Abstract
Background
Increased bronchial responsiveness is characteristic of asthma. Gas cooking, which is a major indoor source of the highly oxidant nitrogen dioxide, has been associated with respiratory symptoms and reduced lung function. However, little is known about the effect of gas cooking on bronchial responsiveness and on how this relationship may be modified by variants in the genes GSTM1, GSTT1 and GSTP1, which influence antioxidant defences.
Methods
The study was performed in subjects with forced expiratory volume in one second at least 70% of predicted who took part in the multicentre European Community Respiratory Health Survey, had bronchial responsiveness assessed by methacholine challenge and had been genotyped for GSTM1, GSTT1 and GSTP1-rs1695. Information on the use of gas for cooking was obtained from interviewer-led questionnaires. Effect modification by genotype on the association between the use of gas for cooking and bronchial responsiveness was assessed within each participating country, and estimates combined using meta-analysis.
Results
Overall, gas cooking, as compared with cooking with electricity, was not associated with bronchial responsiveness (β=−0.08, 95% CI −0.40 to 0.25, p=0.648). However, GSTM1 significantly modified this effect (β for interaction=−0.75, 95% CI −1.16 to −0.33, p=4×10−4), with GSTM1 null subjects showing more responsiveness if they cooked with gas. No effect modification by GSTT1 or GSTP1-rs1695 genotypes was observed.
Conclusions
Increased bronchial responsiveness was associated with gas cooking among subjects with the GSTM1 null genotype. This may reflect the oxidant effects on the bronchi of exposure to nitrogen dioxide.
Keywords: Asthma
Key messages.
What is the key question?
Is the relationship of bronchial responsiveness with use of gas for cooking modified by variants in oxidative stress-related genes (GSTM1, GSTT1, GSTP1)?
What is the bottom line?
In subjects with GSTM1 null genotype, increased bronchial responsiveness was associated with gas cooking, which may reflect the oxidant effects on the bronchi of exposure to gas-derived pollutants.
Why read on?
Since increased bronchial responsiveness is a characteristic feature of asthma, the present findings may help in understanding why some individuals may present asthma-related symptoms when cooking with gas while others do not.
Introduction
Gas cooking is a major source of indoor nitrogen dioxide and, to a lesser extent, of fine particles.1 2 Use of gas for cooking has been inconsistently associated with respiratory symptoms, including wheeze and exacerbation of asthma, and reduced lung function suggestive of airways obstruction,3–6 and only a few studies have examined associations with bronchial responsiveness (BR). In a study of 324 Montreal school children, those exposed to gas cooking were more likely to have increased BR.7 In the Dutch arm of the European Community Respiratory Health Survey (ECRHS), which involved 1921 adults, gas cooking was also associated with increased BR, but only among those with high total immunoglobulin E (IgE) levels.8 In contrast, in a study of 929 eight-year-old Dutch children, exposure to gas cooking was not associated with BR.9
Nitrogen dioxide is an oxidant species that induces upregulation of the expression of T helper type 2 cell cytokines and ICAM1 as well as neutrophilic inflammation in bronchial epithelium.10 11 Changes in air particle number concentrations have also been linked to airway inflammation.12 The extent to which these and other pollutants cause lung damage and inflammation is dependent on the efficacy of internal antioxidant defences and detoxification mechanisms. Glutathione-S-transferases are a group of phase II enzymes involved in the detoxification of xenobiotics in the lung.13 These enzymes play a role in the protection against oxidative stress since they influence the levels of glutathione, an important non-enzymatic antioxidant in the lung.14 15 Variants in genes encoding glutathione-S-transferase mu 1 (GSTM1), theta 1 (GSTT1) and pi 1 (GSTP1) have been linked to decreased lung function and progression from increased BR to asthma,16–18 suggesting that these variants contribute to increased susceptibility to oxidative stress. Thus, the aim of study was to assess whether genetic variants in GSTM1 (null genotype), GSTT1 (null genotype) and GSTP1 (rs1695[G]) modify the association between gas cooking and BR.
Methods
Participants
Data included in the following analysis were collected from subjects participating in the ECRHS, an international multicentre cohort study designed to identify risk factors for asthma.19 In the first survey (ECRHS I), subjects were randomly recruited from community-based sampling frames in each centre after completing a short postal screening questionnaire. A random sample of responders to the postal survey completed an interviewer-led questionnaire between 1992 and 1994 (‘random’ group). A smaller sample of participants with symptoms highly suggestive of asthma, but who had not been randomly selected to take part, was also invited for clinical assessment (‘enriched’ group). Approximately 8 years later, subjects who had participated in the clinical investigations during the first survey were invited for further questionnaires and blood sampling for genotyping (ECRHS II: 1999–2002). The main analysis herein presented is based on data collected in ECRHS II.
Ethical approval for the study from local research ethics committees and written consent from subjects were obtained.
Genotyping
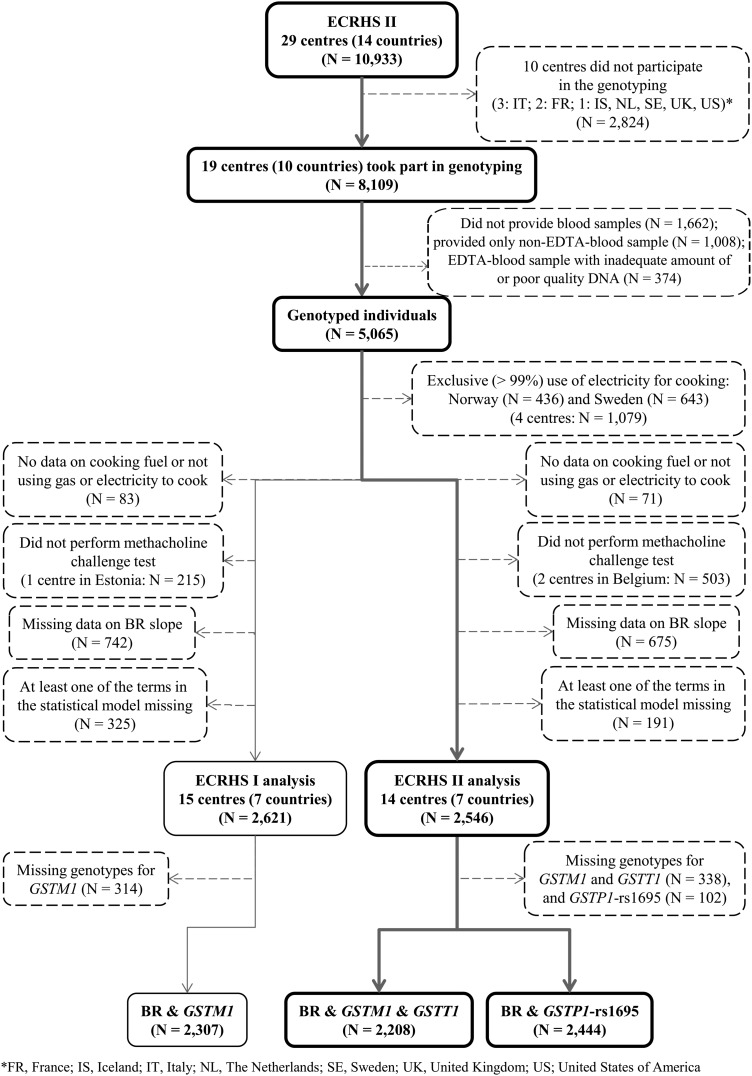
In total, 19 of 29 centres (8109 out of 10 933 subjects) in ECRHS II collected blood samples for genotyping (figure 1). However, not all subjects provided blood samples, and some of the collected samples had inadequate amount of or poor quality DNA. Only 5065 out of the 8109 subjects were genotyped for GSTM1, GSTP1 and GSTT1. Genotyped subjects were slightly older than those who were not genotyped, and as there were some between-country differences in response to genotyping, the distribution by country was not the same between the two groups (see online supplementary T1). DNA was extracted from blood samples using a commercial kit (Puregene, Gentra Inc., MN, USA). At the Centre for Genomic Regulation (Barcelona, Spain), GSTM1 and GSTT1 null genotypes were determined by PCRs, and GSTP1 polymorphism (rs1695—Ile105Val) was genotyped using a specific pyrosequencing assay.20 Genotype frequencies at GSTP1-rs1695 deviated from Hardy–Weinberg equilibrium (HWE) in France and Germany. HWE could not be calculated for GSTM1 and GSTT1 because our data did not distinguish between one and two copies of the variant allele. Population stratification was assessed with 26 unlinked markers using the genomic control approach and the EIGENSTRAT software. There was no evidence of relevant population stratification.20
Figure 1.
Flow diagram showing the selection of subjects included in the analysis.
Exposure to gas cooking
Participants were asked, “What kind of stove do you mostly use for cooking?” Subjects who answered ‘gas (gas from the mains)’ or ‘gas (gas from bottles or other non-mains source)’ were classified as being exposed to gas cooking. Those who answered ‘electric’ or ‘microwave’ were classified as the reference group. Subjects in four centres in Norway (N=436) and Sweden (N=643) were not included in the analysis because the use of gas for cooking in these countries is extremely low (<1%). Subjects who used other types of stove, such as ‘coal, coke or wood (solid fuel)’ or ‘paraffin (kerosene)’, were also excluded (<2% of participants in the analysis, N=71).
Bronchial responsiveness
BR was assessed by methacholine bronchial challenge test as previously described.21 To assess the baseline forced expiratory volume in one second (FEV1) and the forced vital capacity (FVC), each participant was allowed nine attempts to provide at least two technically acceptable expiratory manoeuvres. Subjects with FEV1 at least 70% of predicted, and more than 1.5 L, underwent bronchial challenge unless they had specific contraindications. Bronchial challenge was conducted with increasing amounts of methacholine up to a cumulative dose of 1 mg, with the methacholine solution being administered via a dosimeter. BR was defined by the slope of the dose–response curve obtained with the methacholine test. The ‘slope’ was estimated as rate of change of FEV1 against methacholine dose and, in order to satisfy the assumption of normality and homogeneity of variance, transformed to 100/(log-slope+10).21 22 A low slope is indicative of high BR. In addition, subjects who experienced more than a 20% fall in FEV1 after inhalation of 1 mg of methacholine were identified, that is, those with PD20 <1 mg. In ECRHS II, two centres in Belgium (N=503) did not perform the methacholine challenge test and their data were excluded.
Statistical analysis
Main analysis: ECRHS II
To assess whether genetic variants in GSTM1, GSTT1 and GSTP1 modify the association between gas cooking and BR, linear regression models with the slope as the continuous dependent variable were fitted for each of the three genes and a gene–gas cooking interaction term was entered in the models. GSTM1 and GSTT1 were modelled as dichotomous variables (‘null’ vs ‘present’), whereas GSTP1-rs1695[G] was modelled per number of minor alleles (0, 1, 2) under an additive mode of inheritance. The coefficient (β) for the interaction term between each of the genes and gas cooking was estimated for the whole sample (‘random’ and ‘enriched’) in each country and then combined using a random effects meta-analysis.23 This was repeated adding in the model a term for the type of sample. The analysis was also repeated for both sexes, separately.
Potential confounders considered a priori to be relevant included the following: age, sex, height, smoking in pack-years, specific IgE titre (cat, house dust mite, Timothy-grass, Cladosporium herbarum), total IgE, baseline FEV1 expressed as a standardised difference from an internally derived sex-specific predicted value and baseline FEV1 as a percentage of FVC. An age–sex interaction24 and a gene–smoking interaction, as suggested in the literature,21 were also considered. In addition, the interaction between each genetic variant and BR was further adjusted for the other genetic variants. After excluding subjects with missing slope, genotype and any of the potential confounders included in the models, the total sample consisted of 2208 for models assessing the interaction between gas cooking and GSTM1 or GSTT1, and 2444 for models assessing the interaction with GSTP1 (figure 1). Statistical tests were two-sided, and results were considered significant when nominal p≤0.05. Statistical analyses were performed using STATA/IC V.12.1.25
Sensitivity and post hoc analyses
Details on the sensitivity and post hoc analyses are provided in the online supplementary material.
Results
Main results: ECRHS II
In the present study, both sexes were equally represented, most subjects were ever smokers (42.9% were lifetime non-smokers), most were from Spain, France and Germany, and cooking with gas was more prevalent than cooking with electricity (table 1). GSTM1 and GSTT1 were not present in just above 50% and approximately 20% of the subjects, respectively. Close to half of the subjects were heterozygous for GSTP1-rs1695.
Table 1.
Characteristics of subjects from the two surveys of the European Community Respiratory Health Survey (ECRHS) included in the present analysis
| ECRHS I (N=2621)* | ECRHS II (N=2546)* | |
|---|---|---|
| Age, mean (SD) | 34.2 (7.2) | 42.2 (7.2) |
| Sex (%) | ||
| Males | 50.4 | 49.4 |
| Females | 49.6 | 50.6 |
| Smoking status (%) | ||
| Never smoker | 42.9% | 42.9% |
| Ever smoker | 57.1% | 57.1% |
| Smoking pack-years, mean (SD) | 7.8 (12.5) | 11.0 (17.6) |
| Country (%) | ||
| Australia | 10.7 | 11.5 |
| Belgium | 11.8 | – |
| Estonia | – | 5.1 |
| France | 15.5 | 17.1 |
| Germany | 13.1 | 13.0 |
| Spain | 30.4 | 35.5 |
| Switzerland | 8.8 | 9.4 |
| UK | 9.7 | 8.4 |
| Cooking fuel (%) | ||
| Electricity | 33.5 | 49.4 |
| Gas | 66.5 | 50.6 |
| GSTM1 genotype (%)† | ||
| Null | 51.1 | 51.2 |
| Present | 48.9 | 48.8 |
| GSTT1 genotype (%)† | ||
| Null | 19.8 | 20.2 |
| Present | 80.2 | 79.8 |
| GSTP1-rs1695 genotype (%)‡ | ||
| AA | 42.8 | 41.7 |
| AG | 48.3 | 49.0 |
| GG | 8.9 | 9.3 |
| Bronchial responsiveness, log-slope, mean (SD) | 7.6 (2.3) | 7.4 (2.3) |
| Fall of 20% in FEV1 after inhalation of 1 mg methacholine, PD20 (%) | ||
| No | 84.2 | 83.1 |
| Yes | 15.8 | 16.9 |
| Baseline FEV1, mean (SD) | 3.7 (0.8) | 3.6 (0.8) |
| Baseline FVC, mean (SD) | 4.6 (1.0) | 4.4 (1.0) |
*After exclusion of subjects with missing data on cooking fuel or not using gas or electric stove, who did not perform the methacholine challenge test or had missing data on BR slope, and those with missing data on at least one of the terms in the statistical model.
†314 subjects from ECRHS I and 338 subjects from ECRHS II have missing data on GSTM1 and GSTT1 genotypes.
‡151 subjects from ECRHS I and 102 subjects from ECRHS II have missing data on GSTP1-rs1695 genotype.
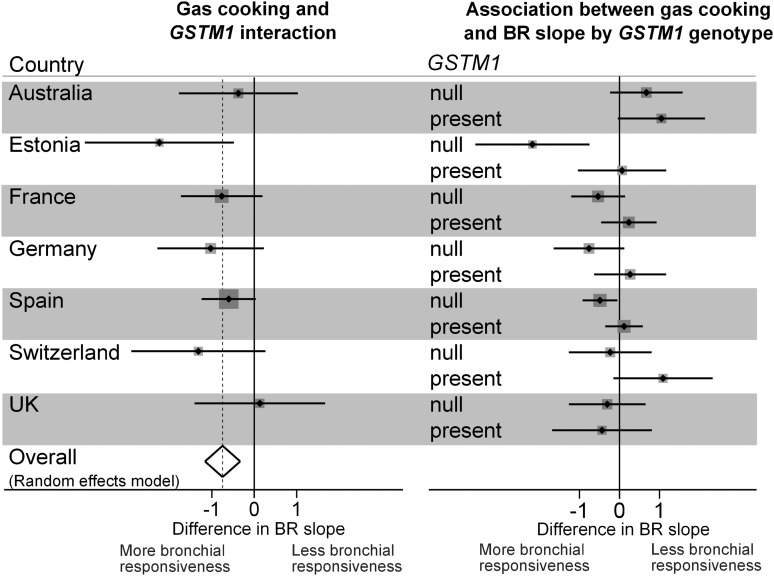
None of the three genetic variants was significantly associated with BR (see online supplementary T2). Overall, gas cooking, versus cooking with electricity, was also not significantly associated with BR (β=−0.08, 95% CI −0.40 to 0.25, p=0.648). However, this association was different depending on GSTM1, with GSTM1 null subjects showing a strong significant association of increased BR with use of gas for cooking (β for interaction=−0.75, 95% CI −1.16 to −0.33, p=4×10−4, Bonferroni-corrected p=0.017) (table 2 and figure 2). There was no evidence of heterogeneity between countries (I2=0%, p=0.521). The interaction was present among males (β for interaction=−0.79, 95% CI −1.63 to 0.06, p=0.067) and females (β for interaction=−0.79, 95% CI −1.40 to −0.19, p=0.010). Adjustment of these models for the type of sample, a priori confounders and for the other genes did not materially alter the estimates for the interaction (see online supplementary T3). The association of BR with use of gas for cooking was not modified by GSTT1 or GSTP1-rs1695 genotypes (table 2; see online supplementary T4).
Table 2.
Estimates for the interaction between gas cooking and genetic variants in GSTM1, GSTT1 and GSTP1 on bronchial responsiveness in the European Community Respiratory Health Survey II
| Genetic variant | Whole sample | ‘Random’ sample | ‘Enriched’ sample* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | β (95% CI) | p Value | N | β (95% CI) | p Value | N | β (95% CI) | p Value | |
| GSTM1 null | 2208 | −0.75 (−1.16 to −0.33) | 4×10−4 | 1843 | −0.81 (−1.34 to −0.28) | 0.003 | 365 | −0.93 (−2.01 to 0.14) | 0.088 |
| GSTT1 null | 2208 | −0.02 (−0.54 to 0.50) | 0.929 | 1843 | −0.27 (−0.83 to 0.28) | 0.336 | 365 | 0.74 (−0.57 to 2.04) | 0.270 |
| GSTP1-rs1695 | 2444 | 0.03 (−0.41 to 0.46) | 0.905 | 2018 | 0.01 (−0.45 to 0.48) | 0.954 | 426 | 0.31 (−0.48 to 1.11) | 0.441 |
*France and Switzerland were considered as one group due to small numbers in those two countries.
Figure 2.
Interaction between gas cooking and GSTM1 on bronchial responsiveness, and association between gas cooking and bronchial responsiveness according to GSTM1 genotype, in the European Community Respiratory Health Survey II.
Sensitivity analysis
The interaction between gas cooking and GSTM1 was present and of the same magnitude in the ‘random’ sample and the smaller sample that was enriched in symptomatic subjects (table 2). The estimate for the interaction was similar in those who had gas hobs (without gas ovens), who used gas mains, who cooked every day in the last 4 weeks, even in a ventilated kitchen, and stronger in those who had gas hobs with gas ovens, who used bottled gas and who cooked every day in the last 4 weeks in an unventilated kitchen (table 3). When the analysis was restricted to lifetime non-smokers only (N=936), the magnitude of interaction reduced slightly, although the direction remained the same (β for interaction=−0.42, 95% CI −1.07 to 0.24, p=0.214).
Table 3.
Estimates for the interaction between gas cooking and GSTM1 null genotype on bronchial responsiveness in the European Community Respiratory Health Survey II, restricting the analysis according to cooking characteristics
| Cooking characteristics | N | β (95% CI) | p Value |
|---|---|---|---|
| Gas hobs (without gas oven) vs electricity | 1565 | −0.53 (−1.06 to −0.001) | 0.050 |
| Gas hobs (with gas oven) vs electricity | 1393 | −0.85 (−1.43 to −0.27) | 0.004 |
| Bottled gas vs electricity | 814 | −1.59 (−2.98 to −0.20) | 0.025 |
| Gas mains vs electricity | 1934 | −0.60 (−1.04 to −0.15) | 0.009 |
| Cooked every day in the last four weeks | 1578 | −0.72 (−1.32 to −0.12) | 0.018 |
| Cooked every day in the last four weeks (ventilated kitchen) | 1205 | −0.50 (−1.04 to 0.04) | 0.071 |
| Cooked every day in the last four weeks (unventilated kitchen) | 373 | −1.47 (−2.54 to −0.40) | 0.007 |
When BR was considered as PD20, the prevalence of increased BR in some centres was very low. Using this outcome, no statistically significant interaction was observed (OR for interaction=1.29, 95% CI 0.64 to 2.62, p=0.478).
Post hoc analysis
Some analyses were repeated using all available data obtained at ECRHS I (ie, including participants from Belgium). The interaction between gas cooking and GSTM1 was not present (β for interaction=−0.001, 95% CI −0.45 to 0.44, p=0.996), and there was some evidence of minor heterogeneity between countries in this relationship (I2=10.6%, p=0.349). As this post hoc analysis included participants from Belgium (who did not conduct BR measures at the follow-up), the analysis was repeated excluding participants from that country but still no interaction was observed (β for interaction=0.12, 95% CI −0.35 to 0.58, p=0.628). Exclusion of Spain (country contributing the most to heterogeneity of results at ECRHS I) did not alter results (β for interaction=−0.19, 95% CI −0.67 to 0.30, p=0.452). Of note, Spain showed the largest change in the use of gas cooking between the two surveys (84.2% to 54.8%, online supplementary T5). Some subjects changed cooking fuel between surveys (gas to electricity: N=483, of whom 35% were from Spain; electricity to gas: N=139). Restriction of the analysis to those who used the same fuel at baseline and follow-up still did not show an interaction between gas cooking and GSTM1 in the ECRHS I. As in the second survey, interactions between gas cooking and GSTT1 and GSTP1-rs1695 were not observed in the baseline survey (p>0.05; data not shown).
Overall, there was no evidence that use of gas over the approximately 8 years of follow-up was associated with greater increases in BR (difference in change in slope comparing gas with electricity: −0.002, 95% CI −0.51 to 0.51, p=0.995, N=1150). However, participants with the GSTM1 null genotype and who cooked with gas, compared with those who cooked with electricity, throughout the period of follow-up showed some sign of greater increase in BR (β for the interaction between gas cooking and GSTM1 on change in BR=−0.25, 95% CI −0.88 to 0.38, p=0.437, N=1150). There was no evidence that GSTT1 or GSTP1-rs1695 genotypes modified the change in BR due to the use of gas (p>0.05; data not shown).
Discussion
In this population-based study of adults of European ancestry and FEV1 at least 70% of predicted, the association of BR with gas cooking was different depending on GSTM1, that is, gas cooking was associated with increased BR among subjects with the null genotype for GSTM1, but not among carriers of that gene. Furthermore, the interaction was stronger among those who cooked in conditions where higher exposure levels are expected (ie, with gas oven and in unventilated kitchens). To our knowledge, this is the first study to assess and report this interaction.
One of the strengths of the present study is its large population sample derived from several areas of Europe. There was some loss to follow-up between the two surveys but little reason to believe that this would result in the detection of false gene–environment interactions as loss to follow-up in subjects with respiratory symptoms and cooking with gas, or electricity, are unlikely to be related to genetic characteristics. The general decrease in prevalence of cooking with gas across countries, from the first to the second survey, probably reflects a change in house building trends and is also unlikely due to genetic makeup. A strength of the study was the use of a standardised protocol across participating centres to perform methacholine bronchial challenge tests.22 The continuous non-censored slope obtained from the bronchial challenge tests performs better than PD20 in indicating BR.26
Despite the fact we were underpowered to detect interactions between gas cooking and GSTT1 and GSTP1, we had enough power (>90%) to detect an interaction with GSTM1 in both surveys. However, we only observed it in ECRHS II. This may be due to heterogeneity between countries being lower in ECRHS II or to lack of information on some environmental exposure that might have confounded the effect of gas cooking in the first survey. It may also be due to better insulated houses and consequently increased concentration of indoor gases in the second survey, but we do not have data to confirm or reject this argument. We could not detect within-survey differences between age groups, although there was some evidence of increasing BR due to gas cooking among older participants with GSTM1 null genotype, when comparing the second with the first survey (data not shown). While the change in cooking fuel between the two surveys may, in part, explain that the interaction was statistically significant only in the most recent survey, we should not exclude the potential role of other genes, epigenetic mechanisms and cellular phenomena during normal ageing. Adjustment for GSTT1 and GSTP1-rs1695 did not make a difference on the estimates for the interaction between GSTM1 and gas cooking, nor did adjusting for NQO1-rs1800566, which has been proposed to interact with GSTM1 and air pollutants on lung function (data not shown).27 Detrimental effects of gene dosage changes (eg, deletion, duplication) may eventually be avoided through compensation at the transcriptional, post-transcriptional and protein levels.28 29 However, the efficiency of this phenomenon may decrease with ageing, and manifestation of the gene dosage changes may arise late in life. This is supported by evidence from studies on autosomal recessive diseases and mitochondrial disorders showing that even among subjects with inherited causal mutations a proportion may live several years or decades without manifesting the disease.30 31 It is also possible that BR is affected by ageing-related decline in baseline lung function; however, we did not observe a relevant change in the estimate for the interaction after adjusting for FEV1 and FEV1/FVC ratio.
There is evidence of the influence of interactions between GSTM1 and some environmental pollutants on asthma and airway obstruction. In a study of school children, prevalence of asthma was associated with maternal smoking during pregnancy, but only among children with GSTM1 deletion.24 In a study of adolescents and young adults with asthma, it was reported that exposure to environmental tobacco smoke significantly reduces peak expiratory flow rate among subjects with no copy of GSTM1, but not among carriers of at least a copy of that gene.32 In a randomised controlled trial with asthmatic children and irrespective of the treatment being studied, forced expiratory flow was significantly reduced due to exposure to ozone among children with no copy of GSTM1, but not among those with at least a copy of GSTM1.33
GSTM1 is located on chromosome 1 where it encodes a phase II enzyme involved in the detoxification of electrophilic xenobiotics, by conjugation with glutathione, and the protection against oxidative and nitrosative stress.13–15 Knockdown of GSTM1 in normal human bronchial epithelial cells has shown that this gene may regulate diesel exhaust particle-induced expression of IL-8 and IL-1β by modulation of reactive oxygen species.34 In vitro and in vivo data have shown that knockout mice for gstm1 have low ability to conjugate 1,2-dichloro-4-nitrobenzene with glutathione.35 Thus, it is biologically plausible that the lack of GSTM1, common in approximately half of the population of European ancestry, may lead to an increased susceptibility to the effects of gas cooking, which is the main source of indoor nitrogen dioxide and particulate matter.1 2
In summary, increased BR was associated with gas for cooking among subjects with the null genotype for GSTM1. This may be an indication of the oxidant effects on the bronchi of exposure to nitrogen dioxide originating from cooking with gas. Further larger studies are recommended to confirm this finding and better understand its mechanism of action.
Supplementary Material
Acknowledgments
The authors would like to thank the participants and field workers of this study for their time and cooperation.
Footnotes
Contributors: AFSA, AR, CM, DJB, and DLJ planned the analysis. AFSA, AR and DLJ analysed the data and drafted the paper. SA, IP, MK, RJ, DN, GV, MO, NPH, CJ, JPZ, JH and DLJ recruited participants and collected data for the epidemiological study. FC-G was responsible for genotyping. All authors discussed and interpreted the results, and revised the paper.
Funding: The coordination of the ECRHS II was supported by the European Commission, as part of their Quality of Life programme. This work was also supported by grants from MaratoTV3, Catalonia, Spain, and from Genome Spain. The following bodies funded the local studies in ECRHS II included in this paper. Albacete: Fondo de Investigaciones Santarias (FIS) (grant code: 97/0035-01, 99/0034-01 and 99/0034-02), Hospital Universitario de Albacete, Consejeria de Sanidad. Antwerp: FWO (Fund for Scientific Research)-Flanders Belgium (grant code: G.0402.00), University of Antwerp, Flemish Health Ministry. Barcelona: SEPAR, Public Health Service (grant code: R01 HL62633-01), Fondo de Investigaciones Sanitarias (FIS) (grant code: 97/0035-01, 99/0034-01 and 99/0034-02) CIRIT (grant code: 1999SGR 00241) ‘Instituto de Salud Carlos III’ Red de Centros RCESP, C03/09 and Red RESPIRA, C03/011. Basel: Swiss National Science Foundation, Swiss Federal Office for Education and Science, Swiss National Accident Insurance Fund (SUVA). Erfurt: GSF-National Research Centre for Environment and Health, Deutsche Forschungsgemeinschaft (DFG) (grant code FR 1526/1-1). Galdakao: Basque Health Dept. Grenoble: Programme Hospitalier de Recherche Clinique-DRC de Grenoble 2000 no. 2610, Ministry of Health, Direction de la Recherche Clinique, Ministere de l'Emploi et de la Solidarite, Direction Generale de la Sante, CHU de Grenoble, Comite des Maladies Respiratoires de l’Isere. Hamburg: GSF-National Research Centre for Environment and Health, Deutsche Forschungsgemeinschaft (DFG) (grant code MA 711/4-1). Ipswich and Norwich: Asthma UK (formerly known as National Asthma Campaign (UK). Huelva: Fondo de Investigaciones Sanitarias (FIS) (grant code: 97/0035-01, 99/0034-01 and 99/0034-02). Oviedo: Fondo de Investigaciones Sanitarias (FIS) (grant code: 97/0035-01, 99/0034-01 and 99/0034-02). Paris: Ministere de l'Emploi et de la Solidarite, Direction Generale de la Sante, UCB-Pharma (France), Aventis (France), Glaxo France, Programme Hospitalier de Recherche Clinique-DRC de Grenoble 2000 no. 2610, Ministry of Health, Direction de la Recherche Clinique, CHU de Grenoble. Tartu: Estonian Science Foundation. AFSA is supported by MRC-PHE Centre for Environment and Health (grant code: G0801056/1).
Competing interests: None.
Ethics approval: Local research ethics committees (eg, NRES Committee London-Stanmore for the UK arm of the study) from the different countries involved in this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lai HK, Bayer-Oglesby L, Colvile R, et al. Determinants of indoor air concentrations of PM2.5, black smoke and NO2 in six European cities (EXPOLIS study). Atmospheric Environ 2006;40:1299–313 [Google Scholar]
- 2.Garcia Algar O, Pichini S, Basagana X, et al. Concentrations and determinants of NO2 in homes of Ashford, UK and Barcelona and Menorca, Spain. Indoor Air 2004;14:298–304 [DOI] [PubMed] [Google Scholar]
- 3.Comstock GW, Meyer MB, Helsing KJ, et al. Respiratory effects on household exposures to tobacco smoke and gas cooking. Am Rev Respir Dis 1981;124:143–8 [DOI] [PubMed] [Google Scholar]
- 4.Jarvis D, Chinn S, Sterne J, et al. The association of respiratory symptoms and lung function with the use of gas for cooking. European Community Respiratory Health Survey. Eur Respir J 1998;11:651–8 [PubMed] [Google Scholar]
- 5.Ostro BD, Lipsett MJ, Mann JK, et al. Indoor air pollution and asthma. Results from a panel study. Am J Respir Crit Care Med 1994;149:1400–6 [DOI] [PubMed] [Google Scholar]
- 6.Moran SE, Strachan DP, Johnston ID, et al. Effects of exposure to gas cooking in childhood and adulthood on respiratory symptoms, allergic sensitization and lung function in young British adults. Clin Exp Allergy 1999;29:1033–41 [DOI] [PubMed] [Google Scholar]
- 7.Ernst P, Ghezzo H, Becklake MR. Risk factors for bronchial hyperresponsiveness in late childhood and early adolescence. Eur Respir J 2002;20:635–9 [DOI] [PubMed] [Google Scholar]
- 8.Kerkhof M, de Monchy JG, Rijken B, et al. The effect of gas cooking on bronchial hyperresponsiveness and the role of immunoglobulin E. Eur Respir J 1999;14:839–44 [DOI] [PubMed] [Google Scholar]
- 9.Lin W, Gehring U, Oldenwening M, et al. Gas cooking, respiratory and allergic outcomes in the PIAMA birth cohort study. Occup Environ Med 2013;70:187–94 [DOI] [PubMed] [Google Scholar]
- 10.Blomberg A, Krishna MT, Bocchino V, et al. The inflammatory effects of 2 ppm NO2 on the airways of healthy subjects. Am J Respir Crit Care Med 1997;156:418–24 [DOI] [PubMed] [Google Scholar]
- 11.Pathmanathan S, Krishna MT, Blomberg A, et al. Repeated daily exposure to 2 ppm nitrogen dioxide upregulates the expression of IL-5, IL-10, IL-13, and ICAM-1 in the bronchial epithelium of healthy human airways. Occup Environ Med 2003;60:892–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strak M, Janssen NA, Godri KJ, et al. Respiratory health effects of airborne particulate matter: the role of particle size, composition, and oxidative potential-the RAPTES project. Environ Health Perspect 2012;120:1183–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strange RC, Spiteri MA, Ramachandran S, et al. Glutathione-S-transferase family of enzymes. Mutat Res 2001;482:21–6 [DOI] [PubMed] [Google Scholar]
- 14.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol 2006;533:222–39 [DOI] [PubMed] [Google Scholar]
- 15.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol 2005;45:51–88 [DOI] [PubMed] [Google Scholar]
- 16.Imboden M, Rochat T, Brutsche M, et al. Glutathione S-transferase genotype increases risk of progression from bronchial hyperresponsiveness to asthma in adults. Thorax 2008;63:322–8 [DOI] [PubMed] [Google Scholar]
- 17.Gilliland FD, Gauderman WJ, Vora H, et al. Effects of glutathione-S-transferase M1, T1, and P1 on childhood lung function growth. Am J Respir Crit Care Med 2002;166:710–16 [DOI] [PubMed] [Google Scholar]
- 18.He JQ, Connett JE, Anthonisen NR, et al. Glutathione S-transferase variants and their interaction with smoking on lung function. Am J Respir Crit Care Med 2004;170:388–94 [DOI] [PubMed] [Google Scholar]
- 19.European Community Respiratory Health Survey IISC. The European Community Respiratory Health Survey II. Eur Respir J 2002;20:1071–9 [DOI] [PubMed] [Google Scholar]
- 20.Castro-Giner F, Kunzli N, Jacquemin B, et al. Traffic-related air pollution, oxidative stress genes, and asthma (ECHRS). Environ Health Perspect 2009;117:1919–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinn S, Burney P, Jarvis D, et al. Variation in bronchial responsiveness in the European Community Respiratory Health Survey (ECRHS). Eur Respir J 1997;10:2495–501 [DOI] [PubMed] [Google Scholar]
- 22.Chinn S, Arossa WA, Jarvis DL, et al. Variation in nebulizer aerosol output and weight output from the Mefar dosimeter: implications for multicentre studies. Eur Respir J 1997;10:452–6 [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88 [DOI] [PubMed] [Google Scholar]
- 24.Gilliland FD, Li YF, Dubeau L, et al. Effects of glutathione S-transferase M1, maternal smoking during pregnancy, and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 2002;166:457–63 [DOI] [PubMed] [Google Scholar]
- 25.StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP, 2011 [Google Scholar]
- 26.Marcon A, Cerveri I, Wjst M, et al. Can an airway challenge test predict respiratory diseases? A population-based international study. J Allergy Clin Immunol 2013 [DOI] [PubMed] [Google Scholar]
- 27.Minelli C, Wei I, Sagoo G, et al. Interactive effects of antioxidant genes and air pollution on respiratory function and airway disease: a HuGE review. Am J Epidemiol 2011;173:603–20 [DOI] [PubMed] [Google Scholar]
- 28.Veitia RA, Bottani S, Birchler JA. Gene dosage effects: nonlinearities, genetic interactions, and dosage compensation. Trends Genet 2013 [DOI] [PubMed] [Google Scholar]
- 29.Stenberg P, Larsson J. Buffering and the evolution of chromosome-wide gene regulation. Chromosoma 2011;120:213–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barthelemy C, Ogier de Baulny H, Diaz J, et al. Late-onset mitochondrial DNA depletion: DNA copy number, multiple deletions, and compensation. Ann Neurol 2001;49:607–17 [PubMed] [Google Scholar]
- 31.Oliveira SA, Scott WK, Martin ER, et al. Parkin mutations and susceptibility alleles in late-onset Parkinson's disease. Ann Neurol 2003;53:624–9 [DOI] [PubMed] [Google Scholar]
- 32.Palmer CN, Doney AS, Lee SP, et al. Glutathione S-transferase M1 and P1 genotype, passive smoking, and peak expiratory flow in asthma. Pediatrics 2006;118:710–16 [DOI] [PubMed] [Google Scholar]
- 33.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, et al. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax 2004;59:8–10 [PMC free article] [PubMed] [Google Scholar]
- 34.Wu W, Peden DB, McConnell R, et al. Glutathione-S-transferase M1 regulation of diesel exhaust particle-induced pro-inflammatory mediator expression in normal human bronchial epithelial cells. Part Fibre Toxicol 2012;9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujimoto K, Arakawa S, Shibaya Y, et al. Characterization of phenotypes in Gstm1-null mice by cytosolic and in vivo metabolic studies using 1,2-dichloro-4-nitrobenzene. Drug Metab Dispos 2006;34:1495–501 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.