Abstract
Objective:
The goal of this study was to explore the prevalence of nonverbal oral apraxia (NVOA), its association with other forms of apraxia, and associated imaging findings in patients with primary progressive aphasia (PPA) and progressive apraxia of speech (PAOS).
Methods:
Patients with a degenerative speech or language disorder were prospectively recruited and diagnosed with a subtype of PPA or with PAOS. All patients had comprehensive speech and language examinations. Voxel-based morphometry was performed to determine whether atrophy of a specific region correlated with the presence of NVOA.
Results:
Eighty-nine patients were identified, of which 34 had PAOS, 9 had agrammatic PPA, 41 had logopenic aphasia, and 5 had semantic dementia. NVOA was very common among patients with PAOS but was found in patients with PPA as well. Several patients exhibited only one of NVOA or apraxia of speech. Among patients with apraxia of speech, the severity of the apraxia of speech was predictive of NVOA, whereas ideomotor apraxia severity was predictive of the presence of NVOA in those without apraxia of speech. Bilateral atrophy of the prefrontal cortex anterior to the premotor area and supplementary motor area was associated with NVOA.
Conclusions:
Apraxia of speech, NVOA, and ideomotor apraxia are at least partially separable disorders. The association of NVOA and apraxia of speech likely results from the proximity of the area reported here and the premotor area, which has been implicated in apraxia of speech. The association of ideomotor apraxia and NVOA among patients without apraxia of speech could represent disruption of modules shared by nonverbal oral movements and limb movements.
Nonverbal oral apraxia (NVOA) refers to impaired volitional oromotor movements due to problems with motor planning and sequencing rather than weakness, language dysfunction, or cognitive impairment.1 The association between NVOA and aphasia, perhaps first described in the medical literature by Hughlings-Jackson in 1878,2 has been primarily explored in stroke patients, most frequently in association with nonfluent aphasia. The same is true of NVOA among patients with apraxia of speech and ideomotor apraxia. Two recent studies3,4 described the presence of apraxia, including facial apraxia, among patients with progressive nonfluent aphasia and logopenic aphasia (LPA). Similarly, NVOA has been mentioned in the setting of disorders dominated by apraxia of speech,5 but little is known about its association with apraxia of speech or ideomotor apraxia. In this study, we evaluated a large group of patients with primary progressive aphasia (PPA) and progressive apraxia of speech (PAOS) to explore the prevalence and severity of NVOA among these patients, and to determine the potential association between NVOA and other forms of apraxia, as well as the neuroanatomical correlate of NVOA through structural imaging. Our hypotheses were that NVOA would be strongly associated with apraxia of speech and hence common among patients with PAOS, that it would be rare in patients with fluent aphasias, and that it would be related to atrophy in the region of the premotor cortex on the left.
METHODS
Subjects.
All patients seen in the Department of Neurology for a speech or language disorder presumed to be due to a degenerative neurologic disease between July 2010 and April 2013 were eligible for inclusion. To be included, subjects had to be older than 18 years, have English as their primary language, and have an informant. Furthermore, subjects had to meet criteria for PPA6 or PAOS.5,7–9 Patients with an alternative or coexisting diagnosis of another degenerative disease such as Alzheimer disease, behavioral variant frontotemporal dementia, progressive supranuclear palsy, or corticobasal syndrome were excluded. Patients who did not fit the operational diagnostic definitions (see below) or where their understanding of the tasks involved in testing for NVOA was judged to be inadequate were excluded.
Standard protocol approvals, registrations, and patient consents.
Informed consent was obtained from subject and informant. The Mayo Clinic Institutional Review Board approved the study.
Speech and language evaluation.
As part of a standard evaluation protocol, all patients were evaluated by a neurologist (K.A.J.) and had video recorded comprehensive speech and language examinations by a speech-language pathologist (J.R.D. or E.A.S.). Details of the evaluation have been described elsewhere.5,9 Speech and language data, along with video recordings of the language tests, were reviewed by both speech-language pathologists. Patients were classified clinically according to operational definitions independent of neurologic, neuropsychological, and neuroimaging results. The Aphasia Quotient from the Western Aphasia Battery–Revised (WAB) Part 110 was used as an indicator of global language function, with lower scores indicating greater language impairment. The upper limb and instrumental apraxia components of the WAB were combined to create an ideomotor apraxia score (30 points maximum), with lower scores indicating more severe apraxia. Apraxia of speech was diagnosed based on published guidelines5,7,8 and severity graded with an Apraxia of Speech Rating Scale (ASRS),5 with higher scores indicating more severe apraxia of speech. NVOA was assessed with an 8-item measure consisting of 4 gestures (“cough,” “click your tongue,” “blow,” “smack your lips”), repeated twice.5 If patients could not complete the task on command, they were asked to imitate the examiner. Each item received 0 to 4 points, with a score of 4 (immediate, accurate response to command) representing best performance. Normative data for the NVOA evaluation tool were obtained from the performance of 29 consecutive patients seen for speech-language assessment who did not have aphasia, NVOA, or apraxia of speech. Ten had normal speech and language, 13 had mild dysarthria, 2 had migraine-related word-finding difficulties, 2 had essential voice tremor, 1 had hoarseness, and 1 had alexia. Mean age ± SD was 57.6 ± 13 years. The average score ± SD on the NVOA tool for these patients was 31.4 ± 0.8 with a range of 29 to 32. Test-retest and interrater reliability of the test were assessed by having the speech and language experts (J.R.D. and E.D.S.) as well as a neurology resident (H.B.) rescore recordings of 10% of the subjects, selected at random. Correlations between the initial scores and rescoring by another judge who reviewed the video recorded test were remarkably high (r = 0.99, 95% confidence interval 0.96–1.00), as was the intrajudge correlation between the examining clinician's first and second ratings (r = 0.97, 95% confidence interval 0.90–0.99). A cutoff of 29 was used to establish the presence of NVOA in our cohort; all but one of the controls scored more than 29. See videos 1–3 on the Neurology® Web site at Neurology.org for examples of different severities of NVOA.
Operational definitions.
PAOS was diagnosed if patients had apraxia of speech as the only feature of their communication disorder,5 or if patients had evidence of apraxia of speech in the context of agrammatism or telegraphic speech but the apraxia of speech was the dominant feature.9 Patients with agrammatism or telegraphic speech with or without apraxia of speech in whom aphasia severity exceeded the severity of any apraxia of speech were diagnosed with agrammatic PPA (agPPA).9 LPA was diagnosed in the presence of word-finding difficulties and anomia without loss of word meaning, as evidenced by recognition of target words on confrontational naming most of the time, along with any combination of impaired sentence repetition, impaired sentence comprehension, or phonemic paraphasias. In addition, performance on tasks involving single-word comprehension had to exceed that of tasks involving sentence comprehension, such as the Token Test.11 Patients were diagnosed with semantic dementia if the dominant features were anomia and loss of single-word comprehension, evidenced by an inability to recognize target words despite semantic and phonemic cues on at least 30% of naming items, or at least one comment during the examination by the patient or informant that the patient no longer recognized the meaning of some words. In addition, performance on tasks involving sentence comprehension, such as the Token Test,11 had to exceed performance involving single-word comprehension. Patients with a progressive aphasia but not meeting any of the operational definitions were excluded. Patients with an Aphasia Quotient of <55 of 100 and patients who, on consensus review, were thought to not understand the NVOA tasks were also excluded.
Neuroimaging.
Two groups of patients, one with NVOA and one without, were selected such that there were an equal number of patients with a specific diagnosis in each group, and with no significant difference in age, sex, Aphasia Quotient, or apraxia of speech severity between groups. All subjects underwent 3-tesla MRI scanning using a protocol that included an magnetization-prepared rapid-acquisition gradient echo sequence as described previously.5 Voxel-based morphometry (VBM)12 with SPM5 was used to assess voxel-level regional differences between the patients with and without NVOA. Standard preprocessing steps were performed, including normalization to a study-specific customized template and segmentation using unified segmentation, modulation, and smoothing at 8-mm full width at half maximum. Results were assessed at p < 0.001 uncorrected for multiple comparisons.
Statistical analysis.
JMP statistical software (version 8.0.0; SAS Institute, Cary, NC) was used, with statistical significance set at p < 0.05. Binary variables were compared across groups using the χ2 test, or Fisher exact test if there were cells with small numbers (<5). Continuous, normal-, or near-normal distributed data were compared across groups using analysis of variance and Student t test, whereas the Wilcoxon rank-sum/Kruskal-Wallis test was used for nonnormally distributed data. Pairwise correlations were used to evaluate which variables correlated with the NVOA score, and multiple linear regression was used to adjust for covariation among the variables. We also performed a logistic regression to determine the contribution of the apraxia of speech and ideomotor apraxia scores to the odds of belonging to the “NVOA present” group.
RESULTS
Clinical results.
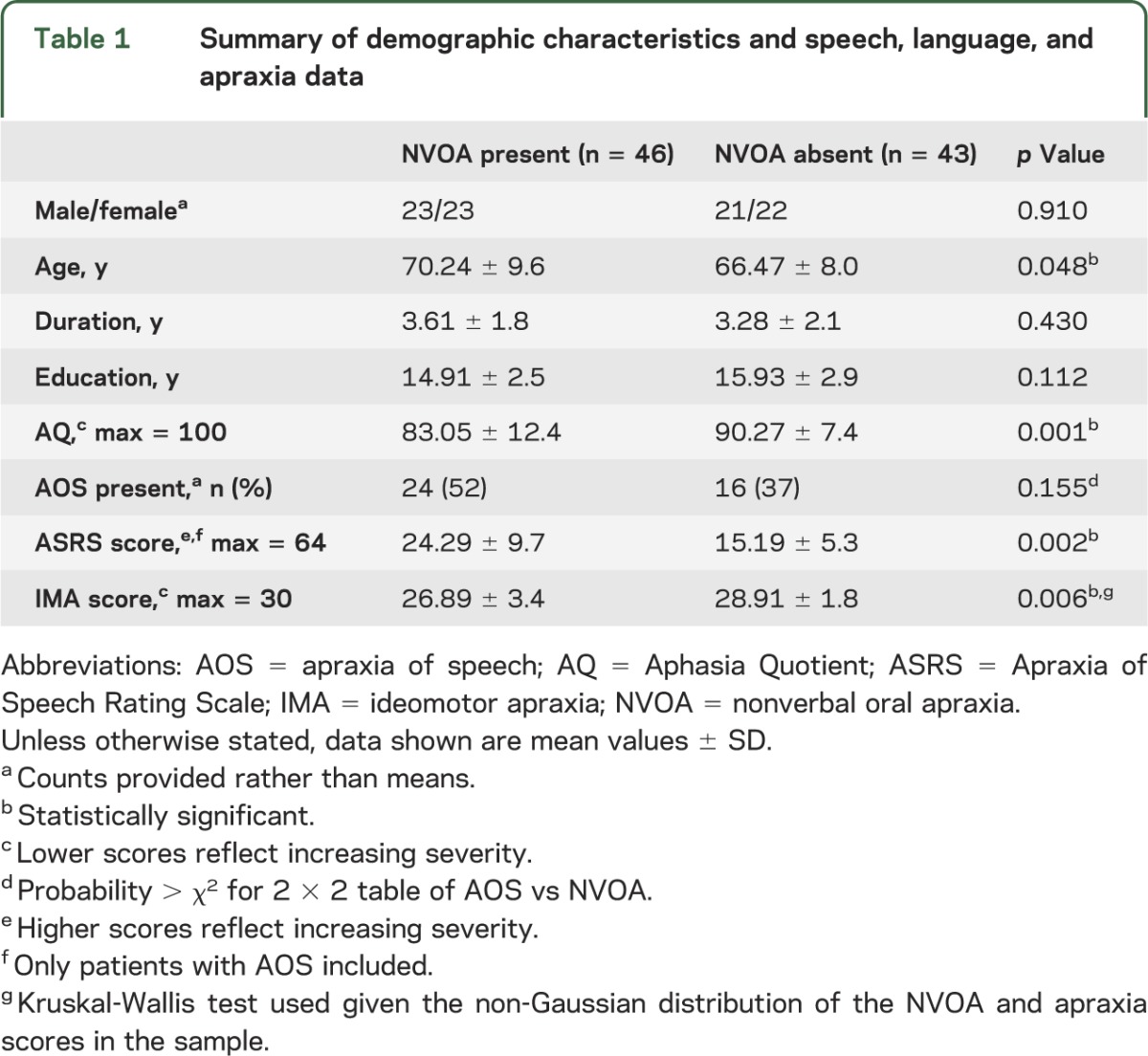
A total of 118 patients were initially included in the cohort. Of these, 29 patients were excluded, 19 because they did not meet criteria for PAOS or a specific PPA subtype and 10 because of poor comprehension of the NVOA tasks. Demographic and speech and language data for patients with and without NVOA are given in table 1. Significant group differences included a higher average age and apraxia of speech severity score, and lower Aphasia Quotient and ideomotor apraxia score among patients with NVOA.
Table 1.
Summary of demographic characteristics and speech, language, and apraxia data
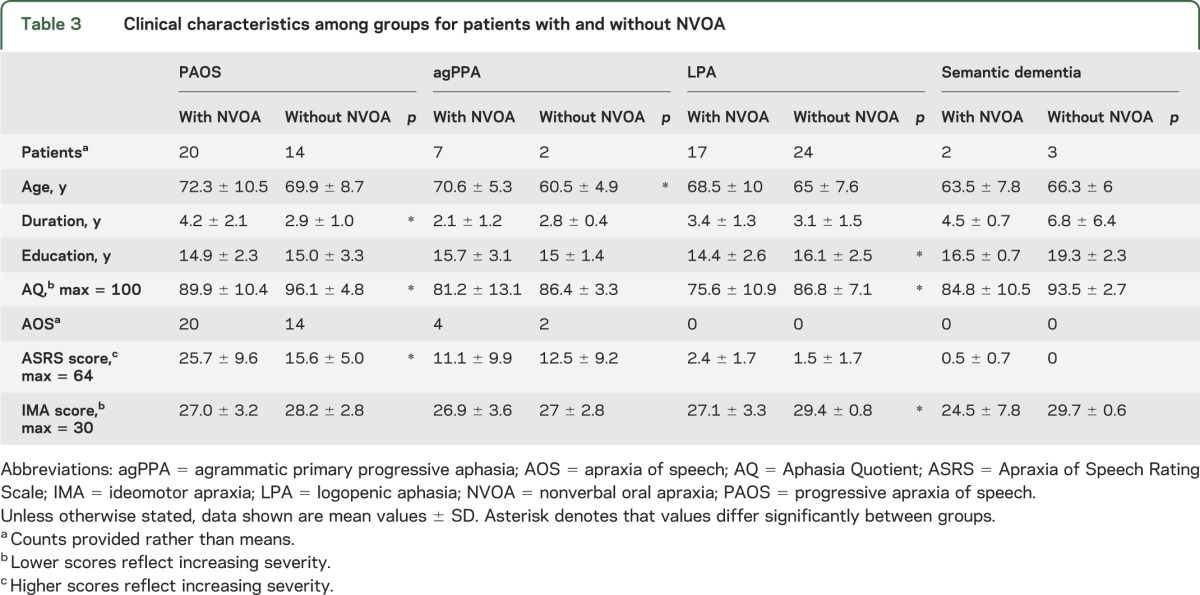
Of the 89 patients diagnosed using the operational definitions, 34 had PAOS, 9 had agPPA, 41 had LPA, and 5 had semantic dementia. Demographic and speech-language data based on diagnosis are shown in tables 2 and 3. Patients with PAOS tended to have a greater severity of NVOA. Patients with agPPA, LPA, or semantic dementia who had NVOA were mostly rated as mildly impaired. For patients with agPPA or semantic dementia, there were no significant differences in speech or language scores or the tests for ideomotor apraxia for patients with and without NVOA. Among patients with PAOS, the ASRS score was significantly higher and the Aphasia Quotient lower (more impaired) among the NVOA group, whereas for the LPA group, the Aphasia Quotient and ideomotor apraxia scores were significantly lower (more impaired) than those among patients without NVOA. Patients with PAOS who had NVOA had a longer duration of illness than those who did not have NVOA.
Table 2.
NVOA scores and severity ratings
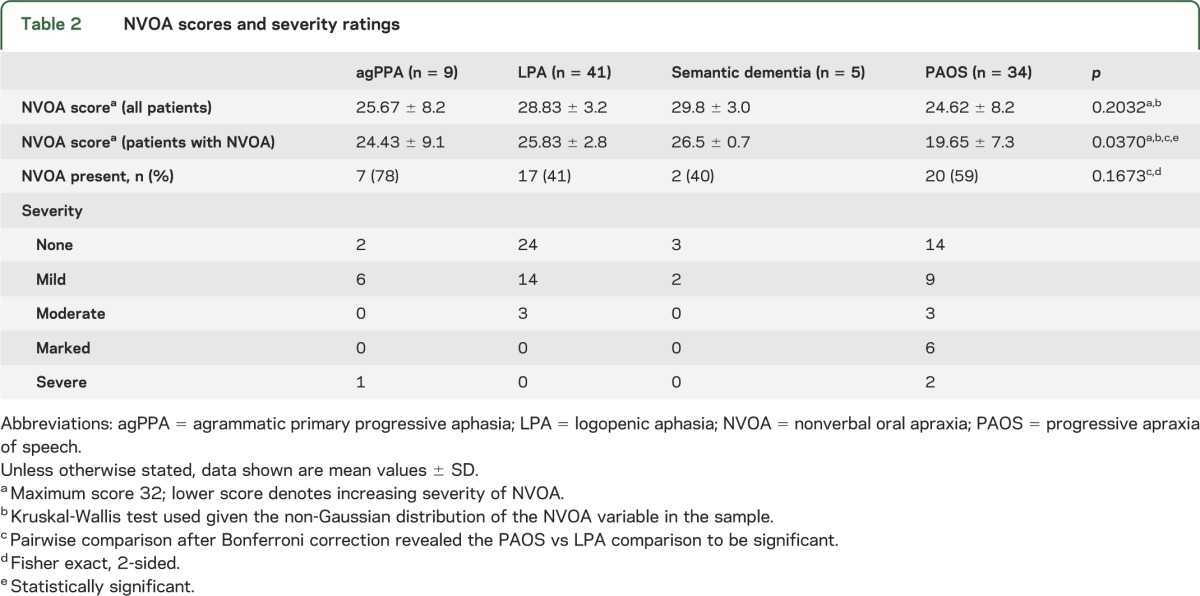
Table 3.
Clinical characteristics among groups for patients with and without NVOA
Associations among NVOA, apraxia of speech, and ideomotor apraxia scores.
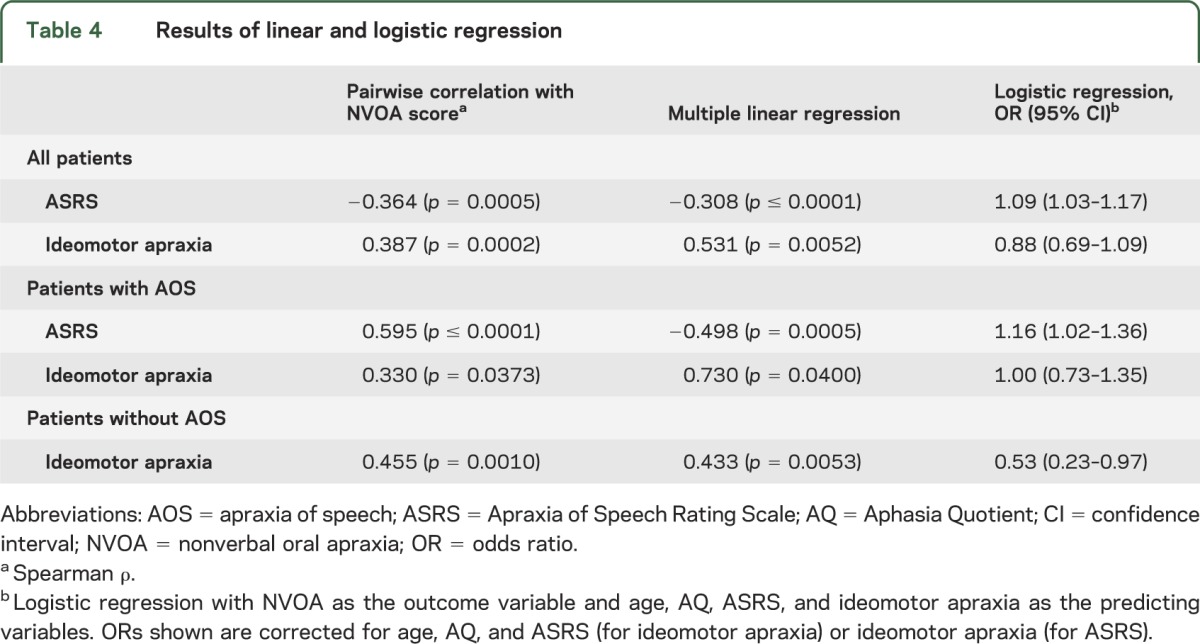
Details of these analyses can be found in table 4. When we included all patients, pairwise analysis revealed a significant correlation between both ASRS and ideomotor apraxia scores and the NVOA score. Because age and Aphasia Quotient differed among the patients with NVOA (table 1), and because the ASRS and ideomotor apraxia scores were correlated with one another, multilinear regression was performed. After correcting for age, Aphasia Quotient, and the ASRS or ideomotor apraxia scores, both the ASRS and ideomotor apraxia scores remained significantly correlated with the NVOA score. We obtained similar results for these analyses when we grouped patients according to the presence of apraxia of speech. A logistic regression was performed with the same variables but with membership to the NVOA group, rather than the raw NVOA score, as the outcome variable. For all patients as well as the subgroup with apraxia of speech, only the odds ratio (OR) for the ASRS score was significant, whereas the OR for ideomotor apraxia was only significant in the subgroup without apraxia of speech. Thus, for example, in a patient without apraxia of speech, a decrease (i.e., worsening) in the ideomotor apraxia score of 1 point nearly doubles the odds of being in the “NVOA present” group, whereas an increase (i.e., worsening) in the ASRS of approximately 5 for a patient with apraxia of speech results in a similar change in OR.
Table 4.
Results of linear and logistic regression
Neuroimaging findings.
There were 27 patients with and 27 patients without NVOA included in the analysis (12 with LPA, 13 with PAOS, and 2 with semantic dementia). The result of the VBM analysis is shown in the figure. At p < 0.001 (uncorrected for multiple comparisons), patients with NVOA had reduced volumes in the bilateral medial and lateral prefrontal cortices.
Figure. Voxel-based morphometric analysis comparing patients with and without NVOA.
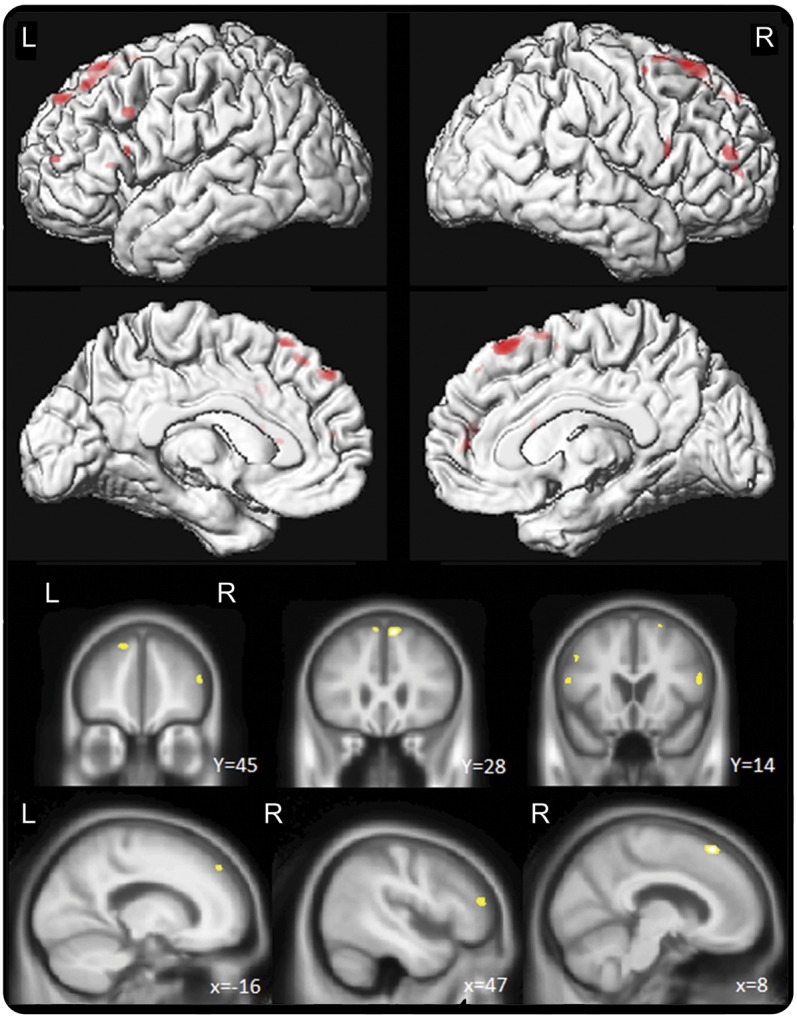
Regions of gray matter loss in the patients with nonverbal oral apraxia (NVOA) compared with the matched patients without NVOA. Results are shown on lateral and medial 3-dimensional renderings of the brain, and on representative coronal and sagittal slices, uncorrected for multiple comparisons at p < 0.001.
DISCUSSION
We have shown that NVOA is common among patients with PAOS. Patients with NVOA tended to have more severe apraxia of speech as measured by the ASRS and poorer scores on ideomotor apraxia testing. However, there were several patients who exhibited NVOA in the absence of apraxia of speech, while others had apraxia of speech without NVOA, suggesting that these are at least partially dissociable. Furthermore, it is worth noting that the ideomotor apraxia score was not predictive of belonging to the NVOA group independent of apraxia of speech severity among patients with apraxia of speech. Lastly, our VBM analysis suggests that bilateral atrophy of the prefrontal cortex anterior to the premotor area and the supplementary motor area is associated with NVOA.
As mentioned before, the associations among aphasia, apraxia of speech, ideomotor apraxia, and NVOA have been studied primarily in stroke patients. Several studies have confirmed a high prevalence of NVOA among patients with nonfluent aphasia,1 and a relative paucity among patients with fluent aphasia.13 The association between NVOA and apraxia of speech in stroke patients is also well known, ranging from 48%14 to 85%.15 The co-occurrence of ideomotor apraxia and NVOA is less clearly established, with some reports suggesting it is as rare as 5%,16 whereas others suggest the association is similar to that of apraxia of speech, approximately 50% to 60%.17,18 Based on data from stroke patients, the structures most often implicated in NVOA are the left anterior insula,1,14,19 putamen,19,20 and the inferior frontal area, including Broca area and the periopercular area.1
A small study of patients with degenerative agrammatic aphasia, which included patients with neurodegenerative disorders other than PPA who had agrammatic aphasia, suggested that limb apraxia among patients with agrammatic aphasia was the result of atrophy of the left inferior parietal lobe, whereas apraxia of speech and NVOA were associated with more anterior atrophy involving the left posterior-inferior frontal gyrus and the middle frontal and premotor cortices, respectively.3 We previously reported4 ideomotor apraxia among patients with agPPA (although this included patients who would now be classified as PAOS) and patients with LPA, and found that the composite apraxia score as measured on the WAB correlated with gray matter loss in the left lateral premotor cortex, especially the middle frontal gyrus, with some extension into motor cortex. Furthermore, a poorer ideomotor apraxia score implied a significantly higher OR for belonging to the agPPA as opposed to the LPA group. We have previously shown that the superior lateral premotor cortex and the supplementary motor area are involved in PAOS,5,9 whereas patients with agPPA have more widespread, frontal predominant atrophy, including the inferior premotor area.21–23 Looking at patients with PAOS specifically, we found that apraxia of speech correlates with left lateral premotor cortex volume.24 Because supplementary motor area atrophy can occur in the absence of apraxia of speech,24 we previously proposed that the premotor cortex has a more important role in apraxia of speech. The areas implicated in NVOA in our study and the fact that it is especially common among patients with PAOS fits well with these prior findings.
The correlation between the ASRS and the NVOA score in our study, together with the fact that patients with PAOS and NVOA had a longer mean disease duration, could suggest that, as the pathology spreads and the apraxia of speech severity increases, there is more involvement of the anterior prefrontal areas resulting in onset or worsening of NVOA. The partial dissociation between NVOA and apraxia of speech noted previously, and the anterior-posterior dissociation between NVOA and apraxia of speech on VBM is supportive of the hypothesis that there are distinct areas within the premotor and prefrontal areas responsible for planning of verbal and nonverbal movements.25,26
Explaining the occurrence of NVOA among patients with LPA or semantic dementia is more difficult. Structural imaging data in these disorders classically show atrophy in the area of the left temporoparietal junction in LPA and the anterior temporal lobe in semantic dementia.23 The significantly poorer Aphasia Quotient and ideomotor apraxia scores among LPA patients with NVOA, and a similar trend in semantic dementia, could be indicative of more widespread pathology in these patients, although disease durations were not significantly different. Furthermore, it is plausible that there is a network underlying the correct planning and spatiotemporal programming of orofacial movements similar to that found in upper limb movements and movements involved in speech.7,27 Lower ideomotor apraxia scores could represent a disruption of a module involved in praxis that might be shared by nonverbal orofacial movements as well as movements of the upper extremity.28
The association between NVOA and right hemispheric atrophy in our study was unexpected. However, some authors have argued that the right hemisphere is important in nonverbal oral movements,29 and it is known that some patients with PAOS have predominantly right-sided pathology.5 Current models for ideomotor apraxia also allow for right-sided lesions as a cause of ideomotor apraxia.27 It is also possible that patients with NVOA in general had more widespread pathology and hence had more right-sided involvement.
The discrepancy between the proposed anatomical substrate of NVOA in prior studies focusing on stroke patients and our findings could be the result of several factors. Several of the patients in the aforementioned studies had large middle cerebral artery strokes, and it is no surprise that areas of overlap included several subcortical structures given their vulnerability in large strokes resulting in aphasia and NVOA. This is perhaps most clearly illustrated by the earlier work on apraxia of speech in stroke patients, which also implicated the insula,14 which was later shown to be less important than the prefrontal cortex.30 Furthermore, a recent case report of “pure” NVOA after a stroke involving the left premotor cortex lends further credence to the idea that the prefrontal cortex is integral to nonverbal oral movements.31
Our study has several limitations. The severity rating scale for apraxia of speech is not yet a fully standardized instrument, potentially limiting the external validity of some of our findings. In addition, our imaging findings did not survive correction for multiple comparisons and hence may require replication with a larger sample size. A further limitation is that the tasks used in assessing for NVOA involve the recognition of “symbols” or gestures as well as selection of the appropriate action from an array of possible stored representations.32 Given that aphasia represents a disruption in the use of representational and linguistic symbols, this complicates the analysis of apraxia among these patients, because aphasia may have contaminated the evaluation in some cases, despite our attempt to exclude patients that clearly did not understand the instructions. Lastly, the nonverbal oral tasks used in our study were all performed following command or imitation, without the use of tools/objects, and all required sound production, and as such did not represent a comprehensive analysis.
Supplementary Material
GLOSSARY
- agPPA
agrammatic primary progressive aphasia
- ASRS
Apraxia of Speech Rating Scale
- LPA
logopenic aphasia
- NVOA
nonverbal oral apraxia
- OR
odds ratio
- PAOS
progressive apraxia of speech
- PPA
primary progressive aphasia
- VBM
voxel-based morphometry
- WAB
Western Aphasia Battery
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Study concept or design: H.B., J.R.D., E.A.S., J.L.W., K.A.J. Analysis or interpretation of data: H.B., J.R.D., E.A.S., M.M.M., J.L.W., K.A.J. Drafting/revising the manuscript for content, including medical writing for content: H.B., J.R.D., E.A.S., J.L.W., K.A.J. Acquisition of data: J.R.D., E.A.S., M.M.M., J.L.W., K.A.J. Statistical analysis: H.B., J.R.D., J.L.W. Study supervision or coordination: J.R.D., K.A.J. Obtaining funding: J.R.D., E.A.S., J.L.W., K.A.J.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
H. Botha reports no disclosures relevant to the manuscript. J. Duffy is funded by NIH grants R01 DC10367 and DC012519. E. Strand is funded by NIH grants R01 DC10367 and DC012519. M. Machulda is funded by NIH grant R01 DC10367. J. Whitwell is funded by NIH grants R01 DC10367, R01 AG37491, and R01 DC012519, and is further supported by funding from The Alzheimer's Foundation. K. Josephs is funded by NIH grants R01 DC10367, R01 AG37491, and R01 DC012519, and is further supported by funding from The Alzheimer's Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Tognola G, Vignolo LA. Brain lesions associated with oral apraxia in stroke patients: a clinico-neuroradiological investigation with the CT scan. Neuropsychologia 1980;18:257–272 [DOI] [PubMed] [Google Scholar]
- 2.Hughlings-Jackson J. On affections of speech from disease of the brain. Brain 1879;2:203–222 [Google Scholar]
- 3.Rohrer JD, Rossor MN, Warren JD. Apraxia in progressive nonfluent aphasia. J Neurol 2010;257:569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adeli A, Whitwell JL, Duffy JR, Strand EA, Josephs KA. Ideomotor apraxia in agrammatic and logopenic variants of primary progressive aphasia. J Neurol 2013;260:1594–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain 2012;135:1522–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol 1982;11:592–598 [DOI] [PubMed] [Google Scholar]
- 7.McNeil M, Doyle P, Wambaugh J. Apraxia of speech: a treatable disorder of motor planning and programming. In: Nadeau S, Rothi L, Crosson B, editors. Aphasia and Language: Theory to Practice. New York: Guilford; 2000:221–266 [Google Scholar]
- 8.Duffy JR. Apraxia of speech in degenerative neurologic disease. Aphasiology 2006;20:511–527 [Google Scholar]
- 9.Josephs KA, Duffy JR, Strand EA, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology 2013;81:337–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kertesz A. Western Aphasia Battery–Revised. San Antonio: PsychCorp; 2007 [Google Scholar]
- 11.De Renzi E, Vignolo LA. The Token Test: a sensitive test to detect receptive disturbances in aphasics. Brain 1962;85:665–678 [DOI] [PubMed] [Google Scholar]
- 12.Ashburner J. Computational anatomy with the SPM software. Magn Reson Imaging 2009;27:1163–1174 [DOI] [PubMed] [Google Scholar]
- 13.Square-Storer P, Qualizza L, Roy EA. Isolated and sequenced oral motor posture production under different input modalities by left-hemisphere damaged adults. Cortex 1989;25:371–386 [DOI] [PubMed] [Google Scholar]
- 14.Dronkers NF. A new brain region for coordinating speech articulation. Nature 1996;384:159–161 [DOI] [PubMed] [Google Scholar]
- 15.LaPointe LL, Wertz RT. Oral-movement abilities and articulatory characteristics of brain-injured adults. Percept Mot Skills 1974;39:39–46 [DOI] [PubMed] [Google Scholar]
- 16.Mohr JP, Pessin MS, Finkelstein S, Funkenstein HH, Duncan GW, Davis KR. Broca aphasia: pathologic and clinical. Neurology 1978;28:311–324 [DOI] [PubMed] [Google Scholar]
- 17.Raade AS, Rothi LJ, Heilman KM. The relationship between buccofacial and limb apraxia. Brain Cogn 1991;16:130–146 [DOI] [PubMed] [Google Scholar]
- 18.Marquardt TP, Sussman H. The elusive lesion: apraxia of speech link in Broca's aphasia. In: Rosenbek JC, McNeil MR, Aronson AE, editors. Apraxia of Speech: Physiology, Acoustics, Linguistics, Management. San Diego: College-Hill Press; 1984 [Google Scholar]
- 19.Mintz T. Lesion Size and Localization in Limb and Buccofacial Apraxia: Retrospective Analysis [MSc]. London, ON: University of Western Ontario; 1989 [Google Scholar]
- 20.Pramstaller PP, Marsden CD. The basal ganglia and apraxia. Brain 1996;119:319–340 [DOI] [PubMed] [Google Scholar]
- 21.Whitwell JL, Avula R, Senjem ML, et al. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology 2010;74:1279–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abe K, Ukita H, Yanagihara T. Imaging in primary progressive aphasia. Neuroradiology 1997;39:556–559 [DOI] [PubMed] [Google Scholar]
- 23.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004;55:335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitwell JL, Duffy JR, Strand EA, et al. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: an MRI and FDG-PET study. Brain Lang 2013;125:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preuss TM, Stepniewska I, Kaas JH. Movement representation in the dorsal and ventral premotor areas of owl monkeys: a microstimulation study. J Comp Neurol 1996;371:649–676 [DOI] [PubMed] [Google Scholar]
- 26.Duffau H, Peggy Gatignol ST, Mandonnet E, Capelle L, Taillandier L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with grade II glioma in the left dominant hemisphere. J Neurosurg 2008;109:461–471 [DOI] [PubMed] [Google Scholar]
- 27.Heilman KM, Watson RT. The disconnection apraxias. Cortex 2008;44:975–982 [DOI] [PubMed] [Google Scholar]
- 28.Fadiga L, Craighero L, Roy A. Broca's region: a speech area? In: Grodzinsky Y, Amunts K, editors. Broca's Region. New York: Oxford University Press; 2006:137–152 [Google Scholar]
- 29.Bizzozero I, Costato D, Sala SD, Papagno C, Spinnler H, Venneri A. Upper and lower face apraxia: role of the right hemisphere. Brain 2000;123:2213–2230 [DOI] [PubMed] [Google Scholar]
- 30.Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain 2004;127:1479–1487 [DOI] [PubMed] [Google Scholar]
- 31.Kwon M, Lee JH, Oh JS, Koh JY. Isolated buccofacial apraxia subsequent to a left ventral premotor cortex infarction. Neurology 2013;80:2166–2167 [DOI] [PubMed] [Google Scholar]
- 32.Square-Storer PA, Hogg SC, Roy EA. The dissociation of aphasia from apraxia of speech, ideomotor limb, and buccofacial apraxia. In: Geoffrey RH, editor. Advances in Psychology. Amsterdam: North-Holland; 1990:451–476 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


