Introduction
The correlation between the red hair/pale skin phenotype and increased melanoma risk has been established for over twenty years [1]. Historically, this correlation has been attributed to redheads’ pale skin and thus poor UV protection. A recent study from our lab, however, has suggested that the very pigment that gives red hair its fiery color may be carcinogenic itself, independent of UV radiation [2].
Specialized skin cells called melanocytes can produce two types of pigment: dark brown eumelanin and red/orange pheomelanin. The enzymes responsible for the production of pigment are activated downstream of the G-protein-coupled receptor MC1R. The switch between production of eumelanin and pheomelanin depends on the strength of MC1R signaling and the availability of the amino acid cysteine. When MC1R signaling is strong, production of pigment precursors outpaces cysteine availability and production of eumelanin (which does not incorporate cysteine) is kinetically favored. When MC1R signaling is weak—such as in redhead melanocytes, 80% of which carry inactivating MCIR polymorphisms—pigment precursors are formed more slowly and cysteine stores keep pace, which leads to formation of cysteine-containing pheomelanin [3,4].
In our study, the most common driver mutation in melanoma—an activating mutation in the kinase BRAF—was induced in the melanocytes of mice. Introducing an inactivating Mc1r mutation into mouse melanocytes caused the mice to have red fur and, in the context of the Braf mutation, develop melanoma. No environmental stressor, such as UV, was necessary for melanoma development. Furthermore, when an inactivating mutation in the rate-limiting pigment production enzyme tyrosinase was introduced to the redhead mouse melanocytes, the mice appeared albino and no longer developed melanoma. This study suggests that the pheomelanin synthetic pathway may be carcinogenic independent of UV radiation.
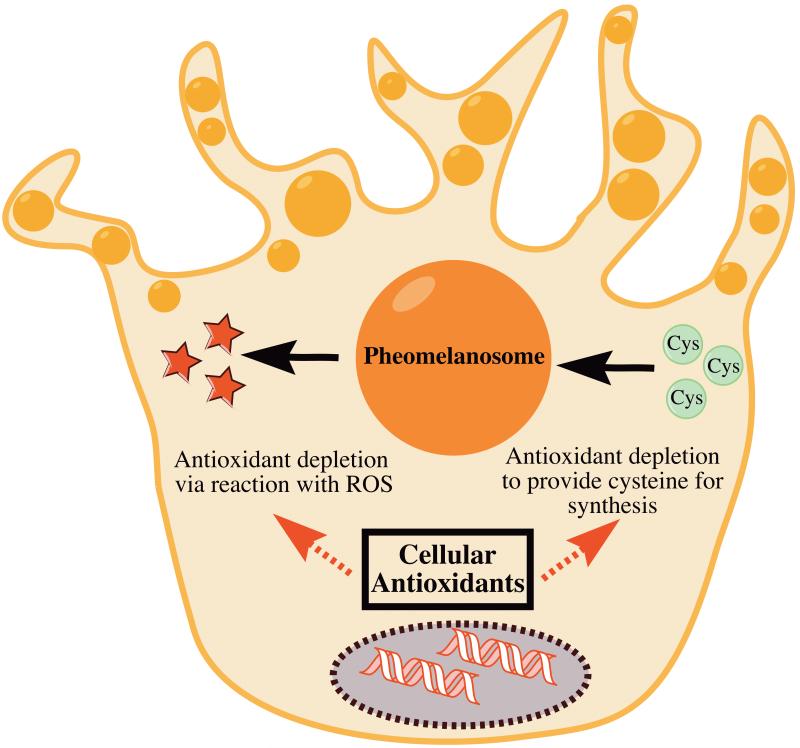
Mechanistically, oxidative stress appears to play a role in pheomelanin-mediated melanomagenesis. In our study, we found that pheomelanin production was associated with increased oxidative lipid damage in mouse skin. We also found that pheomelanotic mouse skin contained more hydroxyl-radical damaged DNA bases. While we demonstrated an association between pheomelanin and oxidative stress, we did not uncover the mechanistic pathway between pheomelanin and the oxidative DNA damage that drives melanomagenesis. We hypothesize two possible pathways. On one hand, pheomelanin might generate reactive oxygen species (ROS) that directly or indirectly cause oxidative DNA damage. On the other hand, pheomelanin synthesis might consume cellular antioxidant stores and make the cell more vulnerable to other endogenous ROS (Fig. 1).
Figure 1. Two distinct mechanisms could explain the increase in oxidative stress associated with pheomelanin production.
On one hand, pheomelanin is known to promote the formation of reactive oxygen species, which could tax cellular antioxidant reserves. On the other hand, the use of cysteine in pheomelanin biosynthesis could deplete cysteine-based cellular antioxidants. These two hypothetical mechanisms are not mutually exclusive.
Hypothesis I. Pheomelanin-generated ROS damage DNA
The first possible pathway between pheomelanin and carcinogenesis involves pheomelanin-generated ROS. Pheomelanin is known to generate ROS when irradiated with UVA [5]. In light of our pheomelanin study, ROS generation may occur without UVA input as well. Although the mechanism of pheomelanin-mediated ROS generation is not thoroughly understood, pheomelanin seems to be unique in its ROS generation ability when compared with eumelanin. Pheomelanin synthesis is known to be associated with lower melanosomal pH than eumelanin synthesis, but it is unclear how low pH could promote ROS formation [6,7]. The most significant structural difference between the two melanins is the presence of sulfur-containing aromatic rings in pheomelanin, but not in eumelanin (Fig. 2). The fact that pheomelanin has a lower ionization potential (energy required to remove an electron from the molecule) than eumelanin suggests that sulfur helps to stabilize a cationic radical form of pheomelanin [8,9]. This suggests that energetically, pheomelanin is more likely to be involved in a radical-producing reaction than eumelanin. The energy input needed to drive such a reaction will be discussed in further detail later.
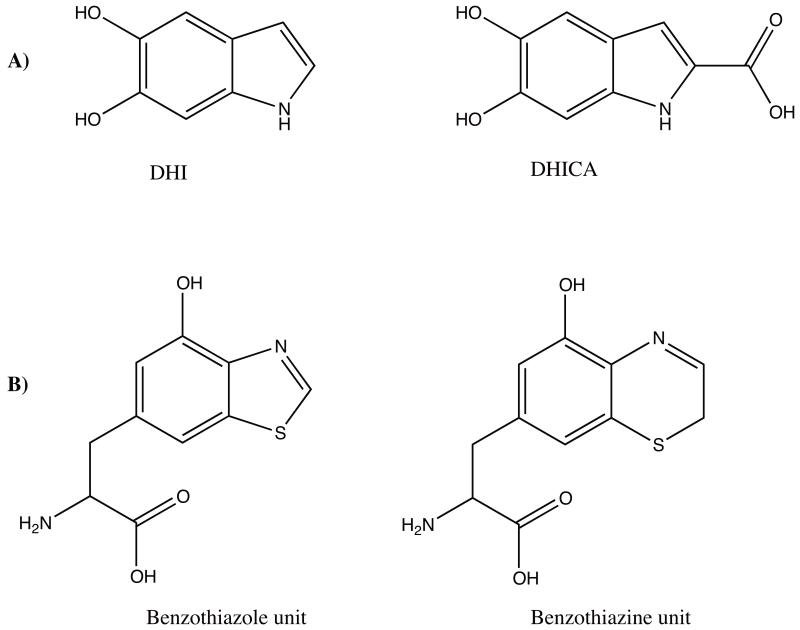
Figure 2. Eumelanin polymers are composed of DHI and DHICA units, while pheomelanin polymers are composed of benzothiazole and benzothiazine units.
Chemical structures of (a) eumelanin monomer units and (b) pheomelanin monomer units are shown above. DHI is 5,6-dihydroxyindole. DHICA is 5,6-dihydroxyindole-2-carboxylic acid. Oligomers of these units constitute the biological pigments [25].
The damaged DNA bases we observed in the skin of pheomelanotic mice could be caused by hydroxyl radicals produced by an interaction between pheomelanin and water. However, typical of ROS, hydroxyl radicals can only travel about 10 Å before reacting [10]. Melanin is synthesized in special organelles called melanosomes, wherein it is deposited along proteinaceous fibrils [11]. Although melanized nuclei are occasionally reported in melanomas, to the authors’ knowledge, there is no known mechanism of melanin transport into the nucleus [12]. Unless evidence of pheomelanin localization in melanocyte nuclei is discovered, it seems unlikely that radicals produced by pheomelanin damage DNA directly.
If pheomelanin is not located in the nucleus, it might still cause damage by generating ROS that interact with free nucleobases in the cytosol. In our study, the damaged DNA bases we found to be enriched in redhead mice were 8,5-cyclo-2′-deoxyadenosine (cdA) and 8,5-cyclo-2′-deoxyguanosine (cdG). Studies in E. coli have found that cdA and cdG can cause A→T and C→G mutations at respective frequencies of 11% and 20% [13]. However, these modified bases are known to block DNA synthesis by mammalian DNA polymerases and thus are unlikely to be incorporated after ROS modification [14]. Nevertheless, cdA and cdG were the only damaged bases we measured specifically. Other types of damaged bases, which can be incorporated post-modification, are likely to be enriched in redhead mouse melanocytes as well. To investigate this hypothesis, it will be necessary to analyze the spectrum of damaged bases that are enriched in pheomelanotic melanocytes.
The most likely effect of pheomelanin-generated radicals is a general toll on cellular antioxidants. Even if pheomelanin ROS do not damage DNA directly, they could still overwhelm the ability of melanocytes to cope with oxidative stress, thus making the nucleus more vulnerable to other endogenous ROS. Our study found elevated levels of damaged lipids in pheomelanotic mouse skin, which is indicative of general oxidative stress. Measuring the total antioxidant capacity of cells in the presence and absence of pheomelanin could provide further evidence that pheomelanin alters the oxidative state of melanocytes.
Just how does pheomelanin generate ROS?
If pheomelanin-generated ROS are the culprit behind pheomelanin-related carcinogenicity, the question arises: what about the structure of pheomelanin causes ROS production? Currently, it is unknown whether pheomelanin needs any input of energy (for example, in the form of electromagnetic radiation) to generate ROS. As previously stated, the ability of pheomelanin to generate ROS in response to UVA is widely cited [5]. In the presence of zinc, pheomelanin can generate ROS when stimulated by visible light as well [15]. On the higher-energy end of the electromagnetic spectrum, ionizing nuclear radiation may also interact with pheomelanin. For example, among birds exposed to radiation at Chernobyl, population declines were more severe among pheomelanotic birds, suggesting that pheomelanin might be toxic when combined with nuclear radiation [16].
Evidence exists that pheomelanin may generate ROS in the absence of electromagnetic radiation as well. One study found that pheomelanotic guinea pigs experienced more ototoxicity (cellular damage from noise) than eumelanotic or albino guinea pigs [17]. The authors of this study hypothesized that pheomelanin may generate ROS upon interacting with noise. However, the result in this study may have been confounded by the use of the drug chloroquine for ototoxicity priming. Pheomelanin is known to bind chloroquine, so the increase in noise damage in pheomelanotic guinea pigs may be attributable to an effect of chloriquine rather than pheomelanin [18]. Nevertheless, in our study, pheomelanin appeared to be carcinogenic without any energy input. Our mice were not exposed to UV or radiation of a higher frequency, and their skin was largely shielded from ambient visible light by fur. Further research on ROS generation by pheomelanin under diverse conditions is necessary to determine whether any energy input is necessary.
Action spectra—or plots of the rate of a physiological activity versus input radiation wavelength—are useful tools for discovering which input wavelengths drive reactions such as radical formation. Development of pheomelanin action spectra has been challenged by uncertainties about the composition of pheomelanin samples and conditions under which spectra should be recorded. Pheomelanin naturally exists as a heterogenous mixture of oligomers of benzothiazine and benzothiazole constituents, but the combination of these constituents varies from sample to sample and is generally poorly understood [19]. For example, the composition of pheomelanin is known to be dependent on ion concentration, but this dependence is poorly characterized [20]. Different constituents of pheomelanin are hypothesized to be responsible for different activities of the pigment (i.e., one portion of the molecule is responsible for light emission, one portion is responsible for oxygen reactivity, etc.), so studying the pigment in a physiologically relevant conformation is essential for obtaining meaningful action spectra [21]. Therefore, better understanding the makeup of pheomelanin as it exists in redhead melanocytes is a prerequisite to studying how pheomelanin interacts with radiation.
Hypothesis II. Pheomelanin synthesis leads to glutathione depletion
An explanation for pheomelanin’s carcinogenicity that concerns pheomelanin synthesis, rather than presence, circumvents the question of how pheomelanin could physically generate ROS. Our study did not clarify whether it is the synthesis or the presence of pheomelanin that predisposes redhead mice to melanoma. Pheomelanin synthesis differs from eumelanin synthesis in that pheomelanin incorporates cysteine into its structure. A major cellular store of cysteine is the molecule glutathione, which is also the most important cellular antioxidant. It is possible that pheomelanin synthesis depletes glutathione stores, which could make melanocytes more susceptible to oxidative damage and carcinogenesis. By this hypothesis, it is not the presence of pheomelanin that is carcinogenic, but rather the absence of glutathione that its synthesis causes.
The glutathione depletion hypothesis has been used to explain other phenomena in animals with pheomelanotic coloring. For example, in wild boars, pheomelanotic coat color is associated with increased levels of oxidative stress in muscle cells and lower muscle glutathione levels [22]. The authors of this study hypothesized that pheomelanin production in the coat globally taxed the boars’ glutathione reserves. A large-scale study of bird species found that pheomelanotic plumage was strongly negatively correlated with brain size [23]. Here, the authors hypothesized that the glutathione tax caused by pheomelanin production limited other oxidatively-costly activities like brain development. As previously discussed, the hypothesis presented by these authors is not the only possibility, as these studies are unable to distinguish between glutathione depletion from pheomelanin synthesis and greater glutathione use due to pheomelanin-caused ROS.
It has been speculated that pheomelanin production evolved as an excretory system for excess cysteine [24]. This hypothesis assumes that under some circumstances, depletion of cysteine is desired. If this hypothesis is true, pheomelanin production is a double-edged sword; in circumstances where cysteine depletion is desired, pheomelanin production is protective, but in circumstances (such as in redhead melanocytes) where when cysteine depletion is not desired, it is a hazard.
The glutathione depletion hypothesis could be tested by measuring gluthathione levels in redhead melanocytes and albino redhead melanocytes, since ablation of pigment production should restore glutathione levels. It could also be tested by measuring indicators of oxidative stress in naturally pheomelanotic cells and comparing those indicators to levels measured in cells which do not produce, but are supplemented with, pheomelanin. Such an experiment would require engineering of melanocytes to reverse their normal melanosome traffic and uptake intact melanosomes into their cytosol. If pheomelanin supplementation does not increase oxidative stress in melanocytes, the synthesis of pheomelanin is likely the carcinogenic culprit.
If glutathione depletion is to blame for pheomelanin’s carcinogenicity, antioxidant supplementation may prove to be a remedy for increased redhead melanoma risk. However, supplementation with a thiol-containing antioxidant such as glutathione may simply feed into the pheomelanin synthesis pathway. In this case, non-thiol antioxidants, such as vitamin E, may be effective where thiol antioxidants are not.
Conclusion
In summary, we have presented two possible explanations for the carcinogenicity of pheomelanin. On one hand, pheomelanin might generate ROS that lead indirectly or directly to DNA damange. On the other hand, pheomelanin synthesis might tax melanocytes’ antioxidant capacites by consuming glutathione stores. It should be noted that these hypotheses are not mutually exclusive, and may act in concert to produce the observed carcinogenic effects of pheomelanin. It should also be noted that the first hypothesis assumes that pheomelanin is able to generate ROS. While this has been proven in response to UVA irradiation, the absolute energy input requirements for ROS generation have not been determined. Further research on the mechanism of pheomelanin’s carcinogenicity should provide insights as to how redheads might prevent melanoma development.
Abbreviations
- MC1R
melanocortin-1 receptor
- ROS
reactive oxygen species
- UVA
Ultraviolet A
- Å
angstroms
- cdA
8,5-cyclo-2′-deoxyadenosine
- cdG
8,5-cyclo-2′-deoxyguanosine
- DHI
5,6-dihydroxyindole
- DHICA
5,6-dihydroxyindole-2-carboxylic acid
References
- 1.Rhodes AR, Weinstock MA, Fitzpatrick TB, Mihm MC, Jr., Sober AJ. Risk factors for cutaneous melanoma: a practical method of recognizing predisposed individuals. JAMA : the journal of the American Medical Association. 1987;258:3146–54. [PubMed] [Google Scholar]
- 2.Mitra D, Luo X, Morgan A, Wang J, Hoang MP. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491:449–53. doi: 10.1038/nature11624. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito S. A chemist’s view of melanogenesis. Pigment cell research. 2003;16:230–6. doi: 10.1034/j.1600-0749.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 4.Flanagan N, Healy E, Ray A, Philips S, Todd C. Pleiotropic effects of the melanocortin 1 receptor (MC1R) gene on human pigmentation. Human molecular genetics. 2000;9:2531–7. doi: 10.1093/hmg/9.17.2531. others. [DOI] [PubMed] [Google Scholar]
- 5.Ranadive NS, Shirwadkar S, Persad S, Menon IA. Effects of melanin-induced free radicals on the isolated rat peritoneal mast cells. The Journal of investigative dermatology. 1986;86:303–7. doi: 10.1111/1523-1747.ep12285496. [DOI] [PubMed] [Google Scholar]
- 6.Cheli Y, Luciani F, Khaled M, Beuret L, Bille K. {alpha}MSH and Cyclic AMP elevating agents control melanosome pH through a protein kinase A-independent mechanism. The Journal of biological chemistry. 2009;284:18699–706. doi: 10.1074/jbc.M109.005819. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ancans J, Tobin DJ, Hoogduijn MJ, Smit NP, Wakamatsu K. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma Cells. Experimental cell research. 2001;268:26–35. doi: 10.1006/excr.2001.5251. others. [DOI] [PubMed] [Google Scholar]
- 8.Ye T, Hong L, Garguilo J, Pawlak A, Edwards GS. Photoionization thresholds of melanins obtained from free electron laser-photoelectron emission microscopy, femtosecond transient absorption spectroscopy and electron paramagnetic resonance measurements of oxygen photoconsumption. Photochemistry and photobiology. 2006;82:733–7. doi: 10.1562/2006-01-02-RA-762. others. [DOI] [PubMed] [Google Scholar]
- 9.Samokhvalov A. Heterogeneous photocatalytic reactions of sulfur aromatic compounds. Chemphyschem. 2011;12:2870–85. doi: 10.1002/cphc.201100101. [DOI] [PubMed] [Google Scholar]
- 10.Pryor WA. Free radial biology: xenobiotics, cancer, and aging. Annals of the New York Academy of Sciences. 1982;393:1–22. doi: 10.1111/j.1749-6632.1982.tb31228.x. [DOI] [PubMed] [Google Scholar]
- 11.Thureau P, Ziarelli F, Thévand A, Martin RW, Farmer PJ. Probing the motional behavior of eumelanin and pheomelanin with solid-state NMR spectroscopy: new insights into the pigment properties. Chemistry. 2012;18:10689–700. doi: 10.1002/chem.201200277. others. [DOI] [PubMed] [Google Scholar]
- 12.Meirowsky E, Freeman LW. Chromatin-melanin eelationships in malignant melanomata. The Journal of investigative dermatology. 1951;16:257–60. doi: 10.1038/jid.1951.32. [DOI] [PubMed] [Google Scholar]
- 13.Yuan B, Wang J, Cao H, Sun R, Wang Y. High-throughput analysis of the mutagenic and cytotoxic properties of DNA lesions by next-generation sequencing. Nucleic acids research. 2011;39:5945–54. doi: 10.1093/nar/gkr159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuraoka I, Bender C, Romieu A, Cadet J, Wood RD. Removal of oxygen free-radical-induced 5,8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proceedings of the National Academy of Sciences. 2000;97:3832–7. doi: 10.1073/pnas.070471597. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panzella L, Szewczyk G, D’Ischia M, Napolitano A, Sarna T. Zinc-induced structural effects enhance oxygen consumption and superoxide generation in synthetic pheomelanins on UVA/visible light irradiation. Photochemistry and photobiology. 2010;86:757–64. doi: 10.1111/j.1751-1097.2010.00726.x. [DOI] [PubMed] [Google Scholar]
- 16.Galván I, Mousseau TA, Moller AP. Bird population declines due to radiation exposure at Chernobyl are stronger in species with pheomelanin-based coloration. Oecologia. 2011;165:827–35. doi: 10.1007/s00442-010-1860-5. [DOI] [PubMed] [Google Scholar]
- 17.Barrenas ML, Holgers KM. Ototoxic interaction between noise and pheomelanin: distortion product otoacoustic emissions after acoustical trauma in chloroquine-treated red, black, and albino guinea pigs. Audiology : official organ of the International Society of Audiology. 2000;39:238–46. [PubMed] [Google Scholar]
- 18.Mars U, Larsson BS. Pheomelanin as a binding site for drugs and chemicals. Pigment cell research. 1999;12:266–74. doi: 10.1111/j.1600-0749.1999.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 19.Huijser A, Pezzella A, Sundstrom V. Functionality of epidermal melanin pigments: current knowledge on UV-dissipative mechanisms and research perspectives. Physical chemistry chemical physics. 2011;13:9119–27. doi: 10.1039/c1cp20131j. [DOI] [PubMed] [Google Scholar]
- 20.Tesema YT, Pham DM, Franz KJ. Counterions influence reactivity of metal ions with cysteinyldopa model compounds. Inorganic chemistry. 2007;47:1087–95. doi: 10.1021/ic701889w. [DOI] [PubMed] [Google Scholar]
- 21.Ye T, Pawlak A, Sarna T, Simon JD. Different molecular constituents in pheomelanin are responsible for emission, transient absorption and oxygen photoconsumption. Photochemistry and photobiology. 2008;84:437–43. doi: 10.1111/j.1751-1097.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- 22.Galván I, Alonso-Alvarez C, Negro JJ. Relationships between hair melanization, glutathione levels, and senescence in wild boars. Physiological and biochemical zoology. 2012;85:332–47. doi: 10.1086/666606. [DOI] [PubMed] [Google Scholar]
- 23.Galván I, Moller AP. Brain size and the expression of pheomelanin-based colour in birds. Journal of evolutionary biology. 2011;24:999–1006. doi: 10.1111/j.1420-9101.2011.02232.x. [DOI] [PubMed] [Google Scholar]
- 24.Galván I, Ghanem G, Møller AP. Has removal of excess cysteine led to the evolution of pheomelanin? BioEssays. 2012;34:565–8. doi: 10.1002/bies.201200017. [DOI] [PubMed] [Google Scholar]
- 25.Ito S, Wakamatsu K. Diversity of human hair pigmentation as studied by chemical analysis of eumelanin and pheomelanin. Journal of the European Academy of Dermatology and Venereology. 2011;25:1369–80. doi: 10.1111/j.1468-3083.2011.04278.x. [DOI] [PubMed] [Google Scholar]