Abstract
We examined the effects of iron oxide nanoparticles (IONPs) on mitochondrial respiratory chain complexes activities and mitochondrial coupling in young (3 months) and middle-aged (18 months) rat liver, organ largely involved in body iron detoxification. Isolated liver mitochondria were extracted using differential centrifugations. Maximal oxidative capacities (V max, complexes I, III, and IV activities), V succ (complexes II, III, and IV activities), and V tmpd, (complex IV activity), together with mitochondrial coupling (V max/V 0) were determined in controls conditions and after exposure to 250, 300, and 350 μg/ml Fe3O4 in young and middle-aged rats. In young liver mitochondria, exposure to IONPs did not alter mitochondrial function. In contrast, IONPs dose-dependently impaired all complexes of the mitochondrial respiratory chain in middle-aged rat liver: V max (from 30 ± 1.6 to 17.9 ± 1.5; P < 0.001), V succ (from 33.9 ± 1.7 to 24.3 ± 1.0; P < 0.01), V tmpd (from 43.0 ± 1.6 to 26.3 ± 2.2 µmol O2/min/g protein; P < 0.001) using Fe3O4 350 µg/ml. Mitochondrial coupling also decreased. Interestingly, 350 μg/ml Fe3O4 in the form of Fe3+ solution did not impair liver mitochondrial function in middle-aged rats. Thus, IONPs showed a specific toxicity in middle-aged rats suggesting caution when using it in old age.
1. Introduction
Because of their unique properties, some nanoparticles have been approved for clinical use like iron oxide nanoparticles (IONPs). IONPs hold immense potential in a vast variety of biomedical applications such as magnetic resonance imaging (MRI), targeted delivery of drugs or genes, tissue engineering, targeted destruction of tumor tissue through hyperthermia, magnetic transfections, iron detection, chelation therapy, and tissue engineering [1–4]. Reports demonstrate that IONPs have the ability to assess focal hepatic lesions [5] and to label human hepatocytes [6].
However, nanoparticles pose a high health risk because of their ability to reach every part of the organs and tissues and their interaction with cellular functions.
Concerning NP clearance, it is known that they are primarily phagocytozed by macrophages in the liver (Kupffer cells) [7]. Thus, the largest detoxification organ of human beings, the liver, is reached by the highest amount of nanoparticles, over all the other tissues [8, 9]. Furthermore, mitochondria are considered a major cell compartment relevant to possible nanoparticle toxicity [10]. Impairment of mitochondria might be a key problem since mitochondrial dysfunction may result in reduced cellular ATP delivery, increased reactive oxygen species production, and triggering of apoptosis pathways. Accordingly, mitochondrial dysfunctions occur early in many acute or chronic diseases such as peripheral arterial or pulmonary diseases [11, 12].
Mitochondrial involvement in IONPs toxicity remains controversial and either no deleterious effects [13, 14] or mitochondrial impairments have been observed [15–17].
Concerning Fe3O4 nanoparticles, we recently reported a lack of IONPs toxicity in liver mitochondria in young rats [18]. Nevertheless, age-related accumulation of iron increases the potential for free redox-active iron, which can promote oxidative stress and mitochondrial damage [19]. More recently, [20] reported that age-dependent accumulation of mitochondrial iron may increase mitochondrial dysfunction and oxidative damage, thereby enhancing the susceptibility to apoptosis. Therefore, age might modify the susceptibility of mitochondria to iron nanoparticles (NPs).
Since, to date, no study investigated the potential effects of iron oxide nanoparticles on middle-aged mitochondria and since the liver is a key organ in iron and NPs detoxification, we investigated and compared for the first time the effects of three different concentrations of Fe3O4 nanoparticles (250, 300, and 350 μg/mL) on young and middle-aged liver mitochondrial respiratory chain complexes activities and on mitochondrial coupling of phosphorylation to oxidation.
2. Materials and Methods
2.1. Materials and Reagents
Iron oxide nanoparticles were acquired from Unit of Research UR11ES30, Faculty of science of Bizerte, Tunisia. They were prepared by the polyol process starting from iron (II) acetate as metal precursor and diethylene glycol (DEG) as solvent purchased from ACROS Organics. All chemicals were used as received without further purification. Deionized water was used in these preparations. For the synthesis of the magnetite (Fe3O4) nanoparticles, an appropriate amount of iron (II) acetate precursor was added to a given volume (125 mL) of DEG to reach nominal iron cations concentration of 0.2 M. The mixture was then refluxed at a rate of +6°C min−1under mechanical stirring up to boiling point and then maintained at this temperature for about 2 h. The powders were washed several times with ethanol and then with acetone under ultrasonication with intermittent centrifugation and then dried in air at 50°C [18].
2.2. Nanoparticle Characterization Using Transmission Electron Microscopy (TEM)
The size and shape of prepared particles were analyzed on a JEOL-100-CX II transmission electron microscope (TEM) operating at 100 kV equipped with an energy dispersive spectrometer (EDX) [21].
TEM imaging of Fe3O4 nanoparticles showed that the powders are constituted by roughly spherical almost nonagglomerated particles. In addition, about 250 particles have been counted for the average particle size and histogram determinations. The calculated average diameter was 9 nm with a SD of 1.6 nm (Figure 1).
Figure 1.
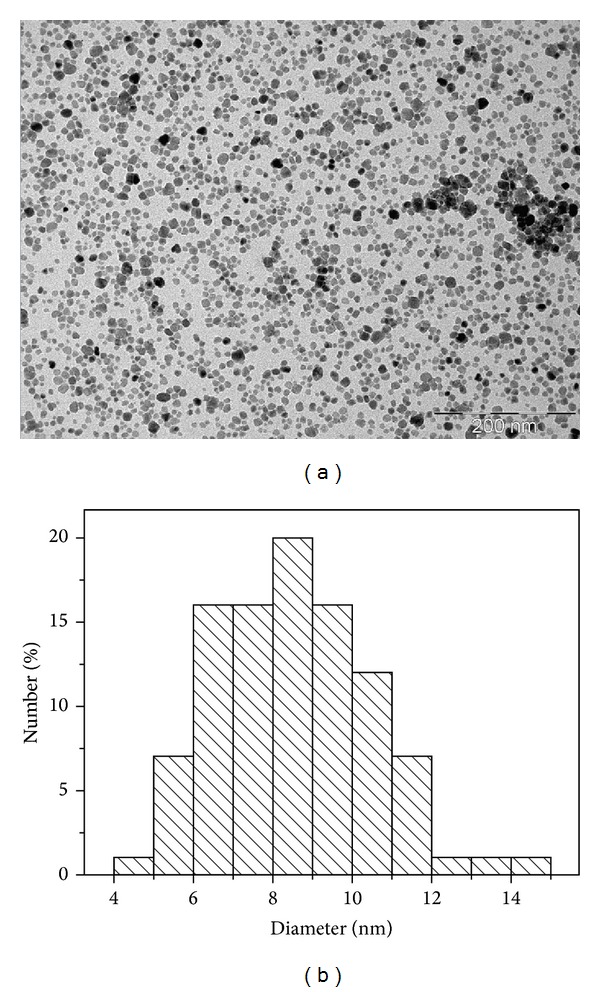
TEM image (a) and size histogram (b) of the Fe3O4 nanoparticles.
2.3. Animals
Young (n = 6, age 3 months) and middle-aged (n = 6, age 18 months) Wistar male rats were housed in a thermoneutral environment (22 ± 2°C), on a 12 : 12 h photoperiod, and were provided food and water ad libitum. This investigation was carried out in accordance with the Guide for the Care and Use of Laboratory published by the US National Institute of Health and approved by the institutional animal care committee (NIH publication number 85-23, revised 1996).
Rats were submitted to general anesthesia with 3% isoflurane and oxygen (1 L/min) in an induction chamber (Minerve, Esternay, France). Anesthesia was maintained with 1.5% isoflurane and 1 L/min oxygen at under spontaneous ventilation. A midline laparotomy was performed and the liver was excised, cleaned, and then immediately used for the study of respiratory parameters.
Similarly, three additional Wistar rats aged 18 months were studied, in order to investigate the potential effect of iron oxide per se.
2.4. Extraction of Mitochondria
All operations were carried on ice. A piece of tissue was placed into buffer A containing 50 mM tris, 1 mM EGTA, 70 mM sucrose, 210 mM mannitol, and pH 7.40 at +4°C. Tissue was finely minced with scissors, placed in buffer A, and homogenized with a Potter-Elvehjem. Then, the homogenate was centrifuged at 1300 ×g for 3 min and 4°C. The supernatant was centrifuged at 10,000 ×g for 10 min and 4°C to sediment mitochondria. Finally, the mitochondrial pellet was washed twice and then suspended in 50 mM tris, 70 mM sucrose, 210 mM mannitol, and pH 7.4 at +4°C. Protein content was routinely quantified with a Bradford assay using bovine serum albumin as a standard [22]. Mitochondria were kept on ice and used within 4 h.
2.5. Exposure of Mitochondria to Nanoparticles or to Iron Oxide
The iron oxide nanoparticles were mixed with a solution of NaCl 9%. The mixture was then stirred vigorously and sonicated for 60 min to break up aggregates. Particle suspensions were vortexed immediately before each use. Before measurement, 3 mL of solution M containing 100 mM KCL, 50 mM mops, 1 mM EGTA, 5 mM Kpi, and 1 mg/mL bovine serum albumin (BSA) was added to the oxygraph chambers for 10 min. Then, 0.50 mg of mitochondrial protein was placed in the oxygraph chambers with 10 mM glutamate and 2.50 mM malate as substrates. The temperature was maintained at +25°C. Isolated liver mitochondria were incubated with different concentrations of Fe3O4 (0, 250, 300, and 350 μg/mL) during 30 min at +25°C.
To discriminate if the results obtained might be due to the size or to a general response pattern in front of iron oxide exposure, we submitted middle-aged liver mitochondria to a Fe3+ solution with concentration of 350 μg/mL Fe3O4. To obtain free Fe3+ ions a precise mass of Fe3O4 nanoparticles was dissolved in a small volume of concentrated HCl under gentle heating [23]. Two experiments were conducted per animal, and thus number of data was six.
2.6. Measurement of the Mitochondrial Respiratory Chain Complexes Activities and Mitochondrial Coupling
Maximal oxidative capacity (V max) was measured by adding adenosine diphosphate (ADP). When V max was recorded, electron flow went through complexes I, III, and IV. Then complex I was blocked with amytal (0.02 mM) and complex II was stimulated with succinate (25 mM). Mitochondrial respiration in these conditions allowed determining complexes II, III, and IV activities (V succ). After that, N, N, N′, N′-tetramethyl-p-phenylenediaminedihydrochloride (TMPD, 0.50 mM) and ascorbate (0.50 mM) were added as artificial electron donors to cytochrome c. In these conditions, the activity of cytochrome c oxidase (complex IV) was determined as an isolated step of the respiratory chain (V tmpd) [24].
Mitochondrial coupling (coupling of phosphorylation to oxidation) was determined by calculating the acceptor control ratio (ACR), ratio between ADP-stimulated respiration (V max) over basal respiration (without ADP) with glutamate and malate as substrate (V 0), as previously reported by Mansour et al. 2012 [25].
2.7. Statistical Analysis
Results are expressed as mean ± SEM. Statistical analyses were performed using one-way ANOVA followed by a Tukey posttest. The unpaired t-test was used to compare young and old rats. Statistical significance required a P < 0.05. Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA).
3. Results
3.1. Effects of Age on Mitochondrial Respiratory Chain Function and on Mitochondrial Coupling
As shown in Table 1, there was no difference in mitochondrial oxygen consumptions between young (3 months) and middle-aged (18 months) control rats liver. Thus, the maximal oxidative capacity was similar in young versus middle-aged rats (29.8 ± 5.6 and 30.0 ± 1.6 μmol O2/min/g protein, respectively). This holded true for V succ (young: 35.0 ± 3.7 versus 33.9 ± 1.7 μmol O2/min/g protein) and for V tmpd (young: 50.0 ± 6.2 versus 43.0 ± 1.6 μmol O2/min/g protein).
Table 1.
Baseline liver mitochondrial respiratory chain complexes activities and mitochondrial coupling.
| Control | Young (3 months) |
Middle-aged (18 months) |
|---|---|---|
| V max (µmol O2/min/g protein) | 29.8 ± 5.6 | 30.0 ± 1.6 |
| V succ (µmol O2/min/g protein) | 35.0 ± 3.7 | 33.9 ± 1.7 |
| V tmpd (µmol O2/min/g protein) | 50.0 ± 6.2 | 43.0 ± 1.6 |
| ACR | 9.7 ± 2.6 | 10.5 ± 1.3 |
Data are means ± SEM.
ACR: acceptor control ratio (V max/V 0).
Finally, mitochondrial coupling was similar in both young and middle-aged rats (9.7 ± 2.6 versus 10.5 ± 1.3; P = NS).
3.2. Effects of IONPs on Young Liver Mitochondrial Respiratory Chain Complexes Activities
Complexes I, III, and IV Activities. The V max in the group treated with increasing doses of IONPs (250, 300, and 350 μg/mL) was not modified as compared to the control group (27.2 ± 4.5; 29.7 ± 4.1; 29.0 ± 3.1 versus 29.8 ± 5.6 μmol O2/min/g protein) (Figure 2(a)).
Figure 2.
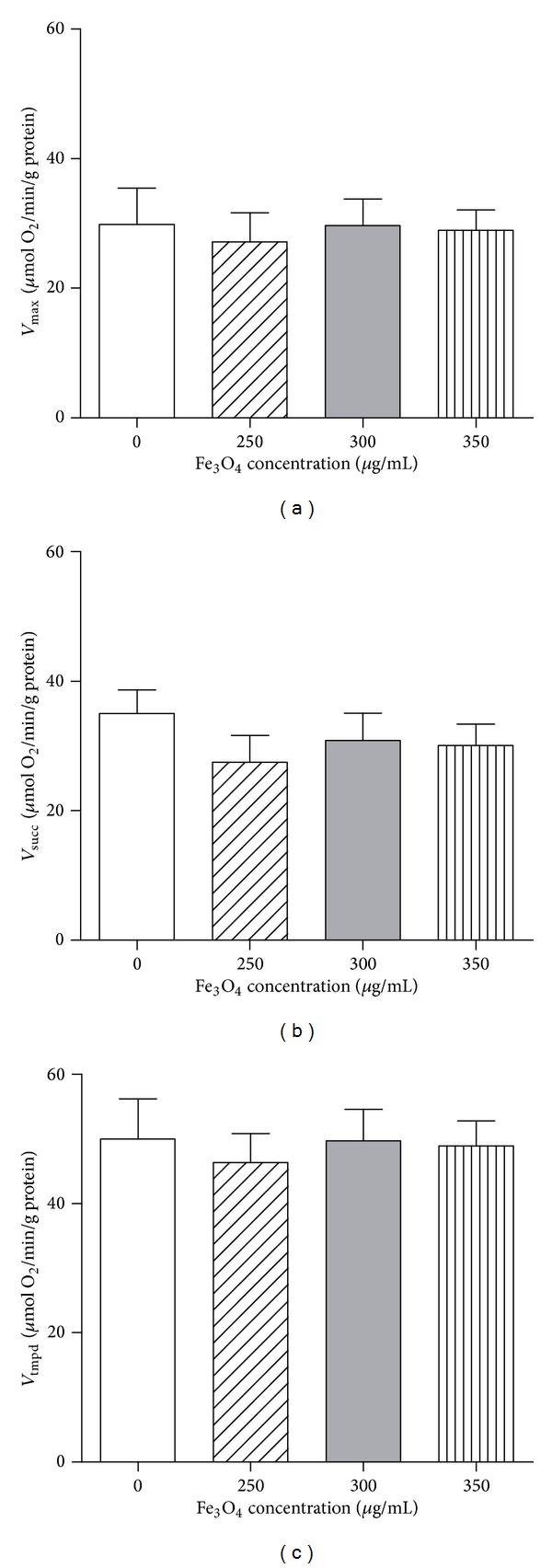
Effects of iron oxide nanoparticles (Fe3O4) on young liver mitochondrial respiratory chain complexes activities. (a) V max reflects complexes I, III, and IV activities and is measured using glutamate and malate. (b) V succ reflects complexes II, III, and IV activities and is measured using succinate. (c) V tmpd reflects complex IV activity and is measured using TMPD and ascorbate as mitochondrial substrates. Data are means ± SEM
Complexes II, III, and IV Activities. V succ was unchanged whatever the dose of IONPs (27.5 ± 4.2, 30.9 ± 4.2, and 30.1 ± 3.3 for Fe3O4 250, 300, and 350, respectively, as compared to control values 35.0 ± 3.7 μmol O2/min/g protein) (Figure 2(b)).
Complex IV Activity. V tmpd, reflecting complex IV activity, was not modified after IONPs treatment as compared with control group (46.3 ± 4.5, 49.7 ± 4.9, and 50.0 ± 3.8 for Fe3O4 250, 300, and 350, respectively, versus 50.0 ± 6.2 μmol O2/min/g protein) (Figure 2(c)).
Taken together, these data support that Fe3O4 nanoparticles, whatever the doses used, failed to alter any of the liver mitochondrial respiratory chain complexes activities in young rats.
3.3. Effects of IONPs on Middle-Aged Liver Mitochondrial Respiratory Chain Complexes Activities
When the middle-aged liver rats were exposed to different concentrations of IONPs, the V max, V succ, and V tmpd decreased significantly.
Complexes I, III, and IV Activities. The maximal oxidative capacities, V max, reflecting I, III, and IV activities significantly decreased whatever the dose of IONPs. At 250 μg/mL, V max was decreased as compared to the control group (24.4 ± 1.4 versus 30 ± 1.6 μmol O2/min/g protein; P < 0.05). V max at 300 and 350 μg/mL were also decreased as compared to control values (23.8 ± 1.0 and 17.9 ± 1.5 for Fe3O4 300 and Fe3O4 350 μg/mL, respectively, versus CON: 30 ± 1.6 μmol O2/min/g protein; P < 0.001).
The V max decrease was greater when using higher IONPs concentration. Thus V max was significantly lower after Fe3O4 350 μg/mL as compared to 300 and 250 μg/mL (Figure 3(a)).
Figure 3.
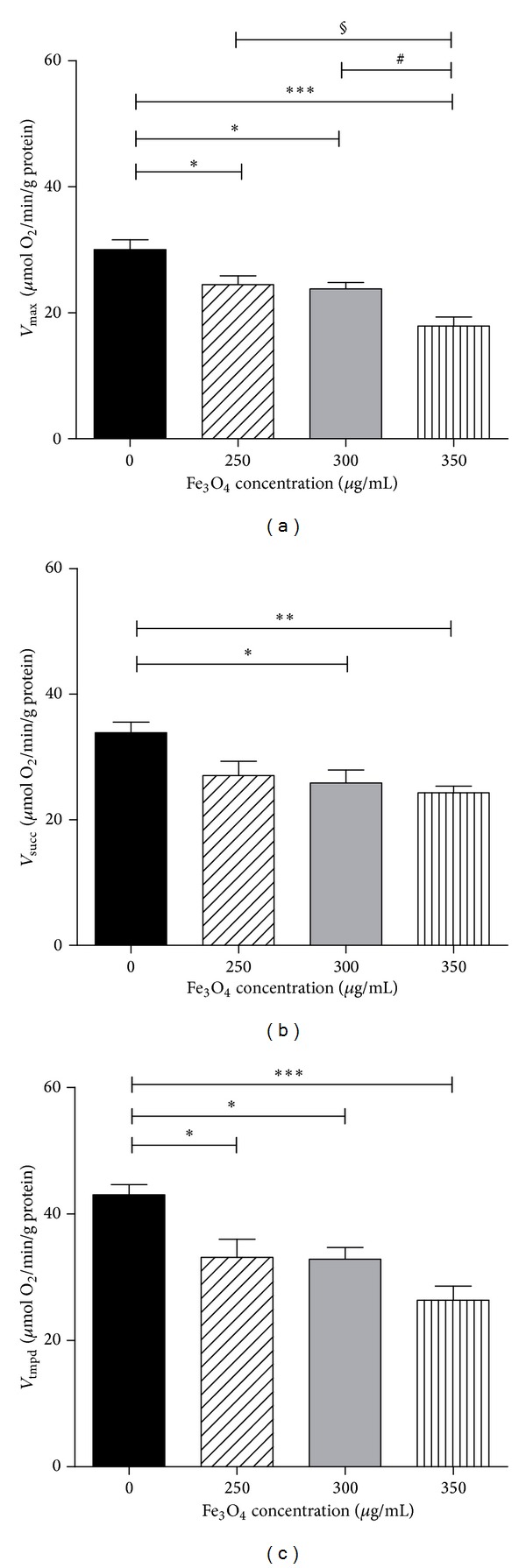
Effects of iron oxide nanoparticles (Fe3O4) on middle-aged liver mitochondrial respiratory chain complexes activities. (a) V max reflects complexes I, III, and IV activities and is measured using glutamate and malate. (b) V succ reflects complexes II, III, and IV activities and is measured using succinate. (c) V tmpd reflects complex IV activity and is measured using TMPD and ascorbate as mitochondrial substrates. Data are means ± SEM (one-way ANOVA followed by Tukey). *P < 0.05; **P < 0.01; ***P < 0.001 compared to control. # P < 0.05 350 μg/mL compared to 300 μg/mL. § P < 0.05 350 μg/mL compared to 250 μg/mL.
Complexes II, III, and IV Activities. At 250 μg/mL, V succ, reflecting complexes II, III, and IV activities, tended to decrease as compared to the control group (27.0 ± 2.3 versus 33.9 ± 1.7 μmol O2/min/g protein). The statistical significance was reached at 300 μg/mL and 350 μg/mL (Fe3O4 300 μg/mL: 25.8 ± 1.0 versus CON: 33.9 ± 1.7 μmol O2/min/g protein; P < 0.05) and Fe3O4 350 μg/mL (24.3 ± 1.0 versus 33.9 ± 1.7 μmol O2/min/g protein; P < 0.01) (Figure 3(b)).
Complex IV Activity. V tmpd, reflecting complex IV activity, decreased significantly after exposure to Fe3O4 250 μg/mL as compared to the control group (33.0 ± 2.9 versus 43.0 ± 1.6 μmol O2/min/g protein; P < 0.05). Similarly, V tmpd, decreased when exposed to 300 μg/mL of Fe3O4 (32.9 ± 1.9 versus 43.0 ± 1.6 μmol O2/min/g protein; P < 0.05) and to 350 μg/mL (26.3 ± 2.2 versus 43.0 ± 1.6 μmol O2/min/g protein; P < 0.001) (Figure 3(c)).
3.4. Effects of IONPs on Liver Mitochondrial Coupling in Young and Middle-Aged Rats
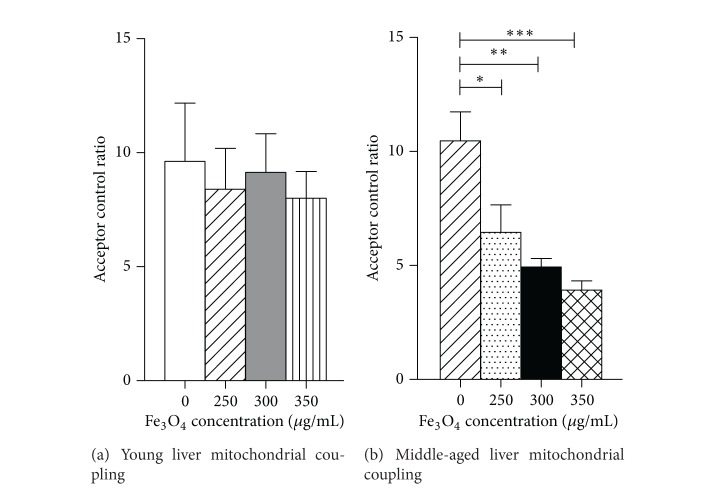
In young liver, the acceptor control ratio (V max/V 0), representing the degree of coupling between oxidation and phosphorylation, was not changed after IONPs treatment (8.4 ± 1.8, 9.1 ± 1.7, and 8.0 ± 1.2 after, respectively, Fe3O4 250, 300, and 350 μg/mL, as compared to control 9.7 ± 2.6).
On the other hand, interestingly, IONPs decreased significantly mitochondrial coupling in middle-aged liver, as compared to controls. Thus, the ACR was lower when mitochondria were exposed to 250 μg/mL Fe3O4, as compared to control (6.4 ± 1.2 versus 10.5 ± 1.3; P < 0.05). Moreover, ACR was also decreased at 300 μg/mL (5.0 ± 0.4 versus 10.5 ± 1.3; P < 0.01) and at 350 μg/mL (Fe3O4 350 μg/mL: 3.9 ± 0.4 versus CON: 10.5 ± 1.3; P < 0.001) (Figure 4).
Figure 4.
Effects of iron oxide nanoparticles (Fe3O4) on (a) young and (b) middle-aged liver mitochondrial coupling. Data are means ± SEM (one-way ANOVA followed by Tukey). *P < 0.05; **P < 0.01; ***P < 0.001 compared to control.
3.5. Effects of Iron Oxide Not in Its Particulate form on Middle-Aged Liver Mitochondrial Respiratory Chain Complexes Activities and Coupling
When the middle-aged liver rats were exposed to 350 μg/mL Fe3O4, V max (41.90 ± 3.93 versus 38.08 ± 3.77 μmol O2/min/g protein), V succ (44.23 ± 3.443 versus 41.42 ± 3.303 μmol O2/min/g protein), V tmpd (84.90 ± 5.75 versus 70.08 ± 4.71 μmol O2/min/g protein), and ACR (7.70 ± 1.15 versus 8.72 ± 1.06) were not significantly modified (Figure 5).
Figure 5.
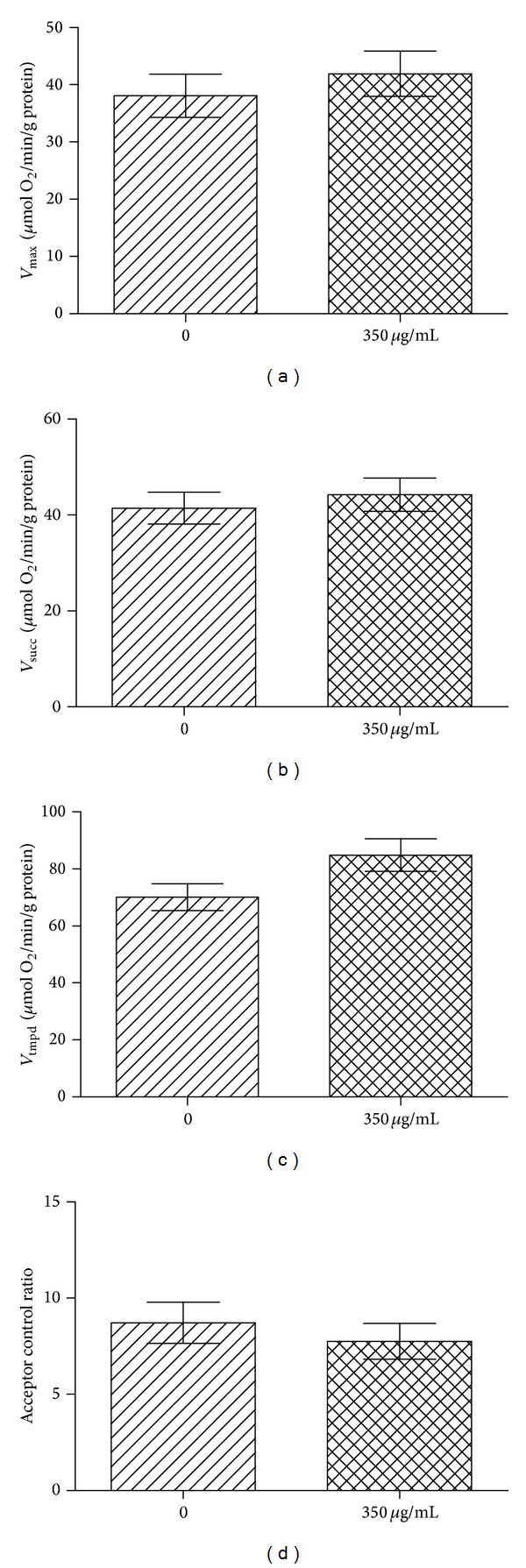
Effects of 350 μg/mL of Fe3O4 but not in its particulate form on middle-aged liver mitochondrial respiration and coupling. (a) V max reflects complexes I, III, and IV activities and is measured using glutamate and malate. (b) V succ reflects complexes II, III, and IV activities and is measured using succinate. (c) V tmpd reflects complex IV activity and is measured using N, N, N′, N′-tetramethyl-p-phenylenediaminedihydrochloride (TMPD) and ascorbate as mitochondrial substrates. (d) Acceptor control ratio reflects the mitochondrial coupling. Data are means ± SEM.
4. Discussion
The main results of this study are to demonstrate that, unlike young animals, middle-aged rats are sensitive to iron oxide nanoparticles (IONPs). Indeed, liver mitochondrial respiration chain complexes I, II, III, and IV activities are altered whatever the concentration of Fe3O4 used. Further, 250, 300, and 350 μg/mL of Fe3O4 specifically impair liver mitochondrial coupling in middle-aged rats. Such impairment is not observed when using 350 μg/mL of Fe3O4 but not in its particulate form.
Young and Middle-Aged Rats Present with Similar Baseline Mitochondrial Respiratory Chain Complexes Activities in the Liver. Several studies indicate that mitochondria are one of the major sources of reactive oxygen species (ROS) and, in turn, are the most adversely affected organelles during aging [26]. In fact, mitochondria from aged tissue use oxygen inefficiently, which impairs ATP synthesis and results in increased oxidant production [27]. Oxidative damage mainly concerns the activities of electron transport complexes of the inner mitochondrial membrane [28], which are specifically modified during aging [29, 30].
Accordingly, in rat liver, previous reports have found aging-related decrease in electron transfer activity in complex I or complexes I and IV in young (4 months) versus old (30 months) rats [31–34]. On the other hand, some studies found no change in the respiratory chain activity with age. Thus, Bakala et al. [35] observed no significant difference in the respiratory chain activity with age between 10-month-old and 27-month-old rats, whatever the substrate used.
This is consistent with our results and might be explained by differences in age range or animals being only middle-aged.
Iron Oxide Nanoparticles Impair Liver Mitochondrial Respiratory Chain Complexes Activities in Middle-Aged but Not in Young Rats. Concerning young rats, iron oxide nanoparticles failed to impair any of the liver mitochondrial respiratory chain complexes activities. Thus, mitochondrial oxygen consumption in young liver was not altered by IONPs and, similarly, mitochondrial coupling remained in the normal range after Fe3O4 exposure. This is in agreement with our previous findings [18].
To the best of our knowledge, no data are available concerning potential related effects of high levels of iron oxide nanoparticles and age on mitochondria. This is particularly interesting since the liver is a major iron storage organ [36, 37]. Very interestingly, the data were different when middle-aged liver rats were exposed to the same IONPs concentrations than the young ones. In middle-aged liver rats, IONPs at 250, 300, and 350 μg/mL significantly decreased V max, V succ, and V tmpd corresponding together to complexes I, II, III, and IV activities of the mitochondrial respiratory chain. IONPs exposure also decreased mitochondrial coupling.
Several mechanisms might explain these results like increased fragility of older mitochondria and iron accumulation.
First, the fragility of mitochondria seems to increase in function of age. Thus, studies reported that mitochondria isolated from the organs of aged animals are also aged in terms of cytosolic and mitochondrial oxidative stress and losses of enzymatic activities [32]. Accordingly, studies demonstrated that aging induces the loss of mitochondrial function in liver of rodents and monkeys [38–40].
Similarly studies investigated the effects of iron accumulation on mitochondrial integrity and function with age [20, 41, 42]. Investigating the pharmacokinetics of IONPs in rats, Schnorr et al. [43] demonstrated that the half-life and the resulting signal changes in blood and liver vary significantly with age. Thus, iron accumulation which is considered a feature of the aging process [44–46] might be associated with a mitochondrial iron increase. In particular under conditions of cellular stress, this may be a potential causative factor of age-related mitochondrial dysfunction [20, 42, 47, 48]. Mitochondrial damage by excessive cellular iron appears as an intrinsic factor contributing to the permeability pore transition and bioenergetics function decline of mitochondria, which can potentially lead to cellular senescence and tissue degeneration [49–51].
Particularly, in accordance with our data of a NPs-related impairment of complex IV, [52] demonstrated that the interaction of bare Fe3O4 NPs with cytochrome c leads to the reduction of the protein. Moreover, [53] using human hepatoma cells showed a dose-dependent decrease in mitochondrial membrane potential after exposure to Fe3O4 NPs and at high concentration (1 mg/mL) the cytochrome c protein expression decreased dramatically. Accordingly, [54] observed that Fe3O4 NPs caused the highest toxic effects on green alga, as compared to other types of NPs.
Taken together, these data confirm that age likely modulates Fe3O4 nanoparticles liver toxicity and one might propose that a threshold of toxicity might play a key role. Indeed, as reported by Bakala et al. [35] and possibly explaining our results, the accumulation of deleterious oxidized and carboxymethylated proteins in the matrix concomitant with loss of the IONPs protease activity may affect the ability of aging mitochondria to respond to additional stress. We could therefore speculate that the increase in glycoxidative and oxidative alteration is relevant only if the damage is severe enough to have an impact on mitochondrial function.
However, whether the observed effects are specific of iron oxide in its particulate form or is independent of its size is not known. We therefore submitted old liver mitochondria to iron oxide but not in its particulate form and, interestingly, no deleterious effect was observed. Thus, it is likely that age specifically modulates Fe3O4 nanoparticles liver toxicity.
5. Conclusion
In summary, we demonstrate for the first time that old rats are more susceptible to IONPs nanoparticle exposure in terms of liver mitochondrial respiration and coupling. These age-related changes in liver mitochondrial respiratory chain activity should perhaps be taken into consideration in preclinical and clinical studies of particulate contrast agents.
Acknowledgments
The authors thank Fabienne Goupilleau, Isabelle Bentz, and Anne-Marie Kasprowicz for their expert biological and secretarial assistance.
Abbreviations
- NPs:
Nanoparticles
- IONPs:
Iron oxide nanoparticles
- Vmax:
Maximal oxidative capacities
- Vsucc:
Complexes II, III, and IV activities
- Vtmpd:
Complex IV activity
- DEG:
Diethylene glycol
- ACR:
Acceptor control ratio.
Conflict of Interests
The authors declare no conflict of interests regarding the publication of this paper.
Authors' Contribution
Yosra Baratli and Anne-Laure Charles contributed equally to the paper.
References
- 1.Alexiou C, Tietze R, Schreiber E, Lyer S. Nanomedicine: magnetic nanoparticles for drug delivery and hyperthermia—new chances for cancer therapy. Bundesgesundheitsblatt—Gesundheitsforschung—Gesundheitsschutz. 2010;53(8):839–845. doi: 10.1007/s00103-010-1097-9. [DOI] [PubMed] [Google Scholar]
- 2.Chen C-L, Zhang H, Ye Q, et al. A new nano-sized Iron oxide particle with high sensitivity for cellular magnetic resonance imaging. Molecular Imaging and Biology. 2011;13(5):825–839. doi: 10.1007/s11307-010-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McBain SC, Yiu HHP, Dobson J. Magnetic nanoparticles for gene and drug delivery. International Journal of Nanomedicine. 2008;3(2):169–180. doi: 10.2147/ijn.s1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rumenapp C, Gleich B, Haase A. Magnetic nanoparticles in magnetic resonance imaging and diagnostics. Pharmaceutical Research. 2012;25(5):1165–1179. doi: 10.1007/s11095-012-0711-y. [DOI] [PubMed] [Google Scholar]
- 5.Corot C, Robert P, Idée J-M, Port M. Recent advances in Iron oxide nanocrystal technology for medical imaging. Advanced Drug Delivery Reviews. 2006;58(14):1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Puppi J, Mitry RR, Modo M, Dhawan A, Raja K, Hughes RD. Use of a clinically approved Iron oxide MRI contrast agent to label human hepatocytes. Cell Transplantation. 2011;20(6):963–975. doi: 10.3727/096368910X543367. [DOI] [PubMed] [Google Scholar]
- 7.Sadauskas E, Wallin H, Stoltenberg M, et al. Kupffer cells are central in the removal of nanoparticles from the organism. Particle and Fibre Toxicology. 2007;4, article 10 doi: 10.1186/1743-8977-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olmedo D, Guglielmotti MB, Cabrini RL. An experimental study of the dissemination of titanium and zirconium in the body. Journal of Materials Science: Materials in Medicine. 2002;13(8):793–796. doi: 10.1023/a:1016131310025. [DOI] [PubMed] [Google Scholar]
- 9.So SJ, Jang IS, Han CS. Effect of micro/nano silica particle feeding for mice. Journal of Nanoscience and Nanotechnology. 2008;8(10):5367–5371. doi: 10.1166/jnn.2008.1347. [DOI] [PubMed] [Google Scholar]
- 10.Unfried K, Albrecht C, Klotz L-O, Von Mikecz A, Grether-Beck S, Schins RPF. Cellular responses to nanoparticles: target structures and mechanisms. Nanotoxicology. 2007;1(1):52–71. [Google Scholar]
- 11.Thaveau F, Zoll J, Rouyer O, et al. Ischemic preconditioning specifically restores complexes I and II activities of the mitochondrial respiratory chain in ischemic skeletal muscle. Journal of Vascular Surgery. 2007;46(3):541–547. doi: 10.1016/j.jvs.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 12.Meyer A, Zoll J, Charles AL, et al. Skeletal muscle mitochondrial dysfunction during chronic obstructive pulmonary disease: central actor and therapeutic target. Experimental Physiology. 2013;98(6):1063–1078. doi: 10.1113/expphysiol.2012.069468. [DOI] [PubMed] [Google Scholar]
- 13.Jeng HA, Swanson J. Toxicity of metal oxide nanoparticles in mammalian cells. Journal of Environmental Science and Health, A: Toxic/Hazardous Substances and Environmental Engineering. 2006;41(12):2699–2711. doi: 10.1080/10934520600966177. [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Marzetti E, Seo AY, Kim J-S, Prolla TA, Leeuwenburgh C. The emerging role of Iron dyshomeostasis in the mitochondrial decay of aging. Mechanisms of Ageing and Development. 2010;131(7-8):487–493. doi: 10.1016/j.mad.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Au C, Mutkus L, Dobson A, Riffle J, Lalli J, Aschner M. Effects of nanoparticles on the adhesion and cell viability on astrocytes. Biological Trace Element Research. 2007;120(1–3):248–256. doi: 10.1007/s12011-007-0067-z. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson HL, Cronholm P, Gustafsson J, Möller L. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chemical Research in Toxicology. 2008;21(9):1726–1732. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]
- 17.Zhu M-T, Wang B, Wang Y, et al. Endothelial dysfunction and inflammation induced by Iron oxide nanoparticle exposure: risk factors for early atherosclerosis. Toxicology Letters. 2011;203(2):162–171. doi: 10.1016/j.toxlet.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Baratli Y, Charles AL, Wolff V, et al. Impact of Iron oxide nanoparticles on brain, heart, lung, liver and kidneys mitochondrial respiratory chain complexes activities and coupling. Toxicology in Vitro. 2013;27(8):2142–2148. doi: 10.1016/j.tiv.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo AY, Xu J, Servais S, et al. Mitochondrial Iron accumulation with age and functional consequences. Aging Cell. 2008;7(5):706–716. doi: 10.1111/j.1474-9726.2008.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basti H, Ben Tahar L, Smiri LS, et al. Catechol derivatives-coated Fe3O4 and γ-Fe2O3 nanoparticles as potential MRI contrast agents. Journal of Colloid and Interface Science. 2010;341(2):248–254. doi: 10.1016/j.jcis.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Cornell RM, Schwertmann U. The Iron Oxides Structure, Properties, Reactions, Occurrence and Uses. Weinheim, Germany: Wiley-VCH; 1996. [Google Scholar]
- 24.Charles A-L, Guilbert A-S, Bouitbir J, et al. Effect of postconditioning on mitochondrial dysfunction in experimental aortic cross-clamping. British Journal of Surgery. 2011;98(4):511–516. doi: 10.1002/bjs.7384. [DOI] [PubMed] [Google Scholar]
- 25.Mansour Z, Bouitbir J, Charles AL, et al. Remote and local ischemic preconditioning equivalently protects rat skeletal muscle mitochondrial function during experimental aortic cross-clamping. Journal of Vascular Surgery. 2012;55(2):497.e1–505.e1. doi: 10.1016/j.jvs.2011.07.084. [DOI] [PubMed] [Google Scholar]
- 26.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Cadenas E, Davies KJA. Mitochondrial free radical generation, oxidative stress, and aging. Free Radical Biology and Medicine. 2000;29(3-4):222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 28.Lejay A, Meyer A, Schlagowski AI, et al. Mitochondria: mitochondrial participation in ischemia-reperfusion injury in skeletal muscle. The International Journal of Biochemistry & Cell Biology. 2014;50:101–105. doi: 10.1016/j.biocel.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Boffoli D, Scacco SC, Vergari R, et al. Ageing is associated in females with a decline in the content and activity of the b-c1 complex in skeletal muscle mitochondria. Biochimica et Biophysica Acta—Molecular Basis of Disease. 1996;1315(1):66–72. doi: 10.1016/0925-4439(95)00107-7. [DOI] [PubMed] [Google Scholar]
- 30.Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Archives of Biochemistry and Biophysics. 2000;373(1):16–22. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- 31.Ventura B, Genova ML, Bovina C, Formiggini G, Lenaz G. Control of oxidative phosphorylation by complex I in rat liver mitochondria: implications for aging. Biochimica et Biophysica Acta—Bioenergetics. 2002;1553(3):249–260. doi: 10.1016/s0005-2728(01)00246-8. [DOI] [PubMed] [Google Scholar]
- 32.Navarro A, Boveris A. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2004;287(5):R1244–R1249. doi: 10.1152/ajpregu.00226.2004. [DOI] [PubMed] [Google Scholar]
- 33.Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. American Journal of Physiology—Cell Physiology. 2007;292(2):C670–C686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- 34.Navarro A, Gomez C, López-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2004;286(3):R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- 35.Bakala H, Delaval E, Hamelin M, et al. Changes in rat liver mitochondria with aging: lon protease-like activity and Nε-carboxymethyllysine accumulation in the-matrix. European Journal of Biochemistry. 2003;270(10):2295–2302. doi: 10.1046/j.1432-1033.2003.03598.x. [DOI] [PubMed] [Google Scholar]
- 36.Angelucci E, Brittenham GM, McLaren CE, et al. Hepatic Iron concentration and total body Iron stores in thalassemia major. The New England Journal of Medicine. 2000;343(5):327–331. doi: 10.1056/NEJM200008033430503. [DOI] [PubMed] [Google Scholar]
- 37.Wood JC. Diagnosis and management of transfusion Iron overload: the role of imaging. American Journal of Hematology. 2007;82(12, supplement):1132–1135. doi: 10.1002/ajh.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castro MDR, Suarez E, Kraiselburd E, et al. Aging increases mitochondrial DNA damage and oxidative stress in liver of rhesus monkeys. Experimental Gerontology. 2012;47(1):29–37. doi: 10.1016/j.exger.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Modi HR, Katyare SS, Patel MA. Ageing-induced alterations in lipid/phospholipid profiles of rat brain and liver mitochondria: implications for mitochondrial energy-linked functions. Journal of Membrane Biology. 2008;221(1):51–60. doi: 10.1007/s00232-007-9086-0. [DOI] [PubMed] [Google Scholar]
- 40.Serviddio G, Bellanti F, Romano AD, et al. Bioenergetics in aging: mitochondrial proton leak in aging rat liver, kidney and heart. Redox Report. 2007;12(1-2):91–95. doi: 10.1179/135100007X162112. [DOI] [PubMed] [Google Scholar]
- 41.Xu J, Knutson MD, Carter CS, Leeuwenburgh C. Iron accumulation with age, oxidative stress and functional decline. PLoS ONE. 2008;3(8) doi: 10.1371/journal.pone.0002865.e2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu J, Marzetti E, Seo AY, Kim J-S, Prolla TA, Leeuwenburgh C. The emerging role of Iron dyshomeostasis in the mitochondrial decay of aging. Mechanisms of Ageing and Development. 2010;131(7-8):487–493. doi: 10.1016/j.mad.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schnorr J, Taupitz M, Wagner S, Pilgrimm H, Hansel J, Hamm B. Age-related blood half-life of particulate contrast material: experimental results with a USPIO in rats. Journal of Magnetic Resonance Imaging. 2000;12(5):740–744. doi: 10.1002/1522-2586(200011)12:5<740::aid-jmri11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 44.Altun M, Edström E, Spooner E, et al. Iron load and redox stress in skeletal muscle of aged rats. Muscle and Nerve. 2007;36(2):223–233. doi: 10.1002/mus.20808. [DOI] [PubMed] [Google Scholar]
- 45.Jung SH, DeRuisseau LR, Kavazis AN, DeRuisseau KC. Plantaris muscle of aged rats demonstrates Iron accumulation and altered expression of Iron regulation proteins. Experimental Physiology. 2008;93(3):407–414. doi: 10.1113/expphysiol.2007.039453. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Hwang JCY, Lees HA, et al. Long-term perturbation of muscle Iron homeostasis following hindlimb suspension in old rats is associated with high levels of oxidative stress and impaired recovery from atrophy. Experimental Gerontology. 2012;47(1):100–108. doi: 10.1016/j.exger.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bulvik BE, Berenshtein E, Konijn AM, et al. Aging is an organ-specific process: changes in homeostasis of Iron and redox proteins in the rat. Age. 2011;34(3):693–704. doi: 10.1007/s11357-011-9268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duvigneau JC, Piskernik C, Haindl S, et al. A novel endotoxin-induced pathway: upregulation of heme oxygenase 1, accumulation of free Iron, and free Iron-mediated mitochondrial dysfunction. Laboratory Investigation. 2008;88(1):70–77. doi: 10.1038/labinvest.3700691. [DOI] [PubMed] [Google Scholar]
- 49.Chen H, Liu B, Lukas TJ, Suyeoka G, Wu G, Neufeld AH. Changes in Iron-regulatory proteins in the aged rodent neural retina. Neurobiology of Aging. 2009;30(11):1865–1876. doi: 10.1016/j.neurobiolaging.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gogvadze V, Walter PB, Ames BN. Fe2+ induces a transient Ca2+ release from rat liver mitochondria. Archives of Biochemistry and Biophysics. 2002;398(2):198–202. doi: 10.1006/abbi.2001.2721. [DOI] [PubMed] [Google Scholar]
- 51.Gogvadze V, Walter PB, Ames BN. The role of Fe2+-induced lipid peroxidation in the initiation of the mitochondrial permeability transition. Archives of Biochemistry and Biophysics. 2003;414(2):255–260. doi: 10.1016/s0003-9861(02)00750-6. [DOI] [PubMed] [Google Scholar]
- 52.Mukhopadhyay A, Joshi N, Chattopadhyay K, De G. A facile synthesis of PEG-coated magnetite (Fe3O4) nanoparticles and their prevention of the reduction of cytochrome C. ACS Applied Materials and Interfaces. 2012;4(1):142–149. doi: 10.1021/am201166m. [DOI] [PubMed] [Google Scholar]
- 53.Kai W, Xiaojun X, Ximing P, Zhenqing H, Qiqing Z. Cytotoxic effects and the mechanism of three types of magnetic nanoparticles on human hepatoma BEL-7402 cells. Nanoscale Research Letters. 2011;6, article 480 doi: 10.1186/1556-276X-6-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barhoumi L, Dewez D. Toxicity of superparamagnetic Iron oxide nanoparticles on green alga Chlorella vulgaris . BioMed Research International. 2013;2013:11 pages. doi: 10.1155/2013/647974.647974 [DOI] [PMC free article] [PubMed] [Google Scholar]