Abstract
Several Bacillus cereus strains possess the genetic fittings to produce two different types of toxins, the heat-stable cereulide or different heat-labile proteins with enterotoxigenic potential. Unlike the diarrheal toxins, cereulide is (pre-)formed in food and can cause foodborne intoxications shortly after ingestion of contaminated food. Based on the widely self-limiting character of cereulide intoxications and rarely performed differential diagnostic in routine laboratories, the real incidence is largely unknown. Therefore, during a 7-year period about 4.300 food samples linked to foodborne illness with a preliminary report of vomiting as well as food analysed in the context of monitoring programs were investigated to determine the prevalence of emetic B. cereus in food environments. In addition, a lux-based real-time monitoring system was employed to assess the significance of the detection of emetic strains in different food matrices and to determine the actual risk of cereulide toxin production in different types of food. This comprehensive study showed that emetic strains are much more volatile than previously thought. Our survey highlights the importance and need of novel strategies to move from the currently taxonomic-driven diagnostic to more risk orientated diagnostics to improve food and consumer safety.
1. Introduction
Cereulide, an emesis-inducing toxin produced by a fairly homogenous group of B. cereus strains called “emetic B. cereus,” is a small heat-stable cyclic peptide [1]. The emetic poisoning caused by cereulide is usually characterized by vomiting starting after 0.5 hour to six hours after consumption of the contaminated food. Intoxications proceed mostly with mild symptoms and last normally not more than one day, but severe cases requiring hospitalization are increasingly reported (for review see Ehling-Schulz et al., 2004 [2], and Ehling-Schulz et al., 2011 [3]).
Because of the short period of illness, the emetic syndrome caused by B. cereus is presumably underreported [4]. In addition, the symptoms of an emetic intoxication caused by B. cereus parallel the symptoms caused by S. aureus enterotoxins, bearing the risk of misdiagnosis of the disease. In the year 2011 the European Food Safety Authority (EFSA) reported an increase of 122.2% in the number of foodborne intoxications and toxicoinfections caused by B. cereus in Europe. The overall reporting rate was 0.04 cases per 100.000 inhabitants [5]. Even if intoxication with the emetic toxin cereulide in most cases produces only mild symptoms, consistently also fatal cases are reported [6–8].
Although B. cereus is an ubiquitous spore former, emetic strains are rarely found in the environment and their natural niches and entrance points into the food production and processing are largely unknown [4]. So far, mostly high-carb food matrices, such as rice and pasta, as well as milk and dairy products, have been investigated for the presence of emetic strains of B. cereus [9–13], whereas other food matrices have rarely been included in the analyses. To improve HACCP-based concepts and prevent foodborne intoxications caused by emetic B. cereus, information on the general prevalence of emetic strains in foods of different origin is of utmost importance and data on the risk of toxin formation in different food categories are required.
This study therefore aimed to (i) investigate the prevalence of emetic B. cereus strains in a wide range of food matrices, covering foods from plant as well as animal origin, to identify potential contamination sources, and to (ii) facilitate hazard identification by exploring the potential of diverse food matrices for the risk of cereulide toxin production. In this context, a perennial survey from 2007 to 2013 was carried out, including food samples connected to emesis-related foodborne illnesses as well as samples not related to foodborne outbreaks. By using an in situ bioassay indicative of cereulide production levels, a general scheme for categorizing foods with respect to their risk of cereulide production was generated.
2. Material and Methods
2.1. Sample Material
Between the years 2007 and 2013 3.564 food samples from Bavaria were analysed for the presence of emetic B. cereus strains in the context of foodborne illness or outbreaks where the consumers showed symptoms of vomiting. The majority of samples were taken from the household of the diseased consumers and from restaurants, canteens, and catering companies. Additionally, the presence of emetic B. cereus strains in different food matrices (n = 742) was investigated in the scope of different monitoring programs. Food categories for the monitoring were chosen from both food of animal origin and food of plant origin. All samples were examined before their expiry date.
2.2. Microbiological Detection of B. cereus and Identification of the Cereulide Synthetase Gene ces
Emetic B. cereus strains were detected with qualitative and quantitative methods (for details see Ehling-Schulz et al., 2011 [3]). The qualitative detection was done weighting 10 g of sample material into 90 mL of tryptone-peptone-glucose-yeast (TPGY) broth and incubating at 30°C under aerobic conditions. After 24 h of cultivation 1 mL of the enrichment broth was taken for the molecular detection of the ces genes, which encode the nonribosomal synthetase responsible for the production of the peptide toxin cereulide. For detection of ces, a previously described probe-based diagnostic real-time-PCR assay was used [9, 15].
The quantitative detection of presumptive B. cereus was carried out using standard reference culture methods recommended by the International Organisation of Standardization (ISO) and the U.S. Food and Drug Administration (FDA). Samples were investigated using spiral plate count method on the Mossel agar [16, 17] or with a 3-tube 3-dilution most probable number (MPN) method [18–21]. Presumptive B. cereus colonies were further differentiated by the detection of the ces gene either by real-time-PCR as described above or by using a conventional PCR system according to Ehling-Schulz et al., 2004 [22]. Depending on the results of these reactions the number of colony-forming units per gram (cfu/g) or MPN of emetic B. cereus cells per gram of sample was calculated following the standard methods recommended by FDA and ISO [16, 17, 21].
2.3. Bioassay-Based Risk Categorization of Foods
Analysis of the potential of food matrices to support cereulide production was performed by artificial contamination of 30 g portions with the bioluminescent B. cereus lux reporter strain F4810/72(pMDX[P1/luxABCDE]) and an IVIS camera system as described earlier [23, 24]. Foods were provided by diverse manufacturers or were obtained from local consumer markets. In the case of powders and freeze-dried products (e.g., infant formulas and instant potato powder) or raw materials (e.g., rice and pasta) foods were prepared according to the manufacturers' instructions thereby simulating common household conditions. The contents of preportioned packaging units (e.g., single-sliced cheese or biscuit snacks) were combined and blended for 3 min with a stomacher to obtain homogenous testing matrices. Dry foods, such as dates, apricots, cocoa powder, and herbal salt, were additionally soaked with sterile water or pasteurized milk (1.5% fat content) as indicated. Matrices were filled into Petri dishes and inoculated to a final reporter strain cell count of 103 CFU per gram. After an incubation step for 24 hours at 24°C, the luciferase signal intensities were quantified with a photon-counting intensified-charge-coupled-device (ICCD) camera (model 2400-32; Hamamatsu Photonics) and are shown as false-color renderings that were superimposed on gray-scale images of the respective food sample.
3. Results and Discussion
This study was designed to get a comprehensive overview of the prevalence of emetic B. cereus strains in both food samples from supposed foodborne intoxications and food samples from general food monitoring programs. These data should provide a profound basis for a better risk assessment concerning the emetic syndrome caused by cereulide producing emetic B. cereus strains. In addition, the influence of food matrix properties on cereulide production was evaluated using a previously established lux reporter system [24].
3.1. Prevalence of Emetic B. cereus in Foods Linked to FoodBorne Intoxications and in Nonfood Intoxication Associated Food Samples
Because most studies hitherto targeted only a very limited range of food matrices, such as rice and pasta (e.g., [25–27]), and samples were collected from very specific sites or during very short sampling periods (see e.g., [13]), prevalence data covering samples from different years and diverse food matrices are still missing. However, in the context of preventive consumer protection policy and for a comprehensive risk assessment, data about the prevalence of emetic strains in different food categories from a perennial sampling period are required. We therefore analysed 3.654 food samples obtained from suspected foodborne illness with a preliminary report of vomiting shortly (within a period from thirty minutes to six hours) after consumption of the suspected meal over a period of 7 years (2007 to 2013). The analysed samples covered a broad variety of food categories (Figure 1). Presumptive B. cereus was detected in 187 samples (5%) and emetic B. cereus strains were detected in 32 samples (1%). Interestingly, emetic strains were not only detected in farinaceous foods commonly linked to cereulide intoxication (e.g., [1, 6, 8]) but also in vegetables, fruit products, sauces, soups, and salads as well as in cheese and meat products (Figure 1). Recently, Doménech-Sánchez et al. [28] reported on an emetic outbreak linked to the consumption of tuna fish. These results emphasize that more data on the prevalence of emetic B. cereus in different types of foods are needed to decipher potential contamination sources.
Figure 1.
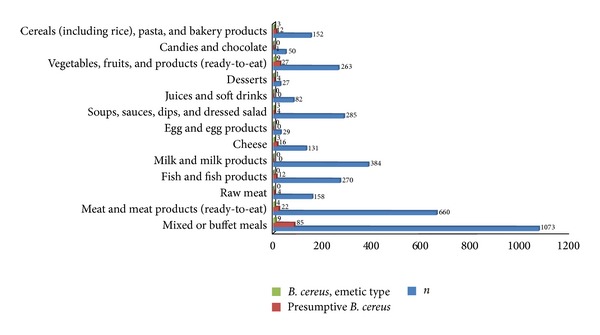
Presumptive Bacillus cereus and emetic strains in different food matrices investigated in the context of supposed foodborne intoxications.
In most samples tested positive for B. cereus, which have been analysed in the context of foodborne intoxications, emetic strains were found in levels ≤102 cfu/g food matrix (see Table 1). All of these samples were tested negative for the presence of other foodborne pathogens, including S. aureus and its enterotoxins (data not shown). It is therefore assumed that B. cereus was indeed the etiological agent of the reported outbreaks. The detection of emetic B. cereus in low levels in samples from suspected foodborne illness could be an indication that the bacteria themselves were reduced by the food production and processing procedure, but the preformed heat- and acid-stable toxin cereulide was not eliminated or inactivated. In addition, it is known that the capability of toxin formation varies significantly among emetic B. cereus strains and the actual toxin production depends on external parameters [11, 23, 29, 30]. These examples highlight the need of novel diagnostic strategies, moving from taxonomy to more risk orientated differential diagnostics (for review see Ehling-Schulz and Messelhäusser, 2013 [31]).
Table 1.
Examples for potentially foodborne diseases caused by emetic B. cereus in Bavaria between the years 2007 and 2013.
| Year | Diseased persons | Place | Food matrix | Level of emetic B. cereus (cfu/g) |
|---|---|---|---|---|
| 2007 | Several students after a cooking lesson at school | School kitchen | Hard cheese | <100 (only positive using a qualitative detection method, but detection of 2 μg cereulid/g*) |
|
| ||||
| 2007 | One adult | Restaurant | Cooked pasta | 3.8 × 105 |
|
| ||||
| 2008 | Several students | School canteen | Paprika filled with meat and rice | <100 (only positive using a qualitative detection method) |
|
| ||||
| 2009 | One adult | Household | Cooked potatoes | <100 (only positive using a qualitative detection method) |
|
| ||||
| 2010 | One adult | Restaurant | Cooked pasta with oysters | <100 (only positive using a qualitative detection method) |
|
| ||||
| 2010 | Several adults | Canteen | Poulard breast in tomato sauce | <100 (only positive using a qualitative detection method) |
|
| ||||
| 2010 | Several adults | Catering | Chana masala (cooked chickpea) with baked potatoes in curry sauce and cooked rice | Cooked rice: 2.8 × 104 (1 μg cereulid/g*) Cooked chickpea: <10 (only positive using a qualitative detection method, but detection of 0.3 μg cereulid/g*); see also Ehling-Schulz and Messelhaeusser, 2012 [14] |
|
| ||||
| 2011 | Several children (1 to 3 years old) | Nursery school | Cooked pasta with tomato sauce | 6.8 × 106 |
|
| ||||
| 2011 | Two adults | Restaurants | Cooked pork meat with potatoes | 1.0 × 102 |
|
| ||||
| 2011 | One adult | Household | Cured and smoked meat | 1.0 × 102 |
|
| ||||
| 2012 | Several students | Canteen | Raspberry quark | 1.4 × 102 |
|
| ||||
| 2012 | One adult | Household | cooked mushrooms | 1.9 × 107 |
|
| ||||
| 2013 | Several adults | Catering at a wedding | Vitello tonnato | 6.1 × 107 |
*Currently, no officially validated method for the quantitative detection of cereulide in food matrices is available; therefore quantitative data on cereulide toxin are only shown for selected samples. However, recently a European initiative has been started to establish appropriate ISO methods (CEN/TC 275/WG 6).
To gain a deeper insight into food associated natural niches of emetic B. cereus and potential contamination sources, 742 food samples of animal and plant origin were investigated for the presence of emetic B. cereus strains within different monitoring programs (Figure 2). For food of animal origin, samples were grouped in categories that have been reported in the context of foodborne illness, for example, ready-to-eat meat products, cheese, and cream. For food of plant origin, food matrices were investigated that could be possible contamination sources for ready-to-eat food, such as herbs, spices, and dried mushrooms or fresh foods, such as lettuce, fruits, and vegetables. Emetic strains were most frequently found in pasta filata cheese obtained from retail level (13%), in dried mushrooms (8%), and in herbal teas (8%). The detection rates in these matrices were even higher than in uncooked rice and pasta (6%), whereof also 78 samples were investigated. Overall, 10% of presumptive B. cereus strains, isolated in the context of monitoring programs, possess the ces gene and therefore the ability to produce cereulide toxin. These prevalence rates are slightly higher than the ones reported from previous studies (e.g., [25, 27]). One explanation might be that emetic B. cereus strains are easily overlooked in routine diagnostic since they frequently show an atypical phenotype and might, in addition, be outcompeted on nonselective agar media often used in microbial diagnostics [32]. The food category investigated could also significantly influence the percentage of emetic isolates detected. For instance, as our study showed (in food categories for which more than 50 samples were investigated) the percentage of emetic strains isolated from different food matrices varied between 10% (dried mushrooms) and 17% (pasta filata cheese) (see Figure 2).
Figure 2.
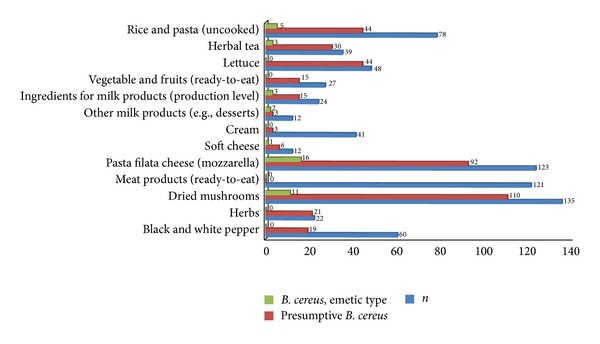
Presumptive and emetic B. cereus in different food matrices investigated in the context of monitoring programs.
However, not only the presence of strains but also the potential of food matrices to support cereulide synthesis should be considered for an accurate risk assessment, since unavoidable low-level contaminations with the spore formers might lead to intoxications or even large-scale outbreaks in cases of improper storage and handling of prepared meals. Previous work showed that the risk of cereulide production is strongly connected with external parameters and varies significantly among different types of model foods that have been investigated so far [23, 24, 30].
3.2. Broad-Scale Risk Categorization of Food Matrices concerning Cereulide Synthesis
Although the EFSA stressed the necessity of identifying categories of foods that may pose a risk for human health with respect to cereulide contamination [4], a comprehensive evaluation of food matrices was hampered due to laborious, time-consuming, and error-prone methods to quantify cereulide amounts in foodstuffs. Recently, a SIDA-based method allowing the quantitative detection of cereulide has been developed [33]. However, alternative high-throughput methods to estimate the risk of toxin production in diverse food matrices are needed. The lux-based reporter system for real-time monitoring of toxin gene expression described by Dommel et al. [24] might represent an interesting tool in the latter context. We previously showed that cereulide production in model food matrices is proportional to the intensity of the bioluminescence signals emitted by the engineered B. cereus reporter strain [23, 24].
In this study, we employed the lux reporter system for the analysis of a total of 70 retail products in order to decipher abiotic and nutritional factors, either promoting or suppressing toxin synthesis. Luciferase signals were quantified with a software-assisted region-of-interest (ROI) analysis and foods were categorized into three main classes regarding their toxin formation capability: high-risk, risk, and low-risk foods (Table 2, Figures S1–S3 in Supplementary Material available online at http://dx.doi.org/10.1155/2014/465603). Derived mean ROI values of each risk category and the corresponding determined threshold values are listed in Table S1. The bioassay revealed that 44% of the foods could be categorized as high-risk foods, while the remaining 20% and 36% were categorized as risk or low-risk foods, respectively (Table 2). Products classified as being insensitive were dairy based, displayed a low pH value (e.g., cream cheese and unsweetened quark), had a high fat content like chocolate and nut spread, and/or were characterized by low water availability or high osmolarity (e.g., dried fruits and 10% herbal salt solution). Earlier studies showed that growth of B. cereus was suppressed in foods with pH values below 5.0 [34–36], which is in line with our low-risk classification of matrices that had pH values around 4.3 to 4.8, such as the whey drinks. The combination of neutral pH values and medium aw-values with high amounts of fat and cocoa was found to be indicative of the group of products being at medium risk of toxin synthesis (Table 2 and Figure S2). Likewise, proteinaceous foodstuff containing high fat amounts, such as minced beef or milk powder-based processed cheeses, fell in the same category. This is in agreement with a previous study [37] showing that cereulide was produced in small quantities in artificially contaminated meat products. The same study also supports our results concerning the pasteurized milk: usually, only low to medium cereulide levels are produced under stationary conditions at room temperature [30, 37]. Additionally, dairy products dulcified with glucose or fructose (quark desserts, cream-filled soft biscuits) fell in the intermediate class in terms of the risk for cereulide production. It was shown previously that glucose had a stimulating effect on cereulide synthesis [4]. The group of high-risk products comprised farinaceous foods, as well as powdered products that were reconstituted with water or milk (Table 2, Figure S3). Dairy- and cereal-based infant food formulas, which were additionally enriched with vitamins or trace elements, promoted exceptional high ces promoter activities. The latter indicates that a combination of readily available saccharides, vitamins, and macronutrients in a pH neutral environment may stimulate toxin formation. Indeed, cereulide was detected in high levels in farinaceous matrices or systems containing high amounts of K+ ions and vitamins [10, 38].
Table 2.
Bioassay-based categorization of 70 retail foods according to their potential for supporting cereulide production. Bioluminescence intensity produced by the cereulide synthesis reporter strain F4810/72(pMDX[P1/luxABCDE]) was measured after 24 hours of incubation at 24°C. Representative images are shown in Figure 3 and Figures S1–S3. Threshold values established for risk categorization are listed in Table S1.
| Low-risk foods | Risk foods | High-risk foods |
|---|---|---|
| Dried apricots | Reconstituted milk powder (organic) | Cereal-based reconstituted infant food (fruit flavour) |
| Dried apricots rehydrated with water | Dried dates rehydrated with water | Cereal-based reconstituted infant food |
| Infant food with yoghurt and fruits | Cheese slices with suisse flavour | Cereal-based reconstituted infant food (whole grain/apple flavour) |
| Crème fraîche | Cheese slices with mozzarella flavour | Dessert creme with cream/coffee flavour |
| Crème fraîche with herbs | Minced pork | Dessert creme with caramel flavour |
| Dried dates | Minced veal | Diet drink with vanilla flavour |
| Diet chocolate with cream filling | Cocoa powder with milk | Muesli with water |
| Cottage cheese (whole fat content) | Herbal salt (1% in water) | Muesli with milk |
| Fresh cheese (natural) | Latte macchiato drink | Semolina pudding (natural) |
| Fresh cheese with herbs | Camembert cheese (60% fat content) | Semolina pudding (vanilla flavour) |
| Fresh cheese with chilli flavour | Chocolate mousse | Semolina pudding (cinnamon flavour) |
| Yoghurt of fresh cheese with fruits | Pasteurized milk (1.5% fat content) | Boiled Jasmin rice (organic grains) |
| Yoghurt of fresh cheese with vanilla/fruit | Pasteurized cream (30% fat content) | Boiled Jasmin rice (parboiled grains) |
| Yoghurt of fresh cheese with raspberry | Chocolate biscuit with milk cream filling | Mashed potatoes (powder reconstituted with water) |
| Cocoa powder reconstituted with water | Mashed potatoes (made from cooked potatoes) | |
| Curd cheese | Reconstituted skim milk powder | |
| Curd cheese with vanilla flavour | Milk drink with nut flavour | |
| Whey drink peach flavour | Rice pudding (natural) | |
| Whey drink cherry/banana flavour | Rice pudding (strawberry flavour) | |
| Nougat creme | Rice pudding (chocolate flavour) | |
| Sauce carbonara | Rice pudding (vanilla flavour) | |
| Chocolate bar with milk/caramel filling | Rice pudding (cinnamon flavour) | |
| Soy bean sprouts | Boiled whole grain rice | |
| Quark | Scrambled egg | |
| Herbal salt (10% in water) | Soy milk | |
| Soy milk-based dessert with caramel flavour | ||
| Soy milk-based dessert with vanilla flavour | ||
| Reconstituted whole milk powder | ||
| Mousse au vanilla | ||
| Pudding with vanilla flavour | ||
| Vanilla sauce |
A summary of food characteristics commonly observed in the three categories is provided in Figure 3. This generalized scheme allows a basic preevaluation of foods and their ingredients concerning their capability to support cereulide formation and should facilitate hazard identification in terms of HACCP concepts.
Figure 3.
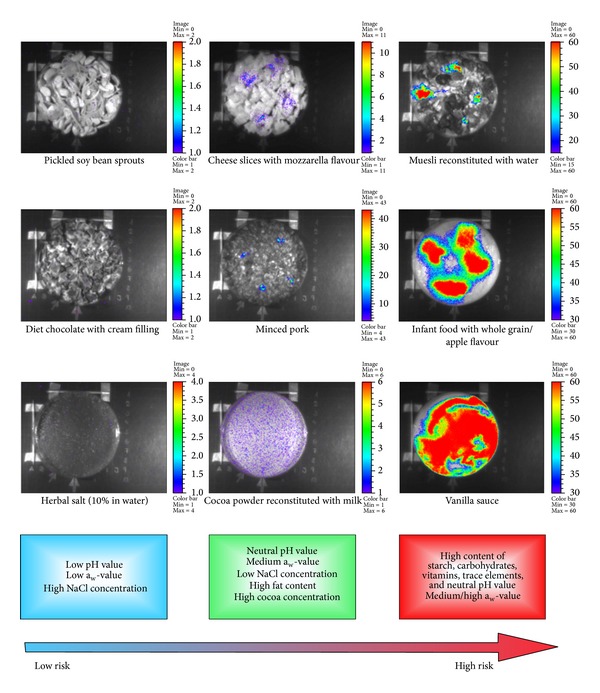
Scheme for abiotic factors influencing the activity of the ces NRPS promoter driving the synthesis of cereulide. The parameters were deduced from the examination of 70 foods and food ingredient using an emetic lux reporter strain [10]. The arrow denotes an increasing toxin formation capability with respect to the food composition. Examples of typical food matrices for each category are shown.
4. Conclusion
Overall, our results indicate that emetic B. cereus strains occur more frequently and in a much broader diversity of foods than noticed so far. In addition, the lux-based real-time monitoring assay turned out to be a valuable tool for assessing the actual risk of cereulide toxin production in different types of food, allowing us to set up a general scheme for the categorizing of foods with respect to their cereulide production risk. Our survey of presumptive emetic B. cereus foodborne outbreaks also showed that the risk of an emetic syndrome caused by the B. cereus cereulide toxin is not restricted to high-carb foods, such as pasta and rice. Much more attention must be paid to other foods, especially the ones supporting cereulide production, as shown by the lux reporter assay.
Supplementary Material
Figures S1, S2, S3: Examples of foods categorized being at low risk (Si), at risk (S2) or at high risk (S3) to promote cereulide formation in the presence of emetic B. cereus strains.
The bioassay-based risk categorization was performed by inoculating 70 retail products and food ingredients with a lux based B. cereus cereulide reporter strain. The luminescence intensity, which corresponds to the amount of synthesized cereulide [24], was quantified in situ after incubation of the samples for 24 hours at 24°C (for details, see Material and Methods). Food samples were grouped into three main classes regarding their toxin formation capability via a software-assisted region-of-interest (ROI) analysis. The bioassay revealed that 44% of the foods could be categorized as high-risk foods, while the remaining 20% and 36% were categorized as risk or low-risk foods, respectively. Derived mean ROI values of each risk category and the corresponding determined threshold values are listed in Table S1.
Acknowledgments
This project was supported by the German Ministry of Economics and Technology (via AiF) and the FEI (Forschungskreis der Ernährungsindustrie e.V., Bonn); Project AiF 15186 N and 16845 N.
Conflict of Interests
The authors have declared no conflict of interests.
References
- 1.Agata N, Ohta M, Mori M, Isobe M. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus . FEMS Microbiology Letters. 1995;129(1):17–20. doi: 10.1016/0378-1097(95)00119-P. [DOI] [PubMed] [Google Scholar]
- 2.Ehling-Schulz M, Fricker M, Scherer S. Bacillus cereus, the causative agent of an emetic type of food-borne illness. Molecular Nutrition & Food Research. 2004;48(7):479–487. doi: 10.1002/mnfr.200400055. [DOI] [PubMed] [Google Scholar]
- 3.Ehling-Schulz M, Messelhäusser U, Granum PE. Bacillus cereus in milk and dairy production. In: Hoorfar J, editor. Rapid Detection, Characterization and Enumeration of Food-Borne Pathogens. Washington, DC, USA: ASM Press; 2011. pp. 275–289. [Google Scholar]
- 4.European Food Safety Authority (EFSA) Opinion of the scientific panel on biological hazards of Bacillus cereus and other Bacillus spp. in foodstuff. The EFSA Journal. 2005;175:1–48. [Google Scholar]
- 5.European Food Safety Authority (EFSA) The European Union Summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2011. The EFSA Journal. 2013;11(4)3129 [PubMed] [Google Scholar]
- 6.Dierick K, van Coillie E, Swiecicka I, et al. Fatal family outbreak of Bacillus cereus-associated food poisoning. Journal of Clinical Microbiology. 2005;43(8):4277–4279. doi: 10.1128/JCM.43.8.4277-4279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pósfay-Barbe KM, Schrenzel J, Frey J, et al. Food poisoning as a cause of acute liver failure. Pediatric Infectious Disease Journal. 2008;27(9):846–847. doi: 10.1097/INF.0b013e318170f2ae. [DOI] [PubMed] [Google Scholar]
- 8.Naranjo M, Denayer S, Botteldoorn N, et al. Sudden death of a young adult associated with Bacillus cereus food poisoning. Journal of Clinical Microbiology. 2011;49(12):4379–4381. doi: 10.1128/JCM.05129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messelhäusser U, Kämpf P, Fricker M, et al. Prevalence of emetic Bacillus cereus in different ice creams in Bavaria. Journal of Food Protection. 2010;73(2):395–399. doi: 10.4315/0362-028x-73.2.395. [DOI] [PubMed] [Google Scholar]
- 10.Shaheen R, Andersson MA, Apetroaie C, et al. Potential of selected infant food formulas for production of Bacillus cereus emetic toxin, cereulide. International Journal of Food Microbiology. 2006;107(3):287–294. doi: 10.1016/j.ijfoodmicro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Svensson B, Monthan A, Shaheen R, Andersson MA, Salkinoja-Salonen M, Christiansson A. Occurrence of emetic toxin producing Bacillus cereus in the dairy production chain. International Dairy Journal. 2006;16(7):740–749. [Google Scholar]
- 12.Ankolekar C, Rahmati T, Labbé RG. Detection of toxigenic Bacillus cereus and Bacillus thuringiensis spores in U.S. rice. International Journal of Food Microbiology. 2009;128(3):460–466. doi: 10.1016/j.ijfoodmicro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Delbrassinne L, Andjelkovic M, Dierick K, Denayer S, Mahillon J, van Loco J. Prevalence and levels of Bacillus cereus emetic toxin in rice dishes randomly collected from restaurants and comparison with the levels measured in a recent foodborne outbreak. Foodborne Pathogens and Disease. 2012;9(9):809–814. doi: 10.1089/fpd.2012.1168. [DOI] [PubMed] [Google Scholar]
- 14.Ehling-Schulz M, Messelhaeusser U. One pathogen but two different types of food borne outbreaks, Bacillus cereus in catering facilities in Germany. In: Hoorfar J, editor. Case Studies in Food Safety and Quality Management: Lessons from Real-Life Situations. Cambridge, UK: Woodhead; 2012. pp. 63–70. [Google Scholar]
- 15.Fricker M, Messelhäusser U, Busch U, Scherer S, Ehling-Schulz M. Diagnostic real-time PCR assays for the detection of emetic Bacillus cereus strains in foods and recent food-borne outbreaks. Applied and Environmental Microbiology. 2007;73(6):1892–1898. doi: 10.1128/AEM.02219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maturin L, Peeler JT. Bacteriological Analytical Manual. chapter 3. Silver Spring, Md, USA: U.S. Food and Drug Administration; 2001. Aerobic plate count. [Google Scholar]
- 17.International Organization of Standardization (ISO) ISO. 6888-1:1999. Geneva, Switzerland: International Organization of Standardization (ISO); 1999. Microbiology of food and animal feeding stuffs—horizontal method for the enumeration of coagulase-positive staphylococci (Staphylococcus aureus and other species)—part 1: technique using Baird-Parker agar medium. [Google Scholar]
- 18.International Organization of Standardization (ISO) EN ISO. 21871:2006. Geneva, Switzerland: International Organization of Standardization (ISO); 2006. Microbiology of food and animal feeding stuffs—horizontal method for the determination of low numbers of presumptive Bacillus cereus—most probable number technique and detection method. [Google Scholar]
- 19.International Organization of Standardization (ISO) EN ISO. 8261:2001. Geneva, Switzerland: International Organization of Standardization (ISO); 2001. Milk and milk products—preparation of test samples and dilutions for microbiological examination. [Google Scholar]
- 20.International Organization of Standardization (ISO) EN ISO. 6887-4:2004. Geneva, Switzerland: International Organization of Standardization (ISO); 2004. Microbiology of food and animal feeding stuffs—preparation of test samples, initial suspension and decimal dilutions for microbiological examination—parts 1–5. [Google Scholar]
- 21.International Organization of Standardization (ISO) PrEN ISO. 7218:2005. Geneva, Switzerland: International Organization of Standardization (ISO); 2005. Microbiology of food and animal feeding stuffs–general requirements and guidance for microbiological examinations. [Google Scholar]
- 22.Ehling-Schulz M, Fricker M, Scherer S. Identification of emetic toxin producing Bacillus cereus strains by a novel molecular assay. FEMS Microbiology Letters. 2004;232(2):189–195. doi: 10.1016/S0378-1097(04)00066-7. [DOI] [PubMed] [Google Scholar]
- 23.Frenzel E, Letzel T, Scherer S, Ehling-Schulz M. Inhibition of cereulide toxin synthesis by emetic Bacillus cereus via long-chain polyphosphates. Applied and Environmental Microbiology. 2011;77(4):1475–1482. doi: 10.1128/AEM.02259-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dommel M, Frenzel E, Straßer B, Blöchinger C, Scherer S, Ehling-Schulz M. Identification of the main promoter directing cereulide biosynthesis in emetic Bacillus cereus and its application for real-time monitoring of ees gene expression in foods. Applied and Environmental Microbiology. 2010;76(4):1232–1240. doi: 10.1128/AEM.02317-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samapundo S, Heyndrickx M, Xhaferi R, Devlieghere F. Incidence, diversity and toxin gene characteristics of Bacillus cereus group strains isolated from food products marketed in Belgium. International Journal of Food Microbiology. 2011;150(1):34–41. doi: 10.1016/j.ijfoodmicro.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Ouoba LI, Thorsen L, Varnam AH. Enterotoxins and emetic toxins production by Bacillus cereus and other species of Bacillus isolated from Soumbala and Bikalga, African alkaline fermented food condiments. International Journal of Food Microbiology. 2008;124(3):224–230. doi: 10.1016/j.ijfoodmicro.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Wijnands LM, Dufrenne JB, Rombouts FM, In’t Veld PH, van Leusden FM. Prevalence of potentially pathogenic Bacillus cereus in food commodities in the Netherlands. Journal of Food Protection. 2006;69(11):2587–2594. doi: 10.4315/0362-028x-69.11.2587. [DOI] [PubMed] [Google Scholar]
- 28.Doménech-Sánchez A, Laso E, Pérez MJ, Berrocal CI. Emetic disease caused by Bacillus cereus after consumption of tuna fish in a beach club. Foodborne Pathogens and Disease. 2011;8(7):835–837. doi: 10.1089/fpd.2010.0783. [DOI] [PubMed] [Google Scholar]
- 29.Dommel M, Lücking G, Scherer S, Ehling-Schulz M. Transcriptional kinetic analyses of cereulide synthetase genes with respect to growth, sporulation and emetic toxin production in Bacillus cereus . Food Microbiology. 2011;28(2):284–290. doi: 10.1016/j.fm.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Rajkovic A, Uyttendaele M, Ombregt S-A, Jaaskelainen E, Salkinoja-Salonen M, Debevere J. Influence of type of food on the kinetics and overall production of Bacillus cereus emetic toxin. Journal of Food Protection. 2006;69(4):847–852. doi: 10.4315/0362-028x-69.4.847. [DOI] [PubMed] [Google Scholar]
- 31.Ehling-Schulz M, Messelhäusser U. Bacillus “next generation” diagnostics: moving from detection toward subtyping and risk-related strain profiling. Frontiers in Microbiology. 2013;4, article 32 doi: 10.3389/fmicb.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fricker M, Reissbrodt R, Ehling-Schulz M. Evaluation of standard and new chromogenic selective plating media for isolation and identification of Bacillus cereus . International Journal of Food Microbiology. 2008;121(1):27–34. doi: 10.1016/j.ijfoodmicro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Bauer T, Stark T, Hofmann T, Ehling-Schulz M. Development of a stable isotope dilution analysis for the quantification of the Bacillus cereus toxin cereulide in foods. Journal of Agricultural and Food Chemistry. 2010;58(3):1420–1428. doi: 10.1021/jf9033046. [DOI] [PubMed] [Google Scholar]
- 34.Valero M, Fernandez PS, Salmeron MC. Influence of pH and temperature on growth of Bacillus cereus in vegetable substrates. International Journal of Food Microbiology. 2003;82(1):71–79. doi: 10.1016/s0168-1605(02)00265-9. [DOI] [PubMed] [Google Scholar]
- 35.Lindsay D, Brözel VS, Mostert JF, Holy A. Physiology of dairy-associated Bacillus spp. over a wide pH range. International Journal of Food Microbiology. 2000;54(1-2):49–62. doi: 10.1016/s0168-1605(99)00178-6. [DOI] [PubMed] [Google Scholar]
- 36.Agata N, Ohta M, Mori M, Shibayama K. Growth conditions of and emetic toxin production by Bacillus cereus in a defined medium with amino acids. Microbiology and Immunology. 1999;43(1):15–18. doi: 10.1111/j.1348-0421.1999.tb02367.x. [DOI] [PubMed] [Google Scholar]
- 37.Agata N, Ohta M, Yokoyama K. Production of Bacillus cereus emetic toxin (cereulide) in various foods. International Journal of Food Microbiology. 2002;73(1):23–27. doi: 10.1016/s0168-1605(01)00692-4. [DOI] [PubMed] [Google Scholar]
- 38.Apetroaie-Constantin C, Shaheen R, Andrup L, Smidt L, Rita H, Salkinoja-Salonen M. Environment driven cereulide production by emetic strains of Bacillus cereus . International Journal of Food Microbiology. 2008;127(1-2):60–67. doi: 10.1016/j.ijfoodmicro.2008.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1, S2, S3: Examples of foods categorized being at low risk (Si), at risk (S2) or at high risk (S3) to promote cereulide formation in the presence of emetic B. cereus strains.
The bioassay-based risk categorization was performed by inoculating 70 retail products and food ingredients with a lux based B. cereus cereulide reporter strain. The luminescence intensity, which corresponds to the amount of synthesized cereulide [24], was quantified in situ after incubation of the samples for 24 hours at 24°C (for details, see Material and Methods). Food samples were grouped into three main classes regarding their toxin formation capability via a software-assisted region-of-interest (ROI) analysis. The bioassay revealed that 44% of the foods could be categorized as high-risk foods, while the remaining 20% and 36% were categorized as risk or low-risk foods, respectively. Derived mean ROI values of each risk category and the corresponding determined threshold values are listed in Table S1.


