Abstract
The importance of TNF-α and its soluble receptors (sTNFR1 and sTNFR2) in the development of kidney disease is being unraveled. Yet, community-based data regarding the role of sTNFRs are lacking. We assessed serum sTNFRs and aspects of kidney damage cross-sectionally in two independent community-based cohorts of elderly participants: Prospective Investigation of the Vasculature in Uppsala Seniors (n=815; mean age, 75 years; 51% women) and Uppsala Longitudinal Study of Adult Men (n=778; mean age, 78 years). Serum sTNFR1 correlated substantially with different aspects of kidney pathology in the Uppsala Longitudinal Study of Adult Men cohort (R=−0.52 for estimated GFR, R=0.22 for urinary albumin-to-creatinine ratio, and R=0.17 for urinary kidney injury molecule-1; P<0.001 for all), with similar correlations in the Prospective Investigation of the Vasculature in Uppsala Seniors cohort. These associations remained significant after adjustment for age, sex, inflammatory markers, and cardiovascular risk factors and were also evident in participants without diabetes. Serum sTNFR2 was associated with all three markers in the Prospective Investigation of the Vasculature in Uppsala Seniors cohort (P<0.001 for all). Our findings from two independent community-based cohorts confirm and extend results of previous studies supporting circulating sTNFRs as relevant biomarkers for kidney damage and dysfunction in elderly individuals, even in the absence of diabetes.
Keywords: albuminuria, chronic inflammation, chronic kidney disease, clinical nephrology, epidemiology and outcomes
TNF-α is a central player in the human immune system, and inflammatory and stress response pathways are activated as soon as TNF-α binds to TNF receptors (TNFRs).1 However, the effects of TNF-α are also regulated by soluble TNFR (sTNFR) in plasma: One effect is that they block TNF-α from binding its target cell surface receptor, and another is a prolonged and delayed effect of TNF-α. Additional unique properties of sTNFRs that do not involve TNF-α are also present.2 Two sTNFRs—sTNFR1 and sTNFR2—are known, and their importance in the development of kidney diseases is being explored.3
Rat models have suggested a causal role for sTNFRs in diabetic nephropathy.4 Interestingly, two recent studies report that soluble TNFRs predict progression of CKD and development of ESRD in patients with diabetes.5,6 Associations between sTNFRs and progression from microalbuminuria to macroalbuminuria,7 and of decline in eGFR8 in type 1 diabetes, have also recently been reported. However, to date, community-based data on the association between sTNFRs and kidney damage are scarce.
We hypothesize that soluble TNFRs play a causal role in the development of kidney damage and dysfunction. Herein, we aimed to explore and validate the cross-sectional associations between soluble TNFRs and markers of kidney damage and dysfunction used in clinical practice (eGFR) and microalbuminuria (albumin-to-creatinine ratio [ACR]) in two independent community-based cohorts of elderly persons. As a secondary aim, we wanted to explore the association between sTNFRs and kidney tubular damage (using the specific tubular damage biomarker urinary kidney injury molecule-1 [KIM-1]).
Results
Baseline Characteristics
Baseline characteristics of the study populations are presented in Table 1. The associations between sTNFRs and CKD stages according to the GFR strata in the Kidney Disease Improving Global Outcomes guidelines are shown in Table 2. Higher levels of sTNFRs were seen in individuals with lower GFR.
Table 1.
Baseline characteristics of PIVUS and ULSAM
| Variable | PIVUS | ULSAM |
|---|---|---|
| Participants (n) | 815 | 778 |
| Women, n (%) | 414 (51) | 0 |
| Age (yr) | 75.3±0.2 | 77.6±0.8 |
| CRP (mg/L) | 3.9±6.3 | 3.9±2.7 |
| IL-6 (pg/ml) | – | 3.9±2.7 |
| sTNFR1 (pg/ml) | 2455±1293 | 2081±865 |
| sTNFR2 (pg/ml) | 6332±2880 | – |
| Urinary KIM-1–to-creatinine ratio (ng/mmol) | 173±1597 | 118±89 |
| GFR (ml/min per 1.73 m2) | 68±19 | 73±17 |
| Urinary ACR (mg/mmol) | 6.1±29 | 4.4±19 |
| Body mass index (kg/m2) | 27±4 | 26±3 |
| Systolic BP (mmHg) | 149±19 | 151±21 |
| Antihypertensive treatment, n (%) | 394 (48) | 365 (47) |
| Serum cholesterol (mmol/L) | 5.4±1.1 | 5.4±1.0 |
| HDL (mmol/L) | 1.49±0.46 | 1.3±0.3 |
| Lipid-lowering treatment, n (%) | 204 (26) | 129 (17) |
| Smoking, n (%) | 50 (6) | 59 (8) |
| Diabetes, n (%) | 112 (14) | 107 (14) |
Data are mean±SD for continuous variables and n (%) for categorical variables
Table 2.
Association between sTNFRs and CKD stages according to the GFR strata in the Kidney Disease Improving Global Outcomes guidelines
| CKD Stage | eGFR (ml/min per 1.73 m2) | ULSAM | PIVUS | |||
|---|---|---|---|---|---|---|
| Participants (n) | Mean sTNFR1±SD | Participants (n) | Mean sTNFR1±SD | Mean sTNFR2±SD | ||
| G1: Normal or high | ≥90 | 108 | 1613±507 | 87 | 1812±356 | 4993±3551 |
| G2: Mildly decreased | 60–89 | 511 | 1973±641 | 450 | 2186±449 | 5727±1692 |
| G3a–b: Mildly to severely decreased | 30–60 | 147 | 2546±834 | 262 | 2924±1425 | 7412±2492 |
| G4: Severely decreased | 15–29 | 11 | 5504±2315 | 10 | 4343±995 | 10,107±2088 |
| G5: Kidney failure | 0–14 | 0 | – | 0 | – | – |
| ANOVA P value | <0.001 | <0.001 | <0.001 | |||
Correlations and Linear Regression Models
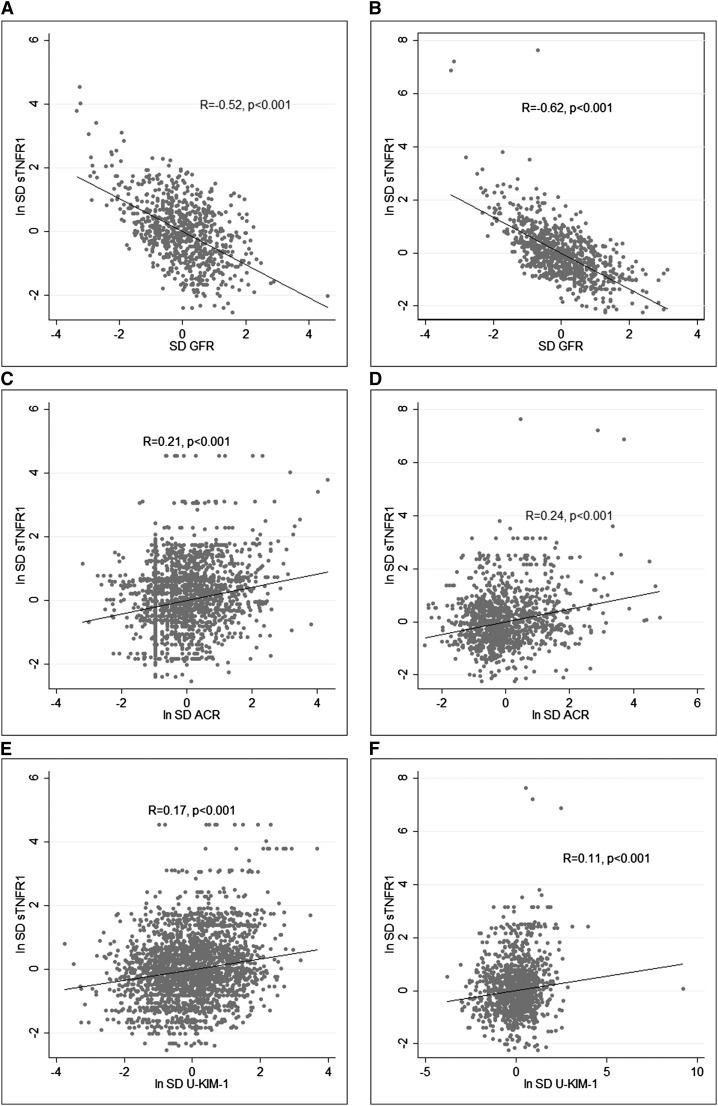
Figure 1, A–F, shows scatterplots of the association between sTNFR1 and eGFR, ACR, and KIM-1 in the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) and Uppsala Longitudinal Study of Adult Men (ULSAM). In these analyses, higher sTNFR1 was significantly associated with lower eGFR, higher ACR, and higher U-KIM-1, with salient similarities in the associations between sTNFR1 and each marker in the two cohorts. The Pearson correlation coefficients and P values are also shown in the figures.
Figure 1.
Significant correlations between sTNFR1 and the following markers of kidney dysfunction in the elderly: GFR in ULSAM (A), GFR in PIVUS (B), ACR in ULSAM (C), ACR in PIVUS (D), U-KIM-1 in ULSAM (E), and U-KIM-1 in PIVUS (F).
Table 3 shows linear regression models of the association between sTNFR1 and markers of kidney and tubular dysfunction in the ULSAM and PIVUS cohorts. ACR, eGFR, and U-KIM-1 were significantly associated with sTNFR1 in all linear regression models tested. The association was strongest with eGFR, followed by ACR and U-KIM-1.
Table 3.
Association between sTNFR1 and kidney markers in the ULSAM and PIVUS cohorts (natural logarithm–transformed variables)
| Kidney Marker | Linear Regression Models, Β-Coefficients per SD Increment (95% CI) | |||||
|---|---|---|---|---|---|---|
| ULSAM | PIVUS | |||||
| Model A | Model B | Model C | Model A | Model B | Model C | |
| GFR | −0.51a (−0.57 to −0.45) | −0.48a (−0.55 to −0.42) | −0.48a (−0.55 to −0.42) | −0.62a (−0.68 to −0.57) | −0.59a (−0.65 to−0.54) | −0.58a (−0.64 to −0.53) |
| ACR | 0.20a (0.13 to 0.28) | 0.18a (0.10 to 0.27) | 0.17a (0.081 to 0.25) | 0.23a (0.16 to 0.30) | 0.21a (0.14 to 0.27) | 0.16a (0.094 to 0.23) |
| U-KIM-1 | 0.16a (0.087 to 0.24) | 0.14a (0.066 to 0.22) | 0.13b (0.048 to 0.20) | 0.11b (0.045 to 0.18) | 0.080c (0.013 to 0.15) | 0.074c (0.0083 to 0.14) |
Model A: age and sex (PIVUS). Model B: inflammation (age, sex [PIVUS], CRP, and IL-6 [ULSAM]). Model C: CVD risk factors (age, sex [PIVUS], CRP, IL-6 [ULSAM], body mass index, smoking, systolic BP, HDL, cholesterol, diabetes, and antihypertensive and lipid treatment).
P<0.001.
P<0.01.
P<0.05.
The linear regression coefficients in a model with all three kidney markers as explanatory variables of sTNFR1 in ULSAM were as follows: eGFR, −0.48 (95% confidence interval [95% CI], −0.54 to −0.42; P<0.001); ACR, 0.068 (95% CI, 0.037 to 0.14; P=0.06); and U-KIM-1, 0.044 (95% CI, −0.026 to 0.11; P=0.21). The corresponding linear regression model with all three kidney markers in PIVUS revealed similar regression coefficients and significant associations between sTNFR1 and the following: eGFR, −0.60 (95% CI, −0.65 to −0.54; P<0.001); ACR, 0.12 (95% CI, 0.062 to 0.17; P<0.001); and U-KIM-1, 0.062 (95% CI, 0.010 to 0.12; P<0.020).
The association between sTNFR1 and eGFR was even more pronounced in participants with eGFR≤60 ml/min per 1.73 m2 (significant multiplicative interaction in both ULSAM and PIVUS, P<0.001). The regression coefficients for a 1-SD increment of eGFR in participants with eGFR<60 ml/min per 1.73 m2 were −1.13 (95% CI, −1.33 to −0.94; P<0.001) in ULSAM and −1.28 (95% CI, −1.50 to −1.06; P<0.001) in PIVUS. The corresponding regression coefficients in individuals with eGFR≥60 ml/min per 1.73 m2 were −0.38 (95% CI, −0.48 to −0.28; P<0.001) in ULSAM and −0.35 (95% CI, −0.42 to −0.28; P<0.001) in PIVUS.
Data on sTNFR2 were available in the PIVUS study only and correlated with TNFR1 (r=0.5937; P<0.001).
Pearson correlations and linear regression models between sTNFR2 and kidney markers are shown in Table 4. ACR and eGFR were associated with sTNFR2 in all linear regression models. KIM-1 was weakly associated with sTNFR2 and was nonsignificant after adjustments for C-reactive protein (CRP) and cardiovascular risk factors. The linear regression coefficients in a model with all three kidney markers as explanatory variables of sTNFR2 were as follows: eGFR, −0.50 (95% CI, −0.55 to −0.44; P<0.001); ACR, 0.064 (95% CI, 0.003 to 0.12; P<0.05); and U-KIM-1, 0.059 (95% CI, −0.018 to 0.12; P=0.06).
Table 4.
Association between sTNFR2 and kidney markers in the PIVUS cohort (natural logarithm–transformed variables)
| Kidney marker | Pearson Correlation Coefficients: All | Linear Regression Models, Β Coefficients (95% CI) | ||
|---|---|---|---|---|
| Model A: All | Model B: All | Model C: All | ||
| GFR | −0.51a | −0.51a (−0.57 to 0.45) | −0.48a (−0.54 to −0.43) | −0.47a (−0.53 to −0.41) |
| ACR | 0.17a | 0.16a (0.088 to 0.22) | 0.14a (0.071 to 0.20) | 0.10b (0.029 to 0.17) |
| U-KIM-1 | 0.090a | 0.093b (0.024–0.16) | 0.065 (−0.002 to 0.13) | 0.059 (−0.007 to 0.13) |
Model A: age and sex (PIVUS). Model B: inflammation (age, sex [PIVUS], CRP, and IL-6 [ULSAM]). Model C: CVD risk factors (age, sex [PIVUS], CRP, IL-6 [ULSAM], body mass index, smoking, systolic BP, HDL, cholesterol, diabetes, and antihypertensive and lipid treatment).
P<0.001.
P<0.01.
P<0.05.
The association between sTNFRs and the different aspects of kidney damage and dysfunction were similar after adjustment for level of physical activity (data not shown).
Finally, there were significant differences in medians and their Bonnet-Price CIs in individuals with and without diabetes (P=0.06 for sTNFR1 in ULSAM; P<0.001 for sTNFR1 and P<0.001 for sTNFR2 in PIVUS). The association between the TNFRs and different aspects of kidney damage and dysfunction were also similar in participants with and without diabetes in both cohorts (Supplemental Table 1).
Discussion
Main Findings
The main finding of this study in two community-based cohorts of elderly persons was that sTNFRs are closely associated with the two most relevant clinical markers defining disease stage and progression risk in CKD: GFR and ACR. The association between sTNFR and eGFR was, however, of a much higher magnitude than that between sTNFRs and ACR, even after adjustment for other inflammatory markers and cardiovascular risk factors. It was also more pronounced in participants with GFR<60 ml/min per 1.73 m2 than in participants with GFR≥60 ml/min per 1.73 m2. Of note, these associations were also evident in participants without diabetes. The association between sTNFR1 and KIM-1, a marker of tubular damage, was present in both cohorts.
Comparisons with Previous Studies
Recent studies have shown that higher sTNFRs are associated with deterioration of kidney function5,8,9 or progression of microalbuminuria to macroalbuminuria7 in patients with type 1 diabetes.5 In this patient group, sTNFRs have been shown to be superior to many inflammatory markers, including IL-6 and TNF-α, as prognostic markers of eGFR decline.8 Furthermore, higher sTNFRs predict ESRD in type 2 diabetes.6 In both cohorts in the present study, circulating levels of sTNFRs were significantly higher in participants with diabetes than in those without diabetes. But more important, the association between sTNFRs and the different aspects of kidney damage and dysfunction were also evident in persons without diabetes. Thus, sTNFRs appear to be a marker for kidney pathology also in the absence of diabetes, a finding that to our knowledge has not been reported before. Our data are in accordance with previous community-based studies that have reported the association between sTNFRs, GFR, and albuminuria.10–12
Moreover, we believe we are the first to report an association between sTNFRs and a specific marker of kidney tubular damage, U-KIM-1. In ULSAM, the association between sTNFRs and U-KIM-1 was attenuated and no longer significant after adjustments for eGFR and albuminuria, whereas in PIVUS the association between sTNFRs remained statistically significant after adjustment for eGFR and albuminuria. Thus, whether sTNFRs are independent markers for specific damages in the proximal tubuli remains to be established.
The correlation between sTNFR1 and sTNFR2 in the present PIVUS study was 0.59, confirming high correlations (0.90 and 0.78) seen in a study of patients with type 2 diabetes, 0.906 and a study of patients with type 1 diabetes,5 respectively. This finding indicates that the strength of association between these two highly correlated markers may depend on the population studied.
Possible Mechanisms for Observed Associations
Several molecular mechanisms may explain our observational findings. Microinflammation driven by interleukins, such as IL-1, IL-6, and IL-18, as well as by TNF-α is directly involved in the pathogenesis and progression of CKD.13 Our findings, and findings by others,5,6,9 indicate that sTNFRs are closely linked to kidney dysfunction and albuminuria,7,8 presumably as direct pathogenic mediators and as markers of high TNF-α activity.14 The fact that sTNFRs were significant after adjustments for IL-6 and CRP in the present study, as well as in other studies of kidney dysfunction and development of ESRD in individuals with diabetes,6–8 further indicates that sTNFRs mirror an independent inflammatory pathway. Specifically, sTNFRs have been shown to be involved in tubulointerstitial fibrosis and thereby contribute to nephropathy.15 Inflammation identified by sTNFRs may also trigger and promote loss of kidney function due to TNFR-driven development of atherosclerosis16 and malnutrition.17
Hyperglycemia has been suggested to affect the levels of oxidative stress and provides one of the main factors explaining the rapid decline in GFR seen in diabetic nephropathy.18,19 Oxidative stress also increases TNF-α activity,18 specifically that of TNFR2.20 Our study, in contrast to a study of patients with diabetes in which sTNFR1 was more linked to development of ESRD than was TNFR2,6 showed equally strong associations for both sTNFRs with kidney function and microalbuminuria.
Physical inactivity is accountable for 15/1000 cardiovascular deaths in individuals with CKD and is as important as traditional risk factors, including systolic BP (14/1000 deaths) and diabetes (14/1000 deaths).21 A possible pathway for the increased mortality in sedentary individuals is a higher inflammatory state, which is associated with IL-6 to a greater extent than CRP in patients with CKD.22 Thus, symptomatic inflammation may decrease ambulation, leading to a vicious circle with more inflammation, progressed CKD, and even less activity. Yet, the present association between sTNFRs and kidney damage and dysfunction remained essentially unaltered in models adjusted for IL-6, CRP, and the level of physical activity, which would argue against this as a major explanation of our findings.
Finally, it is possible that the strong associations between sTNFRs and GFR are partially explained by their impaired renal clearance, but inflammatory mediators are probably the principal cause of their increase in serum that parallels the decline in kidney function.
Clinical Implications
CKD has a major public impact worldwide, with a global prevalence of 10%.23 Soluble TNFRs are promising biomarkers of kidney damage and cardiovascular diseases,24 but more studies are needed to evaluate the clinical value of sTNFR measurements for the detection of kidney damage and for the prediction of GFR decline and the development of cardiovascular disease. The higher correlation between sTNFR and markers of kidney and tubular dysfunction in patients with GFR<60 ml/min per 1.73 m2 in the present study indicates that sTNFR measurements may be more clinically relevant in individuals with established CKD. Longitudinal studies of decline of GFR and its association with sTNFR in the community are warranted.
TNF-α inhibition reduces albuminuria in rats.25 Recombinant antibodies against TNF-α and sTNFR have been suggested as potential drugs that may halt decline in kidney function.26 In fact, studies have demonstrated that progression of CKD can be inhibited by anti-TNF therapy.27,28 Additional clinical studies are warranted to elucidate whether anti-TNF therapy can halt GFR decline and microalbuminuria in patients with rapid nephropathy and high circulating levels of sTNFRs.
Strengths and Limitations
Strengths of our investigation include the validation of our findings in an independent cohort and the detailed characterization of study participants with regard to kidney phenotypes and cardiovascular risk factors. Limitations include the unknown generalizability to other age and ethnic groups. No conclusions regarding causality should be drawn from our cross-sectional observational data; however, the high associations with eGFR and microalbuminuria in the community are of interest because sTNFRs have been shown to be prognostic markers of kidney dysfunction in patients with diabetes.6–8
In conclusion, circulating sTNFRs were associated with different aspects of kidney damage in two independent community-based cohorts of elderly, even in the absence of diabetes. Our findings confirm and extend previous studies in patients with diabetes to the community-based setting, supporting that circulating sTNFRs are relevant biomarkers for kidney damage and dysfunction, and emphasize the importance of microinflammation as a mechanism underpinning kidney damage and nephropathy.
Concise Methods
Description of Study Populations
PIVUS
All 70-year-old men and women living in Uppsala, Sweden, between 2001 and 2004 were eligible for the PIVUS study (described in detail at http://www.medsci.uu.se/pivus/pivus.htm).29 Of 2025 invited individuals, 1016 agreed to participate. In the present study the second examination cycle of PIVUS was used (2006–2009), when participants were 75 years old. Of 964 invited participants, 827 participated (86%); of these, 815 participants had data on sTNFRs.
ULSAM
ULSAM was initiated in 1970. All 50-year-old men, born in 1920–1924 and living in Uppsala, Sweden, were invited to participate in a health survey that focused on identifying cardiovascular risk factors (described in detail at http://www.pubcare.uu.se/ULSAM).30 These analyses are based on the fourth examination cycle, when participants were approximately 77 years of age (1997–2001). Of 1398 invited men, 838 (60%) participated and 778 participants had valid data on sTNFRs.
Ethical Considerations
All participants in both studies gave written informed consent and the Ethics Committee of Uppsala University approved the study protocols. The study was conducted according to the Declaration of Helsinki.
Baseline Investigations
The investigations in PIVUS and ULSAM were performed using similar standardized methods, including anthropometrical measurements; BP; blood sampling; and questionnaires regarding socioeconomic status, medical history, smoking habits, medication, and physical activity level.29–31 Venous blood samples were drawn in the morning after an overnight fast and stored at −70°C until analysis. In the PIVUS cohort, a spot sample of first-morning-void urine was used for analyses. In ULSAM a 24-hour collection of urine was used.
Both sTNFR1 and sTNFR2 were analyzed using a commercially available ELISA kit (DY225 and DY726; R&D Systems, Minneapolis, MN). The assays had a total coefficient of variation of approximately 6%. Soluble TNFR2 was available only in PIVUS.
Cystatin C–based eGFR was calculated as previously described.32,33 Urine albumin was measured by nephelometry (urine albumin; Dade Behring, Deerfield, IL) using a Behring BN ProSpec analyzer (Dade Behring). Urine creatinine was analyzed with a modified kinetic Jaffe reaction on an Architect Ci8200 analyzer (Abbott, Abbot Park, IL), and creatinine-related ACR was calculated. Urinary KIM-1 was analyzed with the commercial sandwich ELISA kit (DY1750; R&D Systems) and adjusted for urinary creatinine (IL test creatinine 181672–00, Monarch 2000 analyzer; Instrumental Laboratories, Lexington, MA).
High-sensitive CRP measurements were performed by latex-enhanced reagent (Siemens) with the use of a BN ProSpec analyzer (Siemens). IL-6 was analyzed in serum using a high-sensitivity IL-6 assay (Quantikine HS ELISA Kit HS600; R&D Systems) according to the instructions of the manufacturer in ULSAM. Diabetes mellitus was diagnosed as fasting plasma glucose level≥7.0 mmol/L (≥126 mg/dl) or use of antidiabetic medication.34
Statistical Analyses
Pearson correlation coefficients were calculated between sTNFR and markers of kidney function (logarithmically transformed variables to promote normal distribution). Linear regression analyses were used to assess cross-sectional associations with log-transformed sTNFR1 and sTNFR2 (PIVUS only) levels as the dependent variable and other parameters as independent variables. The following multivariable models were used:
A: Age and sex (PIVUS).
B: Inflammation (age, sex [PIVUS], and CRP) to test whether sTNFRs add information to models with the clinically most established inflammatory marker, CRP. Additionally, we adjusted for IL-6 in ULSAM.
C: Established cardiovascular disease risk factors (age, sex [PIVUS], CRP, IL-6 [ULSAM], systolic BP, cholesterol, HDL, body mass index, lipid-lowering and antihypertensive treatment) to test whether the associations between sTNFRs are independent of CVD risk factors that may promote kidney damage.
Because of non-normal distributions, Spearman correlation coefficients were used to calculate the correlation between sTNFR1 and sTNFR2 in PIVUS (Spearman correlation was also used so that we could compare our data with those in other studies that used Spearman for this analysis).
In secondary models, we also adjusted for leisure time physical activity. We also used a nonparametric metric method to calculate the differences in medians of sTNFRs and between individuals with and without diabetes due to non-normal distributions, by the use of their Bonnett–Price CIs. Stratified multivariable models (model A) in patients with and without diabetes were also calculated. Finally, we also investigated the association between circulating sTNFR2 and markers of kidney damage in PIVUS using the same multivariable linear regression models.
The statistical software package Stata 11.2 (Stata Corp., College Station, TX) was used.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by The Swedish Research Council, Swedish Heart-Lung Foundation, Thuréus Foundation, the Marianne and Marcus Wallenberg Foundation, Dalarna University, Uppsala-Örebro Regional Research Council, and Uppsala University. The funding sources did not play any role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, and preparation, review, or approval of the manuscript. Dr. Ärnlöv is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013080860/-/DCSupplemental.
References
- 1.Pass HI, Mew D, Pass HA, Temeck BK: The macrophage, TNF, and other cytokines. Chest Surg Clin N Am 5: 73–90, 1995 [PubMed] [Google Scholar]
- 2.Cabal-Hierro L, Lazo PS: Signal transduction by tumor necrosis factor receptors. Cell Signal 24: 1297–1305, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Speeckaert MM, Speeckaert R, Laute M, Vanholder R, Delanghe JR: Tumor necrosis factor receptors: Biology and therapeutic potential in kidney diseases. Am J Nephrol 36: 261–270, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa G, Nakano K, Sawada M, Uno K, Shibayama Y, Ienaga K, Kondo M: Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int 40: 1007–1012, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, Cullere X, Johnson AC, Crabtree G, Smiles AM, Mayadas TN, Warram JH, Krolewski AS: Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 23: 516–524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, Cullere X, Eckfeldt JH, Doria A, Mayadas TN, Warram JH, Krolewski AS: Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 23: 507–515, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopes-Virella MF, Baker NL, Hunt KJ, Cleary PA, Klein R, Virella G, DCCT/EDIC Research Group : Baseline markers of inflammation are associated with progression to macroalbuminuria in type 1 diabetic subjects. Diabetes Care 36: 2317–2323, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krolewski AS, Niewczas MA, Skupien J, Gohda T, Smiles A, Eckfeldt JH, Doria A, Warram JH: Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria [published online ahead of print August 12, 2013]. Diabetes Care 10.2337/dc13-0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyazawa I, Araki S, Obata T, Yoshizaki T, Morino K, Kadota A, Ugi S, Kawai H, Uzu T, Nishio Y, Koya D, Haneda M, Kashiwagi A, Maegawa H: Association between serum soluble TNFα receptors and renal dysfunction in type 2 diabetic patients without proteinuria. Diabetes Res Clin Pract 92: 174–180, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Upadhyay A, Larson MG, Guo CY, Vasan RS, Lipinska I, O'Donnell CJ, Kathiresan S, Meigs JB, Keaney JF, Jr, Rong J, Benjamin EJ, Fox CS: Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol Dial Transplant 26: 920–926, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keller CR, Odden MC, Fried LF, Newman AB, Angleman S, Green CA, Cummings SR, Harris TB, Shlipak MG: Kidney function and markers of inflammation in elderly persons without chronic kidney disease: the health, aging, and body composition study. Kidney Int 71: 239–244, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Keller C, Katz R, Cushman M, Fried LF, Shlipak M: Association of kidney function with inflammatory and procoagulant markers in a diverse cohort: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis (MESA) [published online ahead of print August 5, 2008]. BMC Nephrol 10.1186/1471-2369-9-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navarro-González JF, Mora-Fernández C: The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19: 433–442, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D: Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med 175: 323–329, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo G, Morrissey J, McCracken R, Tolley T, Klahr S: Role of TNFR1 and TNFR2 receptors in tubulointerstitial fibrosis of obstructive nephropathy. Am J Physiol 277: F766–F772, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Svenungsson E, Fei GZ, Jensen-Urstad K, de Faire U, Hamsten A, Frostegard J: TNF-alpha: A link between hypertriglyceridaemia and inflammation in SLE patients with cardiovascular disease. Lupus 12: 454–461, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Pitsiou G, Kyriazis G, Hatzizisi O, Argyropoulou P, Mavrofridis E, Patakas D: Tumor necrosis factor-alpha serum levels, weight loss and tissue oxygenation in chronic obstructive pulmonary disease. Respir Med 96: 594–598, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D: Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 106: 2067–2072, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Evans JL, Goldfine ID, Maddux BA, Grodsky GM: Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr Rev 23: 599–622, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Fernández-Real JM, Broch M, Ricart W, Casamitjana R, Gutierrez C, Vendrell J, Richart C: Plasma levels of the soluble fraction of tumor necrosis factor receptor 2 and insulin resistance. Diabetes 47: 1757–1762, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B: Cardiovascular mortality risk in chronic kidney disease: Comparison of traditional and novel risk factors. JAMA 293: 1737–1745, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Dungey M, Hull KL, Smith AC, Burton JO, Bishop NC: Inflammatory factors and exercise in chronic kidney disease. Int J Endocrinol 2013: 569831, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, Hallan HA, Lydersen S, Holmen J: International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 17: 2275–2284, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Schnabel RB, Yin X, Larson MG, Yamamoto JF, Fontes JD, Kathiresan S, Rong J, Levy D, Keaney JF, Jr, Wang TJ, Murabito JM, Vasan RS, Benjamin EJ: Multiple inflammatory biomarkers in relation to cardiovascular events and mortality in the community. Arterioscler Thromb Vasc Biol 33: 1728–1733, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriwaki Y, Inokuchi T, Yamamoto A, Ka T, Tsutsumi Z, Takahashi S, Yamamoto T: Effect of TNF-alpha inhibition on urinary albumin excretion in experimental diabetic rats. Acta Diabetol 44: 215–218, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Doria A, Niewczas MA, Fiorina P: Can existing drugs approved for other indications retard renal function decline in patients with type 1 diabetes and nephropathy? Semin Nephrol 32: 437–444, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elmarakby AA, Quigley JE, Pollock DM, Imig JD: Tumor necrosis factor alpha blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension 47: 557–562, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC: Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 161: 1679–1693, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lind L, Fors N, Hall J, Marttala K, Stenborg A: A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler Thromb Vasc Biol 25: 2368–2375, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Helmersson J, Vessby B, Larsson A, Basu S: Association of type 2 diabetes with cyclooxygenase-mediated inflammation and oxidative stress in an elderly population. Circulation 109: 1729–1734, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Byberg L, Zethelius B, McKeigue PM, Lithell HO: Changes in physical activity are associated with changes in metabolic cardiovascular risk factors. Diabetologia 44: 2134–2139, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Larsson A, Malm J, Grubb A, Hansson LO: Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest 64: 25–30, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Flodin M, Jonsson AS, Hansson LO, Danielsson LA, Larsson A: Evaluation of Gentian cystatin C reagent on Abbott Ci8200 and calculation of glomerular filtration rate expressed in mL/min/1.73 m2 from the cystatin C values in mg/L. Scand J Clin Lab Invest 67: 560–567, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Wändell PE, Carlsson AC, de Faire U, Hellénius ML: Prevalence of blood lipid disturbances in Swedish and foreign-born 60-year-old men and women in Stockholm, Sweden. Nutr Metab Cardiovasc Dis 21: 173–181, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.