Abstract
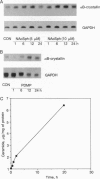
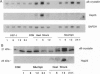
Ceramide has been identified as a potential second messenger that may mediate cell differentiation and apoptosis after exposure to hormonal agonists such as 1 alpha, 25-dihydroxyvitamin D3, tumor necrosis factor alpha, or gamma-interferon. The secondary cellular events that follow ceramide generation remain undefined. We report that in NIH WT-3T3 cells, ceramide induces an enhancement of gene transcription of alpha B-crystallin, a small heat shock protein. The levels of alpha B-crystallin, as measured by Northern blot and immunoblot analyses, were increased by the addition of an exogenous short-chain ceramide, N-acetylsphingosine, or by increasing endogenous intracellular ceramide by inhibition of glucosylceramide synthase. Similar effects were not seen in the expression of the closely related gene, Hsp25. To ascertain whether ceramide-mediated gene transcription was a feature of the heat shock response, cell ceramide was measured in heat shocked cells and observed to be elevated 2-fold immediately upon the return of cells to 37 degrees C. Thus ceramide formed after heat shock treatment of 3T3 cells may mediate the transcription events associated with the cell stress response.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama A., Fröhli E., Schäfer R., Klemenz R. Alpha B-crystallin expression in mouse NIH 3T3 fibroblasts: glucocorticoid responsiveness and involvement in thermal protection. Mol Cell Biol. 1993 Mar;13(3):1824–1835. doi: 10.1128/mcb.13.3.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou L. R., Chao C. P., Holness M. A., Barker S. C., Raghow R. Interleukin-1-mediated PGE2 production and sphingomyelin metabolism. Evidence for the regulation of cyclooxygenase gene expression by sphingosine and ceramide. J Biol Chem. 1992 Oct 5;267(28):20044–20050. [PubMed] [Google Scholar]
- Barenholz Y., Thompson T. E. Sphingomyelins in bilayers and biological membranes. Biochim Biophys Acta. 1980 Sep 30;604(2):129–158. doi: 10.1016/0005-2736(80)90572-6. [DOI] [PubMed] [Google Scholar]
- Betts J. C., Agranoff A. B., Nabel G. J., Shayman J. A. Dissociation of endogenous cellular ceramide from NF-kappa B activation. J Biol Chem. 1994 Mar 18;269(11):8455–8458. [PubMed] [Google Scholar]
- Dobrowsky R. T., Kamibayashi C., Mumby M. C., Hannun Y. A. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993 Jul 25;268(21):15523–15530. [PubMed] [Google Scholar]
- Dobrowsky R. T., Werner M. H., Castellino A. M., Chao M. V., Hannun Y. A. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994 Sep 9;265(5178):1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- Dressler K. A., Mathias S., Kolesnick R. N. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992 Mar 27;255(5052):1715–1718. doi: 10.1126/science.1313189. [DOI] [PubMed] [Google Scholar]
- Dérijard B., Hibi M., Wu I. H., Barrett T., Su B., Deng T., Karin M., Davis R. J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994 Mar 25;76(6):1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Goldkorn T., Dressler K. A., Muindi J., Radin N. S., Mendelsohn J., Menaldino D., Liotta D., Kolesnick R. N. Ceramide stimulates epidermal growth factor receptor phosphorylation in A431 human epidermoid carcinoma cells. Evidence that ceramide may mediate sphingosine action. J Biol Chem. 1991 Aug 25;266(24):16092–16097. [PubMed] [Google Scholar]
- Gopal-Srivastava R., Piatigorsky J. The murine alpha B-crystallin/small heat shock protein enhancer: identification of alpha BE-1, alpha BE-2, alpha BE-3, and MRF control elements. Mol Cell Biol. 1993 Nov;13(11):7144–7152. doi: 10.1128/mcb.13.11.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Campbell D., Dérijard B., Davis R. J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995 Jan 20;267(5196):389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- Head M. W., Corbin E., Goldman J. E. Coordinate and independent regulation of alpha B-crystallin and hsp27 expression in response to physiological stress. J Cell Physiol. 1994 Apr;159(1):41–50. doi: 10.1002/jcp.1041590107. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantorow M., Piatigorsky J. Alpha-crystallin/small heat shock protein has autokinase activity. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3112–3116. doi: 10.1073/pnas.91.8.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. Y., Linardic C., Obeid L., Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991 Jan 5;266(1):484–489. [PubMed] [Google Scholar]
- Klemenz R., Fröhli E., Steiger R. H., Schäfer R., Aoyama A. Alpha B-crystallin is a small heat shock protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J. M., Banerjee P., Nikolakaki E., Dai T., Rubie E. A., Ahmad M. F., Avruch J., Woodgett J. R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994 May 12;369(6476):156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Murano S., Thweatt R., Shmookler Reis R. J., Jones R. A., Moerman E. J., Goldstein S. Diverse gene sequences are overexpressed in werner syndrome fibroblasts undergoing premature replicative senescence. Mol Cell Biol. 1991 Aug;11(8):3905–3914. doi: 10.1128/mcb.11.8.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Abe A., Balazovich K. J., Wu D., Suchard S. J., Boxer L. A., Shayman J. A. Ceramide regulates oxidant release in adherent human neutrophils. J Biol Chem. 1994 Jul 15;269(28):18384–18389. [PubMed] [Google Scholar]
- Obeid L. M., Linardic C. M., Karolak L. A., Hannun Y. A. Programmed cell death induced by ceramide. Science. 1993 Mar 19;259(5102):1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Bell R. M., Hannun Y. A. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989 Nov 15;264(32):19076–19080. [PubMed] [Google Scholar]
- Rani C. S., Abe A., Chang Y., Rosenzweig N., Saltiel A. R., Radin N. S., Shayman J. A. Cell cycle arrest induced by an inhibitor of glucosylceramide synthase. Correlation with cyclin-dependent kinases. J Biol Chem. 1995 Feb 10;270(6):2859–2867. doi: 10.1074/jbc.270.6.2859. [DOI] [PubMed] [Google Scholar]
- Sax C. M., Piatigorsky J. Expression of the alpha-crystallin/small heat-shock protein/molecular chaperone genes in the lens and other tissues. Adv Enzymol Relat Areas Mol Biol. 1994;69:155–201. doi: 10.1002/9780470123157.ch5. [DOI] [PubMed] [Google Scholar]
- Schütze S., Potthoff K., Machleidt T., Berkovic D., Wiegmann K., Krönke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992 Nov 27;71(5):765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- Shayman J. A., Deshmukh G. D., Mahdiyoun S., Thomas T. P., Wu D., Barcelon F. S., Radin N. S. Modulation of renal epithelial cell growth by glucosylceramide. Association with protein kinase C, sphingosine, and diacylglycerol. J Biol Chem. 1991 Dec 5;266(34):22968–22974. [PubMed] [Google Scholar]
- Venable M. E., Blobe G. C., Obeid L. M. Identification of a defect in the phospholipase D/diacylglycerol pathway in cellular senescence. J Biol Chem. 1994 Oct 21;269(42):26040–26044. [PubMed] [Google Scholar]
- Vunnam R. R., Radin N. S. Analogs of ceramide that inhibit glucocerebroside synthetase in mouse brain. Chem Phys Lipids. 1980 Apr;26(3):265–278. doi: 10.1016/0009-3084(80)90057-2. [DOI] [PubMed] [Google Scholar]
- Wilks A. F. Two putative protein-tyrosine kinases identified by application of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1603–1607. doi: 10.1073/pnas.86.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]