Abstract
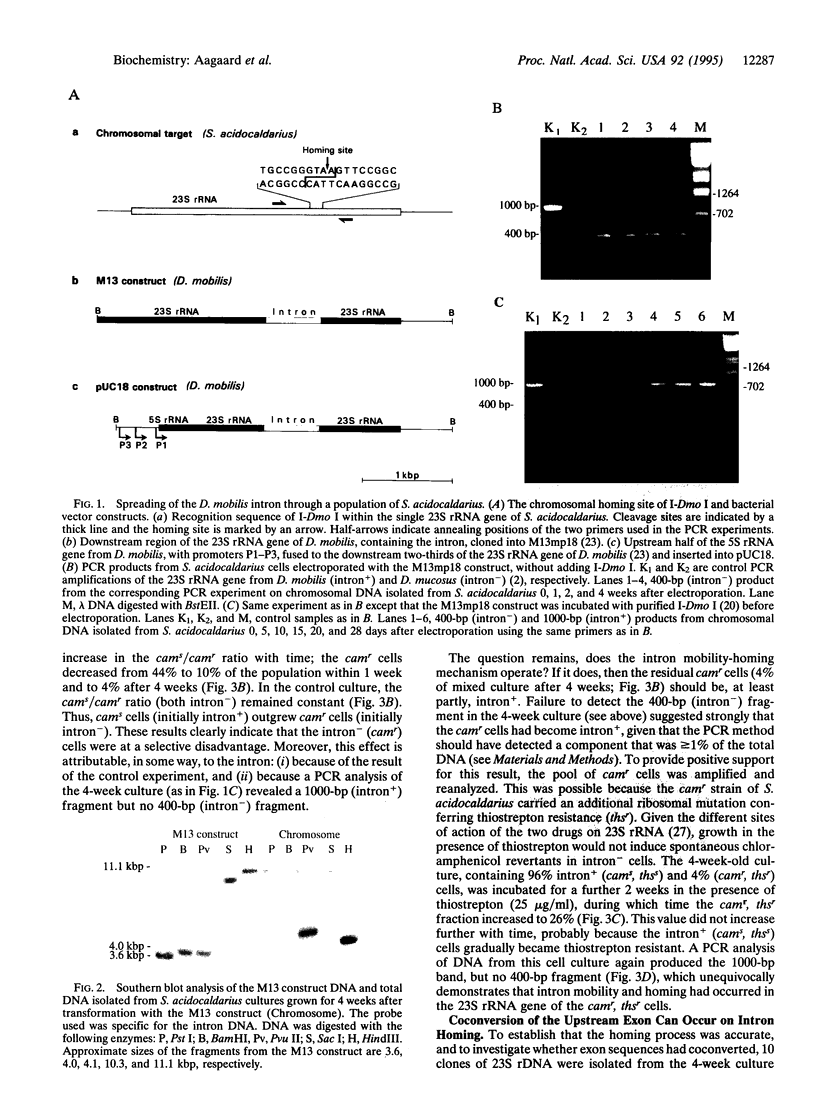
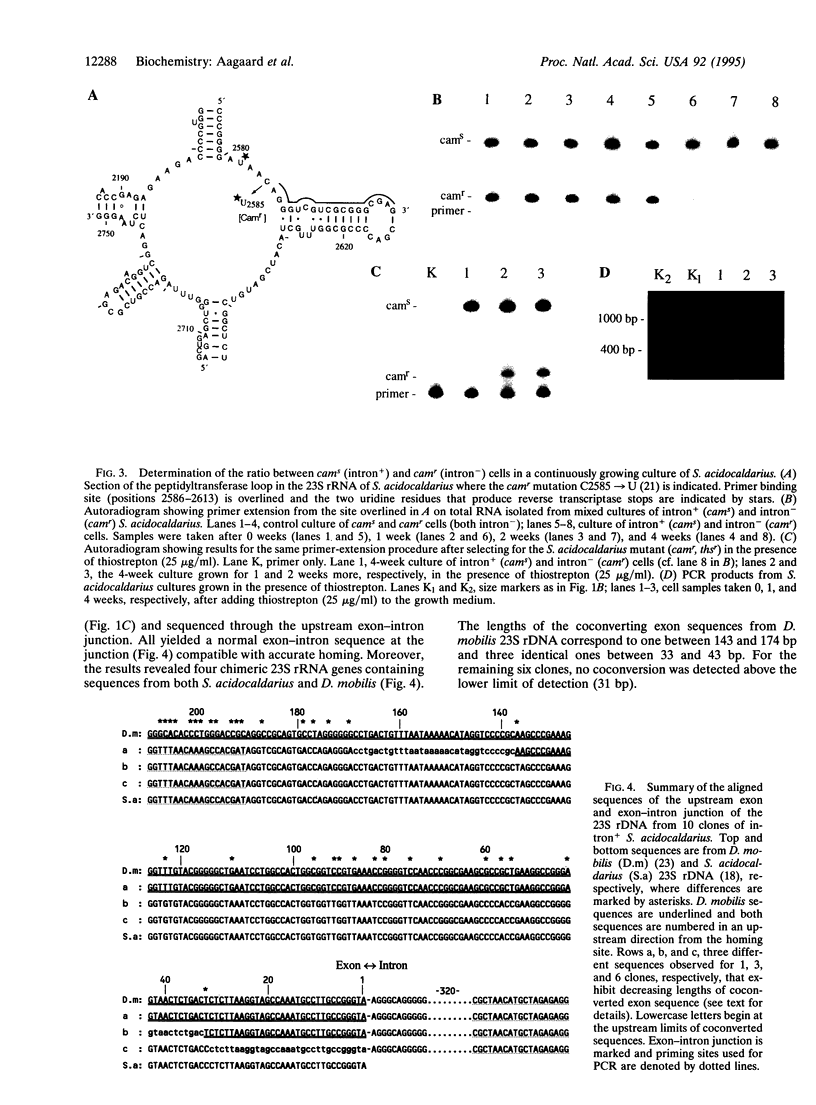
Some intron-containing rRNA genes of archaea encode homing-type endonucleases, which facilitate intron insertion at homologous sites in intron- alleles. These archaeal rRNA genes, in contrast to their eukaryotic counterparts, are present in single copies per cell, which precludes intron homing within one cell. However, given the highly conserved nature of the sequences flanking the intron, homing may occur in intron- rRNA genes of other archaeal cells. To test whether this occurs, the intron-containing 23S rRNA gene of the archaeal hyperthermophile Desulfurococcus mobilis, carried on nonreplicating bacterial vectors, was electroporated into an intron- culture of Sulfolobus acidocaldarius. PCR experiments demonstrated that the intron underwent homing and spread through the culture. By using a double drug-resistant mutant of S. acidocaldarius, it was shown that spreading resulted partly from a selective advantage of intron+ cells and partly from intercellular mobility of the intron and homing.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aagaard C., Phan H., Trevisanato S., Garrett R. A. A spontaneous point mutation in the single 23S rRNA gene of the thermophilic arachaeon Sulfolobus acidocaldarius confers multiple drug resistance. J Bacteriol. 1994 Dec;176(24):7744–7747. doi: 10.1128/jb.176.24.7744-7747.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D., Brock K. M., Belly R. T., Weiss R. L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84(1):54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- Burggraf S., Larsen N., Woese C. R., Stetter K. O. An intron within the 16S ribosomal RNA gene of the archaeon Pyrobaculum aerophilum. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2547–2550. doi: 10.1073/pnas.90.6.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgaard J. Z., Garrett R. A., Belfort M. A site-specific endonuclease encoded by a typical archaeal intron. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5414–5417. doi: 10.1073/pnas.90.12.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgaard J. Z., Garrett R. A., Belfort M. Purification and characterization of two forms of I-DmoI, a thermophilic site-specific endonuclease encoded by an archaeal intron. J Biol Chem. 1994 Nov 18;269(46):28885–28892. [PubMed] [Google Scholar]
- Dalgaard J. Z., Garrett R. A. Protein-coding introns from the 23S rRNA-encoding gene form stable circles in the hyperthermophilic archaeon Pyrobaculum organotrophum. Gene. 1992 Nov 2;121(1):103–110. doi: 10.1016/0378-1119(92)90167-n. [DOI] [PubMed] [Google Scholar]
- Dalgaard J. Z. Mobile introns and inteins: friend or foe? Trends Genet. 1994 Sep;10(9):306–307. doi: 10.1016/0168-9525(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Dujon B. Group I introns as mobile genetic elements: facts and mechanistic speculations--a review. Gene. 1989 Oct 15;82(1):91–114. doi: 10.1016/0378-1119(89)90034-6. [DOI] [PubMed] [Google Scholar]
- Garrett R. A., Dalgaard J., Larsen N., Kjems J., Mankin A. S. Archaeal rRNA operons. Trends Biochem Sci. 1991 Jan;16(1):22–26. doi: 10.1016/0968-0004(91)90011-j. [DOI] [PubMed] [Google Scholar]
- Kaine B. P., Gupta R., Woese C. R. Putative introns in tRNA genes of prokaryotes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3309–3312. doi: 10.1073/pnas.80.11.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjems J., Garrett R. A. Novel expression of the ribosomal RNA genes in the extreme thermophile and archaebacterium Desulfurococcus mobilis. EMBO J. 1987 Nov;6(11):3521–3530. doi: 10.1002/j.1460-2075.1987.tb02678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjems J., Garrett R. A. Novel splicing mechanism for the ribosomal RNA intron in the archaebacterium Desulfurococcus mobilis. Cell. 1988 Aug 26;54(5):693–703. doi: 10.1016/s0092-8674(88)80014-x. [DOI] [PubMed] [Google Scholar]
- Kjems J., Garrett R. A. Ribosomal RNA introns in archaea and evidence for RNA conformational changes associated with splicing. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):439–443. doi: 10.1073/pnas.88.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjems J., Jensen J., Olesen T., Garrett R. A. Comparison of transfer RNA and ribosomal RNA intron splicing in the extreme thermophile and archaebacterium Desulfurococcus mobilis. Can J Microbiol. 1989 Jan;35(1):210–214. doi: 10.1139/m89-033. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Belfort M. Introns as mobile genetic elements. Annu Rev Biochem. 1993;62:587–622. doi: 10.1146/annurev.bi.62.070193.003103. [DOI] [PubMed] [Google Scholar]
- Leviev I., Levieva S., Garrett R. A. Role for the highly conserved region of domain IV of 23S-like rRNA in subunit-subunit interactions at the peptidyl transferase centre. Nucleic Acids Res. 1995 May 11;23(9):1512–1517. doi: 10.1093/nar/23.9.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke-Andersen J., Garrett R. A. Structural characteristics of the stable RNA introns of archaeal hyperthermophiles and their splicing junctions. J Mol Biol. 1994 Nov 11;243(5):846–855. doi: 10.1006/jmbi.1994.1687. [DOI] [PubMed] [Google Scholar]
- Lykke-Andersen J., Thi-Ngoc H. P., Garrett R. A. DNA substrate specificity and cleavage kinetics of an archaeal homing-type endonuclease from Pyrobaculum organotrophum. Nucleic Acids Res. 1994 Nov 11;22(22):4583–4590. doi: 10.1093/nar/22.22.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidak B. L., Larsen N., McCaughey M. J., Overbeek R., Olsen G. J., Fogel K., Blandy J., Woese C. R. The Ribosomal Database Project. Nucleic Acids Res. 1994 Sep;22(17):3485–3487. doi: 10.1093/nar/22.17.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin A. S., Leviev I., Garrett R. A. Cross-hypersensitivity effects of mutations in 23 S rRNA yield insight into aminoacyl-tRNA binding. J Mol Biol. 1994 Nov 25;244(2):151–157. doi: 10.1006/jmbi.1994.1715. [DOI] [PubMed] [Google Scholar]
- Muscarella D. E., Vogt V. M. A mobile group I intron from Physarum polycephalum can insert itself and induce point mutations in the nuclear ribosomal DNA of saccharomyces cerevisiae. Mol Cell Biol. 1993 Feb;13(2):1023–1033. doi: 10.1128/mcb.13.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine I., Tchelet R., Mevarech M. The mechanism of DNA transfer in the mating system of an archaebacterium. Science. 1989 Sep 22;245(4924):1387–1389. doi: 10.1126/science.2818746. [DOI] [PubMed] [Google Scholar]
- Sigmund C. D., Ettayebi M., Borden A., Morgan E. A. Antibiotic resistance mutations in ribosomal RNA genes of Escherichia coli. Methods Enzymol. 1988;164:673–690. doi: 10.1016/s0076-6879(88)64077-8. [DOI] [PubMed] [Google Scholar]
- Thompson L. D., Daniels C. J. A tRNA(Trp) intron endonuclease from Halobacterium volcanii. Unique substrate recognition properties. J Biol Chem. 1988 Dec 5;263(34):17951–17959. [PubMed] [Google Scholar]
- Thompson L. D., Daniels C. J. Recognition of exon-intron boundaries by the Halobacterium volcanii tRNA intron endonuclease. J Biol Chem. 1990 Oct 25;265(30):18104–18111. [PubMed] [Google Scholar]





