Abstract
Etoposide is a topoisomerase II poison that is utilized to treat a broad spectrum of human cancers. Despite its wide clinical use, 2–3% of patients treated with etoposide eventually develop treatment-related acute myeloid leukemias (t-AMLs) characterized by rearrangements of the MLL gene. The molecular basis underlying the development of these t-AMLs is not well understood; however, previous studies have implicated etoposide metabolites (i.e., etoposide quinone) and topoisomerase IIβ in the leukemogenic process. Although interactions between etoposide quinone and topoisomerase IIα have been characterized, the effects of the drug metabolite on the activity of human topoisomerase IIβ have not been reported. Thus, we examined the ability of etoposide quinone to poison human topoisomerase IIβ. The quinone induced ∼4 times more enzyme-mediated DNA cleavage than did the parent drug. Furthermore, the potency of etoposide quinone was ∼2 times greater against topoisomerase IIβ than it was against topoisomerase IIα, and the drug reacted ∼2–4 times faster with the β isoform. Etoposide quinone induced a higher ratio of double- to single-stranded breaks than etoposide, and its activity was less dependent on ATP. Whereas etoposide acts as an interfacial topoisomerase II poison, etoposide quinone displayed all of the hallmarks of a covalent poison: the activity of the metabolite was abolished by reducing agents, and the compound inactivated topoisomerase IIβ when it was incubated with the enzyme prior to the addition of DNA. These results are consistent with the hypothesis that etoposide quinone contributes to etoposide-related leukemogenesis through an interaction with topoisomerase IIβ.
Etoposide is an integral component of chemotherapeutic regimens that are used to treat hematological malignancies, somatic tumors, germ cell tumors, and other human cancers.1−6 The drug targets type II topoisomerases, enzymes that generate transient double-stranded breaks in the double helix.3,5,7−11 These enzymes regulate DNA supercoiling and remove knots and tangles from the genome. Etoposide kills cells by inhibiting the ability of type II topoisomerases to ligate DNA, which leads to the accumulation of double-stranded breaks in the genome.1−6 These breaks induce DNA recombination–repair processes and have the potential to activate apoptosis.3,5,8,12 However, if cells survive drug treatment, they may carry stable chromosomal translocations or other rearrangements.3,8,12−17
Despite the wide use of etoposide, there is a well-established correlation between chemotherapeutic regimens that include the drug and the development of therapy-related acute myeloid leukemias (t-AMLs) that feature rearrangements in the MLL (mixed lineage leukemia) gene at chromosomal band 11q23.12−14,17−24 Initially, as many as 12% of patients treated with etoposide developed t-AMLs.19−22,25 Once high-risk schedules were identified and eliminated, that number subsequently dropped to ∼2–3%.19−22,26
Several studies suggest that therapy-related leukemic translocation breakpoints in MLL are derived directly from chromosomal breaks generated by type II topoisomerases.3,12−15,17,27 However, the molecular events that link the initiating DNA cleavage event and the resulting translocation are not well defined. Recent work indicates that topoisomerase II-mediated DNA strand breaks are processed and eventually resected by an alternative nonhomologous end joining pathway.12,28−32 Furthermore, there is evidence that the induction of t-AMLs following etoposide treatment is influenced by the ability of cells to metabolize the drug and the ability of these metabolites to interact with one of the two topoisomerase II isoforms.12,33,34
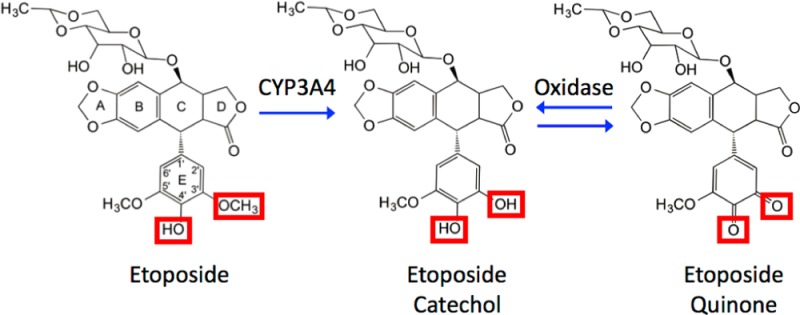
Etoposide can be metabolized by a number of cellular pathways.35−38 In a cytochrome P450-mediated pathway (Figure 1), one of the two methoxy groups on the E-ring of etoposide is converted to a hydroxyl moiety by oxidation by CYP3A4. The resulting etoposide catechol can be further oxidized to a quinone metabolite by the actions of myeloperoxidase and other oxidases (Figure 1).36−41 The high concentration of myeloperoxidase in hematopoietic cells (∼3% of the cell by weight) is consistent with a role for etoposide quinone in the generation of leukemic chromosomal translocations.27,33,42,43 A further epidemiological study has linked a polymorphism in the 5′-promoter region of CYP3A4 (i.e., CYP3A4-V) with a lower risk of t-AMLs that involve MLL gene translocations.33 This polymorphism is believed to decrease the rate of cytochrome P450-mediated production of etoposide catechol, lending further credence to the postulated role for etoposide metabolites in the leukemogenic process.
Figure 1.

Etoposide metabolites. CYP3A4 can metabolize etoposide to the catechol in the liver. In bone marrow progenitor cells, which contain high levels of myeloperoxidase, the catechol can be oxidized further to produce the quinone.
Human cells encode two isoforms of topoisomerase II, α and β.3,8−12,44 These isoforms share extensive amino acid sequence identity (∼70%), but have distinct patterns of expression and separate nuclear functions. Topoisomerase IIα is essential for the survival of proliferating cells, and its expression is proliferation-dependent.3,8−12,45,46 The α isoform functions in growth-related cellular processes and is required for chromosome segregation. In contrast, topoisomerase IIβ is dispensable at the cellular level, and its presence cannot compensate for the loss of topoisomerase IIα in human cells.3,8−12,45,46 The cellular concentration of topoisomerase IIβ is independent of proliferation status, and the enzyme appears to play an important role in transcription.3,8−12,34,44−49
All clinically used topoisomerase II-targeted anticancer drugs affect the activities of both enzyme isoforms.3,4,6,50,51 However, the degree to which topoisomerase IIα and IIβ are targeted by any given drug and the relative contributions of either isoform to the curative effects of drugs are not well understood. The above notwithstanding, in vivo and cellular studies suggest that topoisomerase IIβ is the enzyme primarily responsible for generating the breaks in MLL that initiate t-AMLs.12,34 First, in a skin carcinogenesis model, the incidence of secondary malignancies was greatly diminished in a skin-specific top2b-knockout mouse.52 Second, in a murine cell model, etoposide-induced DNA sequence rearrangements and double-strand breaks were dependent on the presence of topoisomerase IIβ.52 Third, in a human cellular system, the majority of MLL breaks generated by etoposide, as well as the genotoxic effects of the drug, appeared to be mediated primarily by topoisomerase IIβ.17
Despite the proposed roles of etoposide metabolites and topoisomerase IIβ in the induction of t-AMLs, the effects of etoposide quinone on this isoform have not yet been described. Therefore, we characterized the ability of etoposide quinone to alter enzyme-mediated DNA cleavage and ligation. Results indicate that the quinone induces ∼4 times more DNA cleavage than etoposide and appears to function by a different mechanism than that of the parent drug. These findings support a role for etoposide metabolites and topoisomerase IIβ in etoposide-associated t-AMLs.
Experimental Procedures
Enzymes and Materials
Human topoisomerase IIβ was expressed in Saccharomyces cerevisiae JEL1Δtop1 cells and purified as described previously.53−55 The enzyme was stored at −70 °C as a 1.5 mg/mL (4 μM) stock in 50 mM Tris-HCl (pH 7.7), 0.1 mM EDTA, 750 mM KCl, 5% glycerol, and 8 μM DTT (carried from the enzyme preparation). Negatively supercoiled pBR322 DNA was prepared using a Plasmid Mega Kit (Qiagen) as described by the manufacturer. Etoposide was obtained from Sigma-Aldrich. Drugs were prepared as 20 mM solutions in 100% DMSO and stored at −70 °C.
Synthesis of Etoposide Quinone
Etoposide quinone was synthesized and purified according to previously published procedures with slight modifications.27,56,57 The purity was determined to be >99% by liquid chromatography–mass spectrometry analysis at 220 and 254 nm, and the final yield of etoposide quinone was 72%.
DNA Cleavage Mediated by Topoisomerase IIβ
DNA cleavage reactions were performed using the procedure described by Fortune and Osheroff.58 Reaction mixtures contained 100 nM human topoisomerase IIβ and 10 nM negatively supercoiled pBR322 DNA in 20 μL of 10 mM Tris-HCl (pH 7.9), 5 mM MgCl2, 100 mM KCl, 0.1 mM EDTA, and 2.5% (v/v) glycerol. Final reaction mixtures contained ∼0.4 μM DTT, which represents the residual DTT from the enzyme preparation. DNA cleavage reactions were carried out in the absence of the compound or in the presence of 0–30 μM etoposide or etoposide quinone as indicated. In some cases, 50 μM DTT or 1 mM ATP was added to reaction mixtures. Unless stated otherwise, assays were started by the addition of drug, and DNA cleavage mixtures were incubated for 6 min at 37 °C.
DNA cleavage complexes were trapped by the addition of 2 μL of 5% SDS followed by 1 μL of 375 mM Na2EDTA (pH 8.0). Proteinase K was added (2 μL of a 0.8 mg/mL solution), and reaction mixtures were incubated for 30 min at 45 °C to digest topoisomerase IIβ. Samples were mixed with 2 μL of agarose gel loading buffer [60% sucrose in 10 mM Tris-HCl (pH 7.9)], heated for 2 min at 45 °C, and subjected to electrophoresis in 1% agarose gels in 40 mM Tris-acetate (pH 8.3) and 2 mM EDTA containing 0.5 μg/mL ethidium bromide. Double-stranded DNA cleavage was monitored by the conversion of negatively supercoiled plasmid DNA to linear molecules. DNA bands were visualized by UV light and quantified using an Alpha Innotech digital imaging system.
To examine the potential effects of drug–DNA adduction on topoisomerase IIβ-mediated scission, 0.6 μg of pBR322 DNA was incubated with 30 μM etoposide quinone for 6 min at 37 °C in the absence of enzyme. Samples were then applied to a DNA Spin Column (Qiagen) and processed according to the manufacturer’s protocol. DNA was eluted and added to DNA cleavage reaction mixtures.
DNA Ligation Mediated by Topoisomerase IIβ
DNA cleavage–ligation equilibria were established as described above for 6 min at 37 °C in the absence or presence of 30 μM etoposide quinone.57 Ligation was initiated by cooling samples from 37 to 0 °C. Reactions were stopped at time points ranging from 0 to 30 s by the addition of 2 μL of 5% SDS followed by 1 μL of 375 mM Na2EDTA (pH 8.0). Samples were treated with Proteinase K, mixed with agarose gel loading buffer, processed, and analyzed as described above. The amount of linear DNA cleavage product at time zero was set to 100%, and DNA ligation was monitored by the loss of linear DNA.
Results and Discussion
Etoposide is one of the most well studied topoisomerase II-targeted agents in clinical use.1−6 The drug stabilizes covalent topoisomerase II-cleaved DNA complexes (i.e., cleavage complexes) by interacting at the enzyme–DNA interface in a noncovalent manner.2−6,59,60 Once the double helix is cut, the drug slips (i.e., intercalates) between the 3′-hydroxyl and the enzyme-linked 5′-phosphate at the cleaved scissile bond and acts as a physical block to topoisomerase II-mediated DNA ligation.60,61 Etoposide and other drugs that utilize this mechanism are termed “interfacial topoisomerase II poisons”.6,62
The effects of etoposide catechol and etoposide quinone on human topoisomerase IIα have been examined.27,40,41,57,63,64 The catechol displayed properties that were similar to those of the parent drug and appeared to be an interfacial poison. In contrast, the properties of the quinone metabolite differed from those of etoposide, and the quinone appeared to function by a different mechanism.57 Previous studies with quinones and other protein-reactive agents have found that some of these compounds increase levels of topoisomerase II-mediated DNA cleavage by covalently adducting to the enzyme at residues that are distal to the active site.3,65−70 Thus, these agents are termed “covalent topoisomerase II poisons”.70 It is believed that covalent poisons enhance DNA cleavage, at least in part, by closing the N-terminal gate of the protein.68,70−72 Several lines of evidence suggest that etoposide quinone poisons topoisomerase IIα by this latter, covalent mechanism.57
As discussed above, topoisomerase IIβ appears to be the isoform largely responsible for initiating the chromosomal breaks that trigger MLL-associated t-AMLs.12,17,34,52 Because of the proposed role of etoposide quinone in this leukemogenic process, we characterized the effects of the metabolite on the DNA cleavage reaction mediated by human topoisomerase IIβ.
Effects of Etoposide Quinone on DNA Cleavage Mediated by Human Topoisomerase IIβ
The activity of etoposide quinone against topoisomerase IIβ was considerably higher than that of etoposide (Figure 2, left). The quinone increased the relative level of double-stranded DNA cleavage ∼14-fold, which plateaued at ∼17.5 μM, while etoposide increased the level of cleavage only 3-fold at similar concentrations. The efficacy of etoposide quinone against topoisomerase IIβ was similar to that reported with the α isoform.57 However, the metabolite was ∼2 times more potent against topoisomerase IIβ. Furthermore, etoposide quinone reacted more rapidly with the β isoform, inducing maximal DNA cleavage in 2.5–5 min (as opposed to ∼10 min with topoisomerase IIα57) (Figure 2, right).
Figure 2.
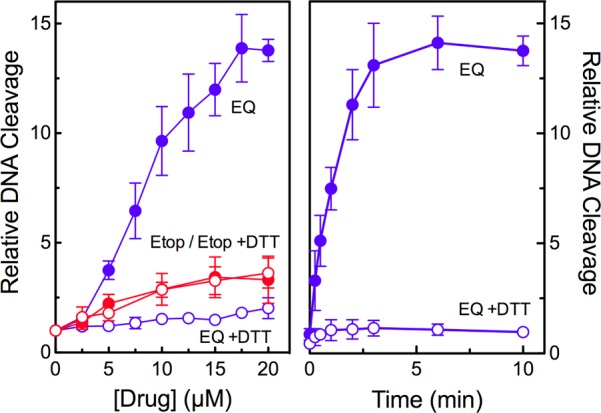
Etoposide quinone enhances DNA cleavage mediated by human topoisomerase IIβ. DNA cleavage was carried out in the presence of etoposide (Etop, red) or etoposide quinone (EQ, blue) in the absence (closed circles) or presence (open circles) of 50 μM dithiothreitol (DTT). The left panel shows drug titrations, and the right panel shows a time course for DNA cleavage in the presence of 15 μM etoposide quinone. Error bars represent the standard deviation of three or more independent experiments.
Because covalent topoisomerase II poisons require protein-reactive groups, their activity can be suppressed by the presence of reducing agents.57,65,66,70,71,73 Therefore, the effects of 50 μM DTT on the activity of etoposide quinone (which should reduce the metabolite to the “unreactive” catechol) were examined. The ability of the quinone to induce topoisomerase IIβ-mediated DNA cleavage decreased precipitously in the presence of the reducing agent (Figure 2). In contrast, DTT had no effect on the activity of the parent drug, which acts by the interfacial mechanism (Figure 2, left). These findings imply that etoposide quinone is a covalent poison of topoisomerase IIβ.
Several control reactions were conducted to ensure that the DNA cleavage enhancement observed with etoposide quinone was mediated by topoisomerase IIβ (Figure 3). No DNA scission was seen in the presence of the quinone when the type II enzyme was omitted from reactions. Moreover, cleaved DNA products were covalently linked to topoisomerase IIβ. In the absence of proteinase K, the linear DNA band disappeared and was replaced by a band that remained at the origin of the gel (not shown). Finally, DNA cleavage induced by the drug metabolite was reversed when the active site Mg2+ ions were chelated with EDTA or the reaction mixture was treated with 0.5 M salt prior to trapping cleavage complexes with SDS.74 EDTA cannot chelate Mg2+ in the cleavage complex and can only sequester the metal ion after the DNA has been ligated. Similarly, the presence of an increased salt concentration will lead to dissociation of the enzyme–DNA complex only after the nucleic acid has been ligated. The fact that EDTA and salt can “reverse” cleavage demonstrates that the DNA scission observed reflects an enzyme-mediated cleavage–ligation equilibrium rather than an enzyme-independent reaction. These results establish that the DNA scission observed in the presence of etoposide quinone is mediated by topoisomerase IIβ.
Figure 3.
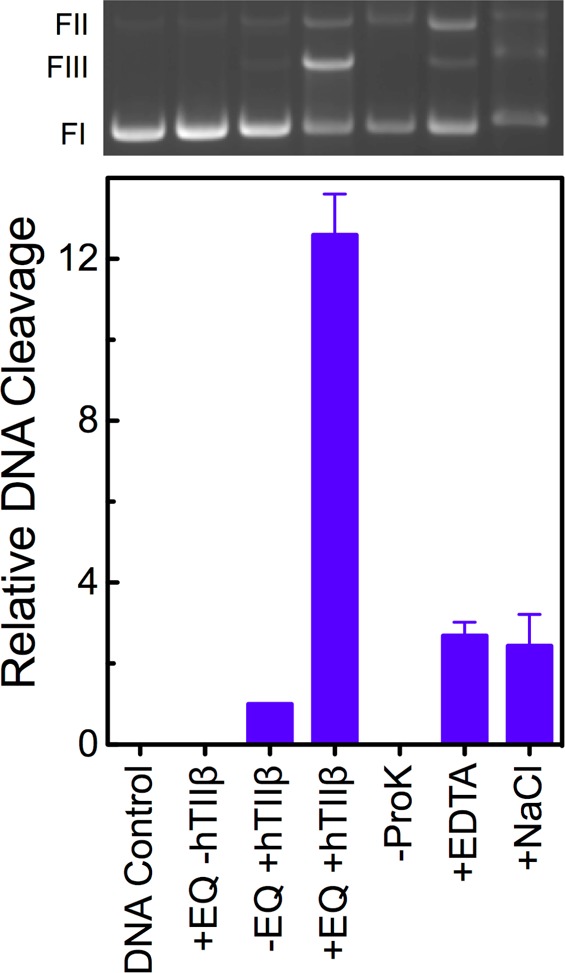
Etoposide quinone induces DNA cleavage via an enzyme-mediated mechanism. Control reactions were conducted in the absence of enzyme or drug (DNA Control), in the presence of 30 μM etoposide quinone without enzyme (+EQ −hTIIβ), or in the presence of topoisomerase IIβ without drug (−EQ +hTIIβ). All other reaction mixtures contained topoisomerase IIβ and 30 μM etoposide quinone. DNA cleavage reactions were terminated by the addition of SDS (+EQ +hTIIβ). To determine whether cleaved DNA was protein-linked, proteinase K treatment was omitted (−ProK). The reversibility of DNA cleavage was examined by adding EDTA (EDTA) or 0.5 M salt (NaCl) prior to SDS. The level of enzyme-mediated DNA cleavage in the absence of etoposide quinone was set to 1 in the bottom panel, and all other reactions were expressed relative to that value. Error bars represent standard deviations for three independent experiments. A representative agarose gel stained with ethidium bromide is shown at the top. The positions of supercoiled (form I, FI), nicked circular (form II, FII), and linear (form III, FIII) molecules are indicated at the left.
Etoposide, as well as many covalent topoisomerase II poisons, increases the level of enzyme–DNA cleavage complexes primarily by inhibiting the DNA ligation activity of the enzyme.57,66,70,71,73 Like its parent compound, etoposide quinone severely inhibited DNA ligation mediated by human topoisomerase IIβ (Figure 4).
Figure 4.

Etoposide quinone inhibits DNA ligation mediated by topoisomerase IIβ. DNA cleavage reactions were initiated in the absence (open circles, ND) or presence (closed circles, EQ) of 30 μM etoposide quinone. The DNA cleavage–ligation equilibrium was established at 37 °C, and ligation was initiated by cooling samples to 0 °C. The level of DNA cleavage observed at equilibrium for each reaction was set to 100% at time zero. Error bars represent the standard deviation of three independent experiments.
Etoposide Quinone Acts Primarily as a Covalent Poison of Human Topoisomerase IIβ
The fact that a reducing agent severely diminishes the activity of etoposide quinone strongly suggests that the drug metabolite is a covalent poison of topoisomerase IIβ. However, the activity of etoposide is highly sensitive to changes in the substituents on the E-ring (Figure 1).59,75,76 Consequently, an alternative hypothesis is that the presence of the 3′- and/or 4′-carbonyl groups on etoposide quinone converts the metabolite into an interfacial poison that is more potent and efficacious than the parent drug (or the catechol).
Therefore, several experiments were performed to resolve this important issue. First, we examined the effects of ATP on the ability of etoposide quinone to induce DNA cleavage mediated by human topoisomerase IIβ. Previous studies have demonstrated that etoposide requires ATP for maximal DNA cleavage activity with the α isoform.57,77 A similar result was seen with topoisomerase IIβ (Figure 5, left). Up to 30 μM etoposide, levels of drug-induced DNA cleavage were 2–5-fold higher in reaction mixtures that contained ATP compared to those that did not. In contrast, no such effect was seen with etoposide quinone and the β isoform (Figure 5, right). In fact, levels of DNA cleavage induced in the presence of ATP were similar to or lower than those seen in the absence of the cofactor.
Figure 5.

Etoposide quinone does not require ATP to induce optimal DNA cleavage mediated by topoisomerase IIβ. DNA cleavage reactions of etoposide (left panel, Etop, red) or etoposide quinone (right panel, EQ, blue) were carried out in the absence (closed bars) or presence (open bars) of 0.25 mM ATP. Control reactions conducted in the absence of drug are shown (ND). Error bars represent the standard deviation of three independent experiments.
Second, we examined the ability of etoposide quinone to generate double-stranded versus single-stranded DNA breaks with topoisomerase IIβ (Figure 6). For the parent drug to stabilize topoisomerase II-generated double-stranded breaks, an etoposide molecule must intercalate between the newly formed DNA termini at each cleaved scissile bond.60,61,78 Because the two drug molecules appear to bind independently, a high proportion of cleavage complexes established at clinically relevant (i.e., subsaturating) concentrations of etoposide contain only one cleaved DNA strand.78−80 Thus, under these conditions, etoposide routinely induces high levels of single-stranded DNA breaks. With human topoisomerase IIα, double-stranded:single-stranded break ratios as low as 0.5:1 frequently are observed in the presence of etoposide.57,78 Although the effect is less dramatic with the β isoform, etoposide still generates approximately equimolar levels of double- and single-stranded DNA breaks (Figure 6 shows results for 30 μM etoposide). In contrast, at a drug concentration (15 μM) that was lower than that used for etoposide, the quinone still generated approximately two double-stranded breaks for every single-stranded cut. Taken with the ATP results, these findings suggest that etoposide quinone induces topoisomerase IIβ-mediated DNA cleavage by a mechanism different than that of the parent drug.
Figure 6.
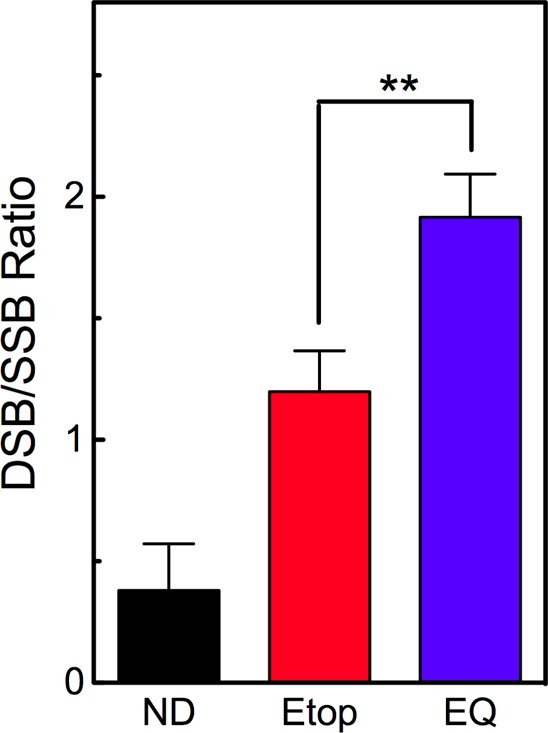
Etoposide quinone induces a high ratio of double-stranded DNA breaks (DSB) to single-stranded DNA breaks (SSB). DNA strand breaks generated by human topoisomerase IIβ were monitored in reaction mixtures containing no drug (ND, black), 30 μM etoposide (Etop, red), or 15 μM etoposide quinone (EQ, blue). Double- and single-stranded DNA cleavage was monitored by the conversion of negatively supercoiled plasmid DNA to linear and nicked molecules, respectively. Error bars represent the standard deviation of three independent experiments. Results of an unpaired two-tailed t test are shown (**p = 0.001).
Third, covalent topoisomerase II poisons display the hallmark characteristic of inactivating the enzyme when the two are incubated prior to the addition of DNA.65,66,70,71 This inactivation is not observed with interfacial poisons. As seen in Figure 7, incubation of 15 μM etoposide quinone with human topoisomerase IIβ rapidly inactivated the enzyme. The DNA cleavage activity was decreased by more than 90% following a 6 min incubation of the drug with the enzyme (t1/2 ≤ 1.5 min). Under parallel conditions (6 min incubation), little enzyme inactivation was observed in the presence of etoposide or a mixture of etoposide quinone and DTT (Figure 7, inset).
Figure 7.

Etoposide quinone inactivates human topoisomerase IIβ when incubated with the enzyme prior to the addition of DNA. The enzyme was incubated in the presence of 15 μM etoposide quinone (closed circles, blue) prior to a DNA cleavage assay. The inset shows cleavage levels established following incubation for 6 min in the absence of drug (ND, black bar) or in the presence of 30 μM etoposide (Etop, red bar), 15 μM etoposide quinone and DTT (EQ+DTT, open blue bar), or 15 μM etoposide quinone in the absence of DTT (EQ, blue bar). Error bars represent the standard deviation of three independent experiments.
Fourth, once a covalent poison has adducted topoisomerase II and stimulated DNA cleavage, the redox state of the poison (quinone vs catechol) no longer appears to matter. Thus, if a reducing agent is added to reaction mixtures after the DNA cleavage–ligation equilibrium has been established in the presence of the poison, it will not reverse the cleavage enhancement.57,66 To determine whether this was the case for etoposide quinone, an order of addition experiment was carried out. As seen in Figure 8 (left), once cleavage complexes were established in the presence of the quinone, the addition of DTT did not affect levels of DNA scission. This is in contrast to results seen when DTT was added to reaction mixtures prior to the generation of cleavage complexes (Figure 2 and Figure 8, left). Once again, these results are consistent with a topoisomerase II adduction mechanism for DNA cleavage enhancement by etoposide quinone.
Figure 8.
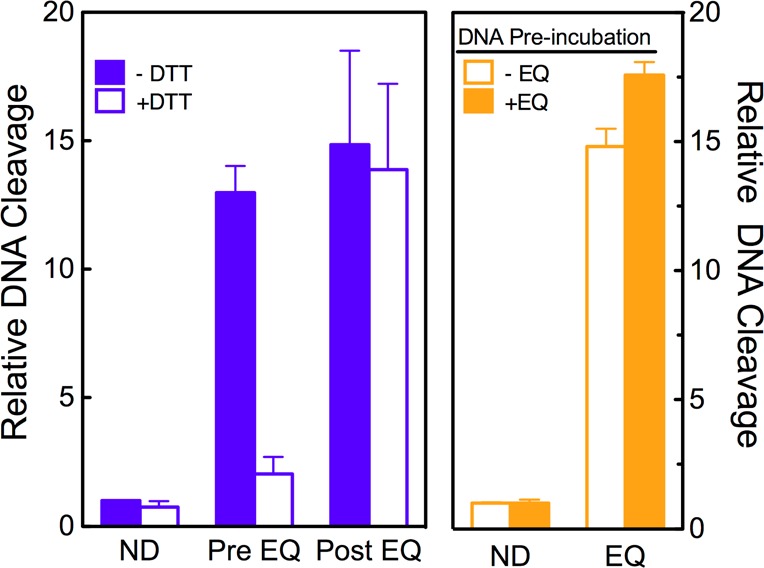
Etoposide quinone is a covalent poison of topoisomerase IIβ. In the left panel, etoposide quinone enhancement of DNA cleavage is not reversed by the addition of reducing agents after DNA cleavage complexes have been established. DNA cleavage reactions were carried out in the absence (blue bars) or presence (open blue bars) of DTT. Reaction mixtures contained no drug (ND) or 30 μM etoposide quinone in mixtures that included DTT at the time of DNA cleavage (Pre EQ) or DTT that was added (for an additional 6 min) after cleavage complexes were formed (Post EQ). In the right panel, etoposide quinone does not form DNA lesions that poison topoisomerase IIβ. DNA was incubated without (−EQ, open orange bars) or with (+EQ, orange bars) 30 μM etoposide quinone. DNA was purified from free drug and used in DNA cleavage reactions mediated by topoisomerase IIβ. DNA cleavage reactions were performed in the absence of drug (ND) or in the presence of 30 μM etoposide quinone (EQ). In all cases, error bars represent the standard deviation of three independent experiments.
In addition to modifying proteins, etoposide quinone also can form covalent nucleic acid adducts, especially with N7 of guanine residues.27 Because alkylated DNA lesions can enhance DNA cleavage mediated by human topoisomerase IIβ,81 it is possible that etoposide quinone stimulates the reaction by a mechanism that involves DNA, rather than protein, adduction. To address this possibility, DNA was incubated with 30 μM etoposide quinone for 6 min at 37 °C and then purified prior to cleavage assays. The incubation had no effect on the ability of topoisomerase IIβ to cleave DNA regardless of whether etoposide quinone was added to final reaction mixtures (Figure 8, right). Together with all of the data presented above, we conclude that etoposide quinone enhances DNA cleavage mediated by human topoisomerase IIβ primarily by a mechanism that involves adduction to the enzyme.
Conclusions
Previous studies have identified a role for etoposide metabolites and topoisomerase IIβ in the initiation of t-AMLs associated with anticancer regimens that contain etoposide. However, the effects of etoposide quinone on the activity of the β isoform have not been described. Results indicate that the quinone is a potent topoisomerase IIβ poison that induces higher levels of enzyme-mediated DNA cleavage than does the parent drug. Etoposide quinone also displays higher reactivity toward topoisomerase IIβ than it does with topoisomerase IIα. Finally, the metabolite induces DNA cleavage primarily by a mechanism that differs from that of etoposide and appears to involve covalent modification of the enzyme. These findings are consistent with the hypothesis that the oxidative environment of hematopoietic progenitor cells generates a highly reactive etoposide metabolite that contributes to the generation of DNA breakpoints with leukemogenic potential.
Acknowledgments
We are grateful to Katie J. Aldred, MaryJean Pendleton, Rachel E. Ashley, Kendra R. Vann, and Joel H. Everett for critical reading of the manuscript. N.A.S. was a participant in the Vanderbilt University School of Medicine Emphasis Program.
The authors declare no competing financial interests.
This research was supported by Grants GM033944 (N.O.) and T35 ES016534 (N.A.S.) from the National Institutes of Health and funds from the Lipscomb University College of Pharmacy and Health Sciences (S.L.M. and J.E.D.).
Funding Statement
National Institutes of Health, United States
References
- Hande K. R. (1998) Etoposide: Four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer 34, 1514–1521. [DOI] [PubMed] [Google Scholar]
- Baldwin E. L.; Osheroff N. (2005) Etoposide, topoisomerase II and cancer. Curr. Med. Chem.: Anti-Cancer Agents 5, 363–372. [DOI] [PubMed] [Google Scholar]
- Deweese J. E.; Osheroff N. (2009) The DNA cleavage reaction of topoisomerase II: Wolf in sheep’s clothing. Nucleic Acids Res. 37, 738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss J. L. (2009) Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 9, 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y.; Leo E.; Zhang H.; Marchand C. (2010) DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 17, 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y.; Marchand C. (2012) Interfacial inhibitors: Targeting macromolecular complexes. Nat. Rev. Drug Discovery 11, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J. (2001) DNA topoisomerases: Structure, function, and mechanism. Annu. Rev. Biochem. 70, 369–413. [DOI] [PubMed] [Google Scholar]
- Nitiss J. L. (2009) DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer 9, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Deibler R. W.; Chan H. S.; Zechiedrich L. (2009) The why and how of DNA unlinking. Nucleic Acids Res. 37, 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos S. M.; Tretter E. M.; Schmidt B. H.; Berger J. M. (2011) All tangled up: How cells direct, manage and exploit topoisomerase function. Nat. Rev. Mol. Cell Biol. 12, 827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry A. C., and Osheroff N. (2013) DNA topoisomerases: Type II. In Encyclopedia of Biological Chemistry, pp 163–168, Elsevier Inc., Amsterdam. [Google Scholar]
- Pendleton M.; Lindsey R. H. Jr.; Felix C. A.; Grimwade D.; Osheroff N. (2014) Topoisomerase II and leukemia. Ann. N.Y. Acad. Sci. 1310, 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix C. A.; Kolaris C. P.; Osheroff N. (2006) Topoisomerase II and the etiology of chromosomal translocations. DNA Repair 5, 1093–1108. [DOI] [PubMed] [Google Scholar]
- Joannides M.; Grimwade D. (2010) Molecular biology of therapy-related leukaemias. Clin. Transl. Oncol. 12, 8–14. [DOI] [PubMed] [Google Scholar]
- Joannides M.; Mays A. N.; Mistry A. R.; Hasan S. K.; Reiter A.; Wiemels J. L.; Felix C. A.; Coco F. L.; Osheroff N.; Solomon E.; Grimwade D. (2011) Molecular pathogenesis of secondary acute promyelocytic leukemia. Mediterranean Journal of Hematology and Infectious Diseases 3, e2011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezoe S. (2012) Secondary leukemia associated with the anti-cancer agent, etoposide, a topoisomerase II inhibitor. Int. J. Environ. Res. Public Health 9, 2444–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell I. G.; Austin C. A. (2012) Mechanism of generation of therapy related leukemia in response to anti-topoisomerase II agents. Int. J. Environ. Res. Public Health 9, 2075–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVore R.; Whitlock J.; Hainsworth J. D.; Johnson D. H. (1989) Therapy-related acute nonlymphocytic leukemia with monocytic features and rearrangement of chromosome 11q. Ann. Int. Med. 110, 740–742. [DOI] [PubMed] [Google Scholar]
- Pui C. H.; Ribeiro R. C.; Hancock M. L.; Rivera G. K.; Evans W. E.; Raimondi S. C.; Head D. R.; Behm F. G.; Mahmoud M. H.; Sandlund J. T.; et al. (1991) Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. N. Engl. J. Med. 325, 1682–1687. [DOI] [PubMed] [Google Scholar]
- Smith M. A.; Rubinstein L.; Ungerleider R. S. (1994) Therapy-related acute myeloid leukemia following treatment with epipodophyllotoxins: Estimating the risks. Med. Pediatr. Oncol. 23, 86–98. [DOI] [PubMed] [Google Scholar]
- Relling M. V.; Yanishevski Y.; Nemec J.; Evans W. E.; Boyett J. M.; Behm F. G.; Pui C. H. (1998) Etoposide and antimetabolite pharmacology in patients who develop secondary acute myeloid leukemia. Leukemia 12, 346–352. [DOI] [PubMed] [Google Scholar]
- Smith M. A.; Rubinstein L.; Anderson J. R.; Arthur D.; Catalano P. J.; Freidlin B.; Heyn R.; Khayat A.; Krailo M.; Land V. J.; Miser J.; Shuster J.; Vena D. (1999) Secondary leukemia or myelodysplastic syndrome after treatment with epipodophyllotoxins. J. Clin. Oncol. 17, 569–577. [DOI] [PubMed] [Google Scholar]
- Pedersen-Bjergaard J.; Philip P. (1991) Balanced translocations involving chromosome bands 11q23 and 21q22 are highly characteristic of myelodysplasia and leukemia following therapy with cytostatic agents targeting at DNA-topoisomerase II. Blood 78, 1147–1148. [PubMed] [Google Scholar]
- Pedersen-Bjergaard J.; Rowley J. D. (1994) The balanced and the unbalanced chromosome aberrations of acute myeloid leukemia may develop in different ways and may contribute differently to malignant transformation. Blood 83, 2780–2786. [PubMed] [Google Scholar]
- Felix C. A. (1998) Secondary leukemias induced by topoisomerase-targeted drugs. Biochim. Biophys. Acta 1400, 233–255. [DOI] [PubMed] [Google Scholar]
- Felix C. A. (2009) A safer regimen for high-risk neuroblastoma. Pediatric Blood & Cancer 53, 3–6. [DOI] [PubMed] [Google Scholar]
- Lovett B. D.; Strumberg D.; Blair I. A.; Pang S.; Burden D. A.; Megonigal M. D.; Rappaport E. F.; Rebbeck T. R.; Osheroff N.; Pommier Y. G.; Felix C. A. (2001) Etoposide metabolites enhance DNA topoisomerase II cleavage near leukemia-associated MLL translocation breakpoints. Biochemistry 40, 1159–1170. [DOI] [PubMed] [Google Scholar]
- Wang M.; Wu W.; Rosidi B.; Zhang L.; Wang H.; Iliakis G. (2006) PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res. 34, 6170–6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray J.; Williamson E. A.; Singh S. B.; Wu Y.; Cogle C. R.; Weinstock D. M.; Zhang Y.; Lee S. H.; Zhou D.; Shao L.; Hauer-Jensen M.; Pathak R.; Klimek V.; Nickoloff J. A.; Hromas R. (2013) PARP1 is required for chromosomal translocations. Blood 121, 4359–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Herreros F.; Romero-Granados R.; Zeng Z.; Alvarez-Quilon A.; Quintero C.; Ju L.; Umans L.; Vermeire L.; Huylebroeck D.; Caldecott K. W.; Cortes-Ledesma F. (2013) TDP2-dependent non-homologous end-joining protects against topoisomerase II-induced DNA breaks and genome instability in cells and in vivo. PLoS Genet. 9, e1003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss J. L.; Nitiss K. C. (2013) Tdp2: A means to fixing the ends. PLoS Genet. 9, e1003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maede Y.; Shimizu H.; Fukushima T.; Kogame T.; Nakamura T.; Miki T.; Takeda S.; Pommier Y.; Murai J. (2014) Differential and common DNA repair pathways for topoisomerase I- and II-targeted drugs in a genetic DT40 repair cell screen panel. Mol. Cancer Ther. 13, 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix C. A.; Walker A. H.; Lange B. J.; Williams T. M.; Winick N. J.; Cheung N. K.; Lovett B. D.; Nowell P. C.; Blair I. A.; Rebbeck T. R. (1998) Association of CYP3A4 genotype with treatment-related leukemia. Proc. Natl. Acad. Sci. U.S.A. 95, 13176–13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell I. G.; Sondka Z.; Smith K.; Lee K. C.; Manville C. M.; Sidorczuk-Lesthuruge M.; Rance H. A.; Padget K.; Jackson G. H.; Adachi N.; Austin C. A. (2012) Model for MLL translocations in therapy-related leukemia involving topoisomerase IIβ-mediated DNA strand breaks and gene proximity. Proc. Natl. Acad. Sci. U.S.A. 109, 8989–8994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Maanen J. M. S.; Retl J.; de Vries J.; Pinedo H. M. (1988) Mechanism of action of antitumor drug etoposide: A review. J. Natl. Cancer Inst. 80, 1526–1533. [DOI] [PubMed] [Google Scholar]
- Relling M. V.; Evans R.; Dass C.; Desiderio D. M.; Nemec J. (1992) Human cytochrome P450 metabolism of teniposide and etoposide. J. Pharmacol. Exp. Ther. 261, 491–496. [PubMed] [Google Scholar]
- Relling M. V.; Nemec J.; Schuetz E. G.; Schuetz J. D.; Gonzalez F. J.; Korzekwa K. R. (1994) O-Demethylation of epipodophyllotoxins is catalyzed by human cytochrome P450 3A4. Mol. Pharmacol. 45, 352–358. [PubMed] [Google Scholar]
- Zhuo X.; Zheng N.; Felix C. A.; Blair I. A. (2004) Kinetics and regulation of cytochrome P450-mediated etoposide metabolism. Drug Metab. Dispos. 32, 993–1000. [PubMed] [Google Scholar]
- Haim N.; Roman J.; Nemec J.; Sinha B. K. (1986) Peroxidative free radical formation and O-demethylation of etoposide(VP-16) and teniposide(VM-26). Biochem. Biophys. Res. Commun. 135, 215–220. [DOI] [PubMed] [Google Scholar]
- Gantchev T. G.; Hunting D. J. (1997) Inhibition of the topoisomerase II-DNA cleavable complex by the ortho-quinone derivative of the antitumor drug etoposide (VP-16). Biochem. Biophys. Res. Commun. 237, 24–27. [DOI] [PubMed] [Google Scholar]
- Gantchev T. G.; Hunting D. J. (1998) The ortho-quinone metabolite of the anticancer drug etoposide (VP-16) is a potent inhibitor of the topoisomerase II/DNA cleavable complex. Mol. Pharmacol. 53, 422–428. [DOI] [PubMed] [Google Scholar]
- Kagan V. E.; Yalowich J. C.; Borisenko G. G.; Tyurina Y. Y.; Tyurin V. A.; Thampatty P.; Fabisiak J. P. (1999) Mechanism-based chemopreventive strategies against etoposide-induced acute myeloid leukemia: Free radical/antioxidant approach. Mol. Pharmacol. 56, 494–506. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Schreiber E. M.; Giorgianni A.; Yalowich J. C.; Day B. W. (2006) Myeloperoxidase-catalyzed metabolism of etoposide to its quinone and glutathione adduct forms in HL60 cells. Chem. Res. Toxicol. 19, 937–943. [DOI] [PubMed] [Google Scholar]
- Austin C. A.; Marsh K. L. (1998) Eukaryotic DNA topoisomerase IIβ. BioEssays 20, 215–226. [DOI] [PubMed] [Google Scholar]
- Woessner R. D.; Mattern M. R.; Mirabelli C. K.; Johnson R. K.; Drake F. H. (1991) Proliferation- and cell cycle-dependent differences in expression of the 170 kDa and 180 kDa forms of topoisomerase II in NIH-3T3 cells. Cell Growth Differ. 2, 209–214. [PubMed] [Google Scholar]
- Christensen M. O.; Larsen M. K.; Barthelmes H. U.; Hock R.; Andersen C. L.; Kjeldsen E.; Knudsen B. R.; Westergaard O.; Boege F.; Mielke C. (2002) Dynamics of human DNA topoisomerases IIα and IIβ in living cells. J. Cell Biol. 157, 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Li W.; Prescott E. D.; Burden S. J.; Wang J. C. (2000) DNA topoisomerase IIβ and neural development. Science 287, 131–134. [DOI] [PubMed] [Google Scholar]
- Ju B. G.; Lunyak V. V.; Perissi V.; Garcia-Bassets I.; Rose D. W.; Glass C. K.; Rosenfeld M. G. (2006) A topoisomerase IIβ-mediated dsDNA break required for regulated transcription. Science 312, 1798–1802. [DOI] [PubMed] [Google Scholar]
- Heng X.; Le W. D. (2010) The function of DNA topoisomerase IIβ in neuronal development. Neurosci. Bull. 26, 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClendon A. K.; Osheroff N. (2007) DNA topoisomerase II, genotoxicity, and cancer. Mutat. Res. 623, 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y. (2013) Drugging topoisomerases: Lessons and challenges. ACS Chem. Biol. 8, 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarova A. M.; Lyu Y. L.; Lin C. P.; Tsai Y. C.; Lau J. Y.; Wang J. C.; Liu L. F. (2007) Roles of DNA topoisomerase II isozymes in chemotherapy and secondary malignancies. Proc. Natl. Acad. Sci. U.S.A. 104, 11014–11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worland S. T.; Wang J. C. (1989) Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 264, 4412–4416. [PubMed] [Google Scholar]
- Elsea S. H.; Hsiung Y.; Nitiss J. L.; Osheroff N. (1995) A yeast type II topoisomerase selected for resistance to quinolones. Mutation of histidine 1012 to tyrosine confers resistance to nonintercalative drugs but hypersensitivity to ellipticine. J. Biol. Chem. 270, 1913–1920. [DOI] [PubMed] [Google Scholar]
- Kingma P. S.; Greider C. A.; Osheroff N. (1997) Spontaneous DNA lesions poison human topoisomerase IIα and stimulate cleavage proximal to leukemic 11q23 chromosomal breakpoints. Biochemistry 36, 5934–5939. [DOI] [PubMed] [Google Scholar]
- Nemec J. (1986) Epipodophyllotoxinquinone glucoside derivatives, method of production and use. U.S. Patent 4609644 A.
- Jacob D. A.; Mercer S. L.; Osheroff N.; Deweese J. E. (2011) Etoposide quinone is a redox-dependent topoisomerase II poison. Biochemistry 50, 5660–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune J. M.; Osheroff N. (1998) Merbarone inhibits the catalytic activity of human topoisomerase IIα by blocking DNA cleavage. J. Biol. Chem. 273, 17643–17650. [DOI] [PubMed] [Google Scholar]
- Wilstermann A. M.; Bender R. P.; Godfrey M.; Choi S.; Anklin C.; Berkowitz D. B.; Osheroff N.; Graves D. E. (2007) Topoisomerase II-drug interaction domains: Identification of substituents on etoposide that interact with the enzyme. Biochemistry 46, 8217–8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. C.; Li T. K.; Farh L.; Lin L. Y.; Lin T. S.; Yu Y. J.; Yen T. J.; Chiang C. W.; Chan N. L. (2011) Structural basis of type II topoisomerase inhibition by the anticancer drug etoposide. Science 333, 459–462. [DOI] [PubMed] [Google Scholar]
- Pommier Y.; Capranico G.; Orr A.; Kohn K. W. (1991) Local base sequence preferences for DNA cleavage by mammalian topoisomerase II in the presence of amsacrine or teniposide. Nucleic Acids Res. 19, 5973–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y.; Marchand C. (2005) Interfacial inhibitors of protein-nucleic acid interactions. Curr. Med. Chem.: Anti-Cancer Agents 5, 421–429. [DOI] [PubMed] [Google Scholar]
- Sinha B. K.; Politi P. M.; Eliot H. M.; Kerrigan D.; Pommier Y. (1990) Structure-activity relations, cytotoxicity and topoisomerase II dependent cleavage induced by pendulum ring analogues of etoposide. Eur. J. Cancer 26, 590–593. [DOI] [PubMed] [Google Scholar]
- Jacob D. A.; Gibson E. G.; Mercer S. L.; Deweese J. E. (2013) Etoposide catechol is an oxidizable topoisomerase II poison. Chem. Res. Toxicol. 26, 1156–1158. [DOI] [PubMed] [Google Scholar]
- Wang H.; Mao Y.; Chen A. Y.; Zhou N.; LaVoie E. J.; Liu L. F. (2001) Stimulation of topoisomerase II-mediated DNA damage via a mechanism involving protein thiolation. Biochemistry 40, 3316–3323. [DOI] [PubMed] [Google Scholar]
- Lindsey R. H. Jr.; Bromberg K. D.; Felix C. A.; Osheroff N. (2004) 1,4-Benzoquinone is a topoisomerase II poison. Biochemistry 43, 7563–7574. [DOI] [PubMed] [Google Scholar]
- Bender R. P.; Ham A. J.; Osheroff N. (2007) Quinone-induced enhancement of DNA cleavage by human topoisomerase IIα: Adduction of cysteine residues 392 and 405. Biochemistry 46, 2856–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R. P.; Osheroff N. (2007) Mutation of cysteine residue 455 to alanine in human topoisomerase IIα confers hypersensitivity to quinones: Enhancing DNA scission by closing the N-terminal protein gate. Chem. Res. Toxicol. 20, 975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R. K.; Zhou N.; Lyu Y. L.; Tsai Y. C.; Lu C. H.; Kerrigan J.; Chen Y. T.; Guan Z.; Hsieh T. S.; Liu L. F. (2011) Dietary isothiocyanate-induced apoptosis via thiol modification of DNA topoisomerase IIα. J. Biol. Chem. 286, 33591–33600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketron A.; Osheroff N. (2014) Phytochemicals as anticancer and chemopreventive topoisomerase II poisons. Phytochem. Rev. 13, 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R. P.; Lehmler H. J.; Robertson L. W.; Ludewig G.; Osheroff N. (2006) Polychlorinated biphenyl quinone metabolites poison human topoisomerase IIα: Altering enzyme function by blocking the N-terminal protein gate. Biochemistry 45, 10140–10152. [DOI] [PubMed] [Google Scholar]
- Mondrala S.; Eastmond D. A. (2010) Topoisomerase II inhibition by the bioactivated benzene metabolite hydroquinone involves multiple mechanisms. Chem.-Biol. Interact. 184, 259–268. [DOI] [PubMed] [Google Scholar]
- Bender R. P.; Lindsey R. H. Jr.; Burden D. A.; Osheroff N. (2004) N-Acetyl-p-benzoquinone imine, the toxic metabolite of acetaminophen, is a topoisomerase II poison. Biochemistry 43, 3731–3739. [DOI] [PubMed] [Google Scholar]
- Osheroff N.; Zechiedrich E. L. (1987) Calcium-promoted DNA cleavage by eukaryotic topoisomerase II: Trapping the covalent enzyme-DNA complex in an active form. Biochemistry 26, 4303–4309. [DOI] [PubMed] [Google Scholar]
- Long B. H. (1987) Structure-activity relationships of podophyllin congeners that inhibit topoisomerase II. Natl. Cancer Inst. Monogr. 123–127. [PubMed] [Google Scholar]
- Bender R. P.; Jablonksy M. J.; Shadid M.; Romaine I.; Dunlap N.; Anklin C.; Graves D. E.; Osheroff N. (2008) Substituents on etoposide that interact with human topoisomerase IIα in the binary enzyme-drug complex: Contributions to etoposide binding and activity. Biochemistry 47, 4501–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Mao Y.; Zhou N.; Hu T.; Hsieh T. S.; Liu L. F. (2001) ATP-bound topoisomerase II as a target for antitumor drugs. J. Biol. Chem. 276, 15990–15995. [DOI] [PubMed] [Google Scholar]
- Bromberg K. D.; Burgin A. B.; Osheroff N. (2003) A two-drug model for etoposide action against human topoisomerase IIα. J. Biol. Chem. 278, 7406–7412. [DOI] [PubMed] [Google Scholar]
- Long B. H.; Musial S. T.; Brattain M. G. (1985) Single- and double-strand DNA breakage and repair in human lung adenocarcinoma cells exposed to etoposide and teniposide. Cancer Res. 45, 3106–3112. [PubMed] [Google Scholar]
- Muslimovic A.; Nystrom S.; Gao Y.; Hammarsten O. (2009) Numerical analysis of etoposide induced DNA breaks. PLoS One 4, e5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin M.; Osheroff N. (2000) Sensitivity of human type II topoisomerases to DNA damage: Stimulation of enzyme-mediated DNA cleavage by abasic, oxidized and alkylated lesions. Nucleic Acids Res. 28, 1947–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]


