Abstract
Background
The study aimed to evaluate the use of positron emission tomography/computed tomography (PET/CT) with 68Ga-PRGD2 as the tracer for imaging of synovial angiogenesis in patients with rheumatoid arthritis (RA).
Methods
Twenty untreated active patients with RA underwent 68Ga-PRGD2 PET/CT and 18F-FDG PET/CT before treatment; two patients with osteoarthritis served as controls. Among the 20 patients with RA, 12 repeated the evaluations after 3-month treatment. The image findings were correlated with core variables of disease activity, including the clinical disease activity index (cDAI).
Results
Our findings demonstrated that 68Ga-PRGD2 specifically accumulated in the synovia with active inflammation rich in neovasculature with high-level αvβ3-integrin expression, but not in the 18F-FDG-avid inflammatory lymph nodes. In patients with intense 18F-FDG uptake in muscles caused by arthritic pain, we observed that 68Ga-PRGD2 PET/CT was better able to evaluate disease severity than 18F-FDG PET/CT. Both 68Ga-PRGD2 accumulation and 18F-FDG uptake changed in response to therapeutic intervention, whereas the changes of 68Ga-PRGD2, not 18F-FDG, significantly correlated with clinical measures of changes in the form of cDAI.
Conclusions
This is the first integrin imaging study conducted in patients with RA that preliminarily indicates the effectiveness of the novel method for evaluating synovial angiogenesis.
Clinical trial registration
This study has been registered online at NIH ClinicalTrial.gov (NCT01940926).
Keywords: Rheumatoid Arthritis, Arthritis, Disease Activity
Introduction
Rheumatoid arthritis (RA), one of the most common rheumatic disorders, is characterised by the onset of synovial angiogenesis and inflammation and eventually leads to pannus formation and joint destruction.1 2 The αvβ3-integrin is a transmembrane heterodimeric receptor that mediates cell–cell and cell–extracellular matrix adhesion.3 The αvβ3-integrin plays a pivotal role in promoting and sustaining angiogenesis and has been identified as a biomarker of angiogenesis.3–5 Cyclic arginine–glycine–aspartic acid (RGD) peptide is the key integrin recognition motif that can strongly bind to the αvβ3-integrin and inhibit new blood vessel formation, which make the RGD-based peptides hold a promise for imaging and treatment of diseases characterised with angiogenesis including RA.6–9
To date, however, no reports have presented the clinical application of integrin imaging for the evaluation of synovial angiogenesis and pannus formation, which are very important for the histopathological analysis of patients with RA. In this prospective cohort study, we evaluated the ability of RGD positron emission tomography/computed tomography (PET/CT) to assess synovial angiogenesis and monitor response to treatment in patients with RA. The results were compared with those generated by FDG PET/CT through clinical case-by-case evaluations.
Patients and methods
Patients
This study was approved by the Institutional Review Board of Peking Union Medical College Hospital and conducted from February 2012 to December 2013. Written informed consent was obtained from each participating patient. All of the patients with RA recruited for the study met the 1987 revised criteria of the American College of Rheumatology (ACR) for RA.10
We recruited 20 patients with RA (n=18 females/2 male; mean age, 49±12 years; disease duration, 36±39 months; the demographic and clinical characteristics of the patients with RA are presented in online supplementary table S1) and two patients with osteoarthritis (OA) as diseased controls. We evaluated all patients using whole-body 68Ga-PRGD2 PET/CT and 18F-FDG PET/CT scans. At the time of enrolment in this study, all patients were assessed for core ACR variables of disease activity, including tender joint count (TJC-28), swollen joint count (SJC-28), pain intensity score (10 cm visual analogue scale (VAS), 0.0 = no pain, 10.0 = very intensive pain), patients’ global assessment of overall well-being (PtGA, 10 cm VAS) and physician's global assessment of disease severity (PyGA, 10 cm VAS); a clinical disease activity index (cDAI) was calculated as follows: cDAI=TJC-28+SJC-28+PtGA+PyGA.11
Twelve patients repeated the 68Ga-PRGD2 PET/CT and 18F-FDG PET/CT scans and the clinical evaluations 3 months after the treatment. Two patients with RA agreed to undergo synovial biopsy before treatment.
Methods
Details of 68Ga-PRGD2 PET/CT scanning, 18F-FDG PET/CT scanning, semi-quantitative analysis, immunohistochemical analysis and statistical analysis are given in online supplementary information.
Results
Comparison of 68Ga-PRGD2 PET/CT with 18F-FDG PET/CT
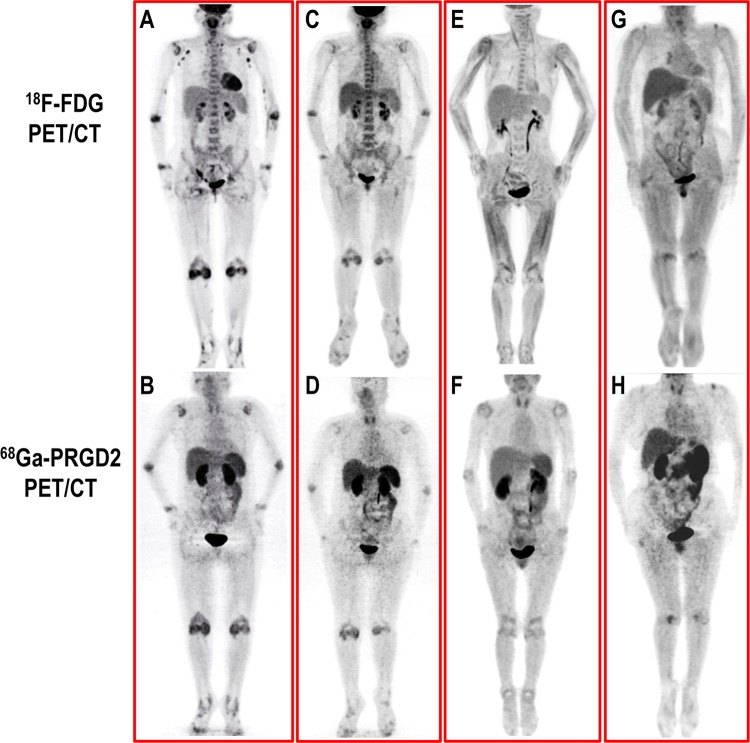
An intense accumulation of 68Ga-PRGD2 occurred in the primary excretory pathways (including the kidneys and bladder) and moderate uptake occurred in the thyroid, liver, spleen and intestinal tract; the distribution of 68Ga-PRGD2 in other parts of the body was low and stable and therefore able to provide an accurate evaluation of joint inflammation in the patients. The patients with RA experienced high levels of 68Ga-PRGD2 accumulation in the involved joints and tendon sheaths and diffuse distribution in the lining of the synovium (figure 1B); in contrast, the OA controls experienced only slight regional tracer uptake in the affected joints (figure 1H). Moreover, the accumulation of 68Ga-PRGD2 decreased with decreasing disease activity after effective treatment (figure 1D).
Figure 1.
Comparison of the distribution of 18F-FDG and 68Ga-PRGD2 in patients with rheumatoid arthritis (RA) and osteoarthritis (OA). (A, B) In a patient with RA (F, 48 years) with a clinical disease activity index (cDAI) of 28.0, intense 18F-FDG uptake was observed in the inflammatory synovia and axillary lymph nodes, whereas 68Ga-PRGD2 accumulated specifically in the synovia. (C, D) After successful treatment (cDAI=6.0), 18F-FDG uptake and 68Ga-PRGD2 accumulation significantly decreased in the joints. (E, F) In another patient with RA, the joint pain caused intense 18F-FDG uptake in the muscles; this accumulation significantly influenced the evaluation of disease using 18F-FDG positron emission tomography/computed tomography (PET/CT) but had no effect on the distribution of 68Ga-PRGD2. (G, H) The regional uptake of 18F-FDG and the accumulation of 68Ga-PRGD2 in the shoulders and knees of an OA patient (M, 60 years) were significantly different from the diffuse synovial involvement in the patients with RA.
In contrast to the results observed in the 18F-FDG PET/CT images, no accumulation of 68Ga-PRGD2 was observed before treatment in the hyperplastic 18F-FDG-avid lymph nodes at the bilateral axillary regions of the patients with RA (figure 1A,C). Moreover, the pain and movement disorder in patients suffering from RA may have caused intense 18F-FDG uptake in the related muscles, which could have significantly influenced the evaluation of disease severity in the joints with18F-FDG PET/CT. However, the distribution of 68Ga-PRGD2 was much less varied in the skeletal muscles, bone marrow and myocardium than that of 18F-FDG; thus,68Ga-PRGD2 introduced less background noise and prevented possible evaluation bias in the assessment of disease severity and treatment response (figure 1E, F).
Correlation of PET/CT images with clinical parameters
The maximum standardised uptake value (SUVmax) of 68Ga-PRGD2 was significantly correlated with the SUVmax of 18F-FDG in the large joints before and after treatment (r=0.60 and 0.36, respectively; both p<0.001). Additionally, the SUVmax of 68Ga-PRGD2 was significantly correlated with TJC and SJC before and after treatment (p<0.001) (table 1).
Table 1 .
Correlation between the uptake of 18F-FDG and the accumulation of 68Ga-PRGD2 and the tender joint count and swollen joint count in patients with rheumatoid arthritis
| Pretreatment (n=20) | Post-treatment (n=12) | ||||
|---|---|---|---|---|---|
| TJC | SJC | TJC | SJC | ||
| SUVmax of 68Ga-PRGD2 | r | 0.44 | 0.22 | 0.43 | 0.33 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | |
| SUVmax of 18F-FDG | r | 0.37 | 0.12 | 0.44 | 0.39 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | |
SJC, swollen joint count; SUVmax, maximal standardised uptake value; TJC, tender joint count.
Three months after treatment, the patients with RA who repeated PET/CT were assigned to a responder group (n=9) or a poor-responder group (n=3) according to the cDAI good response criteria (defined as achieving ≥50% improvement of cDAI or cDAI ≤2.8 after treatment). The SUVmax of 18F-FDG and 68Ga-PRGD2 decreased significantly after treatment in the responder group (p<0.001). In contrast, the SUVmax of 18F-FDG and 68Ga-PRGD2 increased significantly in the poor-responder group (p=0.001 and p=0.002, respectively) (see online supplementary table S2).
We analysed the correlation between the change in SUVmax (ΔSUVmax) and the change in clinical parameters after treatment. We observed that the reduction in 68Ga-PRGD2 uptake in the affected joints was significantly correlated with the ΔPtGA, ΔPyGA and ΔcDAI (p<0.05), whereas the reduction in 18F-FDG uptake after treatment was significantly correlated with ΔPtGA and ΔPyGA (p<0.05) but not with ΔcDAI (p=0.083) (table 2).
Table 2 .
Correlation between the changes in SUVmax in 68Ga-PRGD2 PET/CT and 18F-FDG PET/CT and the changes in clinical parameters of patients with RA (n=12) before and after 3-month treatment
| ΔSUVmax of 68Ga-PRGD2 | ΔSUVmax of 18F-FDG | |||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| ΔVAS | 0.39 | 0.215 | 0.65 | 0.021 |
| ΔPtGA | 0.62 | 0.033 | 0.61 | 0.034 |
| ΔPyGA | 0.60 | 0.040 | 0.72 | 0.009 |
| ΔcDAI | 0.60 | 0.039 | 0.52 | 0.083 |
Δ, changes between the baseline and the post-treatment evaluation; cDAI, clinical disease activity index; r, correlation coefficient; PET, positron emission tomography; PtGA, patient's global assessment of overall well-being; PyGA, physician's global assessment; RA, rheumatoid arthritis; SUVmax, maximal standardised uptake value of a tracer; VAS, visual analogue scale of joint pain.
Histopathological features of the RA synovium
We examined the histopathology and expression of αvβ3-integrin in the synovia of two patients with RA to corroborate relevant findings with the 68Ga-PRGD2 PET/CT findings. In agreement with the intense 68Ga-PRGD2 accumulation in the affected joint synovium (see online supplementary figure S1A,B), high levels of αvβ3-integrin were selectively expressed on the endothelial cells of the synovial blood vessels (see online supplementary figure S1C). An extensive vascular network with ongoing angiogenesis and proliferation was observed in the synovium, as demonstrated by the positive staining of VEGF, CD34 and Ki67 (see online supplementary figure S1D–S1F).
Discussion
Synovial angiogenesis and pannus formation are major histopathological findings in patients with RA.1 2 The development of a new and reliable approach is needed to assess synovial neovasculature and its response to treatment.12 In the last decade, numerous studies have demonstrated that 18F-FDG PET/CT is a sensitive technique for evaluating disease activity and treatment response in patients with RA.13–15 However, the mechanism behind 18F-FDG uptake is only associated with elevated glucose metabolism.16
68Ga-PRGD2 is specifically designed to target the endothelial cells of neovasculature that express αvβ3-integrin at high levels.17 18 Therefore, 68Ga-PRGD2 PET/CT represents a specific method for evaluating angiogenesis. As demonstrated by the present study, 68Ga-PRGD2 was found to be typically distributed in a diffuse manner along the lining of the synovium of the affected joints and tendon sheaths of patients with RA, whereas the accumulation of 68Ga-PRGD2 was confined to a specific diseased area in patients with OA. Interestingly, we also found that 68Ga-PRGD2 did not accumulate in the 18F-FDG-avid axillary lymph nodes commonly observed in patients with RA.19 In patients with intense 18F-FDG uptake in muscles caused by arthritic pain, we observed that 68Ga-PRGD2 PET/CT was better able to evaluate disease severity than 18F-FDG PET/CT. Additionally, in response to therapeutic intervention, the changes of 68Ga-PRGD2, not 18F-FDG, significantly correlated with clinical measures of changes in the form of cDAI.
To the best of our knowledge, this is the first study conducted in humans to investigate the use of integrin imaging (specifically 68Ga-PRGD2 PET/CT) for the non-invasive measurement of synovial angiogenesis in patients with active RA. We compared the findings of this technique with the 18F-FDG PET/CT findings of the same patients. We provided histopathological confirmation showing high-level expression of αvβ3-integrin on the neovasculature endothelial cells of the 68Ga-PRGD2-avid RA synovium that were consistent with previous immunohistochemical findings in synovial tissue.9 20
Some limitations apply to the present study. First, the number of enrolled patients with RA was small. However, each patient underwent 68Ga-PRGD2 PET/CT and 18F-FDG PET/CT scanning, and 12 patients repeated the scans after 3-month treatment; thus, the preliminary results of this study support a proof-of-concept study. Second, the study lacks a sufficient number of control patients with OA or other forms of arthritis. An additional study is required to recruit a wide variety of patients with arthritis to determine the sensitivity, specificity and accuracy of 68Ga-PRGD2 PET/CT in diagnosing RA. Studies with more cases are needed to correlate the image findings related to post-treatment changes with the clinical response and final prognosis of patients with RA.
In conclusion, this prospective cohort study demonstrates that 68Ga-PRGD2 PET/CT is a specific method for identifying and assessing inflammatory synovial angiogenesis in patients with RA. In contrast to 18F-FDG, 68Ga-PRGD2 did not accumulate in areas such as the axillary lymph nodes with reactive hyperplasia and the strenuous skeletal muscles. Therefore, 68Ga-PRGD2 PET/CT is a useful tool for assessing synovial angiogenesis and monitoring treatment responses in patients with RA.
Supplementary Material
Acknowledgments
The authors are grateful to Congxin Li, Haiqun Xing, Na Niu, Yiming Liu, Yufeng Luo and other related staff at the Peking Union Medical College Hospital who helped with the study performance and data collection.
Footnotes
Contributors: XZ, ZZ, FL, XC and FZ were responsible for study design, data analysis and manuscript revision. YY, ZZ and KZ were responsible for patient recruitment, study performance, data collection, image analysis and manuscript drafting.
Funding: This study was funded in part by the National Natural Science Foundation of China (81325019, 81171370, 81172859, 81271614, 81273312, 81371596), the National High Technology Research and Development Project of China (2011AA020111, 2012AA02A513), the National Major Scientific and Technological Special Project (2012ZX09303006-002), the National Basic Research Program of China (973 program 2013CB733802, 2014CB744503), the Research Special Fund for Public Welfare Industry of Health (201202004), the Capital Health Research and Development of Special Foundation (2011-4001-02), the Capital Special Project for Featured Clinical Application (Z121107001012119), the National Laboratory Special Fund (2060204), the Intramural Research Program (IRP), National Institute of Biomedical Imaging and Bioengineering (NIBIB) and National Institutes of Health (NIH) and Beijing Municipal Natural Science Foundation (7141008).
Competing interests: None.
Patient consent: Obtained.
Ethics approval: This study has been approved by the Institutional Review Board of Peking Union Medical College Hospital (S-532).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Koch AE. Angiogenesis as a target in rheumatoid arthritis. Ann Rheum Dis 2003;62(suppl 2):ii60–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conaghan PG, O'Connor P, McGonagle D, et al. Elucidation of the relationship between synovitis and bone damage: a randomized magnetic resonance imaging study of individual joints in patients with early rheumatoid arthritis. Arthritis Rheum 2003;48:64–71 [DOI] [PubMed] [Google Scholar]
- 3.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 1992;69:11–25 [DOI] [PubMed] [Google Scholar]
- 4.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994;264:569–71 [DOI] [PubMed] [Google Scholar]
- 5.Niu G, Chen X. Why integrin as a primary target for imaging and therapy. Theranostics 2011;1:30–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Z, Miao W, Li Q, et al. 99mTc-3PRGD2 for integrin receptor imaging of lung cancer: a multicenter study. J Nucl Med 2012;53:716–22 [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Liu S, Hou Y, et al. MicroPET imaging of breast cancer alphav-integrin expression with 64Cu-labeled dimeric RGD peptides. Mol Imaging Biol 2004;6:350–9 [DOI] [PubMed] [Google Scholar]
- 8.Wilder RL. Integrin alpha V beta 3 as a target for treatment of rheumatoid arthritis and related rheumatic diseases. Ann Rheum Dis 2002; 61(suppl 2):ii96–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Storgard CM, Stupack DG, Jonczyk A, et al. Decreased angiogenesis and arthritic disease in rabbits treated with an alphavbeta3 antagonist. J Clin Invest 1999;103:47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24 [DOI] [PubMed] [Google Scholar]
- 11.Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005;23:S100–8 [PubMed] [Google Scholar]
- 12.Naredo E, Collado P, Cruz A, et al. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum 2007;57:116–24 [DOI] [PubMed] [Google Scholar]
- 13.Goerres GW, Forster A, Uebelhart D, et al. F-18 FDG whole-body PET for the assessment of disease activity in patients with rheumatoid arthritis. Clin Nucl Med 2006;31:386–90 [DOI] [PubMed] [Google Scholar]
- 14.Elzinga EH, van der Laken CJ, Comans EF, et al. 18F-FDG PET as a tool to predict the clinical outcome of infliximab treatment of rheumatoid arthritis: an explorative study. J Nucl Med 2011;52:77–80 [DOI] [PubMed] [Google Scholar]
- 15.Okamura K, Yonemoto Y, Arisaka Y, et al. The assessment of biologic treatment in patients with rheumatoid arthritis using FDG-PET/CT. Rheumatology (Oxford) 2012;51:1484–91 [DOI] [PubMed] [Google Scholar]
- 16.Izuishi K, Yamamoto Y, Sano T, et al. Molecular mechanism underlying the detection of colorectal cancer by 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography. J Gastrointest Surg 2012;16:394–400 [DOI] [PubMed] [Google Scholar]
- 17.Lang L, Li W, Guo N, et al. Comparison study of [18F]FAl-NOTA-PRGD2, [18F]FPPRGD2, and [68Ga]Ga-NOTA-PRGD2 for PET imaging of U87MG tumors in mice. Bioconjug Chem 2011;22:2415–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo N, Lang L, Li W, et al. Quantitative analysis and comparison study of [18F]AlF-NOTA-PRGD2, [18F]FPPRGD2 and [68Ga]Ga-NOTA-PRGD2 using a reference tissue model. PLoS ONE 2012;7:e37506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seldin DW, Habib I, Soudry G. Axillary lymph node visualization on F-18 FDG PET body scans in patients with rheumatoid arthritis. Clin Nuclear Med 2007;32:524–26 [DOI] [PubMed] [Google Scholar]
- 20.Johnson BA, Haines GK, Harlow LA, et al. Adhesion molecule expression in human synovial tissue. Arthritis Rheum 1993;36:137–46 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.