Abstract
Nuclear factor-κB is associated with the pathogenesis of numerous malignancies, and the functional polymorphism −94ins/del ATTG (rs28362491) in the human NFKB1 gene is associated with cancer risk. Previous studies on the association between the −94ins/del ATTG polymorphism and cancer risk reported conflicting results. To clarify this relationship, we performed a meta-analysis of 21 case-control studies involving 6127 cases and 9238 controls. We used pooled odds ratios (ORs) with their 95% confidence intervals (95% CIs) to assess the association. We found that the NFKB1 promoter −94ins/del ATTG polymorphism was significantly associated with cancer risk in four genetic models (ins/ins versus del/del, OR = 1.47, 95% CI = 1.11–1.93; dominant model, OR = 1.26, 95% CI = 1.03–1.53; recessive model, OR = 1.26, 95% CI = 1.05–1.51; ins allele versus del allele, OR = 1.19, 95% CI = 1.05–1.35). Stratified analyses revealed a significant association between the polymorphism and ovarian, oral, and prostate cancers. Similar results were determined in an Asian population and not in a Caucasian population. Thus, our results suggested that the polymorphism can contribute to cancer risk. Moreover, the polymorphism can exert race- and cancer-specific effects on cancer risk. Further large-scale and functional studies are necessary to elucidate this possible effect.
1. Introduction
Cancer is a major public health problem worldwide; it is the primary and secondary causes of death in economically developed and developing countries, respectively [1]. The global concern on cancer continues to intensify as a result of the aging and expanding world population and the increasing adoption of cancer-causing habits. The mechanism of carcinogenesis remains largely unknown although genetic susceptibility is a known possible explanation for the interindividual variation in cancer risk [2].
Nuclear factor-κB (NF-κB) was initially identified in 1986 as a transcription factor which binds to a 10 bp DNA element in kappa immunoglobulin light-chain enhancer in B cells [3]. The NF-κB family consists of p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), c-Rel (Rel), and RelB. The major form of NF-κB is a heterodimer of the p50 and p65/RelA subunits which are encoded by the NFKB1 and NFKB2 genes, respectively [4]. The human NFKB1 gene is mapped to chromosome 4q24 and encodes a 50 kDa DNA-binding protein (p50) that can act as a master regulator of inflammation and cancer development [5–7].
A common insertion/deletion polymorphism (−94ins/del ATTG, rs28362491) in the promoter region of the NFKB1 gene elicits a regulatory effect on the NFKB1 gene [8]. A previous meta-analysis concluded that the deletion allele serves as a risk or protective allele for cancer susceptibility in Caucasian or Asian populations, respectively; however, it revealed no association between the polymorphism and cancer risk [9]. An increasing number of studies have assessed the association between the NFKB1 promoter −94ins/del ATTG polymorphism and cancer risk [10–12]. However, these studies obtained conflicting results. Therefore, we collected all available data to perform an updated meta-analysis that generates a precise estimation to comprehensively and objectively investigate the association between the NFKB1 promoter −94ins/del ATTG polymorphism and cancer risk.
2. Materials and Methods
2.1. Search Strategy and Identification of Relevant Studies
A comprehensive literature search for relevant articles published (last search updated in September 15, 2013) in PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) was performed with the following key words: (“genetic polymorphism,” “polymorphism,” “SNP,” “single nucleotide polymorphism,” “gene mutation,” or “genetic variant”), (“neoplasm,” “cancer,” “tumor,” “carcinoma,” or “carcinogenesis”), and (“NFKB1,” “NF-κB1,” “nuclear factor kappa B1,” “NF kappa B1,” or “nuclear factor κB1”). The search was limited to human studies in English. All eligible studies were retrieved. The reviews and references of eligible studies were hand-searched for additional relevant publications. The most recent or complete study was selected when more than one publications contain overlapping data. A flow diagram of the study selection process is presented in Figure 1.
Figure 1.
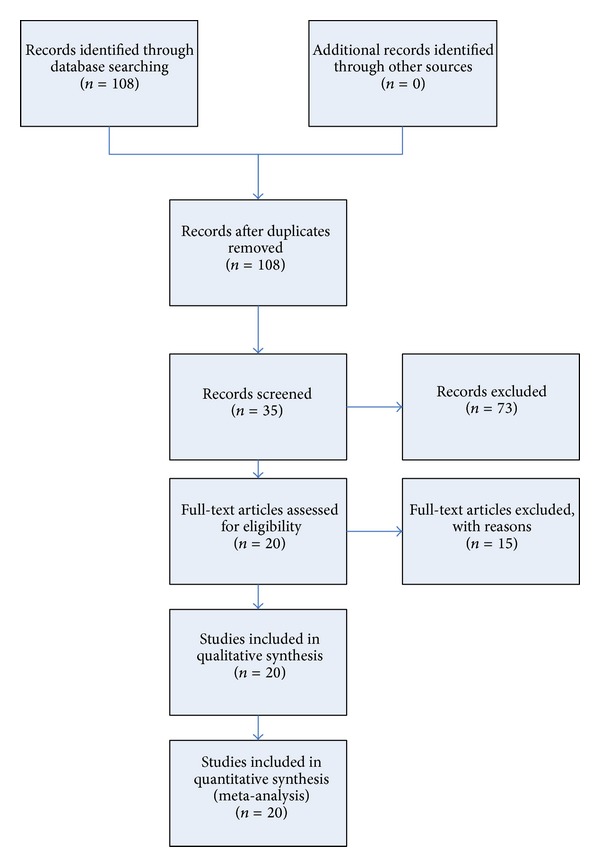
Study selection process.
2.2. Inclusion Criteria
Case-control studies that evaluated the association of the NFKB1 promoter −94ins/del ATTG polymorphism with cancer risk and described in detail the genotype distributions of the polymorphism in cases and controls were included in this meta-analysis.
2.3. Exclusion Criteria
Studies that were not for cancer research, were only case population, and were duplication of previous publication were excluded in this meta-analysis.
2.4. Data Extraction
Information was carefully extracted from eligible studies independently by two investigators (Xiao Yang and Pengchao Li) according to the inclusion criteria listed above, and the result was reviewed by a third investigator (Jun Tao). The following data were collected from each study: surname of first author, year of publication, ethnicity, genotyping method, source of controls, frequencies of the genotypes in cases and controls, cancer type, and Hardy-Weinberg equilibrium (HWE) of genotype distribution among controls. Ethnicity was categorised as “Asian” or “Caucasian.” Studies that investigated more than one type of cancer were regarded as individual datasets only in subgroup analyses according to cancer type. No minimum number of patients was required for this meta-analysis. Articles that reported different ethnic groups and countries or locations were considered different study samples for each category cited above.
2.5. Statistical Analysis
The strength of association between the NFKB1 promoter −94ins/del ATTG polymorphism and cancer risk was estimated through pooled odds ratio (OR) with its corresponding 95% CI. Pooled ORs were calculated for insertion allele versus deletion allele, ins/ins versus del/del, ins/del versus del/del, ins/ins + ins/del versus del/del, and ins/ins versus ins/del + del/del. Subgroup stratification analyses by ethnicity and cancer type were conducted to identify the association of the −94ins/del ATTG polymorphism with cancer susceptibility.
The between-study heterogeneity of the studies included in this meta-analysis was evaluated using the Q and I 2 statistic tests, where I 2 > 50% indicated heterogeneity [13]. The random-effects model was selected when I 2 was significant (>50%); otherwise, the fixed-effects model was selected. The allele frequencies of the NFKB1 promoter −94ins/del ATTG polymorphism from the respective study were determined by allele counting. In addition, a chi-square test was used to determine whether or not the observed frequencies of genotypes conform to HWE. Pooled OR in the current meta-analysis was performed by weighting individual ORs by the inverse of their variance. The significance of the pooled OR was determined by the Z-test. In addition to the comparison among all subjects, we performed stratification analyses by cancer type (if one cancer type contained only one studies, it was combined into the “other cancers” group) and ethnicity. Begg's funnel plot and Egger's test were adopted to evaluate the publication bias in our meta-analysis [14, 15]. All statistical analyses were performed by STATA 10.0 software (StataCorp, College Station, TX, USA).
3. Results
3.1. Eligible Studies and Meta-Analysis Databases
A total of 21 case-control studies involving 6127 cases and 9239 controls were analysed. The characteristics of all studies are presented in Table 1. The allele and genotype frequencies of the NFKB1 promoter −94ins/del ATTG polymorphism were extracted from all eligible studies. In total, this meta-analysis included 3 bladder cancer studies, 4 colorectal cancer studies, 2 ovarian cancer studies, 2 oral cancer studies, 2 prostate cancer studies, and 8 studies with the “other cancers.” Of the 21 studies, 14 were conducted among Asians and 7 were conducted among Caucasians. All cases were clinically pathologically confirmed.
Table 1.
Main characteristics of these studies included in this meta-analysis.
| First Author | Year | Ethnicity | Genotyping method | SC | Genotyping cases | Controls | Cancer type | HWE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ins/ins | ins/del | del/del | ins/ins | ins/del | del/del | |||||||
| Lin [16] | 2006 | Asian | PCR-RFLP | HB | 59 | 103 | 50 | 43 | 100 | 58 | OSCC | 0.993 |
| Riemann [17] | 2007 | Caucasian | Pyrosequencing | HB | 88 | 124 | 30 | 118 | 141 | 48 | Bladder cancer | 0.586 |
| Bu [18] | 2007 | Caucasian | PCR-RFLP | HB | 67 | 84 | 34 | 116 | 255 | 67 | Melanoma | <0.001 |
| Lewander [11] | 2007 | Caucasian | PCR-RFLP | HB | 63 | 323 | 81 | 116 | 256 | 67 | Colorectal cancer | <0.001 |
| Asian | PCR-RFLP | HB | 50 | 101 | 42 | 113 | 266 | 79 | Colorectal cancer | <0.001 | ||
| Lo [19] | 2008 | Asian | PCR-RFLP | HB | 62 | 89 | 31 | 20 | 62 | 34 | Gastric cancer | 0.361 |
| Zhang [20] | 2009 | Asian | PCR-RFLP | HB | 46 | 57 | 14 | 44 | 68 | 31 | Prostate cancer | 0.624 |
| Burnik [21] | 2009 | Caucasian | PCR-RFLP | HB | 18 | 30 | 2 | 30 | 58 | 12 | GNT | 0.047 |
| Zhou [22] | 2009 | Asian | PCR-RFLP | HB | 74 | 67 | 22 | 71 | 90 | 42 | NC | 0.177 |
| Tang [23] | 2009 | Asian | PCR-RFLP | HB | 89 | 92 | 26 | 74 | 108 | 46 | Bladder cancer | 0.565 |
| Andersen [24] | 2010 | Caucasian | Taqman | PB | 121 | 195 | 62 | 307 | 347 | 102 | Colorectal cancer | 0.801 |
| Zhou [25] | 2010 | Asian | PCR-RFLP | HB | 108 | 105 | 20 | 135 | 166 | 64 | CSCC | 0.297 |
| Fan [26] | 2011 | Asian | PCR-RFLP | HB | 78 | 84 | 17 | 76 | 103 | 44 | Ovarian cancer | 0.396 |
| Lin [27] | 2012 | Asian | Taqman | HB | 116 | 246 | 100 | 81 | 271 | 168 | OSCC | 0.099 |
| Vangsted [28] | 2012 | Caucasian | Taqman | PB | 110 | 163 | 55 | 665 | 778 | 253 | Multiple myeloma | 0.303 |
| Cai [10] | 2012 | Asian | Taqman | HB | 401 | 473 | 153 | 379 | 562 | 153 | Renal cell Carcinoma | 0.015 |
| Huo [29] | 2013 | Asian | MassARRAY | HB | 83 | 82 | 22 | 71 | 103 | 47 | Ovarian cancer | 0.399 |
| Cheng [30] | 2013 | Asian | Taqman | HB | 42 | 64 | 29 | 81 | 271 | 168 | HC | 0.099 |
| Mohd Suzairi [31] | 2013 | Asian | PCR-RFLP | HB | 35 | 127 | 75 | 16 | 138 | 83 | Colorectal cancer | <0.001 |
| Kopp [32] | 2013 | Caucasian | Taqman | PB | 128 | 152 | 54 | 109 | 161 | 64 | Prostate cancer | 0.741 |
| Li [12] | 2013 | Asian | Taqman | HB | 189 | 269 | 151 | 223 | 324 | 93 | Bladder cancer | 0.156 |
GNT: Gastroenteropancreatic neuroendocrine tumors; OSCC: oral squamous cell carcinoma; CSCC: cervical squamous cell carcinoma; NC: nasopharyngeal carcinoma; HC: hepatocellular carcinoma; HB: hospital-based study; PB: population-based study; SC: source of controls; HWE: Hardy Weinberg equilibrium.
The results of HWE test for the genotype distribution in the control population are shown in Table 1. Six of the eligible studies were not in HWE [10, 11, 18, 21, 31].
3.2. Quantitative Synthesis
The pooled ORs of the included case-control studies revealed a statistically significant association between the NFKB1 promoter −94ins/del ATTG polymorphism and cancer risk across the four genetic models ins/ins versus del/del, OR = 1.47, 95%, CI = 1.11—1.93; dominant model, OR = 1.26, 95% CI = 1.03—1.53; recessive model, OR = 1.26, 95% CI = 1.05—1.51; and ins allele versus del allele, OR = 1.19, 95%, CI = 1.05–1.35 (Table 2, Figure 2). Stratified analyses also revealed a significant association between the polymorphism and ovarian, oral, and prostate cancers in the various models. Ethnic subgroup analyses revealed significant increases in cancer risk in the four models among Asians but not among Caucasians. The results became prominent when the six studies that deviated from HWE were excluded (see Supplementary Table 1 and Supplementary Figure 1 in Supplementary Material available online at http://dx.doi.org/10.1155/2014/612972).
Table 2.
Meta-analysis of the NFKB1 −94ins/del ATTG promoter polymorphism and cancer risk.
| Variables | n a | Cases/Controls | ins/ins versus del/del | ins/del versus del/del | ins/ins + ins/del versus del/del (dominant) | ins/ins versus ins/del + del/del (recessive) | ins allele versus del allele | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) |
I 2 (%) | OR (95% CI) |
I 2 (%) | OR (95% CI) |
I 2 (%) | OR (95% CI) |
I 2 (%) | OR (95% CI) | I 2 (%) | |||
| Total | 21 | 6127/9239 | 1.47 (1.11–1.93) b | 84.8 | 1.15 (0.97–1.37)b | 67.7 | 1.26 (1.03–1.53) b | 77.5 | 1.26 (1.05–1.51) b | 82.3 | 1.19 (1.05–1.35) b | 84.0 |
| Cancer types | ||||||||||||
| Bladder cancer | 3 | 1058/1175 | 1.07 (0.45–2.53)b | 90.1 | 1.00 (0.46–2.18)b | 88.8 | 1.04 (0.46–2.33)b |
90.7 | 1.04 (0.73–1.48)b | 72.7 | 1.03 (0.70–1.51)b | 89.0 |
| Colorectal cancer | 4 | 1275/1890 | 0.84 (0.47–1.50)b | 82.9 | 0.93 (0.77–1.13) | 0 | 0.88 (0.73–1.06) |
0 | 0.89 (0.51–1.55)b | 88.9 | 0.90 (0.72–1.12)b |
76.2 |
| Ovarian cancer | 2 | 366/444 | 2.57 (1.66–3.98) | 0 | 1.88 (1.23–2.89) | 0 | 2.17 (1.45–3.25) | 0 | 1.59 (1.19–2.11) | 0 | 1.55 (1.27–1.90) | 0 |
| Oral cancer | 2 | 674/721 | 2.10 (1.54–2.87) | 33.0 | 1.42 (1.10–1.83) | 0 | 1.59 (1.25–2.03) | 3.9 | 1.67 (1.29–2.17) | 0 | 1.43 (1.23–1.66) | 6.9 |
| Prostate cancer | 2 | 451/477 | 1.59 (1.09–2.33) | 23.0 | 1.28 (0.89–1.84) | 28.6 | 1.40 (1.00–1.98) | 35.8 | 1.33 (1.01–1.74) | 0 | 1.26 (1.05–1.52) | 0 |
| Other cancers | 8 | 2303/4532 | 1.72 (1.13–2.61) b | 80.9 | 1.16 (0.88–1.53)b | 61.3 | 1.34 (0.99–1.83) b | 72.4 | 1.46 (1.12–1.90) b | 78.0 | 1.29 (1.07–1.57) b | 79.9 |
| Ethnicities | ||||||||||||
| Asian | 14 | 4143/5169 | 1.83 (1.30–2.57) b | 84.8 | 1.23 (0.97–1.58)b | 75.9 | 1.42 (1.08–1.86) b | 82.5 | 1.50 (1.26–1.78) b | 66.8 | 1.32 (1.14–1.54) b | 82.2 |
| Caucasian | 7 | 1984/4070 | 0.90 (0.64–1.27)b | 71.2 | 1.00 (0.85–1.18) | 18.5 | 0.95 (0.81–1.10) | 24.0 | 0.90 (0.66–1.23)b | 83.7 | 0.95 (0.82–1.12)b | 70.9 |
aNumber of comparisons.
bRandom effects estimate.
Figure 2.
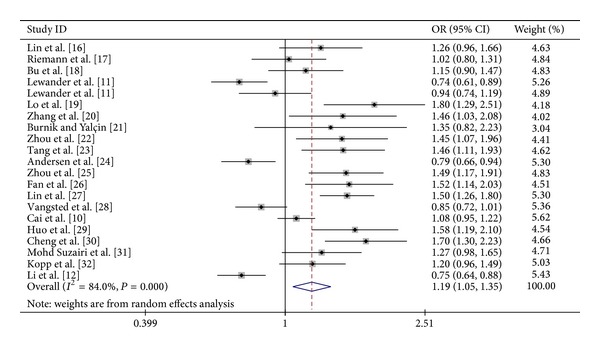
Forest plot of cancer risk associated with NFKB1 promoter −94ins/del ATTG polymorphism (for insertion allele versus deletion allele) among all studies.
3.3. Evaluation of Publication Bias
Publication bias was evaluated by Begg's funnel plot and Egger's test, and the visual asymmetry was determined in the funnel plot analysis (Figure 3). We further evaluated the publication bias in the subgroups. The results of Egger's tests for all genetic models are shown in Supplementary Table 2 (ins allele versus del allele, P = 0.004).
Figure 3.
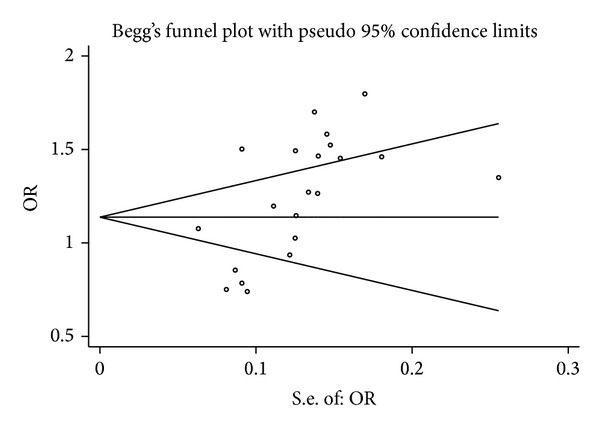
Begg's funnel plot of the association between NFKB1 promoter −94ins/del ATTG polymorphism and cancer risk (ins allele versus del allele).
4. Discussion
NF-κB serves important functions in pathogenetic regulation and influences cancer development and aggressiveness by enhancing tumour angiogenesis, antiapoptosis, and proliferation and by repressing immune response [7, 33, 34]. Several investigators reported the constitutive activation of NF-κB in various malignancies [35, 36], including nonsmall cell lung carcinoma and colon, prostate, breast, bone, and brain cancers. p50 overexpression is frequently observed in various tumour tissues; hence, p50 is potentially involved in tumourigenesis. A polymorphism in the promoter region of NFKB1 encoding the p50 subunit of NF-κB modulates gene activity. This polymorphism has been recently reported to influence cancer risk.
A meta-analysis of all eligible studies in 2010 suggested that the deletion allele serves as a protective or risk allele for cancer susceptibility among Asians or Caucasians, respectively [9]. However, no significant association was detected for the overall population [9]. After the reported study, numerous studies further assessed the relationship between the NFKB1 promoter −94ins/del ATTG polymorphism and cancer among Asians and Caucasians [10, 12, 32]. However, the association remains inconclusive because of the inconsistent results from the published studies. Li et al. [12] found an association between del/del genotype and bladder cancer risk but none between the polymorphism and hepatocellular carcinoma susceptibility [30].
In this study, we analysed 21 eligible case-control studies with 6127 cases and 9239 controls. The results of this meta-analysis revealed a significant association between insertion allele careers and enhanced cancer risk. The probable mechanism behind the observed association may be linked to the enhanced expression and activity of p50 (NF-κB1). The insertion allele is reportedly associated with the increased promoter activity and enhanced NFKB1 mRNA expression [8, 12, 17]. This association might influence cancer development.
The major effect of p50 (NF-κB1) is mediated by its function as a component of the transcription factor NF-κB, which is among the major signalling pathways involved in the cellular response to environmental stress [7]. p50 serves an important function in inhibiting cell apoptosis by modulating the expression levels of several survival genes, such as bcl-2 homologue A1 [37], PAI-2 [38], and IAP gene family [39]. Certain antiapoptosis proteins, such as Bcl-xL and Fas-associated death domain-like IL-1-converting enzyme inhibitor protein, are upregulated through the NF-κB signalling pathway [40–42]. In addition, accumulated evidence illustrated that the p50 (NF-κB1) signalling pathways participate in cellular proliferation by increasing IL-5 [43], promoting MAPK phosphorylation [7, 44], and modulating cyclin D1 expression [45]. Therefore, the observed association between the −94ins/del ATTG polymorphism and cancer risk can be accounted for by the insertion allele that can inhibit apoptosis and promote cellular proliferation by upregulating the expression of p50 (NFKB1) [8, 12, 17], which was implicated in the abovementioned mechanism.
In the stratified analyses, the increased cancer risk remained in subgroups of Asians but not in those of Caucasians. The ethnic differences in the allele frequencies may be caused by natural selection or balance to other related genetic variants. Possible differences in genetic backgrounds and gene environment may also interact with the etiology. The increased cancer risk also remained in the subgroups of ovarian, oral, and prostate cancers. This result suggested that the NFKB1 gene might function as a prominent factor in these cancers. Therefore, further investigations are warranted to validate ethnic difference and cancer specificity in the effect of this functional polymorphism on cancer susceptibility.
This study has several limitations. First, significant between-study heterogeneity was detected in some comparisons and may be distorting the meta-analysis. Second, the genotype distribution among controls did not completely agree with HWE. However, the association between the insertion allele and cancer risk in the overall population and in the Asian population became pronounced when the six studies that deviated from HWE were excluded. Third, the studies included in the analysis used different genotyping methods with different quality control issues that may have also influenced the results. Fourth, publication bias was observed in our study, which may affect the validity of conclusion. In the stratified analysis, we found that the publication bias was significant among the Asian groups and other cancer groups but not significant among the Caucasian, bladder, and colorectal cancer groups. The sample sizes of the included studies were diverse, and most of them were insufficiently large. These conditions might partly interpret the publication bias. Finally, only three controls were population based; thus, they may not represent the general population. Therefore, the results of this study should be interpreted with caution.
In conclusion, the NFKB1 promoter −94ins/del ATTG polymorphism is associated with cancer risk. Well-designed studies with representative sample sizes are necessary to validate these findings.
Supplementary Material
The meta-analysis result of the studies which did not deviated from HWE.
Acknowledgments
This work was supported by the Program for Development of Innovative Research Team of the First Affiliated Hospital of Nanjing Medical University, the Provincial Initiative Program for Excellency Disciplines of Jiangsu Province, the National Natural Science Foundation of China (Grant nos. 81272832 and 81201997), the Natural Science Foundation of Jiangsu Province (Grant no. BK2011848), the Six Major Talent Peak Project of Jiangsu Province (Grant no. 2011-WS-121), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Conflict of Interests
The authors have declared that no conflict of interests exist.
Authors' Contribution
Xiao Yang, Pengchao Li, and Jun Tao contributed equally to this work.
References
- 1.WHO. The GLob L Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 2.Foulkes WD. Inherited susceptibility to common cancers. The New England Journal of Medicine. 2008;359(20):2143–2153. doi: 10.1056/NEJMra0802968. [DOI] [PubMed] [Google Scholar]
- 3.Sen R, Baltimore D. Inducibility of K immunoglobulin enhancer-binding protein NF-kB by a posttranslational mechanism. Cell. 1986;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 4.Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-κB, a ubiquitous transcription factor in the initiation of diseases. Clinical Chemistry. 1999;45(1):7–17. [PubMed] [Google Scholar]
- 5.Sun XF, Zhang H. NFKB and NFKBI polymorphisms in relation to susceptibility of tumour and other diseases. Histology and histopathology. 2007;22(12):1387–1398. doi: 10.14670/HH-22.1387. [DOI] [PubMed] [Google Scholar]
- 6.Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-κB, a ubiquitous transcription factor in the initiation of diseases. Clinical Chemistry. 1999;45(1):7–17. [PubMed] [Google Scholar]
- 7.Yu Y, Wan Y, Huang C. The biological functions of NF-κB1 (p50) and its potential as an anti-cancer target. Current Cancer Drug Targets. 2009;9(4):566–571. doi: 10.2174/156800909788486759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karban AS, Okazaki T, Panhuysen CIM, et al. Functional annotation of a novel NFKB1 promoter polymorphism that increases risk for ulcerative colitis. Human Molecular Genetics. 2004;13(1):35–45. doi: 10.1093/hmg/ddh008. [DOI] [PubMed] [Google Scholar]
- 9.Zou YF, Yuan FL, Feng XL, et al. Association between NFKB1 -94ins/delATTG promoter polymorphism and cancer risk: A meta-analysis. Cancer Investigation. 2011;29(1):78–85. doi: 10.3109/07357907.2010.535054. [DOI] [PubMed] [Google Scholar]
- 10.Cai H, Sun L, Cui L, et al. Functional insertion/deletion polymorphism (-94 ins/del ATTG) in the promoter region of the NFKB1 gene is related to the risk of renal Cell Carcinoma. Urologia Internationalis. 2013;91(2):206–212. doi: 10.1159/000345630. [DOI] [PubMed] [Google Scholar]
- 11.Lewander A, Butchi AKR, Gao J, et al. Polymorphism in the promoter region of the NFKB1 gene increases the risk of sporadic colorectal cancer in Swedish but not in Chinese populations. Scandinavian Journal of Gastroenterology. 2007;42(11):1332–1338. doi: 10.1080/00365520701396026. [DOI] [PubMed] [Google Scholar]
- 12.Li P, Gu J, Yang X, et al. Functional promoter -94 ins/del ATTG polymorphism in NFKB1 gene is associated with bladder cancer risk in a chinese population. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0071604.e71604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37(3-4):256–266. [PubMed] [Google Scholar]
- 14.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuck AE, Rubenstein LZ, Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. British Medical Journal. 1998;316(7129):469–471. [PMC free article] [PubMed] [Google Scholar]
- 16.Lin SC, Liu CJ, Yeh WI, Lui MT, Chang KW, Chang CS. Functional polymorphism in NFKB1 promoter is related to the risks of oral squamous cell carcinoma occurring on older male areca (betel) chewers. Cancer Letters. 2006;243(1):47–54. doi: 10.1016/j.canlet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Riemann K, Becker L, Struwe H, Rübben H, Eisenhardt A, Siffert W. Insertion/deletion polymorphism in the promoter of NFKB1 as a potential molecular marker for the risk of recurrence in superficial bladder cancer. International Journal of Clinical Pharmacology and Therapeutics. 2007;45(8):423–430. doi: 10.5414/cpp45423. [DOI] [PubMed] [Google Scholar]
- 18.Bu H, Rosdahl I, Sun XF, Zhang H. Importance of polymorphisms in NF-kappaB1 and NF-kappaBIalpha genes for melanoma risk, clinicopathological features and tumor progression in Swedish melanoma patients. Journal of Cancer Research and Clinical Oncology. 2007;133(11):859–866. doi: 10.1007/s00432-007-0228-7. [DOI] [PubMed] [Google Scholar]
- 19.Lo SS, Chen JH, Wu CW, Lui WY. Functional polymorphism of NFKB1 promoter may correlate to the susceptibility of gastric cancer in aged patients. Surgery. 2009;145(3):280–285. doi: 10.1016/j.surg.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhang P, Wei Q, Li X, et al. A functional insertion/deletion polymorphism in the promoter region of the NFKB1 gene increases susceptibility for prostate cancer. Cancer Genetics and Cytogenetics. 2009;191(2):73–77. doi: 10.1016/j.cancergencyto.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Burnik FS, Yalçin S. NFKB1 -94 insertion/deletion ATTG polymorphism in gastroenteropancreatic neuroendocrine tumors. Chemotherapy. 2009;55(5):381–385. doi: 10.1159/000237744. [DOI] [PubMed] [Google Scholar]
- 22.Zhou B, Rao L, Li Y, et al. A functional insertion/deletion polymorphism in the promoter region of NFKB1 gene increases susceptibility for nasopharyngeal carcinoma. Cancer Letters. 2009;275(1):72–76. doi: 10.1016/j.canlet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Tang T, Cui S, Deng X, et al. Insertion/deletion polymorphism in the promoter region of NFKB1 gene increases susceptibility for superficial bladder cancer in Chinese. DNA and Cell Biology. 2010;29(1):9–12. doi: 10.1089/dna.2009.0937. [DOI] [PubMed] [Google Scholar]
- 24.Andersen V, Christensen J, Overvad K, Tjønneland A, Vogel U. Polymorphisms in NFkB, PXR, LXR and risk of colorectal cancer in a prospective study of Danes. BMC Cancer. 2010;10, article 484 doi: 10.1186/1471-2407-10-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou B, Qie M, Wang Y, et al. Relationship between NFKB1 -94 insertion/deletion ATTG polymorphism and susceptibility of cervical squamous cell carcinoma risk. Annals of Oncology. 2009;21(3):506–511. doi: 10.1093/annonc/mdp507.mdp507 [DOI] [PubMed] [Google Scholar]
- 26.Fan Y, Yu W, Ye P, et al. NFKB1 insertion/deletion promoter polymorphism increases the risk of advanced ovarian cancer in a Chinese population. DNA and Cell Biology. 2011;30(4):241–245. doi: 10.1089/dna.2010.1107. [DOI] [PubMed] [Google Scholar]
- 27.Lin CW, Hsieh YS, Hsin CH, et al. Effects of NFKB1 and NFKBIA gene polymorphisms on susceptibility to environmental factors and the clinicopathologic development of oral cancer. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035078.e35078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vangsted AJ, Nielsen KR, Klausen TW, Haukaas E, Tjønneland A, Vogel U. A functional polymorphism in the promoter region of the IL1B gene is associated with risk of multiple myeloma. British Journal of Haematology. 2012;158(4):515–518. doi: 10.1111/j.1365-2141.2012.09141.x. [DOI] [PubMed] [Google Scholar]
- 29.Huo ZH, Zhong HJ, Zhu YS, Xing B, Tang H. Roles of functional NFKB1 and β-TrCP insertion/deletion polymorphisms in mRNA expression and epithelial ovarian cancer susceptibility. Genetics and Molecular Research. 2013;12(3):3435–3443. doi: 10.4238/2013.March.11.6. [DOI] [PubMed] [Google Scholar]
- 30.Cheng CW, Su JL, Lin CW, et al. Effects of NFKB1 and NFKBIA gene polymorphisms on hepatocellular carcinoma susceptibility and clinicopathological features. PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0056130.e56130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohd Suzairi MS, Tan SC, Ahmad Aizat AA, et al. The functional -94 insertion/deletion ATTG polymorphism in the promoter region of NFKB1 gene increases the risk of sporadic colorectal cancer. Cancer Epidemiology. 2013;37(5):634–638. doi: 10.1016/j.canep.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Kopp TI, Friis S, Christensen J, Tjønneland A, Vogel U. Polymorphisms in genes related to inflammation, NSAID use, and the risk of prostate cancer among Danish men. Cancer Genetics. 2013;206(7-8):266–78. doi: 10.1016/j.cancergen.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 34.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nature Reviews Immunology. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 35.Mukhopadhyay T, Roth JA, Maxwell SA. Altered expression of the p50 subunit of the NF-κB transcription factor complex in non-small cell lung carcinoma. Oncogene. 1995;11(5):999–1003. [PubMed] [Google Scholar]
- 36.Bours V, Dejardin E, Goujon-Letawe F, Merville MP, Castronovo V. The NF-κB transcription factor and cancer: high expression of NF-κB- and IκB-related proteins in tumor cell lines. Biochemical Pharmacology. 1994;47(1):145–149. doi: 10.1016/0006-2952(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 37.Karsan A, Yee E, Kaushansky K, Harlan JM. Cloning of a human Bcl-2 homologue: inflammatory cytokines induce human A1 in cultured endothelial cells. Blood. 1996;87(8):3089–3096. [PubMed] [Google Scholar]
- 38.Kumar S, Baglioni C. Protection from tumor necrosis factor-mediated cytolysis by overexpression of plasminogen activator inhibitor Type-2. Journal of Biological Chemistry. 1991;266(31):20960–20964. [PubMed] [Google Scholar]
- 39.LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17(25):3247–3259. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]
- 40.Glauert HP, Tharappel JC, Banerjee S, et al. Inhibition of the promotion of hepatocarcinogenesis by 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB-153) by the deletion of the p50 subunit of NF-κB in mice. Toxicology and Applied Pharmacology. 2008;232(2):302–308. doi: 10.1016/j.taap.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cahir-McFarland ED, Davidson DM, Schauer SL, Duong J, Kieff E. NF-κB inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(11):6055–6060. doi: 10.1073/pnas.100119497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernal-Mizrachi L, Lovly CM, Ratner L. The role of NF-κB-1 and NF-κB-2-mediated resistance to apoptosis in lymphomas. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(24):9220–9225. doi: 10.1073/pnas.0507809103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor κB in the induction of eosinophilia in allergic airway inflammation. Journal of Experimental Medicine. 1998;188(9):1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Artis D, Kane CM, Fiore J, et al. Dendritic cell-intrinsic expression of NF-κB1 is required to promote optimal Th2 cell differentiation. Journal of Immunology. 2005;174(11):7154–7159. doi: 10.4049/jimmunol.174.11.7154. [DOI] [PubMed] [Google Scholar]
- 45.Shukla S, MacLennan GT, Marengo SR, Resnick MI, Gupta S. Constitutive activation of PI3K-Akt and NF-κB during prostate cancer progression in autochthonous transgenic mouse model. Prostate. 2005;64(3):224–239. doi: 10.1002/pros.20217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The meta-analysis result of the studies which did not deviated from HWE.


