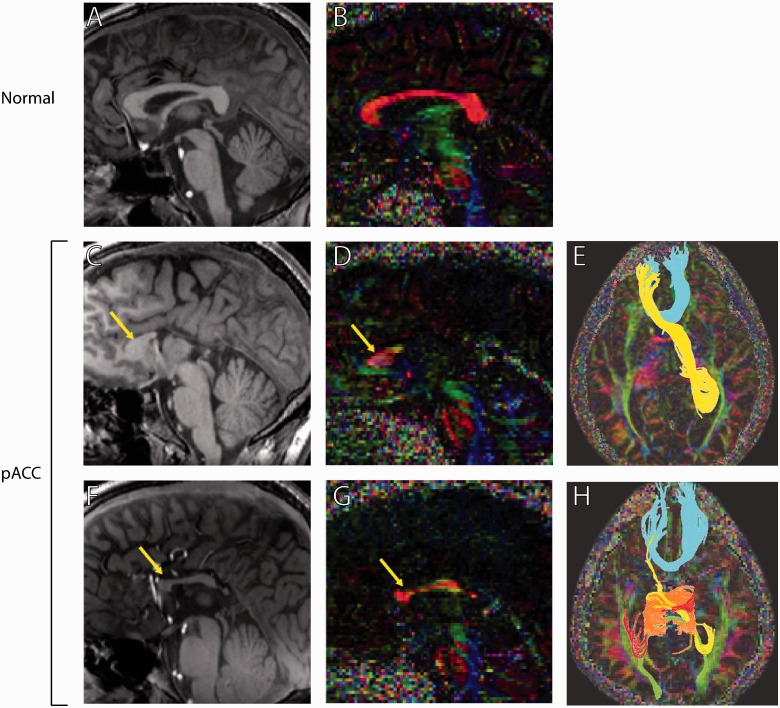
Figure 3.
T1-weighted midsagittal MRI and diffusion tensor imaging tractography of two patients with partial ACC (pACC) and a normal corpus callosum control. (A, C and F) T1-weighted midsagittal MRI scans. (B, D and G) High-angular-resolution diffusion imaging. Arrows indicate callosal fragments present in partial patients with ACC. (E and H) Q-ball tractography of partial patients with ACC reveals callosal connections between homotopic and heterotopic cortical regions. Homotopic connections between anterior frontal lobes are conserved in both partial patients with ACC (blue streamlines in E and H; orange streamlines in H), but the degree of temporal and occipital connectivity varies. Both patients also show ‘sigmoid bundles’ (yellow streamlines in E and H), which connect the anterior frontal lobe with the contralateral parieto-occipital region. Images adapted from Wahl et al. (2009).
Mouse models of callosal development
Mouse models of ACC have proven invaluable in characterizing the cellular and molecular processes underpinning corpus callosum development and the individual genes involved. However, phenotypes in mice cannot always be correlated with human syndromes as it is not usually clear whether developmental mechanisms are conserved between species. Neuroimaging approaches are bridging this gap and provide a means to examine human brain development and structure. A major issue in translating mouse models to humans has been that many single gene mouse models result in embryonic or early post-natal lethality, as the genes regulate multiple developmental processes. These genes may act in a similar manner in humans so patients that completely lack such a gene are not normally seen in the clinic. Instead, point mutations in such genes (both inherited and de novo) are likely to be more common in patients and may decrease or impede the function of the gene without being completely non-functional. Given this, candidate gene approaches, translating directly from mouse null mutations, have not been as successful in identifying the cause of human ACC as might have been expected. However, mouse models have been instrumental in defining the critical processes involved in callosal development and there is reasonable evidence from direct analysis of human foetal brain tissue that similar processes and molecules are involved in human corpus callosum development (Rakic and Yakovlev, 1968; Lent et al., 2005; Ren et al., 2006). Many of the molecules involved in commissure formation throughout the brain and spinal cord are highly evolutionarily conserved across invertebrates and vertebrates (Tessier-Lavigne and Goodman, 1996), providing further compelling evidence for their conservation in humans.
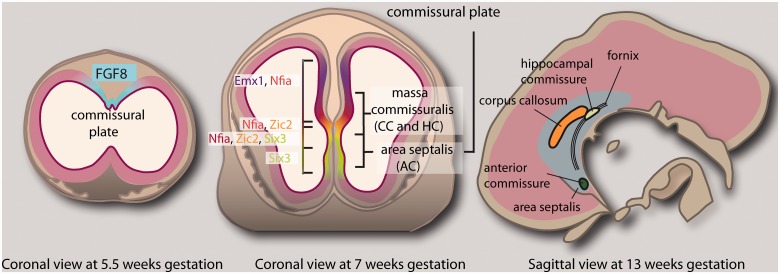
The formation of the corpus callosum follows clear and well-orchestrated developmental events for which we now have a reasonable understanding, even if we are yet to discover the molecular mechanisms underlying these processes. Neurons that give rise to the axons of the corpus callosum reside principally in neocortical layers II/III and V, but also in layer VI (Wise and Jones, 1976; Fame et al., 2011). Disruption of the mechanisms that regulate the production and migration of these neurons causes brain malformations such as microcephaly or pachygyria, which are usually independent of, and occur developmentally before, corpus callosum formation. These processes are therefore discussed in later sections of this review only insofar as they relate to syndromes involving ACC. Perhaps the first step in corpus callosum formation is patterning of the midline, which provides a substrate for callosal axons to traverse. All telencephalic commissures initially cross the midline within a distinct anatomical region termed the commissural plate. In mice, four distinct molecular subdomains of the commissural plate have been identified, through which distinct commissural projections pass (Fig. 4). Expression of the secreted morphogen Fgf8 is crucial in the initial patterning of the forebrain and subsequent development of the commissural plate, and appears to act as an upstream regulator of many midline patterning molecules (Hayhurst et al., 2008; Okada et al., 2008) that correlate anatomically with specific commissures (Moldrich et al., 2010). Dorsally, the corpus callosum passes through an Emx1- and Nfia-expressing domain; the hippocampal commissure passes through domains expressing Nfia, Zic2 and Six3, and the anterior commissure passes through a Six3-expressing domain in the septum. Perturbed development of these subdomains results in disruption of the corresponding commissural projections passing through the domains, suggesting that correct patterning of the commissural plate is a prerequisite for commissure formation (Moldrich et al., 2010).
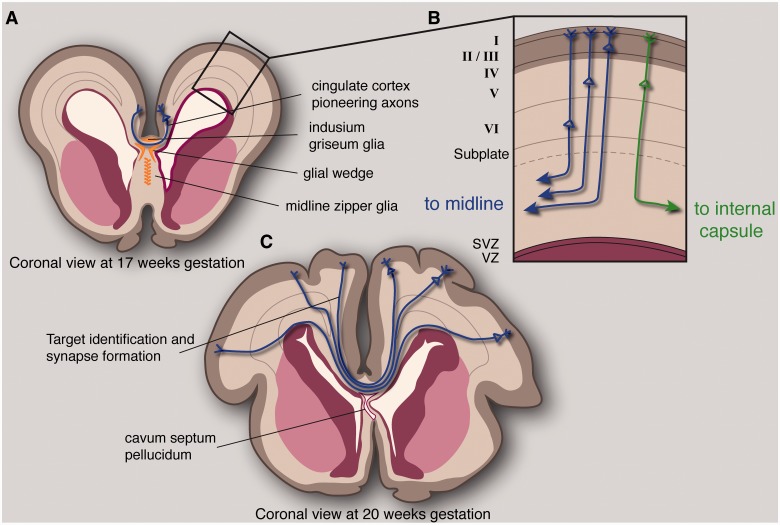
Figure 5.
Processes extrapolated from mouse studies necessary for specification of callosal neurons, correct guidance of axons across the midline, and target identification in the contralateral cortex. Midline zipper glia develop in the septum and may play a role in fusion of the midline, which is correlated with corpus callosum development. As axons reach the midline, they encounter and must correctly interpret multiple attractive and repulsive guidance cues expressed by the glial wedge and indusium griseum. The first axons to cross the midline arise from the cingulate cortex, and these pioneering neurons appear to be necessary for the subsequent crossing of the majority of callosal axons, arising from the neocortex (A). Callosal neurons originate from layers I, II/III, V and VI of the cortex. However, the layer that a neuron resides in is not sufficient for specification as a callosally projecting neuron, and callosal neuron identity seems to coincide with expression of the transcription factor SATB2. These neurons project an axon radially towards the intermediate zone, which must then decide to turn medially rather than laterally (B). Once axons reach the contralateral hemisphere, they must recognize their target area and synapse with target neurons, presumably through molecular-recognition and activity-dependent mechanisms (C). Exuberant axonal growth continues after birth and is accompanied by axonal pruning which continues throughout childhood and adolescence. SVZ = subventricular zone; VZ = ventricular zone.
Figure 4.
Processes underpinning midline patterning in the human foetal brain extrapolated from studies in mouse. Initial expression of the morphogen FGF8 at the midline is necessary for early forebrain patterning, and subsequent development of the commissural plate through which all forebrain commissures pass. The commissural plate can be divided molecularly into four distinct subdomains, each specified by midline patterning molecules that likely act downstream of FGF8. Each forebrain commissure correlates anatomically with a specific subdomain. The corpus callosum (CC) passes through a domain of EMX1 and NFIA expression; the hippocampal commissure (HC) passes through domains expressing NFIA, ZIC2 and SIX3, and the anterior commissure (AC) passes through a SIX3-expressing domain in the septum. Sagittal section at 13 weeks gestation adapted from Rakic and Yakovlev (1968).
The specification of neurons in the cortical plate as callosally projecting neurons, rather than corticofugally or intracortically projecting neurons (Fame et al., 2011), is an essential process in callosal development. There are many genes involved in this specification, as callosal neurons comprise a heterogeneous population (Molyneaux et al., 2009). An important regulator of callosal neuron specification is the transcription factor SATB2 (Alcamo et al., 2008; Britanova et al., 2008). When Satb2 is functionally deleted in mice, the corpus callosum fails to form, and instead the normally callosal neurons project into either the corticofugal tract or the anterior commissure. This latter result is particularly interesting from an evolutionary perspective as marsupials have no corpus callosum, but have a larger anterior commissure that serves the same purpose (Ashwell et al., 1996). Some human patients with ACC also display a larger anterior commissure (Fischer et al., 1992; Barr and Corballis, 2002; Hetts et al., 2006) but neither the underlying cause nor the clinical consequences are yet known.
After callosal neuron specification, these neurons extend an axon into the intermediate zone, which will later become the white matter, and make an axon guidance decision to project medially rather than laterally. Little is known about how this process occurs, but it may be regulated by guidance molecules in the cortical environment. For example, SEMA3A, expressed at the lateral border of the neocortex, repels callosal axons toward the midline, through its receptor neuropilin 1 (Zhao et al., 2011). A different family member, SEMA3C, attracts callosal neurons to the midline (Niquille et al., 2009; Piper et al., 2009). Once callosal neurons reach the midline they encounter glial and neuronal guidepost populations that are crucial for their crossing of the interhemispheric midline. Any perturbation to the development of these structures results in some degree of callosal agenesis. The glial wedge and indusium griseum glia surround the corpus callosum on its dorsal and ventral sides, and both populations secrete repulsive and attractive guidance cues to direct axons across the midline. Current research is focused on how growth cones modulate responsiveness to guidance molecules as they traverse the midline. As axons cross the midline, they must decrease responsiveness to attractive cues at the corticoseptal boundary, and gain responsiveness to repulsive cues in the same region to project dorsally in the contralateral hemisphere. Initial investigations in Xenopus identified the importance of DCC-Robo interactions in silencing axonal attraction at the midline (Stein and Tessier-Lavigne, 2001). Recent research in mice has shown that netrin 1 acts as a chemoattractant for pioneering axons originating in the cingulate cortex, but that it does not attract neocortical axons. Instead, netrin-DCC interactions inhibit Slit2-mediated repulsion until axons have crossed the midline (Fothergill et al., 2013; for a review of axon guidance mechanisms involving interactions between multiple molecular pathways, see Dudanova and Klein, 2013).
Midline zipper glia develop at the medial pial surface of the corticoseptal region, and are thought to have an important role in midline fusion (Silver, 1993; Shu et al., 2003a). Failure of the two hemispheres to fuse is often correlated with ACC, presumably as axons lack the proper substrate to cross the midline (Silver and Ogawa, 1983; Silver, 1993), but experimental evidence for how midline fusion occurs is currently lacking. The subcallosal sling was originally thought to be another midline glial population (Silver et al., 1982), but was later shown to largely comprise neurons (Shu et al., 2003b). Additional populations of glutamatergic and GABAergic neurons exist within and dorsal to the corpus callosum, and together they form a permissive SEMA3C-expressing corridor through which midline-projecting axons pass (Niquille et al., 2009, 2013). This corridor appears particularly crucial for guiding the first axons to cross the midline, which arise from the cingulate cortex. These pioneering cingulate neurons are hypothesized to be necessary for later crossing of axons originating from the neocortex, as supported by a rostral ACC phenotype in Emx2−/− mice. In these mice, the cingulate cortex is not specified and pioneer axons are missing rostrally but not caudally (Piper et al., 2009).
The reliance of neocortical-originating axons on pioneering cingulate axons in both mice and humans points to the importance of axon-axon interactions in callosal development. Before they encounter pioneering cingulate axons, callosally projecting axons fasciculate in part through neuropilin 1-mediated interactions (Hatanaka et al., 2009). The importance of axons from the cingulate cortex appears to be conserved in humans. Decreased size and connectivity of the cingulum bundles has been documented in patients with ACC, and this appears to be correlated with the severity of callosal agenesis (Nakata et al., 2009). However, how this relates to ACC remains to be determined.
Human corpus callosum development
The human commissural plate can be anatomically subdivided into the massa commissuralis through which the corpus callosum and hippocampal commissure pass, and the area septalis through which the anterior commissure crosses (Rakic and Yakovlev, 1968; Fig. 4). For many years, the prevailing theory held that human corpus callosum development occurred in an anterior-to-posterior fashion, with the first callosal axons crossing the midline at the anterior genu, with those in the rostrum added last (Byrd et al., 1978; Barkovich and Kjos, 1988). More recently, neuroimaging studies have suggested that the first axons cross the commissural plate in the hippocampal primordium, with subsequent connections being made bidirectionally (Barkovich et al., 1992; Kier and Truwit, 1996; Huang et al., 2006, 2009; Paul, 2011). Callosal neurons originate from layers II/III, V and VI of the neocortex (Fame et al., 2011), although midline crossing of neocortical neurons in both mouse and human is preceded by crossing of pioneering axons originating from the cingulate cortex (Koester and O’Leary, 1994; Rash and Richards, 2001).
Around Weeks 13 and 14 post-conception, pioneering axons begin to cross the midline; the anterior sections begin to grow by Weeks 14 and 15, whereas growth of the posterior sections occurs during Weeks 18 and 19 (Hewitt, 1962; Rakic and Yakovlev, 1968; Ren et al., 2006). The apparently delayed development of the posterior and most anterior callosal sections led to the assumption that early perturbation of callosal development results in complete ACC, and later developmental disturbances result in partial agenesis confined to the posterior corpus callosum and rostrum. However, current data indicate that connections are first made in two separate loci: the anterior commissure and the hippocampal commissure (for a review see Paul, 2011). The early expansion of the frontal cortex results in the posterior displacement of the hippocampal commissure together with the associated callosal splenium, while the anterior section of the corpus callosum expands. It has therefore been suggested that the absence of the posterior part of the corpus callosum in partial ACC most commonly results from failed dorsoventral expansion of the splenium (Paul, 2011). The two-locus origin of the corpus callosum is to some degree consistent with the anatomic diversity of homotopic and heterotopic connections in the partial ACC brain (Tovar-Moll et al., 2007; Wahl et al., 2009). However, it still fails to account for the great diversity of connectivity seen in structurally similar callosal fragments.
By 20 weeks post-conception, the final shape of the corpus callosum is complete, although exuberant axonal growth continues until 2 months after birth; this is then followed by molecular- and activity-dependent axonal pruning (Innocenti and Price, 2005). Although the number of callosal fibres is more or less determined at birth, structural changes continue throughout post-natal development, and are most marked during childhood and adolescence (Luo and O'Leary, 2005; Luders et al., 2010; Garel et al., 2011).
Single gene syndromes with agenesis of the corpus callosum
Of the 30–45% of cases with ACC with an identifiable genetic cause, 20–35% are caused by a mutation affecting a single gene (Bedeschi et al., 2006; Schell-Apacik et al., 2008). Although some Mendelian syndromes show complete or near complete ACC penetrance, the majority display ACC with incomplete penetrance (Table 3), which suggests that modifying genetic influences are often at play. Autosomal dominant, autosomal recessive, and X-linked causes of ACC have been described; however, no inheritance pattern is found in a significant proportion of cases and it is possible that many arise from de novo mutations. This is consistent with current data from the California Birth Defects Monitoring Programme showing that the risk of giving birth to a child with ACC is 3-fold higher for mothers aged 40 and above (Glass et al., 2008). It is also possible that oligogenic models of inheritance account for a proportion of apparently ‘sporadic’ cases of ACC.
Table 3.
Genes associated with human ACC syndromes
| Syndrome | Gene (HGNC approved symbol) | OMIM number | HGNC ID | Cytogenic Location (human) | Human phenotype |
Mouse phenotype |
Referencesa | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACC penetrance | Salient features | Callosal phenotype |
Associated midline defects |
|||||||||
| Complete ACC | Partial ACC | Midline glia | Hippocampal commissure | Anterior commissure | ||||||||
| GROUP I - Abnormal neuronal and/or glial proliferation | ||||||||||||
| ACC with mental retardation, ocular coloboma, and micrognathia | Immunoglobulin binding protein 1 (IGBP1) | 300139 | 5461 | Xq13.1 | Defining feature | ACC, iris/optic nerve coloboma, mental retardation | n.d | Graham et al., 2003 | ||||
| Alpha thalassemia/mental retardation syndrome X-linked | Alpha thalassemia/mental retardation syndrome X-linked (ATRX) | 300032 | 886 | Xq21.1 | Uncommon | Developmental delay, α-thalassemia, cerebral atrophy | n.d | Gibbons et al., 1995a, b; Villard et al., 1996; Gibbons and Higgs, 2000; Berube et al., 2005 | ||||
| Aniridia | Paired box 6 (PAX6) | 607108 | 8620 | 11p13 | 2/20 (10%) | Aniridia, cataract, glaucoma, anterior commissure agenesis | N | Y | Y | Jones et al., 2002; Bamiou et al., 2007; Abouzeid et al., 2009 | ||
| Chudley-McCullough syndrome | G protein signalling modulator 2 (GPSM2) | 609245 | 29501 | 1p13.3 | Defining feature | Sensorineural deafness, ACC, interhemispheric cyst, cerebral/cerebellar dysplasias | n.d | Nadkarni et al., 2008; Alrashdi et al., 2011; Diaz-Horta et al., 2012; Doherty et al., 2012 | ||||
| Coffin Siris syndrome | AT-rich interaction domain-containing protein 1B (ARID1B) | 614556 | 18040 | 6q25.3 | 9/42 (21%) | Developmental delay, coarse facial appearance, hirsutism, hypoplastic or absent fifth distal phalanges, and microcephaly | n.d | Reversade et al., 2009; Mohamed et al., 2011 Santen et al., 2012; Schrier et al., 2012; Tsurusaki et al., 2012 | ||||
| Cutis laxa, autosomal recessive, type IIB/IIIB | Pyrroline-5-carboxylate reductase 1 (PYCR1) | 179035 | 9721 | 17q25.3 | Common | Microcephaly, failure to thrive | n.d | Reversade et al., 2009; Mohamed et al., 2011 | ||||
| Growth retardation with deafness and mental retardation due to IGF1 deficiency | Insulin-like growth factor I (IGF1) | 147440 | 5464 | 12q23.2 | Uncommon | Poor growth, microcephaly, micrognathia, sensorineural deafness, mental retardation | N | Y | Y | Beck et al., 1995; Ye et al., 2002 | ||
| Lujan-Fryns syndrome | Mediator complex subunit 12 (MED12) | 300188 | 11957 | Xq13.1 | Unknown | Marfanoid habitus, mental retardation, ACC | n.d | Jeret et al., 1987; Lerma-Carrillo et al., 2006 | ||||
| Marshall-Smith syndrome | Nuclear factor I/X (NFIX) | 164005 | 11957 | 19p13.3 | 8/39 (21%) | Macrogyria, cerebral atrophy, ACC | N | Y | N | Driller et al., 2007; Campbell et al., 2008; Malan et al., 2010; Shaw et al., 2010 | ||
| Meckel syndrome | RPGRIP1-Like (RPGRIP1L) | 610937 | 29168 | 16q12.2 | 4/7 (57%) | Chiari malformation, Dandy-Walker malformation, hydrocephalus, cerebral hypoplasia | Y | Y | Paetau et al., 1985; Smith et al., 2006a; Delous et al., 2007 | |||
| TMEM67 | 609884 | 28396 | 8q22.1 | Y (wpk rat) | Y (wpk rat) | |||||||
| MKS1, TMEM216, CEP290, CC2D2A, NPHP3, TCTN2, B9D1, B9D2 | n.d | |||||||||||
| Microcephalic osteodysplastic primordial dwarfism, type I/III | RNA, U4atac small nuclear (U12-dependent splicing) (RNU4ATAC) | 601428 | 34016 | 2q14.2 | 5/9 (56%) | Failure to thrive, short stature, microcephaly, pachygyria, heterotopias, ACC | n.d | Abdel-Salam et al., 2011; Juric-Sekhar et al., 2011 | ||||
| Microcephaly 2, primary, autosomal recessive, with or without cortical malformations | WD repeat domain 62 (WDR62) | 604317 | 24502 | 19q13.12 | Uncommon | Microcephaly, pachygyria, callosal hypoplasia | n.d | Bilguvar et al., 2010; Yu et al., 2010 | ||||
| Microcephaly 5, primary, autosomal recessive | Asp (abnormal spindle) homolog, microcephaly associated (Drosophila) (ASPM) | 605481 | 19048 | 1q31.3 | 3/12 (25%) | Simplified gyral pattern, ventriculomegaly, partial ACC | n.d | Bond et al., 2002; Passemard et al., 2009 | ||||
| Mowat-Wilson syndrome | Zinc finger E-box binding homeobox 2 (ZEB2) | 605802 | 14881 | 2q22.3 | 67/155 (43%) | Mental retardation, seizures, microephaly Hypoplasia of the corpus callosum, ACC, microcephaly | Y | N | Amiel et al., 2001; Cacheux et al., 2001; Mowat et al., 2003; Dastot-Le Moal et al., 2007; Miquelajauregui et al., 2007 | |||
| Opitz–Kaveggia syndrome | Mediator complex subunit 12 (MED12) | 300188 | 11957 | Xq13.1 | 14/28 (50%); 13/13 (100%) for p.R961W mutation | Seizures, hydrocephalus, agenesis of corpus callosum, heterotopia, dysmorphic facies | n.d | Graham et al., 1999; Risheg et al., 2007; Graham et al., 2008; Rump et al., 2011 | ||||
| Perlman syndrome | DIS3 mitotic control homolog (S. cerevisiae)-like 2 (DIS3L2) | 614184 | 267000 | 2q37.1 | Unknown | Polyhydramnios, neonatal macrosomia, visceromegaly, renal dysplasia, Wilms tumour | n.d | Alessandri et al., 2008; Astuti et al., 2012 | ||||
| Rubinstein-Taybi syndrome | CREB-binding protein (CREBBP) | 600140 | 2348 | 16p13.3 | Uncommon | Mental retardation, ACC, post-natal growth deficiency, dysmorphic facies | n.d | Petrij et al., 1995; Tsai et al., 2001; Roelfsema et al., 2005; Wojcik et al., 2010 | ||||
| E1A binding protein p300 (EP300) | 602700 | 3373 | 22q13.2 | n.d | ||||||||
| Seckel syndrome | Ataxia telangiectasia and Rad3 related (ATR) | 601215 | 882 | 3q23 | Common | Microcephaly, cerebellar vermis hypoplasia, dwarfism | Y (100%) | Shanske et al., 1997; Capovilla et al., 2001; Murga et al., 2009; Thapa and Mukherjee, 2010; Juric-Sekhar et al., 2011 | ||||
| RBBP8, SCKL3, CENPJ, CEP152, CEP63, NIN | n.d | |||||||||||
| Septo-optic dysplasia | HESX homeobox 1 (HESX1) | 601802 | 4877 | 3p14.3 | Common | Absent septum pellucidum, ACC, pituitary dysplasia, optic nerve hypoplasia | Y (75%) | Y (25%) | Y (50%) | Y (75%) | Dattani et al., 1998; Kelberman and Dattani, 2008 | |
| Sotos syndrome 1 | Nuclear receptor binding SET domain protein 1 (NSD1) | 606681 | 14234 | 5q35 | 1/51 (2%) | Macrocephaly, mental retardation, seizures, corpus callosum hypoplasia, ventriculomegaly | n.d | Schaefer et al., 1997; Bedeschi et al., 2006; Driller et al., 2007; Campbell et al., 2008; Malan et al., 2010 | ||||
| Sotos syndrome 2 | Nuclear factor I/X (NFIX) | 164005 | 7788 | 19p13.3 | N | Y | N | |||||
| GROUP II - Abnormal midline patterning | ||||||||||||
| Acrocallosal syndrome | GLI family zinc finger 3 (GLI3) | 165240 | 4319 | 7p13 | Defining feature | ACC and/or Dandy-Walker malformation | N | Y | Elson et al., 2002; Putoux et al., 2011; 2012; Wang et al., 2011 | |||
| Kinesin family member 7 (KIF 7) | 611254 | 30497 | 15q26.1 | n.d | ||||||||
| Apert syndrome | Fibroblast growth factor receptor 2 (FGFR2) | 176943 | 3689 | 10q26.13 | 23% | ACC, ventriculomegaly, no septum pellucidum, Chiari I malformation | Y (66%) | Y | Wilkie et al., 1995; Slaney et al., 1996; Quintero-Rivera et al., 2006; Stevens et al., 2010 | |||
| COACH syndrome | Transmembrane protein 67 (TMEM67) | 609884 | 28396 | 8q22.1 | 6/71 (8.5%) | Cerebellar vermis dysplasia, mental retardation, ocular coloboma, hepatic fibrosis | Y (wpk rat) | Y (wpk rat) | Smith et al., 2006a; Arts et al., 2007; Delous et al., 2007; Brancati et al., 2009; Doherty et al., 2010 | |||
| RPGRIP1-like (RPGRIP1L) | 610937 | 29168 | 16q12.2 | Y | Y | |||||||
| Coiled-coil and C2 domain containing 2A (CC2D2A) | 612013 | 29253 | 4p15.33 | n.d | ||||||||
| Donnai-Barrow Syndrome | Low density lipoprotein receptor-related protein 2 (LRP2) | 600073 | 6694 | 2q31.1 | Common | Sensorineural deafness, ACC, congenital diaphragmatic hernia | Y (90%) | Willnow et al., 1996; Kantarci et al., 2007 | ||||
| Greig cephalopolysyndactyly syndrome | GLI family zinc finger 3 (GLI3) | 165240 | 4319 | 7p13 | Uncommon | Hydrocephalus, ACC, polydactyly | N | Y | Hootnick and Holmes, 1972; Marafie et al., 1996; Wild et al., 1997; Kalff-Suske et al., 1999; Wang et al., 2011 | |||
| Hydrolethalus syndrome (HLS) | Kinesin family member 7 (KIF 7) | 611254 | 30497 | 15q26.1 | Defining feature | Hydrocephalus, olfactory aplasia, fused thalami, hypothalamic hamartoma, polymicrogyria, lissencephaly II, ACC | n.d | Mee et al., 2005; Paetau et al., 2008; Putoux et al., 2011 | ||||
| Hydrolethalus syndrome 1 (HYLS1) | 610693 | 26558 | 11q24 | n.d | ||||||||
| Hypogonadotropic hypogonadism with or without anosmia | Heparan sulfate 6-O-sulfotransferase 1 (HS6ST1) | 604846 | 5201 | 2q21 | Uncommon; potentially more common in Kallmann syndrome type 2 | Hypogonadotropic hypogonadism, olfactory lobe agenesis, hyposmia or anosmia, mirror hand movements (bimanual synkinesia), ataxia | Y (100%) | Y | Huffman et al., 2004; Dode et al., 2006; Smith et al., 2006b; Tole et al., 2006; Conway et al., 2011 | |||
| Fibroblast growth factor receptor 1 (FGFR1) | 136350 | 3688 | 8p11.23-p11.22 | Y | Y | Y | Y | |||||
| Fibroblast growth factor 8 (FGF8) | 600483 | 3686 | 10q24.32 | Y | ||||||||
| KAL1, GNRHR, KISS1R, NSMF, TAC3, TACR3,GNRH1, KISS1, WDR11, SEMA3A | ||||||||||||
| Joubert syndrome | Kinesin family member 7 (KIF7) | 611254 | 30497 | 15q26.1 | 6/71 (8.5%) | Dysplasia of brainstem, cerebellar vermis hypoplasia, molar tooth sign, distinctive facies, hypotonia/ataxia | n.d | Smith et al., 2006a; Baala et al., 2007; Doherty et al., 2010; Dafinger et al., 2011; Poretti et al., 2011 | ||||
| RPGRIP1-Like (RPGRIP1L) | 610937 | 29168 | 16q12.2 | Y | Y | |||||||
| Transmembrane protein 67 (TMEM67) | 609884 | 28396 | 8q22.1 | Y (wpk rat) | Y (wpk rat) | |||||||
| INPP5E, TMEM216, AHI1, NPHP1, CEP290, ARL13B, CC2D2A, OFD1, TECT1, TMEM237, CEP41, TMEM138, CTBP1-AS1, TCTN3 | n.d | |||||||||||
| GROUP III- Abnormal callosal neuron migration and/or specification | ||||||||||||
| Complex cortical dysplasia with other brain malformations | Tubulin, beta 3 class III (TUBB3) | 602661 | 20772 | 16q24 | 2/9 (22.2%) | Polymicrogyria, gyral simplification, dysplastic cerebellar vermis, hypoplastic brainstem, ACC, fusion of basal ganglia | n.d | Poirier et al., 2010 | ||||
| Congenital fibrosis of extraocular muscles 3A with extraocular involvement | Tubulin, beta 3 class III (TUBB3) | 602661 | 20772 | 16q24 | 2/8 (25%) | Congenital fibrosis of the extraocular muscles, ACC, peripheral neuropathy | n.d | Tischfield et al., 2010 | ||||
| FG syndrome | Filamin A, aplha (FLNA) | 300017 | 3754 | Xq28 | 50% (14/28) | See Opitz-Kaveggia syndrome | n.d | Graham et al., 1999; Unger et al., 2007 | ||||
| Calcium/calmodulin-dependent serine protein kinase (MAGUK family) (CASK) | 300172 | 1497 | Xp11.4 | n.d | ||||||||
| Lissencephaly 2 | Reelin (RELN) | 600514 | 9957 | 7q22 | 33% | Microcephaly, inversion of cortical layers, thick cerebral cortex | N | N | Kara et al., 2010 | |||
| Lissencephaly 3 | Tubulin alpha 1A (TUBA1A) | 602529 | 20766 | 21q13.2 | 50% (4/8) | Cerebellar and hippocampal dysplasia, ACC, seizures | n.d | Poirier et al., 2007 | ||||
| Lissencephaly 4 | NudE nuclear distribution E homolog 1 (A. nidulans) (NDE1) | 609449 | 17619 | 16p13.11 | 4/6 (67%) | Extreme microcephaly, lissencephaly, brain atrophy | N | N | Alkuraya et al., 2011; Bakircioglu et al., 2011 | |||
| Muscular dystrophy- dystroglycanopathy type A | POMT1, POMGNT1, POMT2, GTDC2, ISPD, FKTN, FKRP, LARGE | Unknown | Eye defects, ACC, cobblestone lissencephaly type 2 | n.d | Dobyns et al., 1989; Villanova et al., 1998; van Reeuwijk et al., 2005; Judas et al., 2009 | |||||||
| Polymicrogyria, symmetric or asymmetric | TUBB2B | 612850 | 30829 | 6p25.2 | 100% (6/6) | Asymmetric polymicrogyria, ACC, cerebellar hypoplasia, brainstem abnormalities | n.d | Jaglin et al., 2009; Romaniello et al., 2012 | ||||
| Proud syndrome | Aristaless related homeobox (ARX) | 300382 | 18060 | Xp21.3 | Defining feature | Mental retardation with ACC, microcephaly, limb contractures, scoliosis, coarse facies, tapered digits, and urogenital abnormalities | N | Y | Y | Kitamura et al., 2002; Kato et al., 2004 | ||
| Schizophrenia | Disrupted in schizophrenia 1 (DISC1) | 605210 | 2888 | 1q42.1 | Uncommon | Multiple loci involved, hallucinations/delusions | N | Y (100%) | N | Shen et al., 2008; Osbun et al., 2011 | ||
| X-linked dominant periventricular heterotopia | Filamin A, alpha (FLNA) | 300017 | 3754 | Xq28 | Uncommon | Mild mental retardation, seizures, subependymal periventricular heterotopic nodules, cardiovascular abnormalities | n.d | Fox et al., 1998; Poussaint et al., 2000; Sheen et al., 2001 | ||||
| X-linked lissencephaly 1 | Doublecortin (DCX) | 300121 | 2714 | Xq22.3-23 | Uncommon | Lissencephaly, subcortical band or laminar heterotopia (in female carriers), malformation of the insula, ACC | Yb | Yb | Yb | Yb | Gleeson et al., 1998; Koizumi et al., 2006a; Chou et al., 2009 | |
| X-linked lissencephaly 2 (XLAG) | Aristaless related homeobox (ARX) | 300382 | 18060 | Xp21.3 | Defining feature | Ambiguous genitalia, mental retardation, neonatal seizures, lissencephaly, pachygyria/agyria, ACC | N | Y | Y | Kitamura et al., 2002; Stromme et al., 2002; Kato et al., 2004; Friocourt et al., 2008; Kara et al., 2010 | ||
| X-linked subcortical laminar heteropia | Doublecortin (DCX) | 300121 | 2714 | Xq22.3-23 | Common | See X-linked lissencephaly 1 | Yb | Yb | Yb | Yb | des Portes et al., 1998a, b; Gleeson et al., 1998; Koizumi et al., 2006b | |
| GROUP IV- Abnormal axon growth and/or guidance | ||||||||||||
| Craniofrontonasal syndrome | Ephrin B1 (EFNB1) | 300035 | 3226 | Xq13.1 | 6/58 (10%) | Developmental delay, corpus callosum hypoplasia, diaphragmiatic and umbilical hernias; more severe phenotype in females | Y | Y | N | Twigg et al., 2004; Wieland et al., 2004; 2005; Wieacker and Wieland, 2005 | ||
| L1 Syndrome spectrum (HSAS/MASA) | L1 cell adhesion molecule (L1CAM) | 308840 | 6470 | Xq28 | Common | Phenotypic spectrum ranging from partial ACC to hydrocephalus and complete ACC | Y (17%) | Y (83%) | N | Demyanenko et al., 1999 | ||
| Syndromic micropthalmia | Ventral anterior homeobox 1 (VAX1) | 604294 | 12660 | 10q26.11 | Unknown | Hypothalamic hamartoma, generalized white matter reduction, ACC, anterior pituitary hypoplasia, cardiovascular abnormalities | Y | N | Y | Y | Bertuzzi et al., 1999; Slavotinek et al., 2012 | |
| BCOR, SOX2, ANOP1, OTX2, BMP4, HCCS, STRA6 | n.d | |||||||||||
| GROUP V - Abnormal post-guidance development | ||||||||||||
| Andermann syndrome | Solute carrier family 12 (potassium/chloride transporters), member 6 (KCC3) | 604878 | 10914 | 15q13 | Defining feature | Peripheral neuropathy and ACC, ventriculomegaly, axonal neuropathy (PNS), dysmorphic facies | N | N | Larbrisseau et al., 1984; Howard et al., 2002; Dupre et al., 2003; Shekarabi et al., 2012 | |||
| Autosomal recessive spastic paraplegia 11 | Spastic paraplegia 11 (autosomal recessive) (SPG11) | 610844 | 11226 | 15q13-q15 | Uncommon | Progressive weakness/ spasticity of lower limbs, mental retardation, corpus callosum hypoplasia | n.d | Stevanin et al., 2007, 2008; Southgate et al., 2010 | ||||
| Desmosterolosis | 24-dehydrocholesterol reductase (DHCR24) | 606418 | 2859 | 1p32.3 | 100% (5/5) | Seizures, ventriculomegaly, hydrocephalus, decreased white matter, partial or complete ACC | n.d | FitzPatrick et al., 1998; Schaaf et al., 2011; Zolotushko et al., 2011 | ||||
| Micropthalmia with linear skin defects (MLS) syndrome | Holocytochrome C synthase (HCCS) | 300056 | 4837 | Xp22.2 | 14/40 (35%) | Bilateral microphthalmia, linear skin defects | n.d | Prakash et al., 2002; Sharma et al., 2008 | ||||
| Pontocerebellar hypoplasia 9 | Adenosine monophosphate deaminase 2 (AMPD2) | 102771 | 469 | 1p13.3 | 100% | Cerehellar and pontin hypoplasia, progressive microcephaly, limb spasticity, ACC | n.d | Akizu et al., 2013a | ||||
| Pyruvate dehydrogenase deficiency | Pyruvate dehydrogenase (lipoamide) alpha 1 (PDHA1) | Xp22.1 | 31% | Lactic acidosis, cerebral atrophy, ventricular dilatation, ACC | n.d | Patel et al., 2012 | ||||||
| Pyruvate dehydrogenase (lipoamide) beta (PDHB) | 179060 | 8808 | 3p21.1-p14.2 | n.d | ||||||||
| Unclear function in corpus callosum development | ||||||||||||
| Coffin-Lowry syndrome | Ribosomal protein S6 kinase, 90 kDa, polypeptide 3 (RPS6KA3) | 300075 | 10432 | Xp22 | Unknown | Sensorineural hearing loss, skeletal malformations, cognitive impairment | n.d | Soekarman and Fryns, 1993 | ||||
| Fumarase deficiency | Fumarate hydratase (FH) | 136850 | 3700 | 1q42.1 | Uncommon | Polymicrogyria, ACC, relative macrocephaly, fumaric aciduria | n.d | Bourgeron et al., 1994; Kerrigan et al., 2000 | ||||
| Genitopatellar syndrome | K(lysine) acetyltransferase 6B (KAT6B) | 605880 | 17582 | 10q22 | 11/14 (77%) | Absent/hypoplastic patellae, lower extremity contractures, urogenital anomalies | n.d | Goldblatt et al., 1988; Cormier-Daire et al., 2000; Penttinen et al., 2009; Brugha et al., 2011; Campeau et al., 2012 | ||||
| Opitz G/BBB syndrome type I (X-linked) | Midline 1 (Opitz/BBB syndrome) (MID1) | 300552 | 7095 | Xp22 | Unknown | Developmental delay, ACC, dysmorphic facies | n.d | Fontanella et al., 2008 | ||||
| Oro–facio–digital syndrome type 1 | Oral-facial-digital syndrome 1 (OFD1) | 300170 | 2567 | Xp22 | Unknown | Oral, facial and digital malformations, polycystic kidney disease | n.d | Towfighi et al., 1985; Connacher et al., 1987 | ||||
| Pitt-Hopkins syndrome | Transcription factor 4 (TCF4) | 602272 | 11634 | 18q21.1 | Unknown | Severe mental retardation, hyperventilation episodes, | n.d | Amiel et al., 2001; Whalen et al., 2012 | ||||
| Smith-Lemli-Opitz syndrome | 7-dehydrocholesterol reductase (DHCR7) | 602858 | 2860 | 11q13.4 | Uncommon | Mental retardation, autistic features, microcephaly, periventricular heterotopia | n.d | Garcia et al., 1973; Fierro et al., 1977; Fitzky et al., 1998 | ||||
| TARP syndrome | RNA-binding motif protein 10 (RBM10) | 300080 | 9896 | Xp11.23 | Uncommon | Congenital heart defect, clubfoot, cleft palate, glossoptosis, micrognathia | Y | Johnston et al., 2010; Gripp et al., 2011 | ||||
| Temtamy syndrome | Chromosome 12 open reading frame 57 (C12ORF57) | 615140 | 29521 | 12p13.31 | Common | Craniofacial dysmorphism, absent corpus callosum, and iris coloboma | n.d | Temtamy and Sinbawy, 1991; Temtamy et al., 1996; Chan et al., 2000; Talisetti et al., 2003; Li et al., 2007; Akizu et al., 2013b | ||||
| Vici syndrome | Ectopic P-granules autophagy protein 5 homolog (C. elegans) (EPG5) | 615068 | 29331 | 18q12.3 | 100% | Combined immunodeficiency, poor post-natal growth, cleft lip and palate, hypopigmentation of skin and hair, ACC | n.d | Vici et al., 1988; del Campo et al., 1999; Chiyonobu et al., 2002; Miyata et al., 2007; Al-Owain et al., 2010; McClelland et al., 2010; Rogers et al., 2011; Callup et al., 2013 | ||||
| Warburg micro syndrome | RAB3GAP1, RAB3GAP2, RAB18 | Unknown | Microcephaly, mental retardation, hypogenitalism | n.d | Aligianis et al., 2005 | |||||||
aReferences in the table that are not included in the reference list can be found in the Supplementary material.
bOnly in DCX/DCLK double knockouts; n.d, no data.
#For syndromes to be considered, the following criteria had to be met: at least three patients with the syndrome had been documented, of whom at least two displayed complete or partial ACC. Syndromes in which callosal abnormalities were secondary to more severe neural defects such as holoprosencephaly were excluded. ACC penetrance was determined by considering case reports and previous imaging studies.
By taking into account the known function of the affected gene, associated mouse models, and neuroanatomical findings in human patients, it is possible to hypothesize a general pathogenic mechanism for callosal agenesis in syndromes commonly associated with ACC. In this review, single gene syndromes associated with ACC have been broadly divided into categories based on abnormalities of important steps in cerebral development: neuronal and glial proliferation, midline patterning, neuronal migration and specification, axon guidance, and post-guidance development.
Abnormal neuronal and glial proliferation
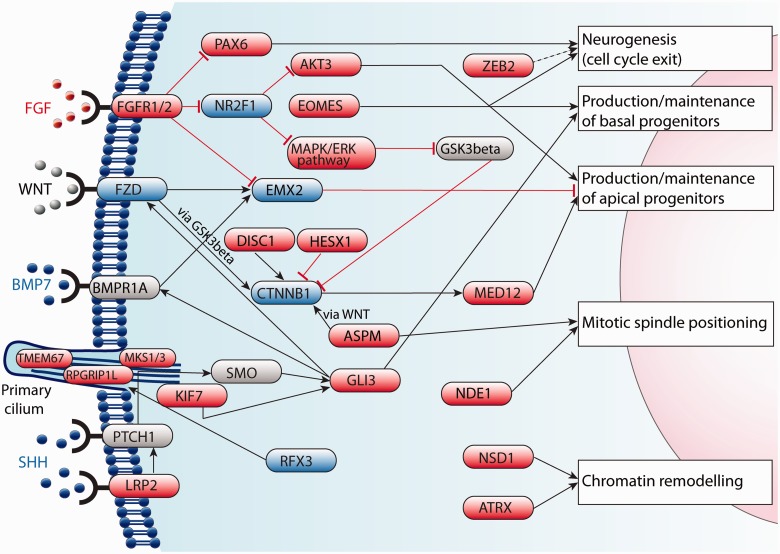
Early cerebral development is associated with cortical patterning, driven by a combination of morphogenetic gradients that together with developing thalamocortical circuits, influence the molecular identity of neuronal progenitors (O'Leary et al., 2007; Kanold and Luhmann, 2010). These influences give rise to spatio-temporal-specific signalling domains called patterning centres, which specify populations of neurons by regulating transcription factor expression. Many molecules involved in neurogenesis have multiple roles in development (Fig. 6), and callosal abnormalities as a result of abnormal neuronal and glial development are never diagnosed in isolation. In these cases, ACC should not be considered a diagnosis in itself, but should rather be cause for detection of additional congenital defects. Glutamatergic cortical neurons are born in the subventricular zone from intermediate progenitor cells, and from radial glia in the ventricular zone (Noctor et al., 2004; Kowalczyk et al., 2009). Multiple transcription factors are necessary for specification of cells in the subventricular zone and ventricular zone, but these are beyond the scope of this review. Intermediate progenitor cells are themselves born from asymmetrical division of radial glia within the ventricular zone (Noctor et al., 2004). To maintain progenitor cell numbers, radial glia may less frequently undergo symmetrical cell division to expand the pool of neuronal precursors (Tamamaki et al., 2001). Whether radial glia produce proliferative or differentiating cells is highly dependent on the orientation of the mitotic spindle relative to the ventricular surface (Shioi et al., 2009), and loss of control over this process results in prenatal microcephaly.
Figure 6.
Major mechanisms underlying neurogenesis in the telencephalon relevant to ACC in humans. Many molecules involved in neurogenesis have multiple functions, and genetic mutations can therefore result in complex neurodevelopmental disorders. Many midline patterning genes functionally interact with primary cilia, and mutations in these genes give rise to a group of overlapping syndromes termed ‘ciliopathies’, which can feature ACC. Genes in red are associated with a human syndrome; genes in blue have a mouse model with ACC but have not yet been associated with a human ACC syndrome, and genes in grey (ligands in black) have not been implicated in either human or mouse ACC.
Autosomal recessive primary microcephaly (MCPH) results from decreased or ineffective proliferation of neurons, generally without disturbance of cortical organization (for a review, see Mahmood et al., 2011). Callosal development is usually not impaired in this group of prenatal microcephalies, so abnormal neuronal proliferation alone cannot always account for ACC. Syndromes that do encompass both ACC and microcephaly represent a broad group, but differ from MCPH in the degree of associated cortical disorganization.
G-protein signalling modulator 2 (GPSM2) is necessary for the planar orientation of the mitotic spindle in symmetrical division, and mutations in GPSM2 result in the autosomal recessive Chudley-McCullough syndrome, which can display complete ACC (Diaz-Horta et al., 2012; Doherty et al., 2012). Cortical malformations in Chudley-McCullough syndrome seem to be principally because of disrupted cortical architecture rather than decreased neuronal proliferation. Mouse models of homozygous Gpsm2 mutations show that vertically aligned divisions of radial glia that would normally produce identical apical progenitor cells instead produce aberrant progenitors that migrate into the cortex (Konno et al., 2008; Shioi et al., 2009). It is possible that a similar disruption to the spatial organization of neurogenesis underlies the two primary microcephaly syndromes in which abnormal cortical architecture and ACC have been well characterized: MCPH5 and MCPH2, caused by mutations in the abnormal spindle-like, microcephaly-associated gene (ASPM) and WD-repeat domain 62 gene (WDR62), respectively. Mutations in ASPM and WDR62 genes together account for at least 55% of MCPH families, and are directly involved in mitotic spindle orientation of neural precursors within the ventricular zone (Mahmood et al., 2011). Along similar lines, homozygous mutations in nudE nuclear distribution E homolog 1 (A. nidulans) (NDE1), which localizes to the centrosome and mitotic spindle poles, results in a severe microlissencephaly syndrome encompassing cortical disorganization and ACC. These patients present with marked architectural defects in the cortex, which is consistent with a combined disorder of neurogenesis and neuronal migration (Feng and Walsh, 2004; Alkuraya et al., 2011; Paciorkowski et al., 2013).
The balance between symmetric and asymmetric division of radial glia is influenced by a series of transcription factors expressed by neuronal precursors and post-mitotic migrating neurons. Mowat-Wilson syndrome results from heterozygous, mostly de novo mutations in the ZEB2 gene encoding SMAD interacting protein 1 (SIP1) (Cacheux et al., 2001; Garavelli and Mainardi, 2007). In neurogenesis, SIP1 is one of several transcription factors expressed specifically in post-mitotic neocortical neurons, and non-cell autonomously controls differentiation of neuronal progenitor cells. Loss of SIP1 function in mice leads to increased superficial layer neuron production and gliogenesis, all at the expense of deep layer neurons (Seuntjens et al., 2009). Callosal agenesis is present in just over 40% of Mowat-Wilson cases (Mowat et al., 2003; Dastot-Le Moal et al., 2007); however, even patients from within the same family show an inconsistent callosal phenotype, suggesting that modifier genes interact with SIP1 to influence callosal development. In addition, SIP1 appears to have earlier roles in telencephalic patterning (Verschueren et al., 1999; Verstappen et al., 2008) and neural crest cell migration, and better genotype-phenotype correlations will improve the accuracy of prognosis in neonates and infants.
The change in expression of a series of transcription factors signals the transition from radial glia to intermediate progenitors to neurons. Expression of the transcription factor eomesodermin (T-box brain protein 2 in mice) in radial glia is sufficient to induce intermediate progenitor cell identity (Sessa et al., 2008). Conversely, expression of PAX6, EMX2 and SOX2 transcription factors maintains radial glia populations (Graham et al., 2003; Englund et al., 2005; Sansom et al., 2009). With the exception of one report of a microcephalic patient with a disruption of the Eomesdermin gene (Baala et al., 2007), no human mutations in these genes have been associated with cortical dysgeneses that recapitulate the severe neurological phenotypes of mouse models. Indeed, for patients with PAX6 or SOX2 mutations, mild callosal hypoplasia is a more common finding than partial or complete ACC (Kelberman et al., 2006).
In syndromes where diffuse thinning of the corpus callosum (callosal hypoplasia) is a frequent finding and ACC occurs occasionally, it is likely that agenesis lies on a spectrum of pathogenic mechanisms underlying hypoplasia. Sotos syndrome is an overgrowth syndrome caused by haploinsufficiency in the NSD1 and NFIX genes (Kurotaki et al., 2002; Malan et al., 2010). Diffuse callosal hypoplasia or thinning of the posterior body is a common finding, whereas callosal agenesis has been reported in only a small proportion of patients (Schaefer et al., 1997; Melo et al., 2002; Horikoshi et al., 2006). It is difficult to tease apart the mechanisms underlying hypoplasia and agenesis; however, it is likely that the underlying mechanisms are similar, and that genetic modifiers influence the severity of the callosal phenotype.
Modifying genetic influences also play an important role in neuropsychiatric disorders such as autism and schizophrenia, in which variable decreases in callosal size and fractional anisotropy suggest underlying abnormalities of white matter microstructure (Woodruff et al., 1995; Downhill et al., 2000; Innocenti et al., 2003). In general, neuropsychiatric disorders such as schizophrenia can be considered polygenic disorders, the inheritance of which is influenced by the combined effect of many genetic modifiers. One possible exception to this rule, however, is mutations in the disrupted in schizophrenia 1 gene (DISC1), which have been implicated in both ACC and a small percentage of schizophrenia cases (Osbun et al., 2011). DISC1 inhibits neuronal progenitor proliferation by inhibiting phosphorylation of β-catenin, which causes cell cycle exit and differentiation (Mao et al., 2009). Following this, DISC1 acts as a molecular switch that, when phosphorylated in post-mitotic neurons, recruits Bardet-Biedl syndrome (BBS) proteins BBS1 and BBS4 to the centrosome and interacts with NDE1-like 1 to promote neuronal migration and neurite outgrowth, respectively (Kamiya et al., 2006; Ishizuka et al., 2011). A mouse model of Disc1 mutation shows high penetrance of partial ACC (Shen et al., 2008), and several rare, potentially pathogenic mutations in DISC1 have been identified in patients with ACC. The number of schizophrenia patients with DISC1 mutations and ACC has not been as widely studied. Given the likelihood that developmental pathways exist that are common to both ACC and schizophrenia, however, it is possible that the link between schizophrenia and callosal development is more widespread than currently thought, and further study may uncover genetic modifiers involved in these disorders (Walterfang et al., 2008; Osbun et al., 2011).
Abnormal midline patterning
Early disruptions in patterning of the prosencephalic vesicle can result in ACC, but this is secondary to more severe pathologies. Failure of invagination of the dorsal prosencephalon to produce two hemispheres results in a single hollow vesicle being formed (holoprosencephaly) and subsequent loss of all midline structures including the corpus callosum. This condition can affect the entire telencephalon, or can be restricted to either rostral or caudal regions, in which case parts of the corpus callosum may still form provided there is a bridge of white matter across which axons can traverse the midline (for a review see Marcorelles and Laquerriere, 2010). Likewise, failure of an established telencephalic midline to fuse invariably results in callosal agenesis because of loss of a substrate through which callosal axons can pass (Silver and Ogawa, 1983; Demyanenko et al., 1999; Brouns et al., 2000; Wahlsten et al., 2006). The BALB/c and 129 mouse strains, for example, display severe retardation of midline fusion in the septal region, but guidance of putative callosal axons is normal to the midline, at which point the axons stall (Wahlsten et al., 2006). Correct patterning of the commissural plate and midline glial populations is essential for commissural axons to cross the midline (Moldrich et al., 2010). Midline glia function primarily as guideposts for callosal axons, and secrete guidance molecules to define migratory boundaries, while each telencephalic commissure must pass through a molecularly distinct region of the commissural plate.
Sonic hedgehog (SHH) is a secreted morphogen that bestows ventral cell identity in the early telencephalon in a concentration-dependent manner. Human mutations in SHH or its receptor patched 1 (PTCH1) cause holoprosencephaly, as a result of disturbances too early in dorsal-ventral patterning to fall within the scope of this review (for a review of the hedgehog signalling network, see Robbins et al., 2012). SHH signalling through PTCH1 is mediated by low density lipoprotein-related protein 2 (LRP2) (Willnow et al., 1996; Spoelgen et al., 2005; Christ et al., 2012), which when mutated, results in the autosomal recessive Donnai-Barrow syndrome (Kantarci et al., 2007). In Lrp2−/− mice, loss of Shh signalling almost always results in holoprosencephaly (Spoelgen et al., 2005), although human cases present with milder ventral patterning defects including ACC (Kantarci et al., 2007), suggesting that there is greater redundancy for the role of LRP2 in SHH signalling in humans.
In recent years, the association between disorders involving primary cilia (ciliopathies) and ACC has been increasingly studied. Primary cilia cooperate with SHH signalling by interacting with the downstream signalling molecules kinesin family member 7 (KIF7) and GLI family zinc finger 3 (GLI3) (Liem et al., 2009; Besse et al., 2011). There are multiple, diverse genetic causes of ciliopathies, but all of the implicated genes are necessary for the normal function of primary cilia (Lee and Gleeson, 2011; Novarino et al., 2011). A summary of the major ciliopathies associated with ACC is given in Table 1. Mice lacking the ciliogenic transcription factor RFX3 display altered patterning of the corticoseptal boundary and abnormal positioning of guidepost neurons associated with expanded FGF8 expression (Benadiba et al., 2012). This is of particular importance because of the well-established role of FGF8 in establishing the commissural plate (Moldrich et al., 2010). However, neurodevelopmental abnormalities are not confined to the corpus callosum. Failure of decussation of superior cerebellar peduncles and absence of the pyramidal decussation (Quisling et al., 1999), in addition to distinctive malformations of the cerebellum (Juric-Sekhar et al., 2012), are consistent with multiple roles for primary cilia throughout brain development.
Table 1.
Major syndromes associated with ACC that are part of the extended ciliopathy spectrum
| Joubert syndrome | Meckel syndrome | Hydrolethalus syndrome | Acrocallosal syndrome | Bardet-Biedl syndrome (JSRD) | |
|---|---|---|---|---|---|
| Selected genes affected | TMEM67, TMEM216, RPGRIP1L, KIF7 | MKS1, MKS3, TMEM67, RPGRIP1L | HYLS1, KIF7, ACLS | GLI3, KIF7, HLS2 | BBS1–12, TMEM67, MKS1 |
| Major neuroanatomical abnormalities | Molar tooth sign (cerebellar vermis hypoplasia/absence, deep interpeduncular fossa, thick elongated superior cerebellar peduncles) | Occipital encephalocele, absence of olfactory bulbs, complete or partial ACC | Severe hydrocephalus, absence of midline structures (ACC) | Exencephaly, hydrocephalus, ACC | Molar tooth sign |
| ACC common/ occasional finding? | Uncommon | Common | Common | Common | Occasional |
GLI3 mutations result in multiple overlapping syndromes including acrocallosal syndrome, Greig cephalopolysyndactyly and metopic craniosynostosis, and some of these affected patients present with callosal anomalies (Vortkamp et al., 1991; Elson et al., 2002; McDonald-McGinn et al., 2010). Specific mutations in different regions of GLI3 have helped to delineate the way in which it transduces SHH signalling, and genotype–phenotype correlations have been made previously (Kang et al., 1997; Johnston et al., 2005; Naruse et al., 2010). The severity of these disorders ranges from polydactyly and hypothalamic hamartoma to holoprosencephaly or neonatal lethality, and neuroanatomical abnormalities appear to correlate with the degree of disruption to the normal dorsal midline patterning function of GLI3. Abnormalities in midline patterning in GLI3 hypomorphic mice are similar to those observed in Rfx3−/− mice, whereby ACC is associated with increased Slit2 and Fgf8 expression (Magnani et al., 2012). Interestingly, FGF signalling has been implicated in Apert syndrome (Wilkie et al., 1995; Slaney et al., 1996; Quintero-Rivera et al., 2006) and a proportion of patients with Kallmann syndrome for whom ACC has occasionally been described (Dode et al., 2003; Falardeau et al., 2008; McCabe et al., 2011). Together, these syndromes represent disruptions of a common developmental pathway (Vaaralahti et al., 2012), and corresponding mouse models all show common midline patterning defects with aberrant positioning of midline glial guideposts.
Abnormal callosal neuron migration and specification
Once born from the subventricular or ventricular zones, post-mitotic neurons migrate outwards along radial glial processes to form six distinct cortical layers in a birth date-dependent inside-out manner (Noctor et al., 2001; Huang, 2009). Early born neurons populate the deeper zones, whereas later born neurons migrate past them to populate more superficial cortical layers. Radial migration from the subventricular and ventricular zones towards the cortical plate is achieved by a recurring cycle of leading process extension, nucleokinesis, and trailing process retraction (Kanatani et al., 2005). Several human ACC syndromes have been associated with the intracellular molecules that underpin neuronal migration. Not surprisingly, mutations in genes known to be involved in microtubule structure (e.g. TUBA1A) and stabilization (e.g. DCX and DCLK1) severely affect early radial migration and post-migrational development of cortical neurons (Gleeson et al., 1998; Deuel et al., 2006; Koizumi et al., 2006a, b; Poirier et al., 2007). The resulting group of human syndromes are often severe, characterized by lissencephaly and periventricular nodular heterotopias, but can also present as disorders mainly of axon guidance (O'Driscoll et al., 2010; Tischfield et al., 2010; Chew et al., 2013).
Mutations in the ARX gene cause a nearly continuous series of syndromes ranging from severe hydranencephaly, lissencephaly and ACC, to syndromes with no brain malformations visible on MRI scans (Kitamura et al., 2002; Weaving et al., 2004; Suri, 2005). ARX comprises an aristaless domain and a prd-like homeodomain (Stromme et al., 2002). In general, non-conservative mutations in either functional domain result in X-linked lissencephaly with an absent corpus callosum and ambiguous genitalia (XLAG), whereas a more severe syndrome is observed when both domains are disrupted (Kato et al., 2004). XLAG is typified by a posterior-to-anterior gradient of lissencephaly, ambiguous genitalia, hypoplastic basal ganglia/hypothalamus, and a slightly thickened cortex comprising three pyramidal neuron layers, epilepsy and complete ACC (Bonneau et al., 2002; Miyata et al., 2009). Abnormal cortical layering is consistent with a radial migration defect of cortical neurons; however, murine Arx is expressed in GABAergic interneurons arising from the ganglionic eminences and the subventricular zone (Friocourt et al., 2008). XLAG is a combined disorder of tangential and radial neuronal migration, and it is likely that defects in neurogenesis also exist (Friocourt et al., 2008). Interestingly, the female XLAG syndrome is less severe than that of the male, suggesting gene dosage effects of ARX mutations; carrier females can exhibit isolated ACC with Probst bundles, variably impaired cognitive function and epilepsy (Bonneau et al., 2002).
The cortical layer that a neuron will inhabit is primarily determined by the time of its birth (Desai and McConnell, 2000; Shen et al., 2006). Once a neuron has migrated to this layer, however, it must continue to be specified by its layer and target area. Callosal neuron identity appears to coincide with expression of the chromatin-remodelling factor Satb2, which has been proposed to specify rostral callosal projecting neurons at the expense of corticofugal projection neurons (Alcamo et al., 2008; Britanova et al., 2008), which are specified by the transcription factors FEZF2 and CTIP2 (Arlotta et al., 2005; Chen et al., 2005; Molyneaux et al., 2005; Chen et al., 2008). SATB2 has recently been shown to functionally interact with the proto-oncogene Ski to specify callosal neurons (Baranek et al., 2012), as discussed later in relation to 1p36 deletion syndrome.
In ACC, the neurons that would have crossed the corpus callosum must be re-specified such that they may project subcortically, intracortically in Probst bundles, or they may preserve some interhemispheric connectivity by projecting to the contralateral cortex through the anterior or hippocampal commissures. In the majority of patients with ACC, the anterior and hippocampal commissures are absent or small, which is consistent with common processes of commissure development (Hetts et al., 2006). In a smaller subset of patients with ACC, but in all cases with ACC with an identified ARX mutation (Hetts et al., 2006; Kara et al., 2010), the anterior commissure is enlarged, and limited evidence suggests that this may represent a compensatory mechanism to maintain inter-cerebral transfer of information (Fischer et al., 1992; Barr and Corballis, 2002). A similar increase in anterior commissure size has been well established in multiple inbred mouse strains, and is accounted for by an increase in unmyelinated axons (Livy et al., 1997). Whether the apparent use of the anterior commissure as a surrogate corpus callosum is compensatory in some patients will depend largely on whether it can transmit information from origins normally exclusive to the corpus callosum (Guenot, 1998), and this is not yet clearly established.
Abnormal axon guidance
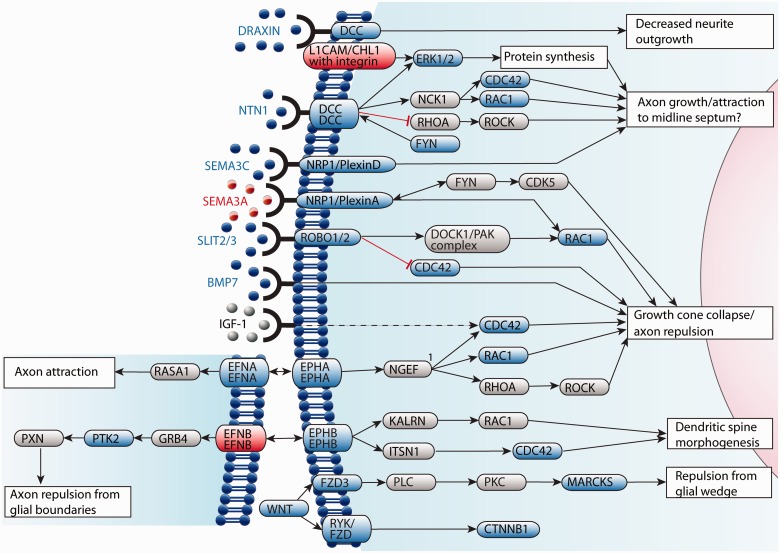
Correct callosal axon guidance is a tightly regulated process that relies on two distinct levels of guidance cue response. First, growth cones must respond to guidance cues specifically and with high fidelity, and this is dependent on correct temporal and spatial expression of receptors. Second, underlying axon migration and the guidance response is a complex network of intracellular actin and microtubule dynamics, and intercellular recognition and fasciculation. Molecules that modulate these processes can be influenced by activation of guidance cue receptors (Fig. 7). The directionality of growth cones can be influenced by long-range attractive or repulsive cues, short-range attractive or repulsive cues, factors affecting axon fasciculation, growth substrate and cellular influences (Lindwall et al., 2007).
Figure 7.
Major mechanisms that potentially underlie guidance of callosal axons in humans. Guidance receptors are expressed on the growth cone of commissural axons, and when bound to their ligand/s, influence microtubule and actin dynamics through second messengers including RHOA, RAC1 and CDC42. Some guidance receptors, such as DCC, have multiple ligands, and the effects of receptor activation depend on the bound ligand. Whereas most ligands are secreted from midline glial populations into the surrounding extracellular matrix, ephrin ligands are membrane-bound and can initiate reverse signalling. The effects of ephrin receptors vary depending on the subtype of receptor activated, and ligands expressed. Genes in red are associated with a human syndrome; genes in blue have a mouse model with ACC but are not associated with a human ACC syndrome, and genes in grey (ligands in black) have not been implicated in human or mouse ACC. 1, based on overexpression studies, NGEF increases RHOA activity relative to RAC1 and CDC42.
Although understanding the mechanisms of axonal guidance has elucidated important aspects of normal corpus callosum development, few patients with ACC syndromes have been identified with mutations in axon guidance genes. This may be because of the fact that broad syndromes as a result of neuronal proliferative or maturational defects display clear neurological disorders, whereas guidance defects could manifest as isolated and less detectable callosal dysgeneses. Indeed, the correct guidance of callosal axons is dependent on a large body of signalling molecules and transcription factors that must be correctly expressed before and during axon guidance. Guidance cues can also act in parallel and compensate for one another, and may therefore exhibit significant redundancy and reduced ACC penetrance. Conversely, homozygous null mice for guidance genes such as netrin 1 (Serafini et al., 1996), Robo1 (Andrews et al., 2006) and Dcc (Fazeli et al., 1997) die as embryos or shortly after birth, and thus human mutations in these genes might be lethal and not actually result in clinically evident syndromes. Interestingly, mutations in DCC have been associated with congenital mirror movements, which is somewhat reminiscent of the hopping gait and mirror movements seen in the DccKanga/Kanga mouse model (Finger et al., 2002; Srour et al., 2010; Djarmati-Westenberger et al., 2011). In addition, a weakly expressing haplotype of ROBO1 has been associated with dyslexia and impaired interhemispheric transfer of auditory signals (Hannula-Jouppi et al., 2005; Lamminmaki et al., 2012).
Craniofrontonasal syndrome, caused by mutations in the EFNB1 gene encoding ephrin-B1, is an exception to the lack of human ACC syndromes associated with axon guidance (Wieland et al., 2004, 2005). Craniofrontonasal syndrome is an atypical X-linked recessive disorder as females are severely affected whereas males show mild or no abnormalities; it typically presents with craniofacial and skeletal abnormalities, and less commonly, ACC (Saavedra et al., 1996; Wieacker and Wieland, 2005). The reason for low ACC penetrance (a review of the literature found ACC in 10% of cases) is likely because of the redundant nature of the ephrin family, which has been verified by mouse models of single and double gene knockouts (Table 2) (Wieacker and Wieland, 2005; Mendes et al., 2006). Ephrins define migratory boundaries in multiple developmental contexts; in callosal development, they are expressed in the glial wedge and redundantly direct axons toward the midline (Mendes et al., 2006). Heterozygous EFNB1 mutations in females seem to have a dominant negative effect owing to the multiple interactions possible between ephrin ligands and receptors of different subclasses. In females, random X-inactivation produces two types of cell, those expressing functional ephrin-B1 and those expressing the mutant ephrin-B1. Mutant ephrin-B1 expressing cells may present alternative ephrin ligands with different receptor affinity, resulting in abnormal cellular cross-talk within these mosaic compartments and unclear migratory boundaries (Twigg et al., 2004; Wieland et al., 2004; Wieacker and Wieland, 2005; Davy et al., 2006).
Table 2.
Genes with ACC mouse models and no human ACC syndrome
| Gene # | OMIM Number | HGNC ID | Location (human) | Mouse phenotype |
Referencesa | ||||
|---|---|---|---|---|---|---|---|---|---|
| Callosal phenotype |
Associated midline defects |
||||||||
| cACCb | pACCb | Midline glia | Hippocampal commissure | Anterior commissure | |||||
| GROUP I - Abnormal neuronal and/or glial proliferation | |||||||||
| Achaete-scute complex homolog 1 (Drosophila) (ASCL1) | 100790 | 738 | 12q22-q23 | Y | Y | N | Y | N | Niquille et al., 2009 |
| Catenin (cadherin-associated protein), beta 1, 88 kDa (CTNNB1) | 116806 | 2514 | 3p22.1 | Y | Machon et al., 2003 | ||||
| Eomesodermin (EOMES) | 604615 | 3372 | 3p24.1 | Y | Y | Y | Y | Arnold et al., 2008; Sessa et al., 2008 | |
| Mitogen-activated protein kinase 1 (MAPK1) | 176948 | 6871 | 22q11.2 | N | Y | Newbern et al., 2008; Satoh et al., 2011 | |||
| Mitogen-activated protein kinase 3 (MAPK3)/Mitogen- activated protein kinase 1 (MAPK1) | 601795/ 176948 | 6877/ 6871 | 16p11.2/ 22q11.2 | N | Y | Satoh et al., 2011 | |||
| Mitogen-activated protein kinase kinase kinase 4 (MAP3K4) | 602425 | 6856 | 6q26 | Y | Y | Chi et al., 2005 | |||
| N-ethylmaleimide-sensitive factor attachment protein, alpha (NAPA) | 603215 | 7641 | 19q13.33 | Y | Y | N | Chae et al., 2004 | ||
| Nuclear receptor subfamily 2, group E, member 1 (NR2E1) | 603849 | 7973 | 6q21 | N | Y | Y | Y | Monaghan et al., 1997; Land and Monaghan, 2003 | |
| Zinc finger and BTB domain containing 18 (ZBTB18) | 608433 | 13030 | 1q44 | Y | Xiang et al., 2011 | ||||
| Zinc finger protein 423 (ZNF423) | 604557 | 16762 | 16q12 | Y | Y | Y | Y | Cheng et al., 2007 | |
| GROUP II – Abnormal midline patterning | |||||||||
| Bone morphogenetic protein 7 (BMP7) | 112267 | 1074 | 20q13 | Y (50%) | Y (50%) | Y | Choe et al., 2012; Sanchez-Camacho et al., 2011 | ||
| Empty spiracles homeobox 1 (EMX1) | 600034 | 3340 | 2p13.2 | Y | Y | Y | N | Y | Qiu et al., 1996; Yoshida et al., 1997 |
| Empty spiracles homeobox 2 (EMX2) | 600035 | 3341 | 10q26.11 | N | Y | Y | Y | Pellegrini et al., 1996; Yoshida et al., 1997 | |
| Nuclear factor I/A (NFIA) | 600727 | 7784 | 1p31.3-p31.2 | Y (100%) | Y | Y | Y | das Neves et al., 1999; Shu et al., 2003a | |
| Nuclear factor I/B (NFIB) | 600728 | 7785 | 9p24.1 | Y | Y | Y | Steele-Perkins et al., 2005; Piper et al., 2009 | ||
| Regulatory factor X, 3 (influences HLA class II expression) (RFX3) | 601337 | 9984 | 9p24.2 | Y (36%) | Y (36%) | Y | N | Y | Benadiba et al., 2012 |
| GROUP III- Abnormal callosal neuron migration and/or specification | |||||||||
| Amyloid Beta A4 precursor protein- binding, family B, member 1 (APBB1) | 602709 | 581/582 | 11p15/4p13 | Y (100%) | Guenette et al., 2006 | ||||
| Amyloid beta (A4) precursor protein (APP) | 104760 | 620 | 21q21.2 | Y | Y | Y | Y | Muller et al., 1996; Magara et al., 1999 | |
| Ankyrin 2, neuronal (ANK2) | 106410 | 493 | 4q25 | N | Y | Scotland et al., 1998 | |||
| Cell adhesion molecule with homology to L1CAM (close homolog of L1) (CHL1) | 607416 | 1939 | 3p26.3 | Y | Demyanenko et al., 1999 | ||||
| Cell division cycle 42 (CDC42) | 116952 | 1736 | 1p36.12 | N | Y | Yokota et al., 2010 | |||
| Cytoplasmic protein tyrosine kinase 2 (PTK2) | 600758 | 9611 | 8q24.3 | N | Y | Beggs et al., 2003 | |||
| Doublecortin-like kinase 1 (DCLK1) | 604742 | 2700 | 13q13.3 | Y | Y | N | Deuel et al., 2006; Koizumi et al., 2006b | ||
| Laminin, gamma 1 (formerly LAMB2) (LAMC1) | 150290 | 6492 | 1q31.1 | N | Y | Chen et al., 2009 | |||
| Myristoylated alanine-rich protein kinase C substrate (MARCKS) | 177061 | 6759 | 6q21 | Y (93%) | Y (7%) | Y | Y | Stumpo et al., 1995 | |
| MARCKS-like 1 (MARCKSL1) | 602940 | 7142 | 1p35.1 | Y (100%) | N | N | Wu et al., 1996; Bjorkblom et al., 2012 | ||
| Mitogen-activated protein kinase 8-interacting protein 3 (MAPK8IP3) | 605431 | 6884 | 16p13.3 | Y (100%) | N | Y | Kelkar et al., 2003; Ha et al., 2005; Cho et al., 2011 | ||
| Rap guanine nucleotide exchange factor (GEF) 1 (RAPGEF1) | 600303 | 4568 | 9q34.13 | N | Y (100%) | Y | Y | Y (100%) | Bilasy et al., 2009; 2011 |
| Rho GTPase-activating protein 5 (ARHGAP5) | 602680 | 675 | 14q12 | N | Y (100%) | Hypoplasia | Y | Matheson et al., 2006 | |
| Rho GTPase activating protein 35 (ARHGAP35) | 605277 | 4591 | 19q13.32 | Y (100%) | Y | Y | Brouns et al., 2000; Matheson et al., 2006 | ||
| Special AT-rich sequence-binding protein-2 (SATB2) | 608148 | 21637 | 2q33.1 | Y (100%) | N | N | N | N | Alcamo et al., 2008; Britanova et al., 2008 |
| V-SKI Avian sarcoma viral oncogene homolog (SKI) | 164780 | 10896 | 1p36.33 | Y | Y | N | Baranek et al., 2012 | ||
| GROUP IV- Abnormal axon growth and/or guidance | |||||||||
| Cyclin-dependent kinase 5, regulatory subunit 1 (p35) (CDK5R1) | 603460 | 1775 | 17q12 | Y | N | Y | Kwon et al., 1999 | ||
| Deleted in colorectal cancer (DCC) | 120470 | 2701 | 18q21.2 | Y (100%) | Y | Y | Fazeli et al., 1997; Ren et al., 2007 | ||
| Dorsal repuslive axon guidance protein (DRAXIN) | 612682 | 25054 | 1p36.22 | Y (42%) | Y (58%) | Y | Y | Y | Islam et al., 2009; Ahmed et al., 2011 |
| Enabled homolog (Drosophila) (ENAH) | 609061 | 18271 | 1q32.2 | Y (55%)c | Y | N | Lanier et al., 1999 | ||
| EFNB3/EPH receptor B1 | 602297/600600 | 3228/3392 | 17p13.1/3q22.2 | Y (87%) | Y (13%) | Mendes et al., 2006 | |||
| EFNB3/EPH receptor B2 | 602297/600997 | 3228/3393 | 17p13.1/1p36.12 | y (45%) | Y (44%) | Mendes et al., 2006 | |||
| EFNB3/EPH receptor A4 | 602297/602188 | 3228/3388 | 17p13.1/2q36.3 | Y (29%) | Y (35%) | Mendes et al., 2006 | |||
| EPH receptor A5 (EPHA5) | 600004 | 3389 | 4q13.1 | N | Y (100%) | N | Y (46%) | Yue et al., 2002; Hu et al., 2003 | |
| EPH receptor B1 (EPH B1) | 600600 | 3392 | 3q22.2 | Y (43%) | Y (44%) | Mendes et al., 2006 | |||
| EPH receptor B2 (EPH B2) | 600997 | 3393 | 1p36.12 | Y (13%)c | Y (48%)c | Y | Orioli et al., 1996; Mendes et al., 2006; Ho et al., 2009 | ||
| EPH receptor B2 (Nuk) | 600997 | 3393 | 1p36.12 | N | Y (20%) | Y (100%) | Henkemeyer et al., 1996; Orioli et al., 1996 | ||
| EPH receptor B3 (EPH B3) (Sek) | 601839 | 3394 | 3q27.1 | Y (37.5%) | N | Orioli et al., 1996 | |||
| Ephrin B3 (EFNB3) | 602297 | 3228 | 17p13.1 | Y (64%) | Y (20%) | Orioli et al., 1996; Mendes et al., 2006 | |||
| EPH receptor B1/EPH receptor B2 | 600600/600997 | 3392/3393 | 3q22.2/1p36.12 | Y (60%) | Y (28%) | Mendes et al., 2006 | |||
| EPH receptor B1/EPH receptor B3 | 600600/601839 | 3392/3394 | 3q22.2/3q27.1 | Y (18%) | Y (90%) | Mendes et al., 2006 | |||
| EPH receptor B1/EPH receptor A4 | 600600/602188 | 3392/3388 | 3q22.2/2q36.3 | Y (12%) | Y (14%) | Mendes et al., 2006 | |||
| EPH receptor B2/EPH receptor B3 | 600997/601839 | 3393/3394 | 1p36.12/3q27.1 | Y (67%) | Y (33%) | Mendes et al., 2006 | |||
| EPH receptor B2/EPH receptor B3 (Nuk/Sek) | 600997/601839 | 3393/3394 | 1p36.12/3q27.1 | Y (89%) | N | Y (100%) | Orioli et al., 1996 | ||
| Exostosin 1 (EXT1) | 608177 | 3512 | 8q24.11 | Y | Y | Y | Inatani et al., 2003 | ||
| FEZ family zinc finger 2 (FEZF2) | 607414 | 13506 | 3p14.2 | Y | Y | N | Chen et al., 2005; Molyneaux et al., 2005 | ||
| Frizzled family receptor 3 (FZD3) | 4041 | 4041 | 8p21.1 | Y | Y | Y | Y | Wang et al., 2002; 2006 | |
| Growth associated protein 43 (GAP43) | 162060 | 4140 | 3q13.31 | Y (100%) | Y | Y (100%) | Y (100%) | Shen et al., 2002; 2004 | |
| Heparan sulphate 6-O-sulfotransferase 1 (HS6ST1) | 604846 | 5201 | 2q21 | Y (100%) | Y | Merry et al., 2001; Conway et al., 2011 | |||
| Microtubule-associated protein 1B (MAP1B) | 157129 | 6836 | 15q13.2 | Y (80%) | Y (20%) | Y | N | Meixner et al., 2000 | |
| Netrin 1 (NTN1) | 601614 | 8029 | 17p13.1 | Y (100%) | Y (100%) | Y | Serafini et al., 1996; Ren et al., 2007 | ||
| Neuropilin 1 (NRP1) | 602069 | 8004 | 10p12 | Y | Y | Y | Piper et al., 2009 | ||
| Nuclear receptor subfamily 2, group F, member 1 (NR2F1) | 132890 | 7975 | 5q15 | Y (63%) | Y (16%) | Y | Y | Armentano et al., 2006 | |
| Pleckstrin homology domain containing, family B (evectins) member 1 (PLEKHB1) | 607651 | 19079 | 11q13.5-q14.1 | N | Y | N | Y | Bloom et al., 2007; Lewcock et al., 2007; Hendricks and Jesuthasan, 2009 | |
| Ras-related C3 botulinum toxin substrate 1 (rho family, small GTP binding protein Rac1) (RAC1) | 602048 | 9801 | 7p22 | Y (100%) | N | Y | Y | Kassai et al., 2008 | |
| Receptor-like tyrosine kinase (RYK) | 600524 | 10481 | 3q22.2 | N | Y (25%) | N | N | N | Keeble et al., 2006 |
| Roundabout, axon guidance receptor, homolog 1 (Drosophila) | 602430 | 10249 | 3p12.3 | N | Y | Y | Y | N | Andrews et al., 2006; Unni et al., 2012 |
| Roundabout, axon guidance receptor, homolog 1 (Drosophila)/roundabout, axon guidance receptor, homolog 2 (Drosophila) | 602430/ 602431 | 10249/ 10250 | 3p12.3/ 3p12.3 | N | Y (100%) | N | Lopez-Bendito et al., 2007 | ||
| Sema domain, immunoglobulin domain (Ig), short basic domain, secreted, (semaphorin) 3C | 602645 | 10725 | 7q21-q31 | Y | Y | N | N | Niquille et al., 2009 | |
| Serum response factor (c-fos serum response element-binding transcription factor) (SRF) | 600589 | 11291 | 6p | N | Y | Y | Y | Lu and Ramanan, 2011 | |
| Slit homolog 2 (Drosophila) | 603746 | 11086 | 4p15.2 | N | Y (100%) | Y | N | N | Bagri et al., 2002; Unni et al., 2012 |
| Slit homolog 3 (Drosophila) | 603745 | 11087 | 5q35 | N | Y (33%) | Y | Unni et al., 2012 | ||
| ST8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 2 (ST8SIA2) | 602546 | 10870 | 15q26 | N | Y | N | Hildebrandt et al., 2009 | ||
| ST8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 2 (ST8SIA2)/ST8 alpha-N-acetyl- neuraminide alpha-2, 8-sialyltransferase 4 (ST8SIA4) | 602546/ 602547 | 10870/ 10871 | 15q26/ 15q21 | N | Y | Y | Hildebrandt et al., 2009 | ||
| Trio Rho guanine nucleotide exchange factor (TRIO) | 601893 | 12303 | 5p15.2 | N | Y (100%) | Y (100%) | Briancon-Marjollet et al., 2008 | ||
| Uronyl-2-sulfotransferase (UST) | 610752 | 17223 | 6q25.1 | Y (50%) | Y (12.5%) | Y | Merry et al., 2001; Conway et al., 2011 | ||
| Vasodilator-stimulated phosphoprotein (VASP) | 601703 | 12652 | 19q13.32 | Y (100%) | Y | Y (100%) | Y (100%) | Menzies et al., 2004 | |
| GROUP V – Abnormal post-guidance development | |||||||||
| cAMP responsive element binding protein 1 (CREB1) | 123810 | 2345 | 2q33.3 | N | Y | N | Y | Rudolph et al., 1998 | |
| Forkhead box C1 (FOXC1) | 601090 | 3800 | 6p25.3 | Y | Zarbalis et al., 2007 | ||||
| Unclear function in corpus callosum development | |||||||||
| Protein tyrosine phosphatase, receptor type, S (PTPRS) | 601576 | 9681 | 19p13.3 | N | Y (100%) | Meathrel et al., 2002 | |||
| Insulin-like growth factor binding protein 1 (IGFBP1) | 146730 | 5469 | 7p12.3 | N | Y (100%) | Doublier et al., 2000 | |||
aReferences in the table that are not included in the reference list can be found in the Supplementary material.
bFor the purposes of this review, complete ACC (cACC) is defined as a complete absence of all callosal axons, or failure of all callosal axons to cross the midline. Partial ACC (pACC) has therefore been defined to encompass incomplete agenesis, where at least part of the corpus callosum can be identified.
cPhenotype varies with mouse strain.
Y = yes; N = no.
Blank cells signify that the given abnormality was not mentioned by the reference/s.
Axonal growth and fasciculation are dependent on cell adhesion molecules (CAMs), and mutations in a member of the immunoglobulin family of CAMs, L1CAM, cause a broad range of X-linked disorders collectively termed L1 syndrome (Fransen et al., 1995). The phenotypic spectrum of the X-linked L1 syndrome comprises partial ACC, CRASH syndrome (corpus callosum hypoplasia, retardation, adducted thumbs, spasticity and hydrocephalus), MASA syndrome (mental retardation, aphasia, shuffling gait and adducted thumbs), X-linked complicated ACC, X-linked complicated spastic paraplegia type 1, and various hydrocephalus-associated syndromes (Rosenthal et al., 1992; Jouet et al., 1994; Vos et al., 2010). In general, males with L1 syndrome display a phenotype at the severe end of the disorder spectrum, which includes macrocephaly, mental retardation and spastic paraparesis. Other individuals can have ACC with Probst bundles and subcortically projecting tracts in the absence of cortical dysplasia, which is consistent with a role for L1CAM in axonal guidance and growth (Chow et al., 1985; Halliday et al., 1986; Graf et al., 2000). The genotype-phenotype correlations for neurological abnormalities in L1 syndrome are well characterized (Vos et al., 2010), and generally depend on whether homophilic L1CAM interactions or heterophilic interactions are disrupted (De Angelis et al., 1999, 2002; Itoh et al., 2011).
In addition to genetic causes, it is likely that a significant proportion of cases with ACC are caused by environmental insults. One example of this is foetal alcohol spectrum disorders, which can present with either complete or partial ACC, or callosal hypoplasia (Riley et al., 1995). Early exposure to alcohol has been proposed to result in an overall decrease in white matter volume and organization, and structural abnormalities including ACC (Spadoni et al., 2007). Alcohol exposure silences growth cone responses to guidance cues such as SEMA3A and netrin 1, which are involved in corpus callosum development (Sepulveda et al., 2011). These features are similar to the L1 syndrome spectrum; ethanol inhibits L1CAM-mediated cell–cell adhesion (Charness et al., 1994; Ramanathan et al., 1996) and neurite outgrowth (Bearer et al., 1999), suggesting that a comparable axon growth/guidance defect is common to both syndromes.
Abnormal post-guidance development
Synaptogenesis and synaptic specificity are usually achieved by a combination of molecular recognition and activity-dependent signals that prune initially formed synapses. The mechanisms by which callosal axons make specific synaptic connections are likely to be dependent on the origin and target of callosal axons and the functional information that will be transmitted. Andermann syndrome is one of a small group of neurodevelopmental disorders known to result from an ion transporter defect, namely homozygous mutations in SLC12A6 encoding the K–Cl transporter KCC3 (Howard et al., 2002). It is also a member of an interesting group of ACC-associated syndromes that feature nervous system degeneration post-natally. Andermann syndrome has presented in neuroimaging studies as a primary defect in axonal growth/guidance (Dupre et al., 2003). It has been suggested that loss of KCC3 in migrating callosal neurons increases their susceptibility to damage early in development. In support of this hypothesis, homozygous SLC12A6 loss of function mice display callosal hypoplasia, but no specific abnormality in callosal development has been identified (Shekarabi et al., 2012). It may also be the case that activity-dependent mechanisms are one aspect of callosal development in which humans and mice differ.
ACC has been noted in several enzyme deficiencies affecting cellular metabolism, including pyruvate dehydrogenase deficiency (Patel et al., 2012), fumarase deficiency (Coughlin et al., 1998; Mroch et al., 2012), desmosterolosis (Zolotushko et al., 2011) and Smith-Lemli-Opitz syndrome (Garcia et al., 1973; Fierro et al., 1977). The causative link between cellular metabolism disorders and callosal agenesis is unclear, although the majority of callosal abnormalities are hypoplastic, and may be secondary to post-natal CNS development or white matter injury (Bamforth et al., 1988; Weinstein et al., 2003). Deficient cholesterol synthesis, in particular, has been linked with abnormal neurological development. Desmosterolosis results from homozygous or compound heterozygous mutations in DHCR24, and ACC has been reported in all cases where imaging has been performed (Zolotushko et al., 2011). Desmosterolosis shares midline neurological defects with Smith-Lemli-Opitz syndrome, which results from homozygous mutations in DHCR7 (Fitzky et al., 1998; Jira et al., 2003). In addition to its role in myelination, cholesterol is required for post-translational modification of the ventral morphogen SHH (Grover et al., 2011), and therefore has a direct role in neural patterning.
Agenesis of the corpus callosum as a result of copy number variations
Despite the progress made in identifying and characterizing single-gene Mendelian disorders associated with ACC, a clear genetic cause will not be identified in the majority of patients (Bedeschi et al., 2006; Schell-Apacik et al., 2008). Improved and increased use of microarray comparative genomic hybridization has resulted in the identification of multiple rare copy number variants associated with ACC, and this genotype-to-phenotype diagnostic approach has resulted in a series of new recognizable disorder spectrums (Table 4 and Supplementary Table 1). A recent analysis of cytogenic, fluorescence in situ hybridization and microarray studies of 374 patients with reported or confirmed ACC identified many new loci associated with ACC and demonstrated the power of this approach (O'Driscoll et al., 2010).
Table 4.
Major copy number variants associated with ACC in humans
| Candidate genes for ACC | MIM Phenotype number | Callosal abnormality penetrance | Salient features | Referencesa | |
|---|---|---|---|---|---|
| 1p36 deletion | TMEM52, C1ORF222, KIAA1751, GABRD, PRKCZ, SKI | 607872 | 5.8% | Corpus callosum hypoplasia, diffuse white matter reduction, periventricular nodular heterotopia | Neal et al., 2006; Gajecka et al., 2007; Bahi-Buisson et al., 2008; Battaglia et al., 2008; O'Driscoll et al., 2010; Rosenfeld et al., 2010 |
| 1p32-p31 deletion | NFIA | 613735 | 2/6 (33%) | Hypoplasia or agenesis of the corpus callosum, tethered spinal cord, urinary tract defects | Lu et al., 2007; Koehler et al., 2010 |
| 1q42-q44 deletion | AKT3, DISP1, HNRNPU, FAM36A, ZBTB18, NCRNA00201 | 612337 | 12/15 (80%) | Micrognathia, post-natal microcephaly, ACC | De Vries et al., 2001; Tschopp et al., 2005; Boland et al., 2007; Hill et al., 2007; Merritt et al., 2007; van Bon et al., 2008; Thierry et al., 2012; Zaki et al., 2012 |
| 3q24-q25.3 deletion | ZIC1, GSK3B | N/A | Unknown | Not common enough to have a clear cluster of features | O'Driscoll et al., 2010 |
| 4p16.3 deletion (Wolf-Hirschhorn syndrome) | WHSC1, LETM1, TACC3, SLBP, HSPX153, WHSC2, YOL027, MSR7, FGFR3 CPLX1, DGKQ, FGFRL1, CTBP1 | 194190 | 17/24 (71%) | Prenatal and post-natal growth deficiency, developmental disability, characteristic craniofacial features and seizures, microcephaly, dysgenic corpus callosum | Battaglia et al., 1999; Tutunculer et al., 2004; Bergemann et al., 2005; Balci et al., 2006; Righini et al., 2007 |
| 6pter-p24 deletion (6p25 deletion included) | FOXC1, FOXF2, FOXQ1, TUBB2A, TUBB2B | 612582 | Unknown | Hydrocephalus, hypoplasia of the cerebellum, brainstem, and corpus callosum, Dandy-Walker malformation | Nishimura et al., 1998; Maclean et al., 2005; Aldinger et al., 2009; O'Driscoll et al., 2010 |
| 6q2 deletion | MARCKS, MAP3K4, NRE1, ARID1B, UST, TIAM2, SYNJ2 | 612863 | 5/20 (25%); 6q25.2–q25.3 microdeletion: 5/11 (45%) | Periventricular nodular heterotopia, polymicrogyria, cerebellar malformations, hydrocephalus, and ACC | Hopkin et al., 1997; Eash et al., 2005; Sherr et al., 2005; Nagamani et al., 2009 |
| 8p rearrangements | ARHGEF10, FZD3, FGFR1, FGF17, FGF20, NRG1 | N/A | 25%; 66% for mosaic tetrasomy 8p | Varies with rearrangement type; ACC appears commonly, with hydrocephaly | O'Driscoll et al., 2010; Wilson et al., 2010 |
| 9q34.3 deletion (Kleefstra syndrome) | EHMT1 | 610253 | Uncommon (hypoplasia more common) | Ventriculomegaly, microcephaly, abnormal myelination | Schimmenti et al., 1994; Knight et al., 1999; Anderlid et al., 2002; Dawson et al., 2002; Cormier-Daire et al., 2003; Font-Montgomery et al., 2004; Harada et al., 2004; Iwakoshi et al., 2004; Stewart et al., 2004; Yatsenko et al., 2005 |
| 11q23-q25 duplication | NCAM1, ANKK1 | N/A | Unknown | ACC, microcephaly and cerebellar malformations | Pihko et al., 1981; O'Driscoll et al., 2010 |
| 13q14 deletion syndrome | NUFIP1, HTR2A, PCDH8, PCDH17 | 613884 | 1/3 | Hypoplasia of the corpus callosum, retinoblastoma, mental impairment | Caselli et al., 2007; O'Driscoll et al., 2010 |
| 13q32.3-q33.1 deletion | ZIC2, ZIC5, FGF14, TMTC4 | N/A | Unknown | ACC, holoprosencephaly, cerebellum abnormalities | Brown et al., 1993, 1995; Ballarati et al., 2007; O'Driscoll et al., 2010 |
| 13q34 duplication | COL4A1, COL4A2, ARHGEF7, SOX1, ATP11A, MCF2L | N/A | Unknown | ACC, unspecified brain malformations | Witters et al., 2009; O'Driscoll et al., 2010 |
| 14q11-q22 deletion | FOXG1B, ARHGAP5 | 613457 | Unknown | Corpus callosum hypoplasia and abnormal myelination | Schwarzbraun et al., 2004; Ariani et al., 2008; O'Driscoll et al., 2010; Kortum et al., 2011; Torgyekes et al., 2011 |
| 14qter deletion | GARNL1 | N/A | Unknown (hypoplasia more common) | Corpus callosum hypoplasia, polymicrogyria, heterotopia, and microcephaly | Schwarzbraun et al., 2004; Schneider et al., 2008; O'Driscoll et al., 2010; Engels et al., 2012 |
| 17p13.3 deletion (Miller-Dieker lissencephaly syndrome) | LIS1, YWHAE | 613457 | 74% (27 patients) | Lissencephaly, microcephaly, micrognathia, bitemporal narrowing, short nose with upturned nares, protuberant upper lip and a thin vermilion border, severe mental retardation and seizures | Dobyns et al., 1991; Cardoso et al., 2003; Toyo-oka et al., 2003; Nagamani et al., 2009; Bruno et al., 2010 |
| 21q22.3 deletion | DYRK1A | 145410 | Unknown | Microcephaly, pachygyria, polymicrogyria, colpocephaly, corpus callosum hypoplasia and generalized white matter reduction | Moller et al., 2008; O'Driscoll et al., 2010 |
| DiGeorge syndrome (22q11.2 deletion) | TBX1, COMT, UFD1L, RANBP1 | 188400 | Uncommon | Delayed development, tetany, seizures | Kraynack et al., 1999; Maynard et al., 2003 |
| Opitz G/BBB syndrome, autosomal dominant (22q11.2 deletion; Opitz phenotype) | TBX1, COMT, UFDL1, RANBP1 | 145410 | Uncommon | Cerebellar vermal hypoplasia, cortical atrophy, ventriculomegaly, widened cavum septum pellucidum | Robin et al., 1995; 1996; Maynard et al., 2003 |
| Xp21 deletion (Complex glycerol kinase deficiency) | GK, DMD, NR0B1 | 300679 | 2/10 (20%) | Mental retardation, severe developmental delay, hypotonia, seizures and progressive microcephaly | Stanczak et al., 2007 |
| Xq28 duplication syndrome | GDI1, MECP2 | 300815 | Uncommon | Microcephaly, Dandy-Walker malformation, with agenesis of the cerebellar vermis and corpus callosum hypoplasia | Vandewalle et al., 2009 |
aReferences in the table that are not included in the reference list can be found in the Supplementary material.
One of the most notable copy number variants associated with callosal agenesis is 1q42-q44 deletion syndrome, which is strongly associated with ACC of variable severity and post-natal microcephaly (O'Driscoll et al., 2010). The major locus within this region appears to be 1q44, which contains the AKT3 gene. Over 90% of patients with ACC and microcephaly were found to have a disrupted AKT3, a gene shown to promote neuronal survival in mouse models (Tschopp et al., 2005; Boland et al., 2007; Hill et al., 2007; Merritt et al., 2007). Although dysregulation of the PI3K/AKT-signalling pathway may explain the apparent proliferative/apoptotic abnormality, some patients have presented with 1q42-44 deletions outside the AKT3 gene (Poot et al., 2007; van Bon et al., 2008; Malan et al., 2010), suggesting that at least one more neurodevelopmental gene exists within the locus. Haploinsufficiency of other genes, such as DISP1 located in 1q41, has been suggested as a cause of midline developmental defects. In particular, ZBTB18 is a promising candidate, as one patient with post-natal microcephaly and ACC was found to have a reciprocal translocation with a breakpoint between AKT3 and ZBTB18 (Boland et al., 2007; Perlman et al., 2013). In reality, there are likely multiple genes involved, reflecting combined defects in both midline and lateral axis patterning (Filges et al., 2010; O'Driscoll et al., 2010).
In some cases, phenotypic effects of microdeletions or microduplications are likely to result from the disruption of the synergistic action of two or more genes. Miller-Dieker lissencephaly syndrome is a contiguous gene deletion syndrome involving genes within the chromosome 17p13.3 region (Cardoso et al., 2003; Nagamani et al., 2009; Bruno et al., 2010; Mignon-Ravix et al., 2010). Miller-Dieker syndrome is characterized by a combination of classic lissencephaly, microcephaly, seizures and facial dysmorphisms, and is more severe than isolated lissencephaly. In both isolated lissencephaly and Miller-Dieker syndrome, the LIS1 gene is affected, and the more severe phenotype in Miller-Dieker syndrome has been attributed to deletion of the YWHAE gene distal to LIS1 (Bruno et al., 2010). Both genes are involved in neuronal migration, and interact indirectly through the CDK5 substrate NDEL1 (Niethammer et al., 2000; Toyo-oka et al., 2003). Interestingly, patients with 17p13.3 microduplications present within the autistic spectrum, which is more severe when LIS1, but not YWHAE, is duplicated, suggesting that interactions between the proteins are related to the pathogenesis of the syndrome (Bruno et al., 2010).
8p rearrangements are frequently associated with brain malformations (Robinow et al., 1989; Newton et al., 1993; Schrander-Stumpel et al., 1994; Winters et al., 1995; O'Driscoll et al., 2010). The 8p inverted duplication/deletion is one of the most common and results in brain malformations including ACC and speech problems (O'Driscoll et al., 2010). Fifteen cases of mosaic tetrasomy of 8p have also been described, of which ACC was identified in 10 (Wilson et al., 2010). A recent review of the imaging literature confirmed ACC in 25% of published 8p rearrangements reported with callosal agenesis (O'Driscoll et al., 2010), although variations in penetrance exist depending on the type of rearrangement. As ACC is apparently most common in inversion duplication/deletions (O'Driscoll et al., 2010), it is likely that at least two loci exist, one that contributes to ACC when deleted, and another that contributes to ACC when duplicated. This explanation is supported by the recent description of ACC in two patients with 8p duplications only (Nieh et al., 2012; Sajan et al., 2013).
1p36 deletion syndrome (monosomy 1p36) is one of the most common chromosome deletions (incidence is 1 in 5000), but has a relatively low penetrance of ACC (5.8%; Table 3) (Gajecka et al., 2007; Bahi-Buisson et al., 2008; Battaglia et al., 2008). The phenotypic diversity of this syndrome and apparent lack of genotype-phenotype correlations illustrate the complexity of contiguous gene syndromes. Common neurological features include pachygyria, polymicrogyria, hydrocephalus and ACC (Gajecka et al., 2007; Battaglia et al., 2008). It has been suggested that haploinsufficiency of functionally unrelated but contiguous genes is responsible for some phenotypic variability (Redon et al., 2005; Rosenfeld et al., 2010); however, the expression of long-distance genes may also be affected through a positional effect of the deletion (Giannikou et al., 2012). Epigenetic and modifier factors may contribute to the phenotype, and herein lies a major difficulty in pinpointing causative genes in contiguous deletions. The haploinsufficiency of one gene in 1p36 deletions, SKI, is of particular interest for ACC (Colmenares et al., 2002; Rosenfeld et al., 2010) as it was recently reported to functionally interact with SATB2 to specify callosally projecting neuron identity (Baranek et al., 2012).
Agenesis of the corpus callosum syndromes of unknown aetiology
Several ACC syndromes are yet to have causative genetic mutations identified (Table 5). Confirming the underlying genetic cause of inheritable syndromes is complicated by the high incidence of de novo mutations, genetic heterogeneity and difficulties achieving consistent clinical diagnosis.
Table 5.
ACC syndromes for which a causative gene has not been identified
| Inheritance | MIM Phenotype number | ACC penetrance | Salient features | Referencesa | |
|---|---|---|---|---|---|
| Aicardi syndrome | X-linked dominant | 304050 | 100% | Microcephaly, periventricular and subcortical heterotopia, ACC | Aicardi et al., 1965; Donnenfeld et al., 1989; Smith et al., 1996; Yamagata et al., 1990; Barkovich et al., 2001; Palmer et al., 2006; Hopkins et al., 2008 |
| Cerebrofrontofacial syndrome | Unknown | 608578 | Unknown (few clearly delineated cases) | Grey matter heterotopia, white matter cysts, | Guion-Almeida and Richieri-Costa, 1992, 2001; Masuno et al., 2000; Winter, 2001 |
| Craniosynostosis-mental retardation syndrome of Lin and Gettig | Unknown | 218649 | 3/3 (100%) | ACC, Chiari malformation type I | Lin and Gettig, 1990; Hedera and Innis, 2002 |
| Curry-Jones syndrome | Unknown | 601707 | 5/9 (56%) | ACC, polysyndactyly, skin defects | Temple et al., 1995; Mingarelli et al., 1999; Thomas et al., 2006; Grange et al., 2008 |
| Ectodermal dysplasia, hypohidrotic, with hypothyroidism and agenesis of the corpus callosum | X-linked recessive^ | 225040 | 3/3 (100%) | ACC, primary hypothyroidism, hypohidrotic ectodermal dysplasia | Fryns et al., 1989; Soekarman and Fryns, 1993; Devriendt et al., 1996 |
| Fryns syndrome | Autosomal recessive | 229850 | 13.5% | Congenital diaphragmatic hernia, distal limb hypoplasia, pulmonary hypoplasia | Pinar et al., 1994; Neville et al., 2002; Slavotinek, 2004; Ludmiła et al., 2010 |
| Hartsfield syndrome | X-linked inheritance | 300571 | 3/11 (27%) | Holoprosencephaly, etrodactyly, cleft lip/palate, complete or partial ACC | Imaizumi et al., 1998; Corona-Rivera et al., 2000; Vilain et al., 2009; Zechi-Ceide et al., 2009 |
| Ivemark syndrome | Autosomal recessive | 208530 | Unknown | Asplenia, cardiovascular anomalies, ACC, malposition and maldevelopment of the abdominal organs | Kiuchi et al., 1988; Rodriguez et al., 1991; Devriendt et al., 1997; Noack et al., 2002 |
| Marden-Walker syndrome | Autosomal recessive^ | 248700 | Unknown | Microcephaly, hydrocephaly, ACC, cerebellar vermis hypoplasia, enlarged cisterna magna | Begum and Nayek, 2002; Ozbek et al., 2005; Theys et al., 2011 |
| Macrocephaly with multiple epiphyseal dysplasia and distinctive facies | Autosomal recessive^ | 607131 | 2/4 (50%) | Dysmorphic facies, genu valgum, and swelling of the joints | al-Gazali and Bakalinova, 1998 |
| Neu-Laxova Syndrome | Autosomal recessive | 256520 | 24/71 (34%) | Microcephaly, lissencephaly, cerebellar hypoplasia and ACC | Manning et al., 2004; Ugras et al., 2006; Coto-Puckett et al., 2010 |
| Oculocerebrocutaneous syndrome | Unclear | 164180 | 10/10 (100%) | Orbital cyst, ACC, frontal polymicrogyria, periventricular nodular heterotopia, ventriculomegaly or hydrocephalus | Moog et al., 2005 |
| Pai syndrome | Autosomal dominant^ | 155145 | 8/16 (50%) | Nasal cleft, facial skin polyps and CNS lipomas; ACC | Pai et al., 1987; Preece et al., 1988; Morgan and Evans, 1990; Rudnik-Schoneborn and Zerres, 1994; Mishima et al., 1999; Al-Mazrou et al., 2001; Coban et al., 2003; Castori et al., 2007; Guion-Almeida et al., 2007; Vaccarella et al., 2008 |
| Sakoda complex | Unknown (likely X-linked) | 610871 | 24/24 (100%) | Sphenoethmoidal encephalomeningocele, complete or partial ACC | Sakoda et al., 1979; Ehara et al., 1998; Dempsey et al., 2007 |
| Shapiro syndrome | Unknown | N/A | Defining feature | Recurrent episodes of hypothermia, hyperhidrosis, ACC | Shapiro et al., 1969; Tambasco et al., 2005; Shenoy, 2008 |
| Toriello-Carey syndrome | Autosomal recessive^ | 217980 | 100% | Mental retardation, ACC, post-natal growth delay, cardiac defects, distal limb defects, micrognathia, microcephaly, facial abnormalities, | Toriello et al., 2003; McGoey et al., 2010 |
| Lissencephaly type III with bone dysplasia | Autosomal recessive^ | 601160 | 4/7 (57%) | ACC and vermis agenesis, lissencephaly, hypoplastic brainstem, cystic cerebellum, ventriculomegaly and multicystic periventricular lesions | Encha Razavi et al., 1996; Plauchu et al., 2001 |
aReferences in the table that are not included in the reference list can be found in the Supplementary material.
Many of these syndromes are of interest because of the diversity of organ systems affected, which may allude to their underlying genetic aetiology. Curry-Jones syndrome is a rare disorder associated with ACC and ventriculomegaly, polysyndactyly, eye defects and malformations of the skin and gastrointestinal tract (Temple et al., 1995). Importantly, the association of this syndrome with the development of skin and CNS neoplasias has implicated the SHH signalling pathway in its pathogenesis. In addition, the defects in limb development seen in patients with Curry-Jones syndrome are similar to those reported in patients with confirmed mutations in the SHH signalling pathway (Johnston et al., 2005). If nothing else, Curry-Jones syndrome illustrates the necessity of investigating multiple organ systems if ACC is identified, as it often serves as a relatively easily identifiable phenotypic marker for wider developmental disturbances.
Aicardi syndrome is another multisystem disorder with a complex neurological phenotype, and is only observed in females (and XXY males). Neurological features incorporate severely disordered neuronal migration, ACC, infantile spasms and chorioretinal lacunae (Aicardi et al., 1965; Hopkins et al., 2008; Fig. 8). The interhemispheric and intrahemispheric mis-wirings that result from aberrant neuronal migration are profound. Diffusion tensor imaging has shown widespread disruption of corticocortical tracts not replicated in matched subjects with callosal agenesis and cortical malformations (Wahl et al., 2010). Pachygria and periventricular and subcortical heterotopias are consistent with an interruption of radial neuronal migration, although the extent of corticocortical disorganization suggests that the neuronal migration defect is almost universal. The presence of type 2 interhemispheric cysts in some patients is intriguing, and may be secondary to failure of midline formation resulting from a related abnormality in migration and positioning of midline glial and neuronal populations. Given the widespread migration defects, it seems unlikely that the formation of Probst bundles in Aicardi syndrome is adaptive or compensatory, but rather suggests that they may represent multiple aetiologies and functions that differ depending on the developmental processes that are disturbed.
Figure 8.
Associated malformations commonly seen in patients with ACC. (A) T1-weighted axial MRI scan showing complete ACC associated with a third ventricle cyst (asterisk) and periventricular nodular heterotopia (arrowheads). (B) T2-weighted axial MRI scan showing ACC (asterisk) associated with polymicrogyria (PMG) (arrowheads) and copolcephaly (+). (C) T2-weighted axial MRI scan showing ACC with subcortical heterotopia (SCH) (arrowheads) and marked asymmetry of the cerebral hemispheres. Midsagittal (D) and axial (E) T1-weighted MRI scan of a patient with Aicardi syndrome revealing a constellation of neuroradiological features, including complete ACC (arrow), grey matter heterotopia (white arrowhead), cystic dilation of the left lateral ventricle (asterisk) and enlarged fourth ventricle (+). In addition, there is marked asymmetry of the cerebral hemispheres.
Increased use of array comparative genomic hybridization has highlighted the genetic heterogeneity of disorders such as Toriello-Carey syndrome, which has ACC as a defining feature. It is possible that several syndromes previously considered distinct are in fact a cluster of clinical features that are aetiologically unrelated. In Toriello-Carey syndrome, microdeletions at 22q12 (Hatchwell et al., 2007; Said et al., 2011) and 1q42 (Hatchwell et al., 2007), an unbalanced translocation t(8;18)(p12;q22) (Martin-Denavit et al., 2004), and a cryptic translocation t(10q;16p) (Martin et al., 2002) have all been reported to produce a similar phenotype. These diverse findings may also be an artefact of the difficulties in diagnosing a complex syndrome based on clinical features alone.
Conclusion
ACC remains one of the most complicated neurological birth defects described, given the sheer number of developmental processes that may be disrupted. As a corollary, callosal agenesis rarely occurs in isolation, and is a specific and relatively easy-to-detect phenotypic marker for developmental disorders. Mouse models have vastly improved our understanding of the mechanisms of normal corpus callosum formation, and have paved the way for a developmental classification system based on the clinical and genetic features of human ACC syndromes.
Callosal development can be affected by the disruption of neurogenesis, telencephalic midline patterning, neuronal migration and specification, axon guidance and post-guidance development. Recent genetic studies have identified an abundance of copy number variations and single gene mutations in patients with ACC, but have also highlighted the underlying genetic complexity of many ACC syndromes. Meanwhile, continually improving neuroimaging data are allowing us to understand how genetic mutations affect brain connectivity, and in turn how the brain responds to developmental perturbations. These approaches have, in combination with animal models, improved our understanding of the mechanisms involved in callosal agenesis, and may pave the way for future therapies tailored towards individual patients.
Supplementary Material
Acknowledgements
We thank Ilse Buttiens for illustration and preparation of the figures and Rowan Tweedale for reading and editing the text.
Glossary
Abbreviations
- ACC
agenesis of the corpus callosum
- MCPH
autosomal recessive primary microcephaly
Funding
This work was supported by the National Health and Medical Research Council (NHMRC) [grant numbers APP1048849 and APP1043045 and Principal Research Fellowship to L.J.R.]; Drs. Sherr and Barkovich were supported in part by a grant from the National Institutes of Health/National Institute of Neurological Disorders and Stroke R01NS058721 and the University of Queensland (Summer Research Scholarship) [T.J.E.]. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NHMRC.
Supplementary material
Supplementary material is available at Brain online.
References
- Aicardi J, Lefebvre J, Lerique-Koechlin A. A new syndrome: spasms in flexion, callosal agenesis, ocular abnormalities. Electroencephalogr Clin Neurophysiol. 1965;19:609–10. [Google Scholar]
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, Grosschedl R, et al. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–77. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Alkuraya FS, Cai X, Emery C, Mochida GH, Al-Dosari MS, Felie JM, et al. Human mutations in NDE1 cause extreme microcephaly with lissencephaly [corrected] Am J Hum Genet. 2011;88:536–47. doi: 10.1016/j.ajhg.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, Mori S, et al. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–52. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–21. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Ashwell KW, Waite PM, Marotte L. Ontogeny of the projection tracts and commissural fibres in the forebrain of the tammar wallaby (Macropus eugenii): timing in comparison with other mammals. Brain Behav Evol. 1996;47:8–22. doi: 10.1159/000113225. [DOI] [PubMed] [Google Scholar]
- Baala L, Briault S, Etchevers HC, Laumonnier F, Natiq A, Amiel J, et al. Homozygous silencing of T-box transcription factor EOMES leads to microcephaly with polymicrogyria and corpus callosum agenesis. Nat Genet. 2007;39:454–6. doi: 10.1038/ng1993. [DOI] [PubMed] [Google Scholar]
- Bahi-Buisson N, Guttierrez-Delicado E, Soufflet C, Rio M, Daire VC, Lacombe D, et al. Spectrum of epilepsy in terminal 1p36 deletion syndrome. Epilepsia. 2008;49:509–15. doi: 10.1111/j.1528-1167.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- Bamforth F, Bamforth S, Poskitt K, Applegarth D, Hall J. Abnormalities of corpus callosum in patients with inherited metabolic diseases. Lancet. 1988;2:451. doi: 10.1016/s0140-6736(88)90437-0. [DOI] [PubMed] [Google Scholar]
- Baranek C, Dittrich M, Parthasarathy S, Bonnon CG, Britanova O, Lanshakov D, et al. Protooncogene Ski cooperates with the chromatin-remodeling factor Satb2 in specifying callosal neurons. Proc Natl Acad Sci USA. 2012;109:3546–51. doi: 10.1073/pnas.1108718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135 (Pt 5):1348–69. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Kjos BO. Normal postnatal development of the corpus callosum as demonstrated by MR imaging. AJNR Am J Neuroradiol. 1988;9:487–91. [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Lyon G, Evrard P. Formation, maturation, and disorders of white matter. AJNR Am J Neuroradiol. 1992;13:447–61. [PMC free article] [PubMed] [Google Scholar]
- Barr MS, Corballis MC. The role of the anterior commissure in callosal agenesis. Neuropsychology. 2002;16:459–71. [PubMed] [Google Scholar]
- Battaglia A, Hoyme HE, Dallapiccola B, Zackai E, Hudgins L, McDonald-McGinn D, et al. Further delineation of deletion 1p36 syndrome in 60 patients: a recognizable phenotype and common cause of developmental delay and mental retardation. Pediatrics. 2008;121:404–10. doi: 10.1542/peds.2007-0929. [DOI] [PubMed] [Google Scholar]
- Bearer CF, Swick AR, O'Riordan MA, Cheng G. Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells. J Biol Chem. 1999;274:13264–70. doi: 10.1074/jbc.274.19.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedeschi MF, Bonaglia MC, Grasso R, Pellegri A, Garghentino RR, Battaglia MA, et al. Agenesis of the corpus callosum: clinical and genetic study in 63 young patients. Pediatr Neurol. 2006;34:186–93. doi: 10.1016/j.pediatrneurol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Benadiba C, Magnani D, Niquille M, Morle L, Valloton D, Nawabi H, et al. The ciliogenic transcription factor RFX3 regulates early midline distribution of guidepost neurons required for corpus callosum development. PLoS Genet. 2012;8:e1002606. doi: 10.1371/journal.pgen.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse L, Neti M, Anselme I, Gerhardt C, Ruther U, Laclef C, et al. Primary cilia control telencephalic patterning and morphogenesis via Gli3 proteolytic processing. Development. 2011;138:2079–88. doi: 10.1242/dev.059808. [DOI] [PubMed] [Google Scholar]
- Boland E, Clayton-Smith J, Woo VG, McKee S, Manson FD, Medne L, et al. Mapping of deletion and translocation breakpoints in 1q44 implicates the serine/threonine kinase AKT3 in postnatal microcephaly and agenesis of the corpus callosum. Am J Hum Genet. 2007;81:292–303. doi: 10.1086/519999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau D, Toutain A, Laquerriere A, Marret S, Saugier-Veber P, Barthez MA, et al. X-linked lissencephaly with absent corpus callosum and ambiguous genitalia (XLAG): clinical, magnetic resonance imaging, and neuropathological findings. Ann Neurol. 2002;51:340–9. doi: 10.1002/ana.10119. [DOI] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–92. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Brouns MR, Matheson SF, Hu KQ, Delalle I, Caviness VS, Silver J, et al. The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development. 2000;127:4891–903. doi: 10.1242/dev.127.22.4891. [DOI] [PubMed] [Google Scholar]
- Brown WS, Jeeves MA, Dietrich R, Burnison DS. Bilateral field advantage and evoked potential interhemispheric transmission in commissurotomy and callosal agenesis. Neuropsychologia. 1999;37:1165–80. doi: 10.1016/s0028-3932(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Bruno DL, Anderlid BM, Lindstrand A, van Ravenswaaij-Arts C, Ganesamoorthy D, Lundin J, et al. Further molecular and clinical delineation of co-locating 17p13.3 microdeletions and microduplications that show distinctive phenotypes. J Med Genet. 2010;47:299–311. doi: 10.1136/jmg.2009.069906. [DOI] [PubMed] [Google Scholar]
- Byrd SE, Harwood-Nash DC, Fitz CR. Absence of the corpus callosum: computed tomographic evaluation in infants and children. J Can Assoc Radiol. 1978;29:108–12. [PubMed] [Google Scholar]
- Cacheux V, Dastot-Le Moal F, Kaariainen H, Bondurand N, Rintala R, Boissier B, et al. Loss-of-function mutations in SIP1 Smad interacting protein 1 result in a syndromic Hirschsprung disease. Hum Mol Genet. 2001;10:1503–10. doi: 10.1093/hmg/10.14.1503. [DOI] [PubMed] [Google Scholar]
- Cardoso C, Leventer RJ, Ward HL, Toyo-Oka K, Chung J, Gross A, et al. Refinement of a 400-kb critical region allows genotypic differentiation between isolated lissencephaly, Miller-Dieker syndrome, and other phenotypes secondary to deletions of 17p13.3. Am J Hum Genet. 2003;72:918–30. doi: 10.1086/374320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charness ME, Safran RM, Perides G. Ethanol inhibits neural cell-cell adhesion. J Biol Chem. 1994;269:9304–9. [PubMed] [Google Scholar]
- Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17184–89. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Wang SS, Hattox AM, Rayburn H, Nelson SB, McConnell SK. The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. Proc Natl Acad Sci USA. 2008;105:11382–7. doi: 10.1073/pnas.0804918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew S, Balasubramanian R, Chan W, Kang PB, Andrews C, Webb BO. A novel syndrome caused by the E410K amino acid substitution in the neuronal β-tubulin isotype 3. Brain. 2013;136:522–35. doi: 10.1093/brain/aws345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CW, Halliday JL, Anderson RM, Danks DM, Fortune DW. Congenital absence of pyramids and its significance in genetic diseases. Acta Neuropathol. 1985;65:313–7. doi: 10.1007/BF00687014. [DOI] [PubMed] [Google Scholar]
- Christ A, Christa A, Kur E, Lioubinski O, Bachmann S, Willnow TE, et al. LRP2 is an auxiliary SHH receptor required to condition the forebrain ventral midline for inductive signals. Dev Cell. 2012;22:268–78. doi: 10.1016/j.devcel.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Colmenares C, Heilstedt HA, Shaffer LG, Schwartz S, Berk M, Murray JC, et al. Loss of the SKI proto-oncogene in individuals affected with 1p36 deletion syndrome is predicted by strain-dependent defects in Ski-/- mice. Nat Genet. 2002;30:106–9. doi: 10.1038/ng770. [DOI] [PubMed] [Google Scholar]
- Coughlin EM, Christensen E, Kunz PL, Krishnamoorthy KS, Walker V, Dennis NR, et al. Molecular analysis and prenatal diagnosis of human fumarase deficiency. Mol Genet Metab. 1998;63:254–62. doi: 10.1006/mgme.1998.2684. [DOI] [PubMed] [Google Scholar]
- Dastot-Le Moal F, Wilson M, Mowat D, Collot N, Niel F, Goossens M. ZFHX1B mutations in patients with Mowat-Wilson syndrome. Hum Mutat. 2007;28:313–21. doi: 10.1002/humu.20452. [DOI] [PubMed] [Google Scholar]
- Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4:e315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis E, MacFarlane J, Du JS, Yeo G, Hicks R, Rathjen FG, et al. Pathological missense mutations of neural cell adhesion molecule L1 affect homophilic and heterophilic binding activities. EMBO J. 1999;18:4744–53. doi: 10.1093/emboj/18.17.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis E, Watkins A, Schafer M, Brummendorf T, Kenwrick S. Disease-associated mutations in L1 CAM interfere with ligand interactions and cell-surface expression. Hum Mol Genet. 2002;11:1–12. doi: 10.1093/hmg/11.1.1. [DOI] [PubMed] [Google Scholar]
- Demyanenko GP, Tsai AY, Maness PF. Abnormalities in neuronal process extension, hippocampal development, and the ventricular system of L1 knockout mice. J Neurosci. 1999;19:4907–20. doi: 10.1523/JNEUROSCI.19-12-04907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–72. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- Deuel TA, Liu JS, Corbo JC, Yoo SY, Rorke-Adams LB, Walsh CA. Genetic interactions between doublecortin and doublecortin-like kinase in neuronal migration and axon outgrowth. Neuron. 2006;49:41–53. doi: 10.1016/j.neuron.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Diaz-Horta O, Sirmaci A, Doherty D, Nance W, Arnos K, Pandya A, et al. GPSM2 mutations in Chudley-McCullough syndrome. Am J Med Genet A. 2012;158A:2972–3. doi: 10.1002/ajmg.a.35636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djarmati-Westenberger A, Bruggemann N, Espay AJ, Bhatia KP, Klein C. A novel DCC mutation and genetic heterogeneity in congenital mirror movements. Neurology. 2011;77:1580. doi: 10.1212/WNL.0b013e318230b140. [DOI] [PubMed] [Google Scholar]
- Dode C, Levilliers J, Dupont JM, De Paepe A, Le Du N, Soussi-Yanicostas N, et al. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet. 2003;33:463–5. doi: 10.1038/ng1122. [DOI] [PubMed] [Google Scholar]
- Doherty D, Chudley AE, Coghlan G, Ishak GE, Innes AM, Lemire EG, et al. GPSM2 mutations cause the brain malformations and hearing loss in Chudley-McCullough syndrome. Am J Hum Genet. 2012;90:1088–93. doi: 10.1016/j.ajhg.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downhill JE, Jr, Buchsbaum MS, Wei T, Spiegel-Cohen J, Hazlett EA, Haznedar MM, et al. Shape and size of the corpus callosum in schizophrenia and schizotypal personality disorder. Schizophr Res. 2000;42:193–208. doi: 10.1016/s0920-9964(99)00123-1. [DOI] [PubMed] [Google Scholar]
- Dudanova I, Klein R. Integration of guidance cues: parallel signaling and crosstalk. Trends Neurosci. 2013;36:295–304. doi: 10.1016/j.tins.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Dupre N, Howard HC, Mathieu J, Karpati G, Vanasse M, Bouchard JP, et al. Hereditary motor and sensory neuropathy with agenesis of the corpus callosum. Ann Neurol. 2003;54:9–18. doi: 10.1002/ana.77777. [DOI] [PubMed] [Google Scholar]
- Elson E, Perveen R, Donnai D, Wall S, Black GC. De novo GLI3 mutation in acrocallosal syndrome: broadening the phenotypic spectrum of GLI3 defects and overlap with murine models. J Med Genet. 2002;39:804–6. doi: 10.1136/jmg.39.11.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, et al. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–51. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, et al. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest. 2008;118:2822–31. doi: 10.1172/JCI34538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fame RM, MacDonald JL, Macklis JD. Development, specification, and diversity of callosal projection neurons. Trends Neurosci. 2011;34:41–50. doi: 10.1016/j.tins.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, et al. Phenotype of mice lacking functional deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Feng Y, Walsh CA. Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron. 2004;44:279–93. doi: 10.1016/j.neuron.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Fierro M, Martinez AJ, Harbison JW, Hay SH. Smith-Lemli-Opitz syndrome: neuropathological and ophthalmological observations. Dev Med Child Neurol. 1977;19:57–62. doi: 10.1111/j.1469-8749.1977.tb08021.x. [DOI] [PubMed] [Google Scholar]
- Filges I, Rothlisberger B, Boesch N, Weber P, Wenzel F, Huber AR, et al. Interstitial deletion 1q42 in a patient with agenesis of corpus callosum: phenotype-genotype comparison to the 1q41q42 microdeletion suggests a contiguous 1q4 syndrome. Am J Med Genet A. 2010;152A:987–93. doi: 10.1002/ajmg.a.33330. [DOI] [PubMed] [Google Scholar]
- Finger JH, Bronson RT, Harris B, Johnson K, Przyborski SA, Ackerman SL. The netrin 1 receptors Unc5h3 and Dcc are necessary at multiple choice points for the guidance of corticospinal tract axons. J Neurosci. 2002;22:10346–56. doi: 10.1523/JNEUROSCI.22-23-10346.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Ryan SB, Dobyns WB. Mechanisms of interhemispheric transfer and patterns of cognitive function in acallosal patients of normal intelligence. Arch Neurol-Chicago. 1992;49:271–7. doi: 10.1001/archneur.1992.00530270085023. [DOI] [PubMed] [Google Scholar]
- Fitzky BU, Witsch-Baumgartner M, Erdel M, Lee JN, Paik YK, Glossmann H, et al. Mutations in the Delta7-sterol reductase gene in patients with the Smith-Lemli-Opitz syndrome. Proc Natl Acad Sci USA. 1998;95:8181–6. doi: 10.1073/pnas.95.14.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill T, Donahoo AL, Douglass A, Zalucki O, Yuan J, Shu T, et al. Netrin-DCC signaling regulates corpus callosum formation through attraction of pioneering axons and by modulating slit2-mediated repulsion. Cereb Cortex. 2013 doi: 10.1093/cercor/bhs395. in press. [DOI] [PubMed] [Google Scholar]
- Fransen E, Lemmon V, Van Camp G, Vits L, Coucke P, Willems PJ. CRASH syndrome: clinical spectrum of corpus callosum hypoplasia, retardation, adducted thumbs, spastic paraparesis and hydrocephalus due to mutations in one single gene, L1. Eur J Hum Genet. 1995;3:273–84. doi: 10.1159/000472311. [DOI] [PubMed] [Google Scholar]
- Friocourt G, Kanatani S, Tabata H, Yozu M, Takahashi T, Antypa M, et al. Cell-autonomous roles of ARX in cell proliferation and neuronal migration during corticogenesis. J Neurosci. 2008;28:5794–805. doi: 10.1523/JNEUROSCI.1067-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajecka M, Mackay KL, Shaffer LG. Monosomy 1p36 deletion syndrome. Am J Med Genet C Semin Med Genet. 2007;145C:346–56. doi: 10.1002/ajmg.c.30154. [DOI] [PubMed] [Google Scholar]
- Garavelli L, Mainardi PC. Mowat-Wilson syndrome. Orphanet J Rare Dis. 2007;2:42. doi: 10.1186/1750-1172-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CA, McGarry PA, Voirol M, Duncan C. Neurological involvement in the Smith-Lemli-Opitz syndrome: clinical and neuropathological findings. Dev Med Child Neurol. 1973;15:48–55. doi: 10.1111/j.1469-8749.1973.tb04865.x. [DOI] [PubMed] [Google Scholar]
- Garel C, Cont I, Alberti C, Josserand E, Moutard ML, Ducou le Pointe H. Biometry of the corpus callosum in children: MR imaging reference data. AJNR Am J Neuroradiol. 2011;32:1436–43. doi: 10.3174/ajnr.A2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghi T, Carletti A, Contro E, Cera E, Falco P, Tagliavini G, et al. Prenatal diagnosis and outcome of partial agenesis and hypoplasia of the corpus callosum. Ultrasound Obstet Gynecol. 2010;35:35–41. doi: 10.1002/uog.7489. [DOI] [PubMed] [Google Scholar]
- Giannikou K, Fryssira H, Oikonomakis V, Syrmou A, Kosma K, Tzetis M, et al. Further delineation of novel 1p36 rearrangements by array-CGH analysis: narrowing the breakpoints and clarifying the “extended” phenotype. Gene. 2012;506:360–8. doi: 10.1016/j.gene.2012.06.060. [DOI] [PubMed] [Google Scholar]
- Glass HC, Shaw GM, Ma C, Sherr EH. Agenesis of the corpus callosum in California 1983–2003: a population-based study. Am J Med Genet A. 2008;146A:2495–500. doi: 10.1002/ajmg.a.32418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Graf WD, Born DE, Shaw DW, Thomas JR, Holloway LW, Michaelis RC. Diffusion-weighted magnetic resonance imaging in boys with neural cell adhesion molecule L1 mutations and congenital hydrocephalus. Ann Neurol. 2000;47:113–7. [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–65. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Grover VK, Valadez JG, Bowman AB, Cooper MK. Lipid modifications of Sonic hedgehog ligand dictate cellular reception and signal response. PloS One. 2011;6:e21353. doi: 10.1371/journal.pone.0021353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenot M. Interhemispheric transfer and agenesis of the corpus callosum. Capacities and limitations of the anterior commissure. Neurochirurgie. 1998;44(suppl 1):113–5. [PubMed] [Google Scholar]
- Halliday J, Chow CW, Wallace D, Danks DM. X linked hydrocephalus: a survey of a 20 year period in Victoria, Australia. J Med Genet. 1986;23:23–31. doi: 10.1136/jmg.23.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula-Jouppi K, Kaminen-Ahola N, Taipale M, Eklund R, Nopola-Hemmi J, Kaariainen H, et al. The axon guidance receptor gene ROBO1 is a candidate gene for developmental dyslexia. PLoS Genet. 2005;1:e50. doi: 10.1371/journal.pgen.0010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka Y, Matsumoto T, Yanagawa Y, Fujisawa H, Murakami F, Masu M. Distinct roles of neuropilin 1 signaling for radial and tangential extension of callosal axons. J Comp Neurol. 2009;514:215–25. doi: 10.1002/cne.22021. [DOI] [PubMed] [Google Scholar]
- Hatchwell E, Wang C, O'Brien JE, Allen W, Pettanati M, Montagna C, Nowak NJ, Toriello HV. Nashville: American College of Medical Genetics meeting; 2007. Application of a novel tiling-path array CGH platform reveals additional genetic heterogeneity in Toriello Carey syndrome. [Google Scholar]
- Hayhurst M, Gore BB, Tessier-Lavigne M, McConnell SK. Ongoing sonic hedgehog signaling is required for dorsal midline formation in the developing forebrain. Dev Neurobiol. 2008;68:83–100. doi: 10.1002/dneu.20576. [DOI] [PubMed] [Google Scholar]
- Hetts SW, Sherr EH, Chao S, Gobuty S, Barkovich AJ. Anomalies of the corpus callosum: an MR analysis of the phenotypic spectrum of associated malformations. AJR Am J Roentgenol. 2006;187:1343–8. doi: 10.2214/AJR.05.0146. [DOI] [PubMed] [Google Scholar]
- Hewitt W. The development of the human corpus callosum. J Anat. 1962;96:355–8. [PMC free article] [PubMed] [Google Scholar]
- Hill AD, Chang BS, Hill RS, Garraway LA, Bodell A, Sellers WR, et al. A 2-Mb critical region implicated in the microcephaly associated with terminal 1q deletion syndrome. Am J Med Genet A. 2007;143A:1692–8. doi: 10.1002/ajmg.a.31776. [DOI] [PubMed] [Google Scholar]
- Hopkins B, Sutton VR, Lewis RA, Van den Veyver I, Clark G. Neuroimaging aspects of Aicardi syndrome. Am J Med Genet A. 2008;146A:2871–8. doi: 10.1002/ajmg.a.32537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi H, Kato Z, Masuno M, Asano T, Nagase T, Yamagishi Y, et al. Neuroradiologic findings in Sotos syndrome. J Child Neurol. 2006;21:614–8. doi: 10.1177/08830738060210071001. [DOI] [PubMed] [Google Scholar]
- Howard HC, Mount DB, Rochefort D, Byun N, Dupre N, Lu J, et al. The K-Cl cotransporter KCC3 is mutant in a severe peripheral neuropathy associated with agenesis of the corpus callosum. Nat Genet. 2002;32:384–92. doi: 10.1038/ng1002. [DOI] [PubMed] [Google Scholar]
- Huang H, Xue R, Zhang J, Ren T, Richards LJ, Yarowsky P, et al. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci. 2009;29:4263–73. doi: 10.1523/JNEUROSCI.2769-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards LJ, et al. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage. 2006;33:27–38. doi: 10.1016/j.neuroimage.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Huang Z. Molecular regulation of neuronal migration during neocortical development. Mol Cell Neurosci. 2009;42:11–22. doi: 10.1016/j.mcn.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Ansermet F, Parnas J. Schizophrenia, neurodevelopment and corpus callosum. Mol Psychiatr. 2003;8:261–74. doi: 10.1038/sj.mp.4001205. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6:955–65. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- Ishizuka K, Kamiya A, Oh EC, Kanki H, Seshadri S, Robinson JF, et al. DISC1-dependent switch from progenitor proliferation to migration in the developing cortex. Nature. 2011;473:92–6. doi: 10.1038/nature09859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Fujisaki K, Watanabe M. Human L1CAM carrying the missense mutations of the fibronectin-like type III domains is localized in the endoplasmic reticulum and degraded by polyubiquitylation. J Neurosci Res. 2011;89:1637–45. doi: 10.1002/jnr.22695. [DOI] [PubMed] [Google Scholar]
- Jira PE, Waterham HR, Wanders RJ, Smeitink JA, Sengers RC, Wevers RA. Smith-Lemli-Opitz syndrome and the DHCR7 gene. Ann Hum Gen. 2003;67:269–80. doi: 10.1046/j.1469-1809.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- Johnston JJ, Olivos-Glander I, Killoran C, Elson E, Turner JT, Peters KF, et al. Molecular and clinical analyses of Greig cephalopolysyndactyly and Pallister-Hall syndromes: robust phenotype prediction from the type and position of GLI3 mutations. Am J Hum Genet. 2005;76:609–22. doi: 10.1086/429346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouet M, Rosenthal A, Armstrong G, MacFarlane J, Stevenson R, Paterson J, et al. X-linked spastic paraplegia (SPG1), MASA syndrome and X-linked hydrocephalus result from mutations in the L1 gene. Nat Genet. 1994;7:402–7. doi: 10.1038/ng0794-402. [DOI] [PubMed] [Google Scholar]
- Juric-Sekhar G, Adkins J, Doherty D, Hevner RF. Joubert sydrome: brain and spinal cord malformations in genotyped cases and implications for neurodevelopmental functions of primary cilia. Acta Neuropathol. 2012;123:695–709. doi: 10.1007/s00401-012-0951-2. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Tomoda T, Chang J, Takaki M, Zhan C, Morita M, et al. DISC1-NDEL1/NUDEL protein interaction, an essential component for neurite outgrowth, is modulated by genetic variations of DISC1. Hum Mol Genet. 2006;15:3313–23. doi: 10.1093/hmg/ddl407. [DOI] [PubMed] [Google Scholar]
- Kanatani S, Tabata H, Nakajima K. Neuronal migration in cortical development. J Child Neurol. 2005;20:274–9. doi: 10.1177/08830738050200040201. [DOI] [PubMed] [Google Scholar]
- Kang S, Graham JM, Jr, Olney AH, Biesecker LG. GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat Genet. 1997;15:266–8. doi: 10.1038/ng0397-266. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Kantarci S, Al-Gazali L, Hill RS, Donnai D, Black GC, Bieth E, et al. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat Genet. 2007;39:957–9. doi: 10.1038/ng2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara S, Jissendi-Tchofo P, Barkovich AJ. Developmental differences of the major forebrain commissures in lissencephalies. AJNR Am J Neuroradiol. 2010;31:1602–7. doi: 10.3174/ajnr.A2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Das S, Petras K, Kitamura K, Morohashi K, Abuelo DN, et al. Mutations of ARX are associated with striking pleiotropy and consistent genotype-phenotype correlation. Hum Mutat. 2004;23:147–59. doi: 10.1002/humu.10310. [DOI] [PubMed] [Google Scholar]
- Kelberman D, Rizzoti K, Avilion A, Bitner-Glindzicz M, Cianfarani S, Collins J, et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest. 2006;116:2442–55. doi: 10.1172/JCI28658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier EL, Truwit CL. The normal and abnormal genu of the corpus callosum: an evolutionary, embryologic, anatomic, and MR analysis. AJNR Am J Neuroradiol. 1996;17:1631–41. [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32:359–69. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- Koester SE, O’Leary DD. Axons of early generated neurons in cingulate cortex pioneer the corpus callosum. J Neurosci. 1994;14:6608–20. doi: 10.1523/JNEUROSCI.14-11-06608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Higginbotham H, Poon T, Tanaka T, Brinkman BC, Gleeson JG. Doublecortin maintains bipolar shape and nuclear translocation during migration in the adult forebrain. Nat Neurosci. 2006a;9:779–86. doi: 10.1038/nn1704. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Tanaka T, Gleeson JG. Doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron. 2006b;49:55–66. doi: 10.1016/j.neuron.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, et al. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- Kowalczyk T, Pontious A, Englund C, Daza RA, Bedogni F, Hodge R, et al. Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex. 2009;19:2439–50. doi: 10.1093/cercor/bhn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurotaki N, Imaizumi K, Harada N, Masuno M, Kondoh T, Nagai T, et al. Haploinsufficiency of NSD1 causes Sotos syndrome. Nat Genet. 2002;30:365–6. doi: 10.1038/ng863. [DOI] [PubMed] [Google Scholar]
- Lamminmaki S, Massinen S, Nopola-Hemmi J, Kere J, Hari R. Human ROBO1 regulates interaural interaction in auditory pathways. J Neurosci. 2012;32:966–71. doi: 10.1523/JNEUROSCI.4007-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau YC, Hinkley LB, Bukshpun P, Strominger ZA, Wakahiro ML, Baron-Cohen S, et al. Autism traits in individuals with agenesis of the corpus callosum. J Autism Dev Disord. 2013;43:1106–18. doi: 10.1007/s10803-012-1653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Gleeson JG. Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurol. 2011;24:98–105. doi: 10.1097/WCO.0b013e3283444d05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lent R, Uziel D, Baudrimont M, Fallet C. Cellular and molecular tunnels surrounding the forebrain commissures of human fetuses. J Comp Neurol. 2005;483:375–82. doi: 10.1002/cne.20427. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr, He M, Ocbina PJ, Anderson KV. Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proc Natl Acad Sci USA. 2009;106:13377–82. doi: 10.1073/pnas.0906944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall C, Fothergill T, Richards LJ. Commissure formation in the mammalian forebrain. Curr Opin Neurobiol. 2007;17:3–14. doi: 10.1016/j.conb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Schalomon PM, Roy M, Zacharias MC, Pimenta J, Lent R, et al. Increased axon number in the anterior commissure of mice lacking a corpus callosum. Exp Neurol. 1997;146:491–501. doi: 10.1006/exnr.1997.6564. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthy human brain. J Neurosci. 2010;30:10985–90. doi: 10.1523/JNEUROSCI.5122-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, O'Leary DD. Axon retraction and degeneration in development and disease. [Review]. Annu Rev Neurosci. 2005;28:127–56. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Magnani D, Hasenpusch-Theil K, Benadiba C, Yu T, Basson MA, Price DJ, et al. Gli3 controls corpus callosum formation by positioning midline guideposts during telencephalic patterning. Cereb Cortex. 2012;24:186–98. doi: 10.1093/cercor/bhs303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood S, Ahmad W, Hassan MJ. Autosomal Recessive Primary Microcephaly (MCPH): clinical manifestations, genetic heterogeneity and mutation continuum. Orphanet J Rare Dis. 2011;6:39. doi: 10.1186/1750-1172-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan V, Rajan D, Thomas S, Shaw AC, Louis Dit Picard H, Layet V, et al. Distinct effects of allelic NFIX mutations on nonsense-mediated mRNA decay engender either a Sotos-like or a Marshall-Smith syndrome. Am J Hum Genet. 2010;87:189–98. doi: 10.1016/j.ajhg.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–31. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcorelles P, Laquerriere A. Neuropathology of holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C:109–19. doi: 10.1002/ajmg.c.30249. [DOI] [PubMed] [Google Scholar]
- Martin-Denavit T, Till M, Plauchu H. Toriello-Carey syndrome and unbalanced translocation t(8;18)(p12;q22) Am J Med Genet A. 2004;128A:219–21. doi: 10.1002/ajmg.a.30043. [DOI] [PubMed] [Google Scholar]
- Martin CL, Waggoner DJ, Wong A, Uhrig S, Roseberry JA, Hedrick JF, et al. Molecular rulers for calibrating phenotypic effects of telomere imbalance. J Med Genet. 2002;39:734–40. doi: 10.1136/jmg.39.10.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe MJ, Gaston-Massuet C, Tziaferi V, Gregory LC, Alatzoglou KS, Signore M, et al. Novel FGF8 mutations associated with recessive holoprosencephaly, craniofacial defects, and hypothalamo-pituitary dysfunction. J Clin Endorinol Metab. 2011;96:E1709–18. doi: 10.1210/jc.2011-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Feret H, Nah HD, Bartlett SP, Whitaker LA, Zackai EH. Metopic craniosynostosis due to mutations in GLI3: a novel association. Am J Med Genet A. 2010;152A:1654–60. doi: 10.1002/ajmg.a.33495. [DOI] [PubMed] [Google Scholar]
- Melo DG, Acosta AX, Salles MA, Pina-Neto JM, Castro JD, Santos AC. Sotos syndrome (cerebral gigantism): analysis of 8 cases. Arq Neuropsiquiatr. 2002;60:234–8. doi: 10.1590/s0004-282x2002000200009. [DOI] [PubMed] [Google Scholar]
- Mendes SW, Henkemeyer M, Liebl DJ. Multiple Eph receptors and B-class ephrins regulate midline crossing of corpus callosum fibers in the developing mouse forebrain. J Neurosci. 2006;26:882–92. doi: 10.1523/JNEUROSCI.3162-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt JL, II, Zou Y, Jalal SM, Michels VV. Delineation of the cryptic 1qter deletion phenotype. Am J Med Genet A. 2007;143:599–603. doi: 10.1002/ajmg.a.31611. [DOI] [PubMed] [Google Scholar]
- Mignon-Ravix C, Cacciagli P, El-Waly B, Moncla A, Milh M, Girard N, et al. Deletion of YWHAE in a patient with periventricular heterotopias and pronounced corpus callosum hypoplasia. J Med Genet. 2010;47:132–6. doi: 10.1136/jmg.2009.069112. [DOI] [PubMed] [Google Scholar]
- Miyata R, Hayashi M, Miyai K, Akashi T, Kato M, Kohyama J. Analysis of the hypothalamus in a case of X-linked lissencephaly with abnormal genitalia (XLAG) Brain Dev. 2009;31:456–60. doi: 10.1016/j.braindev.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Moldrich RX, Gobius I, Pollak T, Zhang J, Ren T, Brown L, et al. Molecular regulation of the developing commissural plate. J Comp Neurol. 2010;518:3645–61. doi: 10.1002/cne.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–31. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Fame RM, MacDonald JL, MacQuarrie KL, Macklis JD. Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J Neurosci. 2009;29:12343–54. doi: 10.1523/JNEUROSCI.6108-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutard ML, Kieffer V, Feingold J, Kieffer F, Lewin F, Adamsbaum C, et al. Agenesis of corpus callosum: prenatal diagnosis and prognosis. Childs Nerv Syst. 2003;19:471–476. doi: 10.1007/s00381-003-0781-6. [DOI] [PubMed] [Google Scholar]
- Mowat DR, Wilson MJ, Goossens M. Mowat-Wilson syndrome. J Med Genet. 2003;40:305–10. doi: 10.1136/jmg.40.5.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroch AR, Laudenschlager M, Flanagan JD. Detection of a novel FH whole gene deletion in the propositus leading to subsequent prenatal diagnosis in a sibship with fumarase deficiency. Am J Med Genet A. 2012;158A:155–8. doi: 10.1002/ajmg.a.34344. [DOI] [PubMed] [Google Scholar]
- Nagamani SC, Zhang F, Shchelochkov OA, Bi W, Ou Z, Scaglia F, et al. Microdeletions including YWHAE in the Miller-Dieker syndrome region on chromosome 17p13.3 result in facial dysmorphisms, growth restriction, and cognitive impairment. J Med Genet. 2009;46:825–33. doi: 10.1136/jmg.2009.067637. [DOI] [PubMed] [Google Scholar]
- Nakata Y, Barkovich AJ, Wahl M, Strominger Z, Jeremy RJ, Wakahiro M, et al. Diffusion abnormalities and reduced volume of the ventral cingulum bundle in agenesis of the corpus callosum: a 3T imaging study. AJNR Am J Neuroradiol. 2009;30:1142–8. doi: 10.3174/ajnr.A1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse I, Ueta E, Sumino Y, Ogawa M, Ishikiriyama S. Birth defects caused by mutations in human GLI3 and mouse Gli3 genes. Congenit Anom (Kyoto) 2010;50:1–7. doi: 10.1111/j.1741-4520.2009.00266.x. [DOI] [PubMed] [Google Scholar]
- Newton D, Hammond L, Wiley J, Kushnick T. Mosaic tetrasomy 8p. Am J Med Genet. 1993;46:513–6. doi: 10.1002/ajmg.1320460510. [DOI] [PubMed] [Google Scholar]
- Nieh SE, Fernandez L, Sajan S, Rider E, Bukshpun P, Wakahiro M, et al. Copy number variations and agenesis of the corpus callosum, narrowing the recurrent 8p duplication interval (2389) Presented at the 62nd Annual Meeting of The American Society of Human Genetics, 2012. San Francisco, California. [Google Scholar]
- Niethammer M, Smith DS, Ayala R, Peng J, Ko J, Lee MS, et al. NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron. 2000;28:697–711. doi: 10.1016/s0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Niquille M, Garel S, Mann F, Hornung JP, Otsmane B, Chevalley S, et al. Transient neuronal populations are required to guide callosal axons: a role for semaphorin 3C. PLoS Biol. 2009;7:e1000230. doi: 10.1371/journal.pbio.1000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niquille M, Minocha S, Hornung J, Ruger N, Valloton D, Kessaris N, et al. Two specific populations of GABAergic neurons originating from the medial and the caudal ganglionic eminences aid in proper navigation of callosal axons. Dev Neurobiol. 2013;73:647–72. doi: 10.1002/dneu.22075. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–20. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–44. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Novarino G, Akizu N, Gleeson JG. Modeling human disease in humans: the ciliopathies. Cell. 2011;147:70–9. doi: 10.1016/j.cell.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll MC, Black GC, Clayton-Smith J, Sherr EH, Dobyns WB. Identification of genomic loci contributing to agenesis of the corpus callosum. Am J Med Genet A. 2010;152A:2145–59. doi: 10.1002/ajmg.a.33558. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–69. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Okada T, Okumura Y, Motoyama J, Ogawa M. FGF8 signaling patterns the telencephalic midline by regulating putative key factors of midline development. Dev Biol. 2008;320:92–101. doi: 10.1016/j.ydbio.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Osbun N, Li J, O'Driscoll MC, Strominger Z, Wakahiro M, Rider E, et al. Genetic and functional analyses identify DISC1 as a novel callosal agenesis candidate gene. Am J Med Genet A. 2011;155A:1865–76. doi: 10.1002/ajmg.a.34081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorkowski AR, Keppler-Noreuli K, Robinson L, Sullivan C, Sullivan C, Sajan S, et al. Deletion 16p13.11 uncovers NDE1 mutations on the non-deleted homolog and extends the spectrum of severe microcephaly to include fetal brain disruption. Am J Med Genet A. 2013;161A:1523–30. doi: 10.1002/ajmg.a.35969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini D, Pastore G, Cavallaro A, Massaro M, Nappi C. Corpus callosum agenesis in the fetus. Sonographic signs change with advancing gestational age. Ultrasound Obstet Gynecol. 2013;42:687–90. doi: 10.1002/uog.12506. [DOI] [PubMed] [Google Scholar]
- Patel KP, O'Brien TW, Subramony SH, Shuster J, Stacpoole PW. The spectrum of pyruvate dehydrogenase complex deficiency: clinical, biochemical and genetic features in 371 patients. Mol Genet Metab. 2012;106:385–94. doi: 10.1016/j.ymgme.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LK. Developmental malformation of the corpus callosum: a review of typical callosal development and examples of developmental disorders with callosal involvement. J Neurodev Disord. 2011;3:3–27. doi: 10.1007/s11689-010-9059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LK, Van Lancker-Sidtis D, Schieffer D, Dietrich R, Brown WS. Communicative deficits in agenesis of the corpus callosum: nonliteral language and affective prosody. Brain Lang. 2003;85:313–24. doi: 10.1016/s0093-934x(03)00062-2. [DOI] [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, et al. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 2007;8:287–99. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- Perlman SJ, Kulkarni S, Manwaring L, Shinawi M. Haploinsufficiency of ZNF238 is associated with corpus callosum abnormalities in 1q44 deletions. Am J Med Genet A. 2013;161:711–6. doi: 10.1002/ajmg.a.35779. [DOI] [PubMed] [Google Scholar]
- Piper M, Plachez C, Zalucki O, Fothergill T, Goudreau G, Erzurumlu R, et al. Neuropilin 1-Sema signaling regulates crossing of cingulate pioneering axons during development of the corpus callosum. Cereb Cortex. 2009;19(Suppl 1):i11–21. doi: 10.1093/cercor/bhp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier K, Keays DA, Francis F, Saillour Y, Bahi N, Manouvrier S, et al. Large spectrum of lissencephaly and pachygyria phenotypes resulting from de novo missense mutations in tubulin alpha 1A (TUBA1A) Hum Mutat. 2007;28:1055–64. doi: 10.1002/humu.20572. [DOI] [PubMed] [Google Scholar]
- Poot M, Kroes HY, V D Wijst SE, Eleveld MJ, Rooms L, Nievelstein RA, et al. Dandy-Walker complex in a boy with a 5 Mb deletion of region 1q44 due to a paternal t(1;20)(q44;q13.33) Am J Med Genet A. 2007;143A:1038–44. doi: 10.1002/ajmg.a.31690. [DOI] [PubMed] [Google Scholar]
- Quintero-Rivera F, Robson CD, Reiss RE, Levine D, Benson CB, Mulliken JB, et al. Intracranial anomalies detected by imaging studies in 30 patients with Apert syndrome. Am J Med Genet A. 2006;140:1337–8. doi: 10.1002/ajmg.a.31277. [DOI] [PubMed] [Google Scholar]
- Quisling RG, Barkovich AJ, Maria BL. Magnetic resonance imaging features and classification of central nervous system malformations in Joubert syndrome. J Child Neurol. 1999;14:628–35; discussion 669–72. doi: 10.1177/088307389901401002. [DOI] [PubMed] [Google Scholar]
- Rakic P, Yakovlev PI. Development of the corpus callosum and cavum septi in man. J Comp Neurol. 1968;132:45–72. doi: 10.1002/cne.901320103. [DOI] [PubMed] [Google Scholar]
- Ramanathan R, Wilkemeyer MF, Mittal B, Perides G, Charness ME. Alcohol inhibits cell-cell adhesion mediated by human L1. J Cell Biol. 1996;133:381–90. doi: 10.1083/jcb.133.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash BG, Richards LJ. A role for cingulate pioneering axons in the development of the corpus callosum. J Comp Neurol. 2001;434:147–57. doi: 10.1002/cne.1170. [DOI] [PubMed] [Google Scholar]
- Redon R, Rio M, Gregory SG, Cooper RA, Fiegler H, Sanlaville D, et al. Tiling path resolution mapping of constitutional 1p36 deletions by array-CGH: contiguous gene deletion or “deletion with positional effect” syndrome? J Med Genet. 2005;42:166–71. doi: 10.1136/jmg.2004.023861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren T, Anderson A, Shen WB, Huang H, Plachez C, Zhang J, et al. Imaging, anatomical, and molecular analysis of callosal formation in the developing human fetal brain. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:191–204. doi: 10.1002/ar.a.20282. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19:1198–202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Robbins DJ, Fei DL, Riobo NA. The hedgehog signal transduction network. Sci Signal. 2012;5:re6. doi: 10.1126/scisignal.2002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinow M, Haney N, Chen H, Sorauf T, Van Dyke DL, Babu VR, et al. Secondary trisomy or mosaic “tetrasomy” 8p. Am J Med Genet. 1989;32:320–4. doi: 10.1002/ajmg.1320320309. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JA, Crolla JA, Tomkins S, Bader P, Morrow B, Gorski J, et al. Refinement of causative genes in monosomy 1p36 through clinical and molecular cytogenetic characterization of small interstitial deletions. Am J Med Genet A. 2010;152A:1951–9. doi: 10.1002/ajmg.a.33516. [DOI] [PubMed] [Google Scholar]
- Rosenthal A, Jouet M, Kenwrick S. Aberrant splicing of neural cell adhesion molecule L1 mRNA in a family with X-linked hydrocephalus. Nat Genet. 1992;2:107–12. doi: 10.1038/ng1092-107. [DOI] [PubMed] [Google Scholar]
- Saavedra D, Richieri-Costa A, Guion-Almeida ML, Cohen MM., Jr Craniofrontonasal syndrome: study of 41 patients. Am J Med Genet. 1996;61:147–51. doi: 10.1002/(SICI)1096-8628(19960111)61:2<147::AID-AJMG8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Said E, Cuschieri A, Vermeesch J, Fryns JP. Toriello-Carey syndrome with a 6Mb interstitial deletion at 22q12 detected by array CGH. Am J Med Genet A. 2011;155A:1390–2. doi: 10.1002/ajmg.a.33961. [DOI] [PubMed] [Google Scholar]
- Sajan SA, Fernandez L, Esmaeeli Nieh S, Rider E, Bukshpun P, Wakahiro M, et al. Both rare and de novo copy number variants are prevalent in agenesis of the corpus callosum but not in cerebellar hypoplasia or polymicrogyria. PLoS Genet. 2013;9:e1003823. doi: 10.1371/journal.pgen.1003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom SN, Griffiths DS, Faedo A, Kleinjan DJ, Ruan Y, Smith J, et al. The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS Genet. 2009;5:e1000511. doi: 10.1371/journal.pgen.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo S, D’Antonio F, Homfray T, Rich P, Pilu G, Bhide A, et al. Counseling in fetal medicine: agenesis of the corpus callosum. Ultrasound Obstet Gynecol. 2012;40:513–21. doi: 10.1002/uog.12315. [DOI] [PubMed] [Google Scholar]
- Schaefer GB, Bodensteiner JB, Buehler BA, Lin A, Cole TR. The neuroimaging findings in Sotos syndrome. Am J Med Genet. 1997;68:462–5. doi: 10.1002/(sici)1096-8628(19970211)68:4<462::aid-ajmg18>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Schell-Apacik CC, Wagner K, Bihler M, Ertl-Wagner B, Heinrich U, Klopocki E, et al. Agenesis and dysgenesis of the corpus callosum: clinical, genetic and neuroimaging findings in a series of 41 patients. Am J Med Genet A. 2008;146A:2501–11. doi: 10.1002/ajmg.a.32476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrander-Stumpel CT, Govaerts LC, Engelen JJ, van der Blij-Philipsen M, Borghgraef M, Loots WJ, et al. Mosaic tetrasomy 8p in two patients: clinical data and review of the literature. Am J Med Genet. 1994;50:377–80. doi: 10.1002/ajmg.1320500416. [DOI] [PubMed] [Google Scholar]
- Sepulveda B, Carcea I, Zhao B, Salton SR, Benson DL. L1 cell adhesion molecule promotes resistance to alcohol-induced silencing of growth cone responses to guidance cues. Neuroscience. 2011;180:30–40. doi: 10.1016/j.neuroscience.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–14. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Sessa A, Mao CA, Hadjantonakis AK, Klein WH, Broccoli V. Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron. 2008;60:56–69. doi: 10.1016/j.neuron.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seuntjens E, Nityanandam A, Miquelajauregui A, Debruyn J, Stryjewska A, Goebbels S, et al. Sip1 regulates sequential fate decisions by feedback signaling from postmitotic neurons to progenitors. Nat Neurosci. 2009;12:1373–80. doi: 10.1038/nn.2409. [DOI] [PubMed] [Google Scholar]
- Shekarabi M, Moldrich RX, Rasheed S, Salin-Cantegrel A, Laganiere J, Rochefort D, et al. Loss of neuronal potassium/chloride cotransporter 3 (KCC3) is responsible for the degenerative phenotype in a conditional mouse model of hereditary motor and sensory neuropathy associated with agenesis of the corpus callosum. J Neurosci. 2012;32:3865–76. doi: 10.1523/JNEUROSCI.3679-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–51. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Shen S, Lang B, Nakamoto C, Zhang F, Pu J, Kuan SL, et al. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J Neurosci. 2008;28:10893–904. doi: 10.1523/JNEUROSCI.3299-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr EH, Owen R, Albertson DG, Pinkel D, Cotter PD, Slavotinek AM, et al. Genomic microarray analysis identifies candidate loci in patients with corpus callosum anomalies. Neurology. 2005;65:1496–8. doi: 10.1212/01.wnl.0000183066.09239.b6. [DOI] [PubMed] [Google Scholar]
- Shioi G, Konno D, Shitamukai A, Matsuzaki F. Structural basis for self-renewal of neural progenitors in cortical neurogenesis. Cereb Cortex. 2009;19(suppl 1):i55–61. doi: 10.1093/cercor/bhp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Butz KG, Plachez C, Gronostajski RM, Richards LJ. Abnormal development of forebrain midline glia and commissural projections in Nfia knock-out mice. J Neurosci. 2003a;23:203–12. doi: 10.1523/JNEUROSCI.23-01-00203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Li Y, Keller A, Richards LJ. he glial sling is a migratory population of developing neurons. Development. 2003b;130:2929–37. doi: 10.1242/dev.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siffredi V, Anderon V, Leventer RJ, Spencer-Smith MM. Neuropsychological profile of agenesis of the corpus callosum: a systematic review. Dev Neuropsychol. 2013;38:36–57. doi: 10.1080/87565641.2012.721421. [DOI] [PubMed] [Google Scholar]
- Silver J. Glia-neuron interactions at the midline of the developing mammalian brain and spinal cord. Perspect Dev Neurobi. 1993;1:227–36. [PubMed] [Google Scholar]
- Silver J, Lorenz SE, Wahlsten D, Coughlin J. Axonal guidance during development of the great cerebral commissures: descriptive and experimental studies, in vivo, on the role of preformed glial pathways. J Comp Neurol. 1982;210:10–29. doi: 10.1002/cne.902100103. [DOI] [PubMed] [Google Scholar]
- Silver J, Ogawa MY. Postnatally induced formation of the corpus callosum in acallosal mice on glia-coated cellulose bridges. Science. 1983;220:1067–9. doi: 10.1126/science.6844928. [DOI] [PubMed] [Google Scholar]
- Slaney SF, Oldridge M, Hurst JA, Moriss-Kay GM, Hall CM, Poole MD, et al. Differential effects of FGFR2 mutations on syndactyly and cleft palate in Apert syndrome. Am J Hum Genet. 1996;58:923–32. [PMC free article] [PubMed] [Google Scholar]
- Sotiriadis A, Makrydimas G. Neurodevelopment after prenatal diagnosis of isolated agenesis of the corpus callosum: an integrative review. Am J Obstet Gynecol. 2012;206:337 e1–5. doi: 10.1016/j.ajog.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, McGee CL, Fryer SL, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Neurosci Biobehav R. 2007;31:239–45. doi: 10.1016/j.neubiorev.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelgen R, Hammes A, Anzenberger U, Zechner D, Andersen OM, Jerchow B, et al. LRP2/megalin is required for patterning of the ventral telencephalon. Development. 2005;132:405–14. doi: 10.1242/dev.01580. [DOI] [PubMed] [Google Scholar]
- Srour M, Riviere JB, Pham JM, Dube MP, Girard S, Morin S, et al. Mutations in DCC cause congenital mirror movements. Science. 2010;328:592. doi: 10.1126/science.1186463. [DOI] [PubMed] [Google Scholar]
- Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–38. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- Stromme P, Mangelsdorf ME, Shaw MA, Lower KM, Lewis SM, Bruyere H, et al. Mutations in the human ortholog of Aristaless cause X-linked mental retardation and epilepsy. Nat Genet. 2002;30:441–5. doi: 10.1038/ng862. [DOI] [PubMed] [Google Scholar]
- Suri M. The phenotypic spectrum of ARX mutations. Dev Med Child Neurol. 2005;47:133–7. doi: 10.1017/s001216220500023x. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Nakamura K, Okamoto K, Kaneko T. Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci Res. 2001;41:51–60. doi: 10.1016/s0168-0102(01)00259-0. [DOI] [PubMed] [Google Scholar]
- Tang PH, Bartha AI, Norton ME, Barkovich AJ, Sherr EH, Glenn OA. Agenesis of the corpus callosum: an MR imaging analysis of associated abnormalities in the fetus. AJNR Am J Neuroradiol. 2009;30:257–63. doi: 10.3174/ajnr.A1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple IK, Eccles DM, Winter RM, Baraitser M, Carr SB, Shortland D, et al. Craniofacial abnormalities, agenesis of the corpus callosum, polysyndactyly and abnormal skin and gut development - the Curry Jones syndrome. Clin Dysmorphol. 1995;4:116–29. [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–33. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L, He W. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Moll F, Moll J, de Oliveira-Souza R, Bramati I, Andreiuolo PA, Lent R. Neuroplasticity in human callosal dysgenesis: a diffusion tensor imaging study. Cereb Cortex. 2007;17:531–41. doi: 10.1093/cercor/bhj178. [DOI] [PubMed] [Google Scholar]
- Toyo-oka K, Shionoya A, Gambello MJ, Cardoso C, Leventer R, Ward HL, et al. 14-3-3epsilon is important for neuronal migration by binding to NUDEL: a molecular explanation for Miller-Dieker syndrome. Nat Genet. 2003;34:274–85. doi: 10.1038/ng1169. [DOI] [PubMed] [Google Scholar]
- Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, et al. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–54. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- Twigg SR, Kan R, Babbs C, Bochukova EG, Robertson SP, Wall SA, et al. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc Natl Acad Sci USA. 2004;101:8652–7. doi: 10.1073/pnas.0402819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaaralahti K, Raivio T, Koivu R, Valanne L, Laitinen EM, Tommiska J. Genetic Overlap between Holoprosencephaly and Kallmann Syndrome. Mol Syndromol. 2012;3:1–5. doi: 10.1159/000338706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bon BW, Koolen DA, Borgatti R, Magee A, Garcia-Minaur S, Rooms L, et al. Clinical and molecular characteristics of 1qter microdeletion syndrome: delineating a critical region for corpus callosum agenesis/hypogenesis. J Med Genet. 2008;45:346–54. doi: 10.1136/jmg.2007.055830. [DOI] [PubMed] [Google Scholar]
- Verschueren K, Remacle JE, Collart C, Kraft H, Baker BS, Tylzanowski P, et al. SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5′-CACCT sequences in candidate target genes. J Biol Chem. 1999;274:20489–98. doi: 10.1074/jbc.274.29.20489. [DOI] [PubMed] [Google Scholar]
- Verstappen G, van Grunsven LA, Michiels C, Van de Putte T, Souopgui J, Van Damme J, et al. Atypical Mowat-Wilson patient confirms the importance of the novel association between ZFHX1B/SIP1 and NuRD corepressor complex. Hum Mol Genet. 2008;17:1175–83. doi: 10.1093/hmg/ddn007. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Gessler M, Grzeschik KH. GLI3 zinc-finger gene interrupted by translocations in Greig syndrome families. Nature. 1991;352:539–40. doi: 10.1038/352539a0. [DOI] [PubMed] [Google Scholar]
- Vos YJ, de Walle HE, Bos KK, Stegeman JA, Ten Berge AM, Bruining M, et al. Genotype-phenotype correlations in L1 syndrome: a guide for genetic counselling and mutation analysis. J Med Genet. 2010;47:169–75. doi: 10.1136/jmg.2009.071688. [DOI] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, et al. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. Neuroscience. 2007;27:12132–8. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Strominger Z, Jeremy RJ, Barkovich AJ, Wakahiro M, Sherr EH, et al. Variability of homotopic and heterotopic callosal connectivity in partial agenesis of the corpus callosum: a 3T diffusion tensor imaging and Q-ball tractography study. AJNR Am J Neuroradiol. 2009;30:282–9. doi: 10.3174/ajnr.A1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Strominger ZA, Wakahiro M, Jeremy RJ, Mukherjee P, Sherr EH. Diffusion tensor imaging of Aicardi syndrome. Pediatr Neurol. 2010;43:87–91. doi: 10.1016/j.pediatrneurol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D, Bishop KM, Ozaki HS. Recombinant inbreeding in mice reveals thresholds in embryonic corpus callosum development. Genes Brain Behav. 2006;5:170–88. doi: 10.1111/j.1601-183X.2005.00153.x. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Wood AG, Reutens DC, Wood SJ, Chen J, Velakoulis D, et al. Morphology of the corpus callosum at different stages of schizophrenia: cross-sectional study in first-episode and chronic illness. Br J Psychiatry. 2008;192:429–34. doi: 10.1192/bjp.bp.107.041251. [DOI] [PubMed] [Google Scholar]
- Weaving LS, Christodoulou J, Williamson SL, Friend KL, McKenzie OL, Archer H, et al. Mutations of CDKL5 cause a severe neurodevelopmental disorder with infantile spasms and mental retardation. Am J Hum Genet. 2004;75:1079–93. doi: 10.1086/426462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein AS, Goldstein RB, Barkovich J. In utero disappearance of the corpus callosum secondary to extensive brain injury. J Ultrasound Med. 2003;22:837–40. doi: 10.7863/jum.2003.22.8.837. [DOI] [PubMed] [Google Scholar]
- Wieacker P, Wieland I. Clinical and genetic aspects of craniofrontonasal syndrome: towards resolving a genetic paradox. Mol Genet Metab. 2005;86:110–6. doi: 10.1016/j.ymgme.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Wieland I, Jakubiczka S, Muschke P, Cohen M, Thiele H, Gerlach KL, et al. Mutations of the ephrin-B1 gene cause craniofrontonasal syndrome. Am J Hum Genet. 2004;74:1209–15. doi: 10.1086/421532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland I, Reardon W, Jakubiczka S, Franco B, Kress W, Vincent-Delorme C, et al. Twenty-six novel EFNB1 mutations in familial and sporadic craniofrontonasal syndrome (CFNS) Hum Mutat. 2005;26:113–8. doi: 10.1002/humu.20193. [DOI] [PubMed] [Google Scholar]
- Wilkie AO, Slaney SF, Oldridge M, Poole MD, Ashworth GJ, Hockley AD, et al. Apert syndrome results from localized mutations of FGFR2 and is allelic with Crouzon syndrome. Nat Genet. 1995;9:165–72. doi: 10.1038/ng0295-165. [DOI] [PubMed] [Google Scholar]
- Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, et al. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci USA. 1996;93:8460–4. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BT, Harikumar C, Fisher RB. Agenesis of the corpus callosum in mosaic tetrasomy 8p. Clin Dysmorphol. 2010;19:215–7. doi: 10.1097/MCD.0b013e32833c5efa. [DOI] [PubMed] [Google Scholar]
- Winters J, Markello T, Nance W, Jackson-Cook C. Mosaic “tetrasomy” 8p: case report and review of the literature. Clin Genet. 1995;48:195–8. doi: 10.1111/j.1399-0004.1995.tb04088.x. [DOI] [PubMed] [Google Scholar]
- Wise SP, Jones EG. The organization and postnatal development of the commissural projection of the rat somatic sensory cortex. J Comp Neurol. 1976;168:313–43. doi: 10.1002/cne.901680302. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Woodruff PW, McManus IC, David AS. Meta-analysis of corpus callosum size in schizophrenia. J Neurol Neurosur PS. 1995;58:457–61. doi: 10.1136/jnnp.58.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Maruyama T, Hattori Y, Sugo N, Takamatsu H, Kumanogoh A, et al. A molecular mechanism that regulates medially oriented axonal growth of upper layer neurons in the developing neocortex. J Comp Neurol. 2011;519:834–48. doi: 10.1002/cne.22536. [DOI] [PubMed] [Google Scholar]
- Zolotushko J, Flusser H, Markus B, Shelef I, Langer Y, Heverin M, et al. The desmosterolosis phenotype: spasticity, microcephaly and micrognathia with agenesis of corpus callosum and loss of white matter. Eur J Hum Genet. 2011;19:942–6. doi: 10.1038/ejhg.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.