Abstract
Importance
Post-traumatic stress disorder (PTSD) has been associated in cross-sectional studies with peripheral inflammation. It is not known whether this observed association is due to PTSD predisposing to inflammation (as sometimes postulated) or to inflammation predisposing to PTSD.
Objective
To determine whether plasma concentration of the inflammatory marker, C-reactive protein (CRP), helps predict future PTSD symptoms.
Design and Setting
The Marine Resiliency Study (MRS), a prospective study of ~2,600 war zone-deployed Marines, during which PTSD symptomatology and various physiological and psychological parameters were determined pre-deployment and at approximately three and six months following a seven month deployment.
Participants
Subjects were recruited from four all-male infantry battalions imminently deploying to a war zone. Participation was requested of 2,978 subjects, of whom 2,610 (87.6%) consented and 2,555 (85.8%) were included in the current analysis. Post-deployment data on combat exposure were included from 2,215 subjects (86.7% of the 2,555 included subjects), and on PTSD symptomatology from 1,861 (72.8%) and 1,609 subjects (63.0%) at three and six months following deployment, respectively.
Main Outcome Measure(s)
PTSD symptoms three months after deployment, assessed by the Clinician Administered PTSD Scale (CAPS).
Results
We determined the effects of baseline plasma CRP concentration on post-deployment CAPS using Zero-inflated negative binomial regression (ZINBR), a procedure designed for distributions, such as CAPS in this study, which have an excess of zeros in addition to being positively skewed. Adjusting for baseline CAPS, trauma exposure, and other relevant covariates, we found baseline plasma CRP concentration to be a highly significant overall predictor of post-deployment CAPS scores (p=0.002): each 10-fold increment in CRP concentration was associated with an odds ratio of non-zero outcome (presence vs. absence of any PTSD symptoms) of 1.51 (95% CI, 1.15–1.97; p = 0.003) and a fold increase in outcome when non-zero (extent of symptoms when present) of 1.062 (95% CI, 0.99–1.14; p = 0.086).
Conclusions and Relevance
A marker of peripheral inflammation, plasma CRP, may be prospectively associated with PTSD symptom emergence, suggesting that inflammation may predispose to PTSD.
Introduction
Observational studies largely support an association of post-traumatic stress disorder (PTSD 1) with increased peripheral inflammation (for a recent review of the overall evidence, see 2). For instance, one large cross-sectional community-based study found that patients with PTSD had about twice the odds of those without this disorder of elevation in the inflammatory marker, C-reactive protein (CRP) 3. Similarly, while some case-control studies have had negative or equivocal findings (e.g., 4,5), in most such studies PTSD cohorts have had significantly greater plasma levels of CRP or IL-6, among other inflammatory markers, than did controls (e.g., 6–11). This association is of prognostic significance because low grade inflammation is likely involved in the pathophysiology of the metabolic syndrome 12–14, a major cardiovascular risk factor 15,16; and, indeed, PTSD has been found to be associated with this syndrome 17–24.
It is plausible that the observed association between PTSD and inflammation is due to PTSD-related stress hormone dysregulation leading to alterations in immune, and therefore inflammatory signaling (see, e.g., 7,25–27). However, given the cross-sectional nature of the evidence at hand, it remains possible that rather than PTSD promoting inflammation, inflammation places individuals at heightened risk for developing PTSD in the setting of trauma – in other words, the direction of causality runs from inflammation to PTSD rather than from PTSD to inflammation.
Service members serving in the Iraq and Afghanistan conflicts endure substantial combat stress and consequent PTSD 28. The Marine Resiliency Study (MRS) is a prospective field study of approximately 2,600 Marines and Sailors deployed to Iraq or Afghanistan, during which PTSD severity and various physiological and psychological parameters were determined pre- and post-deployment, affording an outstanding opportunity to investigate the causal relationship between inflammation and PTSD. In the current study, we have determined whether baseline peripheral inflammation, assessed by plasma CRP in the MRS, contributes to post-deployment PTSD symptomatology, assessed by scores on the Clinician Administered PTSD Scale (CAPS), adjusting for trauma exposure and other relevant covariates.
Methods
Subjects
MRS is a prospective, longitudinal study of biological and neuropsychological modulators of combat stress-related PTSD in Marines 29. Approval was received and has been maintained since August 2007 from the Institutional Review Boards of the University of California, San Diego, VA San Diego Research Service, and Naval Health Research Center. Subjects were recruited from four all-male infantry battalions that were imminently deploying to a war zone. Participation was requested of 2,978 subjects, of whom 2,610 (87.6%) provided written informed consent and were enrolled. Assessment of enrolled subjects began in July 2008 and continued through May 2012. Fifty-five of the enrollees were excluded from the current analysis because they did not deploy with their cohort or withdrew prior to completing the pre-deployment visit, so that the number of subjects included was 2,555. The demographics of these subjects are summarized in Table 1.
Table 1.
Selected baseline and post-deployment characteristics of study subjects. CRP, C-reactive protein; BMI, body mass index; AUDIT-C, Alcohol Use Disorders Identification Test–consumption; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; CAPS, Clinician Administered PTSD Scale; CES, Combat Exposure Scale; PBE, post-battle experience; visit 0, baseline; visit 2, three months post-deployment; visit 3, six months post-deployment. See Methods for definition of variables and for additional details concerning subjects’ demographics and military characteristics.
| N | Min. | Max. | Mean or Percentagea | Median | Std. Deviation | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age | 2548 | 18 | 48 | 22.78 | 21.83 | 3.51 |
| Ethnicitya | 2540 | |||||
| Non-Hispanic | 76.5% | |||||
| Hispanic | 23.2% | |||||
| Racea | 2503 | |||||
| European-American | 82.7% | |||||
| African-American | 4.7% | |||||
| Asian-American | 2.7% | |||||
| American Indian | 1.4% | |||||
| Pacific Islanders | 1.5% | |||||
| Mixed | 5.0% | |||||
| Educational Levela | 2538 | |||||
| High School Graduate | 64.3% | |||||
| College | 32.3% | |||||
| Post-Graduate | 0.5% | |||||
| Marital Statusa | ||||||
| Never Married | 61.1% | |||||
| Married | 34.8% | |||||
| Divorced or Separated | 3.5% | |||||
| Military Characteristics | ||||||
| Service Length, months | 2538 | 0 | 324 | 36.29 | 26.00 | 24.45 |
| Previously Deployed | 2541 | -- | -- | 51.3% | -- | -- |
| Enlisted | 2540 | 97.4% | ||||
| Plasma CRP, mg/l | 2484 | 0.03 | 28.53 | 1.93 | 0.79 | 3.31 |
| Waist circumference, inches | 2533 | 25.75 | 48.63 | 33.62 | 33.25 | 3.00 |
| BMI | 2533 | 18.83 | 41.41 | 27.60 | 27.42 | 3.24 |
| Mean arterial blood pressure, mm Hg | 2527 | 64.67 | 148.33 | 90.38 | 90.00 | 7.98 |
| AUDIT-C | 2527 | 0 | 12 | 5.06 | 5.00 | 3.61 |
| BAI, visit 0 | 2519 | 0 | 53 | 6.79 | 4.00 | 7.85 |
| BAI, visit 2 | 1850 | 0 | 57 | 4.79 | 2.00 | 7.36 |
| BAI, visit 3 | 1609 | 0 | 63 | 4.22 | 1.00 | 7.26 |
| BDI, visit 0 | 2526 | 0 | 51 | 6.59 | 4.00 | 7.67 |
| BDI, visit 2 | 1854 | 0 | 54 | 5.05 | 2.00 | 6.80 |
| BDI, visit 3 | 1612 | 0 | 46 | 4.79 | 2.00 | 6.82 |
| CAPS, visit 0 | 2533 | 0 | 101 | 14.89 | 10.00 | 15.37 |
| CAPS, visit 2 | 1861 | 0 | 120 | 17.40 | 12.00 | 18.01 |
| CAPS, visit 3 | 1617 | 0 | 107 | 15.41 | 10.00 | 17.39 |
| PTSD, visit 0 | 2533 | 4.7% | ||||
| PTSD, visit 2 | 1861 | 6.3% | ||||
| PTSD, visit 3 | 1617 | 5.1% | ||||
| CES | 2189 | 0 | 64 | 13.57 | 9.00 | 11.39 |
| PBE | 2204 | 0 | 15 | 5.65 | 4.00 | 4.79 |
A small proportion of subjects did not provide data on these demographic traits so that the percentages do not add up to 100%.
Data were collected approximately 1 month before a seven month-deployment (baseline; visit 0) and at 1 week, and 3 and 6 months following the deployment (visits 1, 2, & 3). Among the 2,555 included subjects, baseline plasma CRP concentrations were included from 2,484 subjects (97.2%) and baseline CAPS score from 2,533 (99.1%). For the other specific baseline variables utilized in the current statistical analyses (anthropometrics, psychometrics, and demographics; see below), individuals with included data ranged from 2,503 to 2,548 (98.0% to 99.7%). Data on combat exposure were obtained at visit 1 and were included from 2,215 subjects (86.7%); visit 2 CAPS scores from 1,861 subjects (72.8%); and visit 3 CAPS scores from 1,609 subjects (63.0%).
Measures
CAPS 30, a gold standard PTSD symptom scale, was the primary outcome measure for our analyses, since as a 136-point numerical scale it would be expected to yield greater discriminant power than the binary outcome of PTSD diagnosis. Trauma exposure occurring during combat was assessed with the Deployment Risk and Resilience Inventory Combat Experiences Scale (CES; http://www.ptsd.va.gov/), and exposure occurring in the aftermath of combat with the DRRI post-battle experience scale (PBE; http://www.ptsd.va.gov/). Baseline high-sensitive CRP plasma levels were measured using an enzyme-linked immunosorbent assay (ALPCO Diagnostics, Salem, NH). Measures for variables not included in the final regression model are described in the Supplementary Material (eMethods).
Statistical Methods
The association of our predictors of interest with CAPS was determined using zero-inflated negative binomial regression (ZINBR). Please see the Supplementary Material for a description of this method and the rationale for its choice. Potential confounders were selected for inclusion in regression modeling on the basis of their univariate association, at a lenient significance threshold (p<0.2), with both the outcome (post-deployment CAPS) and the predictor of interest (plasma CRP concentration) (determined by ANOVA, linear regression, or ZINBR as appropriate). The values for plasma CRP concentrations were skewed and were therefore log-transformed prior to analyses. Ordinal and binomial logistic regression were used to determine the effects of the same predictors as in the final ZINBR model (Table 2) on the categorical outcomes at visit 2 of full PTSD (as defined in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition [DSM-IV]) 31, partial PTSD 32–34, or no PTSD. Statistical analyses were performed with either SPSS v20.0 (IBM, Armonk, NY) or (in the case of ZINBR) with the R statistical package (http://cran.r-project.org/). All p values reported are two-tailed.
Table 2.
Zero-inflated negative binomial regression (ZINBR) model of post-deployment (visit 2) Clinician Administered PTSD Scale score. The number of subjects in this model was 1719. OR, odds ratio; CI, confidence interval; CAPS0, Clinician Administered PTSD Scale score at visit 0 (baseline); CES, Combat Exposure Scale score; PBE, post-battle experience score; CRP, baseline plasma C-reactive protein concentration.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Zero model | Count model | ||||||
|
| |||||||
| Variable | ORa,b | 95% CI | P value | Fold changec,d | 95% CI | P value | Overall P valuee |
| Interceptb,d | 1.254 | 0.765 – 2.054 | 0.369 | 10.569 | 9.288 – 12.026 | 0.000 | |
|
| |||||||
| Cohort 1 | 1.922 | 1.077 – 3.429 | 0.027 | 1.031 | 0.895 – 1.186 | 0.676 | 0.000 |
| Cohort 2 | 0.569 | 0.359 – 0.902 | 0.016 | 0.943 | 0.833 – 1.068 | 0.354 | 0.000 |
| Cohort 3 | 0.632 | 0.413 – 0.968 | 0.035 | 0.778 | 0.699 – 0.865 | 0.000 | 0.000 |
| Cohort 4 | 0f | ||||||
| CAPS0 | 1.097 | 1.075 – 1.119 | 0.000 | 1.020 | 1.018 – 1.023 | 0.000 | 0.000 |
| CES | 1.029 | 1.005 – 1.054 | 0.019 | 1.009 | 1.004 – 1.014 | 0.001 | 0.000 |
| PBE | 1.077 | 1.026 – 1.131 | 0.003 | 1.039 | 1.026 – 1.053 | 0.000 | 0.000 |
| log CRP | 1.507 | 1.153 – 1.969 | 0.003 | 1.062 | 0.991 – 1.138 | 0.086 | 0.002 |
Ratio of approximate odds of non-zero outcome (computed by exponentiating the corresponding coefficient in the regression model and adjusted for the variables listed in the table).
Value for the intercept indicates approximate odds of non-zero outcome at baseline (cohort equals 4 and all other parameters have zero values).
Approximate fold change in outcome, in the event of a non-zero outcome (computed by exponentiating the corresponding coefficient in the regression model and adjusted for the variables listed in the table).
Value for the intercept indicates approximate outcome, in the event of a non-zero outcome, at baseline (cohort equals 4 and all other parameters have zero values).
By the likelihood ratio test
This parameter is set to zero because it is redundant.
Results
Choice of Outcomes and Model Covariates
Baseline and post-deployment values of subjects for the variables included in the statistical models are listed in Table 1, along with selected additional characteristics. PTSD symptomatology, assessed by CAPS scores (see Methods), increased significantly between the baseline and three month post-deployment visits used for our analysis (visits 0 and 2), and then trended back towards baseline in line with findings in a recent systematic review 35. Of note, in contrast to CAPS, subjects’ scores on the anxiety and depression scales, BAI and BDI (see eMethods for description of these metrics), dropped markedly after completion of deployment (Table 1), potentially reflecting the relief experienced by service personnel upon return from combat. Thus, the observed post-deployment increases in PTSD symptomatology were not attributable to broad psychopathology or general psychological distress.
We included baseline CAPS scores (hereafter, termed CAPS0) as a covariate in all statistical analyses of the outcome of visit 2 CAPS (hereafter, CAPS2) so as to adjust for any differences between subjects in CAPS2 that were attributable to pre-existing differences in CAPS0; this also adjusted for any effects of baseline PTSD symptomatology on the subsequent trajectory of the disorder. In addition to CAPS0, CES and PBE scores (determined at visit 1 immediately following deployment) were also included as covariates in regression models to adjust for differences between subjects in traumatic exposure during and after combat, respectively (please see Methods for details). Moreover, since the four MRS battalions differed from one another in their war zone experiences and in the timing of their training regimen relative to the period of data collection, cohort assignment of each subject was set as a factor in regression analysis. Multiple other potential confounders were evaluated, including several previously associated with both PTSD and peripheral inflammation (baseline depression, anxiety, and alcohol and tobacco use 36–52) and various anthropometric and demographic variables (listed in Table 1); however, none met criteria for inclusion in the regression models.
Zero-Inflated Negative Binomial Regression of Post-Deployment CAPS
In accordance with the analyses described above, our ZINBR model (please see Methods) of CAPS2 comprised plasma CRP concentration, baseline CAPS, cohort assignment, and CES and PBE scores. CRP was a highly significant overall predictor of CAPS2 in this model (p = 0.002 by likelihood-ratio test), as were each of the other predictors (Table 2). (CRP was also a highly significant predictor in the analogous linear regression model with the same covariates [with a similar p value; p = 0.002]; however, as noted in the Supplementary Material, ZINBR is significantly superior to linear regression when modeling the outcome of CAPS.) We assessed all two-way interactions with CRP; none were statistically significant. Of note, based on analysis of the scores on CAPS subscales, the greatest effect of CRP appeared to be in the domain of hyper-arousal (subscale D of CAPS; overall p = 0.0004), with less of an effect on numbing (subscale CN; p = 0.032), and lesser effects still on re-experiencing (subscale B; p = 0. 277) and avoidance (subscale CA; p = 0.571)
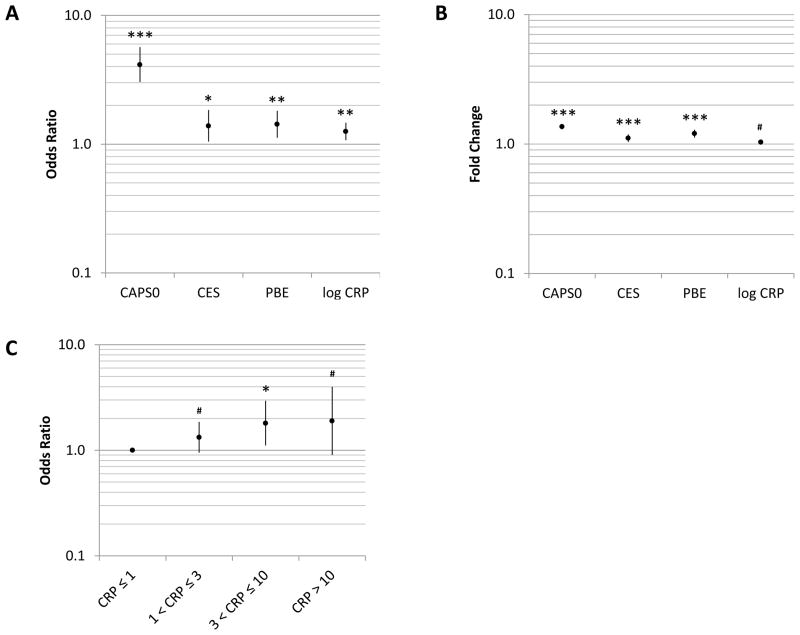
In the zero component of the ZINBR model, CRP was a positive predictor of CAPS2: each one unit increment in log10 plasma CRP concentration (i.e., each 10-fold increase in CRP concentration) was associated with a fold change in the approximate odds of obtaining a CAPS2 score greater than zero (hereafter referred to as odds ratio of non-zero CAPS2) of 1.507 (95% confidence interval: 1.153–1.969; p = 0.003) (Table 2). Stated in the context of the range of CRP concentrations in our study population, a one standard deviation increase in log10 CRP (corresponding to a 3.57-fold increase in CRP concentration) was associated with an odds ratio (OR) of 1.254 (1.082–1.454; Figure 1A). By comparison, one standard deviation increases in other measures were associated with ORs of 4.149 (3.060–5.626; p < 0.001); 1.388 (1.055–1.826; p = 0.019), and 1.428 (1.131–1.802; p = 0.003) in the case of CAPS0, CES, and PBE, respectively. Consistent with the findings obtained with CRP treated as a continuous predictor, categorization of CRP revealed a trend toward greater OR of non-zero outcome with increasing CRP category (though, there was, as expected, a loss of statistical power) (Figure 1C).
Figure 1.
A, Adjusted odds ratios of a non-zero Clinician Administered PTSD Scale score at visit 2 (CAPS2) associated with one standard deviation increases in the indicated variables. B, Adjusted fold changes in CAPS2 associated with one standard deviation increases in the indicated variables. Data in A and B are from the zero inflated negative binomial regression (ZINBR) model summarized in Table 2. C, Odds ratios of non-zero CAPS2 by baseline plasma C-reactive protein concentration category (≤1 [reference category], 1–3, 3–10, and >10 mg/l), as determined by ZINBR and adjusted for the same covariates as the model in Table 2. CAPS0, Clinician Administered PTSD Scale at visit 0 (baseline); CES, Combat Exposure Scale; PBE, post-battle experience; CRP, baseline plasma C-reactive protein concentration. Error bars delineate 95% CIs; ***, p<0.001; **, p<0.01, *, p<0.05, #, p<0.1 (all values two-tailed). Please refer to the text for details.
Likewise, CRP was a positive predictor of CAPS2 scores in the count component of the ZINBR model (which approximately predicts the extent of the outcome when it is non-zero; please see Methods): each 10-fold increase in CRP concentration was associated with a 1.062-fold increase in CAPS2. However, this effect was statistically significant only at the trend level (95% confidence interval: 0.991–1.138; p = 0.086 [two-tailed]) (Table 2). Accounting for the ranges in values of the predictors, a one standard deviation increase in log10 CRP was associated with 1.034-fold change (0.995–1.074) in CAPS2, while one standard deviation increases were associated with fold changes of 1.362 (1.310–1.416; p < 0.001), 1.110 (1.046–1.177; p = 0.001), and 1.203 (1.131–1.280; p < 0.001), in the cases of CAPS0, CES, and PBE, respectively (Figure 1B).
Logistic Regression of Post-Deployment PTSD
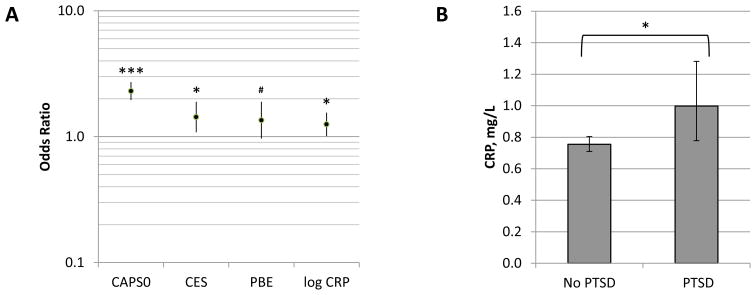
In addition to analysis of the continuous outcome of visit 2 CAPS, we performed logistic regression of the categorical outcome of PTSD (DSM-IV definition 31) at visit 2, using the same covariates as in the ZINBR model. CRP was a significant predictor in this analysis as well, albeit not to as great a degree as in the ZINBR model: each 10-fold increment in CRP concentration was associated with an OR of PTSD of 1.501 (1.017 – 2.215; p = 0.041). Taking into account the ranges of predictor values, a one standard deviation increase in log10 CRP was associated with an OR of PTSD of 1.252, while, by comparison, one standard deviation increases in CAPS0, CES, and PBE were associated with ORs of 2.303, 1.431, and 1.351 for respectively (Table 3 and Figure 2A). Conversely, adjusted baseline CRP values for subjects with and without PTSD at visit 2 were 0.998 and 0.755 mg/l, respectively (adjusted for the same covariates as the logistic regression model) (Figure 2B). CRP was similarly a significant predictor in ordinal logistic regression of the diagnostic categories of PTSD, partial PTSD 32–34, or neither (p = 0.029; please see eResults and eFigure 1).
Table 3.
Binomial logistic regression of post-deployment (visit 2) PTSD diagnosis. The number of subjects in this model was 1719. OR, odds ratio; CI, confidence interval; CAPS0, visit 0 (baseline) Clinician Administered PTSD Scale score; CES, Combat Exposure Scale score; PBE, post-battle experience score; CRP, baseline plasma C-reactive protein concentration.
| Variable | ORa,b | 95% CI | P value |
|---|---|---|---|
| Interceptb | 0.009 | 0.004 – 0.020 | <0.001 |
|
| |||
| Cohort 1 | 0.999 | 0.407 – 2.452 | 0.999 |
| Cohort 2 | 1.130 | 0.539 – 2.366 | 0.747 |
| Cohort 3 | 0.809 | 0.449 – 1.457 | 0.480 |
| Cohort 4 | 0c | ||
| CAPS0 | 1.056 | 1.044 – 1.067 | <0.001 |
| CES | 1.032 | 1.007 – 1.058 | 0.012 |
| PBE | 1.065 | 0.993 – 1.142 | 0.079 |
| log CRP | 1.501 | 1.017 – 2.215 | 0.041 |
Odds ratio of PTSD (computed by exponentiating the corresponding coefficient in the regression model and adjusted for the variables listed in the table).
Value for intercept indicates the odds of PTSD at baseline (cohort equals 4 and all other parameters have zero values).
This parameter is set to zero because it is redundant.
Figure 2.
A, Adjusted odds ratios of PTSD at visit 2 associated with one standard deviation increases in the indicated variables. Data are from the binomial logistic regression model summarized in Table 3. B, Baseline plasma C-reactive protein concentration of subjects without or with PTSD at visit 2, adjusted for the same covariates as the logistic regression model in Table 3. CAPS0, Clinician Administered PTSD Scale at visit 0 (baseline); CES, Combat Exposure Scale; PBE, post-battle experience; CRP, baseline plasma C-reactive protein concentration. Error bars delineate 95% CIs; ***, p<0.001; *, p<0.05, #, p<0.1 (all values two-tailed). Please refer to the text for details.
We also performed sub-group analyses excluding subjects at various thresholds of the model variables – plasma CRP, baseline CAPS, CES, and PBE. CRP effects in these subsets were generally similar to those obtained when considering all subjects, indicating that the effects are not being “driven” by subjects at the extremes of baseline PTSD symptomatology, plasma CRP, or combat exposure (please see eResults and eFigure 2). Moreover, CRP was not significantly associated with baseline CAPS or PTSD diagnosis (p = 0.518 and 0.217, respectively), indicating that CRP is not a mediator or proxy for the effects of one of these other predictors on CAPS2.
Comment
We report here a significant effect of baseline CRP on post-deployment PTSD symptom emergence in Marine and Navy combatants, suggesting that levels of this inflammatory marker may be prospectively associated with resilience versus risk for PTSD. CRP predominantly influenced the likelihood of subjects endorsing the presence vs. absence of PTSD symptoms rather than the extent of symptoms when present (as indicated by its greater statistical significant in the zero model of the ZINBR compared with the count and logistic regression models), and had a greater effect on the hyper-arousal and numbing symptom clusters than on the other clusters. Conceivably, high CRP levels mark a state of vulnerability to developing these symptoms of PTSD, while the influences of other factors prevail in determining the severity of symptoms once they are manifested.
It is sometimes postulated that the observed association between PTSD and peripheral inflammation owes to the former disorder predisposing to the latter, plausibly due to PTSD-induced dysregulation of the stress axis resulting in disinhibition of pro-inflammatory pathways (see, e.g., 7,25–27). Our data raise the converse possibility – that individuals with lesser inflammation may be relatively resilient and those with greater inflammation relatively vulnerable to developing PTSD symptoms. In other words, a characteristic conventionally considered to be ‘physiological’ rather than ‘psychological’ may significantly influence the tendency to develop PTSD. However, the possibility that higher CRP levels at baseline resulted from preceding trauma cannot be excluded, This supposition is also supported by the recent finding that the risk for PTSD following medical illness during military deployment is comparable to that following physical injury 53,54.
The underlying mechanism may involve the actions of inflammatory cytokines, which in addition to their well-characterized adverse effects on metabolic and therefore cardiovascular health 12–14 also have adverse effects on mental health 55–57. In particular, depression, has long been known to be associated with increased peripheral inflammation 36,46–50 (with some recent studies suggesting that baseline inflammation may predict subsequent depression 37,38) and, notably, inflammatory cytokines are known to elicit symptoms of depression 58–63 (reviewed in 2,56,57,64). Furthermore, peripheral inflammation has been associated with impairments in memory and executive function 65–69. Significantly, inflammatory cytokines have been demonstrated to suppress hippocampal neurogenesis in animals 70 and have been associated with low hippocampal volume in humans 71, a neuroanatomical trait that might mark vulnerability to PTSD – in studies of identical twins discordant for combat trauma exposure, twin pairs in which the combat-exposed member developed PTSD had smaller hippocampi than the other twin pairs 72,73.
Nevertheless, the causal relationships between psychiatric disorders and inflammation are likely to be complex. For instance, in one recent large, prospective, population-based study, cumulative episodes of depression predicted subsequent CRP levels 74, although this effect was attenuated after controlling for BMI and smoking, suggesting that it might be attributable in part to depression-related lifestyle changes rather than directly to the neurophysiology of depression. Importantly with respect to PTSD, much work in animal models supports the conclusion that chronic stress induces immunological changes that culminate in a pro-inflammatory phenotype (see, e.g., 75–77). Thus, inflammation may both contribute to PTSD and be a consequence of the stressors that led to the disorder.
Strengths of our study include its size, prospective design, and adjustment for multiple potential confounders. Moreover, owing to the youth of the study participants (mean age, 22.8 years) and their relative physical fitness (given the requirements for combat deployment), it is unlikely for chronic physical illness to have confounded the observed effects of baseline CRP on post-deployment PTSD symptomatology. However, certain limitations merit discussion. First, the relative fitness of our cohort also limits the generalizability of our findings, as does the absence of females. In addition, whereas CRP concentrations fluctuate substantially in response to transient inflammatory states (such as minor infections; 78), values in our subjects were determined only once and thus may be relatively “noisy”. Moreover, use of anti-inflammatory medications, which might also have contributed to variability in CRP levels, was not ascertained in our study. However, it should be noted that such variability would generally be expected to bias towards the null hypothesis.
Finally, with regard to missing data, as noted in the Methods, 27.2% of subjects did not have determination of CAPS2. However, CRP values did not differ significantly between subjects for whom CAPS2 scores were present vs. absent; and, conversely, CAPS2 was not significantly different when comparing subjects for whom CRP values were present vs. absent (not shown). Moreover, we have found the “effect size” of CRP on visit 2 CAPS or PTSD diagnosis (the CRP-associated odds ratio or fold change) to be generally similar across subsets of subjects having markedly different mean values for the various covariates in our regression models (please see eResults). This suggests that even if the subjects with missing data were considerably different from the other subjects with regard to CES, PBE, or baseline CAPS scores, the CRP effect sizes that we observed are likely to be similar to those that would have been obtained had their data not been missing. Taken together, these results suggest that missing data might not have appreciably biased our findings concerning CRP effects on PTSD symptomatology.
Our results, if validated by future studies, could have important clinical implications. If peripheral inflammation contributes to the development of PTSD, interventions to decrease inflammation, such as dietary or life-style modifications 79–81, might ameliorate the severity of this disorder. At minimum, our findings are consistent with the old adage: mens sana in corpore sano, a healthy mind in a healthy body.
Supplementary Material
Acknowledgments
Funding/Support: Dr. Eraly was funded by a UCSD Department of Medicine Career Award. Drs. Baker, Nievergelt, O’Connor and MRS personnel were supported by VA Health Service Research and Development Project Number SDR 09-0128, National Institutes of Health MH093500, the Marine Corps, and the Navy Bureau of Medicine and Surgery.
Role of the Sponsors: The funding sources had no role in the design and conduct of the study; collection, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Financial Disclosures: None reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the federal government.
Additional Contributions: We acknowledge all MRS co-investigators, Mark A. Geyer, Ph.D. (Department of Psychiatry, UCSD) Victoria B. Risbrough, Ph.D. (VA Center of Excellence for Stress and Mental Health, CESAMH, and Department of Psychiatry, UCSD), Gerald E. Larson Ph.D., (Naval Health Research Center), Brett T. Litz Ph.D.(Dept. of Psychiatry, Boston University School of Medicine, VA Boston Healthcare System,), William P. Nash M.D. (Boston VA Research Institute, BVARI), Nicholas J. Schork (Scripps Translational Research Institute, San Diego) Jennifer J. Vasterling Ph.D.(Dept. of Psychiatry, Boston University School of Medicine, VA Boston Healthcare System), Paul S. Hammer, M.D. (Defense Center of Excellence for Psychological Health and Traumatic Brain Injury, DeCO), Jennifer A. Webb-Murphy, Ph.D. (Naval Center for Combat and Operational Stress Control, NC COSC), as well as members of the administrative core, Anjana Patel, Andrew De La Rosa, and Patricia Gorman, and members of the MRS Team, including logistic coordinators, clinician-interviewers, and data collection staff listed in our methods paper 29. We also thank Michael G. Ziegler, M.D. (Department of Medicine, UCSD), Deborah L. Wingard, Ph.D. (Department of Family and Preventative Medicine, UCSD), Felix K. Yam, Pharm. D. (School of Pharmacy, UCSD), and Florin Vaida Ph.D. (Department of Family and Preventative Medicine, UCSD) for helpful discussions. Finally, we thank the Marine and Navy Corpsmen volunteers for their military service and participation in this study.
Author Contributions: Drs. Nievergelt, and Baker had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Eraly, Nievergelt, O’Connor, and Baker. Acquisition of data: Biswas, Baker, and Nievergelt. Analysis and interpretation of the data: Eraly, Nievergelt, Maihofer, Barkauskas, Agorastos, O’Connor, and Baker. Drafting of the manuscript: Eraly and Barkauskas. Critical revision of the manuscript for important intellectual content: Eraly, Nievergelt, Barkauskas, Agorastos, O’Connor, and Baker. Statistical analysis: Eraly, Nievergelt, Maihofer, and Barkauskas. Obtaining funding: Eraly, Nievergelt, O’Connor, and Baker. Administrative, technical and material support: Nievergelt, Biswas, O’Connor, and Baker. Study supervision: Nievergelt, O’Connor, and Baker. All authors approved the final version of the manuscript.
References
- 1.Yehuda R. Post-Traumatic Stress Disorder. New England Journal of Medicine. 2002;346(2):108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- 2.Baker DG, Nievergelt CM, O’Connor DT. Biomarkers of PTSD: neuropeptides and immune signaling. Neuropharmacology. 2012;62(2):663–673. doi: 10.1016/j.neuropharm.2011.02.027. Epub 2011 Mar 2022. [DOI] [PubMed] [Google Scholar]
- 3.Spitzer C, Barnow S, Völzke H, et al. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: Evidence from the general population. Journal of Psychiatric Research. 2010;44(1):15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 4.von Kanel R, Begre S, Abbas CC, Saner H, Gander ML, Schmid JP. Inflammatory biomarkers in patients with posttraumatic stress disorder caused by myocardial infarction and the role of depressive symptoms. Neuroimmunomodulation. 2010;17(1):39–46. doi: 10.1159/000243084. Epub 2009 Oct 2005. [DOI] [PubMed] [Google Scholar]
- 5.von Kanel R, Hepp U, Kraemer B, et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41(9):744–752. doi: 10.1016/j.jpsychires.2006.06.009. Epub 2006 Aug 2009. [DOI] [PubMed] [Google Scholar]
- 6.Aurer A, Aurer-Kozelj J, Stavljenic-Rukavina A, Kalenic S, Ivic-Kardum M, Haban V. Inflammatory mediators in saliva of patients with rapidly progressive periodontitis during war stress induced incidence increase. Coll Antropol. 1999;23(1):117–124. [PubMed] [Google Scholar]
- 7.Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26(5):447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- 8.Tucker P, Jeon-Slaughter H, Pfefferbaum B, Khan Q, Davis NJ. Emotional and biological stress measures in Katrina survivors relocated to Oklahoma. Am J Disaster Med. 2010;5(2):113–125. doi: 10.5055/ajdm.2010.0013. [DOI] [PubMed] [Google Scholar]
- 9.Vidovic A, Gotovac K, Vilibic M, et al. Repeated assessments of endocrine- and immune-related changes in posttraumatic stress disorder. Neuroimmunomodulation. 2011;18(4):199–211. doi: 10.1159/000322869. Epub 2011 Feb 2019. [DOI] [PubMed] [Google Scholar]
- 10.Guo M, Liu T, Guo JC, Jiang XL, Chen F, Gao YS. Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pac J Trop Med. 2012;5(4):323–325. doi: 10.1016/S1995-7645(12)60048-0. [DOI] [PubMed] [Google Scholar]
- 11.Maes M, Lin AH, Delmeire L, et al. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry. 1999;45(7):833–839. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 12.Shoelson SE, Herrero L, Naaz A. Obesity, Inflammation, and Insulin Resistance. Gastroenterology. 2007;132(6):2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 13.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 14.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15(5):635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM. Pre-Diabetes, Metabolic Syndrome, and Cardiovascular Risk. Journal of the American College of Cardiology. 2012;59(7):635–643. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 17.Agyemang C, Goosen S, Anujuo K, Ogedegbe G. Relationship between post-traumatic stress disorder and diabetes among 105_180 asylum seekers in the Netherlands. The European Journal of Public Health. 2011 Sep 27; doi: 10.1093/eurpub/ckr138. [DOI] [PubMed] [Google Scholar]
- 18.Boyko EJ, Jacobson IG, Smith B, et al. Risk of Diabetes in U.S. Military Service Members in Relation to Combat Deployment and Mental Health. Diabetes Care. 2010 Aug 1;33(8):1771–1777. doi: 10.2337/dc10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qureshi S, Pyne J, Magruder K, Schulz P, Kunik M. The Link Between Post-traumatic Stress Disorder and Physical Comorbidities: A Systematic Review. Psychiatric Quarterly. 2009;80(2):87–97. doi: 10.1007/s11126-009-9096-4. [DOI] [PubMed] [Google Scholar]
- 20.Jin H, Lanouette NM, Mudaliar S, et al. Association of posttraumatic stress disorder with increased prevalence of metabolic syndrome. J Clin Psychopharmacol. 2009;29(3):210–215. doi: 10.1097/JCP.0b013e3181a45ed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heppner P, Crawford E, Haji U, et al. The association of posttraumatic stress disorder and metabolic syndrome: a study of increased health risk in veterans. BMC Medicine. 2009;7(1):1. doi: 10.1186/1741-7015-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Violanti JM, Fekedulegn D, Hartley TA, et al. Police trauma and cardiovascular disease: association between PTSD symptoms and metabolic syndrome. Int J Emerg Ment Health. 2006;8(4):227–237. [PubMed] [Google Scholar]
- 23.Goodwin RD, Davidson JR. Self-reported diabetes and posttraumatic stress disorder among adults in the community. Preventive Medicine. 2005;40(5):570–574. doi: 10.1016/j.ypmed.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Heppner PS, Lohr JB, Kash TP, Jin H, Wang H, Baker DG. Metabolic Syndrome: Relative Risk Associated with Post-Traumatic Stress Disorder (PTSD) Severity and Antipsychotic Medication Use. Psychosomatics. 2012;53(6):550–558. doi: 10.1016/j.psym.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Rohleder N, Karl A. Role of endocrine and inflammatory alterations in comorbid somatic diseases of post-traumatic stress disorder. Minerva Endocrinol. 2006;31(4):273–288. [PubMed] [Google Scholar]
- 26.Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care. 2009;45(4):262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- 27.Jiang JX. Posttraumatic stress and immune dissonance. Chin J Traumatol. 2008;11(4):203–208. doi: 10.1016/s1008-1275(08)60044-9. [DOI] [PubMed] [Google Scholar]
- 28.Seal K, Bertenthal D, Miner CR, Sen S, Marmar C. Bringing the war back home: Mental health disorders among 103 788 us veterans returning from iraq and afghanistan seen at department of veterans affairs facilities. Archives of Internal Medicine. 2007;167(5):476–482. doi: 10.1001/archinte.167.5.476. [DOI] [PubMed] [Google Scholar]
- 29.Baker DG, Nash WP, Litz BT, et al. Predictors of risk and resilience for posttraumatic stress disorder among ground combat Marines: methods of the Marine Resiliency Study. Prev Chronic Dis. 2012;9:E97. doi: 10.5888/pcd9.110134. Epub 2012 May 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 31.Diagnostic and Statistical Manual of Mental Disorders IV, text revision. 2000.
- 32.Schnyder U, Moergeli H, Klaghofer R, Buddeberg C. Incidence and prediction of posttraumatic stress disorder symptoms in severely injured accident victims. Am J Psychiatry. 2001 Apr;158(4):594–599. doi: 10.1176/appi.ajp.158.4.594. [DOI] [PubMed] [Google Scholar]
- 33.Pietrzak RH, Goldstein MB, Malley JC, Johnson DC, Southwick SM. Subsyndromal posttraumatic stress disorder is associated with health and psychosocial difficulties in veterans of Operations Enduring Freedom and Iraqi Freedom. Depress Anxiety. 2009;26(8):739–744. doi: 10.1002/da.20574. [DOI] [PubMed] [Google Scholar]
- 34.Adams RE, Boscarino JA, Galea S. Alcohol use, mental health status and psychological well-being 2 years after the World Trade Center attacks in New York City. Am J Drug Alcohol Abuse. 2006;32(2):203–224. doi: 10.1080/00952990500479522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santiago PN, Ursano RJ, Gray CL, et al. A systematic review of PTSD prevalence and trajectories in DSM-5 defined trauma exposed populations: intentional and non-intentional traumatic events. PloS one. 2013;8(4):e59236. doi: 10.1371/journal.pone.0059236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duivis HE, de Jonge P, Penninx BW, Na BY, Cohen BE, Whooley MA. Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: prospective findings from the heart and soul study. Am J Psychiatry. 2011;168(9):913–920. doi: 10.1176/appi.ajp.2011.10081163. doi: 910.1176/appi.ajp.2011.10081163 Epub 10082011 Jul 10081161. [DOI] [PubMed] [Google Scholar]
- 37.Wium-Andersen MK, Orsted DD, Nielsen SF, Nordestgaard BG. Elevated C-reactive protein levels, psychological distress, and depression in 73 131 individuals. JAMA Psychiatry. 2013;70(2):176–184. doi: 10.1001/2013.jamapsychiatry.102. doi: 110.1001/2013.jamapsychiatry.1102. [DOI] [PubMed] [Google Scholar]
- 38.Gimeno D, Kivimaki M, Brunner EJ, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39(3):413–423. doi: 10.1017/S0033291708003723. doi: 410.1017/S0033291708003723 Epub 0033291708002008 Jun 0033291708003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitfield JB, Heath AC, Madden PA, Pergadia ML, Montgomery GW, Martin NG. Metabolic and Biochemical Effects of Low-to-Moderate Alcohol Consumption. Alcohol Clin Exp Res. 2012;7(10):12015. doi: 10.1111/acer.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alkerwi A, Boutsen M, Vaillant M, et al. Alcohol consumption and the prevalence of metabolic syndrome: a meta-analysis of observational studies. Atherosclerosis. 2009;204(2):624–635. doi: 10.1016/j.atherosclerosis.2008.10.036. doi: 610.1016/j.atherosclerosis.2008.1010.1036 Epub 2008 Nov 1011. [DOI] [PubMed] [Google Scholar]
- 41.Pai JK, Hankinson SE, Thadhani R, Rifai N, Pischon T, Rimm EB. Moderate alcohol consumption and lower levels of inflammatory markers in US men and women. Atherosclerosis. 2006;186(1):113–120. doi: 10.1016/j.atherosclerosis.2005.06.037. Epub 2005 Aug 2001. [DOI] [PubMed] [Google Scholar]
- 42.Deverts DJ, Cohen S, Kalra P, Matthews KA. The prospective association of socioeconomic status with C-reactive protein levels in the CARDIA study. Brain Behav Immun. 2012;26(7):1128–1135. doi: 10.1016/j.bbi.2012.07.017. 1110.1016/j.bbi.2012.1107.1017 Epub 2012 Aug 1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131(5):1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 44.Fu SS, McFall M, Saxon AJ, et al. Post-traumatic stress disorder and smoking: a systematic review. Nicotine Tob Res. 2007;9(11):1071–1084. doi: 10.1080/14622200701488418. [DOI] [PubMed] [Google Scholar]
- 45.Schafer I, Najavits LM. Clinical challenges in the treatment of patients with posttraumatic stress disorder and substance abuse. Curr Opin Psychiatry. 2007;20(6):614–618. doi: 10.1097/YCO.0b013e3282f0ffd9. [DOI] [PubMed] [Google Scholar]
- 46.Deverts DJ, Cohen S, DiLillo VG, et al. Depressive symptoms, race, and circulating C-reactive protein: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosom Med. 2010;72(8):734–741. doi: 10.1097/PSY.0b013e3181ec4b98. Epub 2010 Jul 2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elovainio M, Keltikangas-Jarvinen L, Pulkki-Raback L, et al. Depressive symptoms and C-reactive protein: the Cardiovascular Risk in Young Finns Study. Psychol Med. 2006;36(6):797–805. doi: 10.1017/S0033291706007574. Epub 2006 Apr 2020. [DOI] [PubMed] [Google Scholar]
- 48.Morris AA, Zhao L, Ahmed Y, et al. Association between depression and inflammation--differences by race and sex: the META-Health study. Psychosom Med. 2011;73(6):462–468. doi: 10.1097/PSY.0b013e318222379c. Epub 2011 Jun 2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. Epub 2009 Feb 2002. [DOI] [PubMed] [Google Scholar]
- 50.De Berardis D, Campanella D, Gambi F, et al. The role of C-reactive protein in mood disorders. Int J Immunopathol Pharmacol. 2006;19(4):721–725. doi: 10.1177/039463200601900402. [DOI] [PubMed] [Google Scholar]
- 51.Baker DG, Heppner P, Afari N, et al. Trauma exposure, branch of service, and physical injury in relation to mental health among U.S. veterans returning from Iraq and Afghanistan. Mil Med. 2009;174(8):773–778. [PubMed] [Google Scholar]
- 52.Rasmusson AM, Schnurr PP, Zukowska Z, Scioli E, Forman DE. Adaptation to extreme stress: post-traumatic stress disorder, neuropeptide Y and metabolic syndrome. Experimental Biology and Medicine. 2010 Oct 1;235(10):1150–1162. doi: 10.1258/ebm.2010.009334. [DOI] [PubMed] [Google Scholar]
- 53.Forbes HJ, Jones N, Woodhead C, et al. What are the effects of having an illness or injury whilst deployed on post deployment mental health? A population based record linkage study of UK army personnel who have served in Iraq or Afghanistan. BMC Psychiatry. 2012;12:178. doi: 10.1186/1471-1244X-1112-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McFarlane AC. Health surveillance of deployed military personnel occasionally leads to unexpected findings. BMC Med. 2012;10:126. doi: 10.1186/1741-7015-1110-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raison CL, Borisov AS, Majer M, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65(4):296–303. doi: 10.1016/j.biopsych.2008.08.010. Epub 2008 Sep 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. Epub 2009 Jan 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58(5):445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 58.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66(5):407–414. doi: 10.1016/j.biopsych.2009.03.015. Epub 2009 May 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68(8):748–754. doi: 10.1016/j.biopsych.2010.06.010. Epub 2010 Aug 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19(4):345–350. doi: 10.1016/j.bbi.2004.10.003. Epub 2004 Dec 2008. [DOI] [PubMed] [Google Scholar]
- 61.Miyaoka H, Otsubo T, Kamijima K, Ishii M, Onuki M, Mitamura K. Depression from interferon therapy in patients with hepatitis C. Am J Psychiatry. 1999;156(7):1120. doi: 10.1176/ajp.156.7.1120. [DOI] [PubMed] [Google Scholar]
- 62.Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344(13):961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 63.Valentine AD, Meyers CA, Kling MA, Richelson E, Hauser P. Mood and cognitive side effects of interferon-alpha therapy. Semin Oncol. 1998;25(1 Suppl 1):39–47. [PubMed] [Google Scholar]
- 64.Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13(6):467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Komulainen P, Lakka TA, Kivipelto M, et al. Serum high sensitivity C-reactive protein and cognitive function in elderly women. Age Ageing. 2007;36(4):443–448. doi: 10.1093/ageing/afm051. Epub 2007 May 2030. [DOI] [PubMed] [Google Scholar]
- 66.Wersching H, Duning T, Lohmann H, et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74(13):1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- 67.Cavalieri M, Ropele S, Petrovic K, et al. Metabolic syndrome, brain magnetic resonance imaging, and cognition. Diabetes Care. 2010;33(12):2489–2495. doi: 10.2337/dc10-0851. Epub 2010 Sep 2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bettcher BM, Wilheim R, Rigby T, et al. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun. 2012;26(1):103–108. doi: 10.1016/j.bbi.2011.07.240. Epub 2011 Aug 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elderkin-Thompson V, Irwin MR, Hellemann G, Kumar A. Interleukin-6 and memory functions of encoding and recall in healthy and depressed elderly adults. Am J Geriatr Psychiatry. 2012;20(9):753–763. doi: 10.1097/JGP.0b013e31825d08d6. doi: 710.1097/JGP.1090b1013e31825d31808d31826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goshen I, Kreisel T, Ben-Menachem-Zidon O, et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13(7):717–728. doi: 10.1038/sj.mp.4002055. Epub 2007 Aug 2014. [DOI] [PubMed] [Google Scholar]
- 71.Frodl T, Carballedo A, Hughes MM, et al. Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: high IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Transl Psychiatry. 2012;2:e88. doi: 10.1038/tp.2012.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pitman RK, Gilbertson MW, Gurvits TV, et al. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Ann N Y Acad Sci. 2006;1071(11):242–254. doi: 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry. 2012;71(1):15–21. doi: 10.1016/j.biopsych.2011.09.023. Epub 2011 Nov 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chida Y, Sudo N, Sonoda J, Hiramoto T, Kubo C. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am J Respir Crit Care Med. 2007;175(4):316–322. doi: 10.1164/rccm.200607-898OC. Epub 2006 Nov 2016. [DOI] [PubMed] [Google Scholar]
- 76.Wei L, Simen A, Mane S, Kaffman A. Early life stress inhibits expression of a novel innate immune pathway in the developing hippocampus. Neuropsychopharmacology. 2012;37(2):567–580. doi: 10.1038/npp.2011.239. doi: 510.1038/npp.2011.1239 Epub 2011 Oct 1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Powell ND, Bailey MT, Mays JW, et al. Repeated social defeat activates dendritic cells and enhances Toll-like receptor dependent cytokine secretion. Brain Behav Immun. 2009;23(2):225–231. doi: 10.1016/j.bbi.2008.09.010. Epub 2008 Sep 2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pepys MB, Hirschfield GM, Tennent GA, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440(7088):1217–1221. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 79.Kao PC, Shiesh SC, Wu TJ. Serum C-reactive protein as a marker for wellness assessment. Ann Clin Lab Sci. 2006;36(2):163–169. [PubMed] [Google Scholar]
- 80.Shen J, Ordovas JM. Impact of genetic and environmental factors on hsCRP concentrations and response to therapeutic agents. Clin Chem. 2009;55(2):256–264. doi: 10.1373/clinchem.2008.117754. doi: 210.1373/clinchem.2008.117754 Epub 112008 Dec 117712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beavers KM, Nicklas BJ, Shen J, et al. Effects of lifestyle interventions on inflammatory markers in the metabolic syndrome. Front Biosci (Schol Ed) 2011;3(2):168–177. doi: 10.2741/s142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.